Abstract
Coagulation proteases have been suggested to trigger a diversity of inflammatory responses in addition to their critical role in the coagulation cascade. It has been well established that the inflammatory and coagulation pathways are invariably linked. However, the mechanisms through which coagulation protease factor Xa (FXa) causes inflammation remain unclear. Thus, we assessed the pro-inflammatory effects of FXa in RAW 264.7 macrophages. We show that FXa elicits signal transduction in RAW 264.7 macrophages. FXa-induced signal transduction was dependent on the activation of protease-activated receptor 2 (PAR-2), PAR-2 desensitization but not PAR-1 desensitization abolished FXa-induced ERK1/2 phosphorylation. The PAR-2-dependent cellular effects of FXa led to the expression of pro-inflammatory cytokines IL-6, IL-8, TNF-α and IFN-γ in RAW 264.7 macrophages. Furthermore, a specific inhibitor of the ERK1/2 pathway, U0126, decreased the FXa-induced pro-inflammatory cytokines expression significantly. Taken together, our data indicate that FXa induces PAR-2-dependent pro-inflammatory activity in RAW 264.7 macrophages through the ERK1/2 pathway.
Keywords: Factor Xa, inflammation, protease-activated receptor 2, ERK1/2 pathway
Introduction
Inflammation is a physiological response of a body to stimuli, including infections and tissue injury, and protects a body from these inflammatory stimuli [1]. Macrophages play critical roles in immune reaction, allergy, and inflammation. These cells induce inflammatory reaction, and initiate and maintain specific immune responses by releasing different types of cytokines [2,3]. Over-expression of the inflammatory mediators in macrophages is involved in many inflammation related diseases, such as atherosclerosis, rheumatoid arthritis, chronic obstructive pulmonary disease, and autoimmune diabetes [4-6].
It has been well established that the inflammatory and coagulation pathways are invariably linked. Various coagulation proteases trigger a diversity of pro-inflammatory responses, in addition to their critical role in the coagulation cascade [7]. Such as factor Xa (FXa) and thrombin, can induce multiple cellular effects via activation of protease activated receptors (PARs). PARs are the family of G-protein–coupled receptors that are activated by proteolytic cleavage. PAR-1, -3, and -4 are cleaved by thrombin, whereas FXa can activate both PAR-1 and PAR-2 [8-11]. It has been demonstrated that coagulation protease-dependent activation of PARs contributes to inflammation in many vascular disorders [12-14]. FXa triggers signaling pathways involved in the regulation of inflammation, it can induce the expression of pro-inflammatory mediators, such as IL-6, IL-8, and MCP-1 [15].
PAR-2 occupies a crucial position in inflammation and regulates vascular function [16,17]. Pro-inflammatory mediators, such as TNF-α and IFN-γ, can induce the expression of PAR-2, in turn, PAR-2 activation promotes the production of TNF-α, IFN-γ, IL-8 and IL-18 in various cell types [18]. Indeed, The expression of IFN-γ and IL-18 is significantly decreased in PAR-2 knockout mice [18,19], whereas endotoxin-stimulated macrophages show significantly greater IL-10 expression and enhanced IL-4 secretion in PAR-2 knockout mice [20,21].
Taken together, these data support the hypothesis that FXa-induced intracellular signaling is associated with inflammation. Therefore, in this study, we sought to investigate the pro-inflammatory effect of FXa in RAW 264.7 macrophages. We showed that FXa enhanced the expression of pro-inflammatory cytokines in RAW 264.7 macrophages. The pro-inflammatory effect of FXa in RAW 264.7 macrophages was found to be largely dependent on PAR-2 activation through the ERK1/2 pathway.
Materials and methods
Cell culture
The murine macrophage cell line RAW 264.7 was purchased from Lonza (Walkersville, USA). RAW 264.7 macrophages were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 100 μg/ml of penicillin, 100 μg/ml of streptomycin and 10% fetal bovine serum (FBS). The cells were incubated in an atmosphere of 5% CO2 at 37°C and were routinely passaged every 3 days. For all experiments, cells were washed twice with phosphate-buffered saline (PBS), serum-starved in DMEM with 0.1% FBS for 24 hours and subsequently stimulated as described.
Reagents
DMEM, FBS and PBS were obtained from Invitrogen (Carlsbad, USA). Thrombin, Hirudin, ERK1/2 inhibitor U0126 and the antibiotics (penicillin and streptomycin) were purchased from Sigma-Aldrich (Shanghai, China). Human FXa was purchased from Merck Group (Darmstadt, Germany). Tick anticoagulant peptide (TAP) was purchased from American Custom Chemicals Corporation (San Diego, USA). PAR-1 neutralizing antibody (ATAP-2) and PAR-2 neutralizing antibody (SAM11) were obtained from Santa Cruz Biotechnology (Santa Cruz, USA). Antibodies: Phospho-p44/42, total p44/42 and β-actin antibodies were purchased from Cell Signaling Technology (Beverly, USA). Goat anti-mouse IRD 700 and goat IRD anti-rabbit 800 were purchased from LI-COR Biotechnology (Lincoln, USA).
Western blot
Cells were rinsed with ice-cold Dulbecco’s phosphate buffered saline (dPBS) and lysed in 100 ml of cell lysis buffer containing proteinase inhibitor cocktail and phosphatase-inhibitor cocktail followed by scraping with a cell scraper. Cell debris was removed by centrifugation (12000 rpm for 15 min) and protein was quantified by the Bradford method using bovine serum albumin as standard. Samples containing equal amounts of total cell protein were separated by 10% SDS-PAGE, and transferred onto a nitrocellulose membrane using the Bio-Rad wet transfer system at 100 v for 1 hour. The membranes were blocked with 2.5% skim milk in TBST (50 mM Tris, pH 7.6, 0.15 mM NaCl, 0.1% Tween 20) for 1 h at room temperature. Membranes were then incubated with antibodies against phospho-p44/42 MAPK, total p44/42 MAPK over night at 4°C with gentle shaking. After several washes in TBST, membranes were incubated with goat anti-mouse IRD 700 and goat IRD anti-rabbit 800 for 1 hour at room temperature. Fluorescent signal was imaged using the Li-COR Odyssey Infrared imaging system (Li-COR biosciences, Lincoln, USA). Densitometry was used to quantify all bands. Membranes were then re-probed with mAb β-actin for 1 hour at room temperature and incubated with goat anti-mouse IRD 800 for 45 minutes. The relative levels of phospho-p44/42 MAPK is expressed as the ratio to β-actin.
RNA extraction and relative quantitative PCR assay
After the treatment, the total RNA of the RAW 264.7 macrophages was extracted with the TRIzol reagent (Invitrogen, Carlsbad, USA), according to the manufacturer’s guidelines. The RNA pellets were suspended in RNase-Free water, and the DNA contamination of the RNA was removed by DNase treatment (Promega, Madison, USA). cDNA was obtained from 4 μg of RNA by reverse transcription using AMV reverse transcriptase and the oligo-dT primer (300 pmol) in a total reaction volume of 40 μl. Relative quantitative PCR was performed to measure the mRNA levels of pro-inflammatory cytokines (IL-6, IL-8, TNF-α, and IFN-γ) using a SYBR green PCR Kit (Roche, Basel, Switzerland) and the ABI ViiA 7 instrument. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the reference gene.
Statistics
All statistical analyses were carried out using the statistical analysis software package SPSS 19. Data are presented as mean ± standard deviation. Two-tailed t-test was used to compare treatment groups with the controls. P<0.05 was considered as a statistical significance.
Results
PAR-1 and PAR-2 are expressed and functional in RAW 264.7 macrophages
As shown in Figure 1A, the RAW 264.7 macrophages used in this study constitutively expressed PAR-1 and PAR-2. As we all know, the activation of the cellular receptors is the essential condition for FXa-induced efficient signal transduction. Therefore, we next assessed whether the expression of PAR-1 and PAR-2 was involved in functional responses in RAW 264.7 macrophages. Cells were serum-starved for 24 hours, then stimulated with PBS (negative control), 1 U/mL thrombin (specific PAR-1 agonist) and 200 nmol/L trypsin (specific PAR-2 agonist), respectively. Finally, we examined the phosphorylation of ERK1/2, which is widely used as a surrogate marker for PAR-1 and PAR-2 activation [22]. As shown in Figure 1B, both thrombin and trypsin induced strong phosphorylation of ERK1/2 as compared to the negative control. Hence, we conclude that both PAR-1 and PAR-2 are functionally active in RAW 264.7 macrophages.
Figure 1.
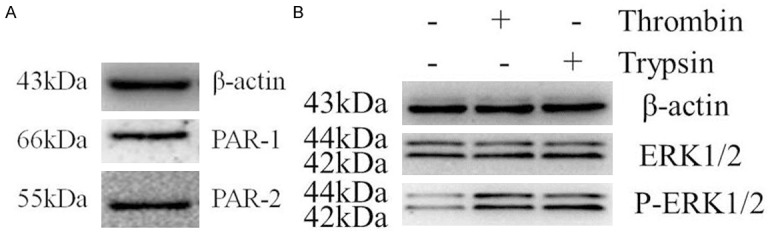
PAR-1 and PAR-2 are expressed and functional in RAW 264.7 macrophages. A. Western blot analysis on cell lysates of RAW 264.7 macrophages shows that macrophages constitutively express PAR-1 and PAR-2. B. Serum-starved cells (24 hours) treated with PBS, thrombin (1 U/mL), or trypsin (200 nmol/L) for 30 minutes. Western blot analysis of phospho-ERK1/2 indicates that expression of PAR-1 and PAR-2 in macrophages was functional. ERK1/2 phosphorylation is used as a surrogate marker for PAR-1 and PAR-2 activation. Protein loading was verified and normalized using β-actin.
FXa elicits signal transduction in RAW 264.7 macrophages
We next determined whether FXa could induce signal transduction in RAW 264.7 Macrophages. We examined FXa-induced phosphorylation of ERK1/2 to assess the capacity of FXa. As shown in Figure 2, the phosphorylation of ERK1/2 was detectable after stimulation with 0.25 U/mL FXa, whereas maximal level was obtained at a concentration of 0.75 U/mL. To verify that FXa-induced ERK1/2 phosphorylation is specific, cells were pre-incubated with 200 nmol/L TAP (specific FXa inhibitor) or 100 nmol/L hirudin (specific thrombin inhibitor), then stimulated with 0.75 U/mL FXa. As shown in Figure 3, pretreatment with TAP, but not with hirudin, almost completely inhibited FXa-induced ERK1/2 phosphorylation. Hence, we conclude that FXa elicits signal transduction in RAW 264.7 macrophages and the intracellular signaling is specific, independent of thrombin formation.
Figure 2.
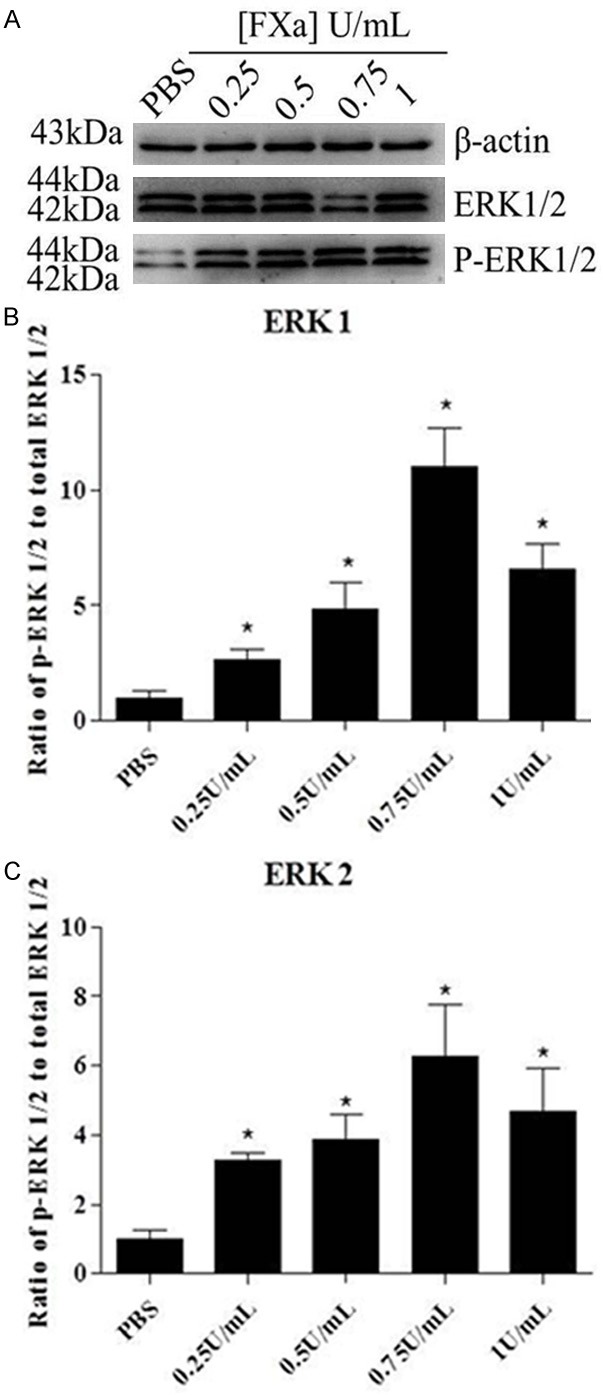
FXa elicits signal transduction in RAW 264.7 macrophages. Cells were exposed to PBS and different concentrations of FXa ranging from 0.25-1 U/mL for 30 minutes. Western blot analysis of phospho-ERK1/2 indicates that signal transduction was detectable after stimulation with 0.25 U/mL FXa, whereas maximal level was obtained at a concentration of 0.75 U/mL. Relative levels of ERK1/2 phosphorylation are expressed as a ratio of phospho-ERK1/2 to total ERK1/2. Protein loading was verified and normalized using β-actin. Data represent mean ± SEM. *P<0.05.
Figure 3.
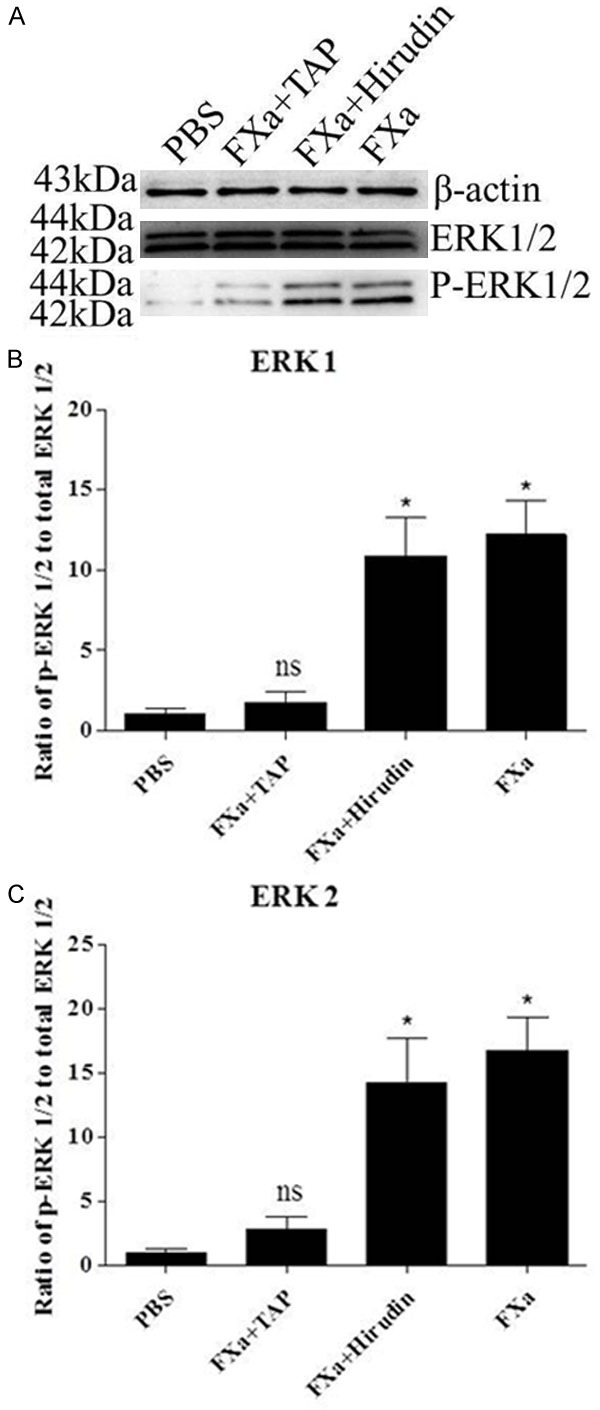
Specificity of FXa-induced ERK1/2 phosphorylation. Cells were pretreated with TAP (200 nmol/L) or hirudin (100 nmol/L) for 30 minutes prior to incubation with FXa (0.75 U/mL) for 30 minutes. Western blot analysis of phospho-ERK1/2 indicates that TAP but not hirudin almost completely inhibited FXa-induced ERK1/2 phosphorylation. Relative levels of ERK1/2 phosphorylation are expressed as a ratio of phospho-ERK1/2 to total ERK1/2. Protein loading was verified and normalized using β-actin. Data represent mean ± SEM. *P<0.05.
FXa signals via PAR-2 activation
We next determined which receptor was essential in FXa-induced signal transduction. First, we tested whether the activation of PAR-1 is required for FXa-induced signal transduction. Cells were pre-incubated with PBS (negative control) or 5 μg/mL ATAP2 (PAR-1 neutralizing antibody), then treated with 0.75 U/mL FXa or 1 U/mL thrombin. As shown in Figure 4, both FXa and thrombin induced the phosphorylation of ERK1/2 strongly as compared to the negative control. The PAR-1 neutralizing antibody didn’t impact the strong phosphorylation of ERK1/2 induced by FXa. In contrast, pretreatment with ATAP2 almost completely inhibited thrombin-induced ERK1/2 phosphorylation. These results suggest that PAR-1 activation is essential in thrombin-induced signal transduction, but not required for FXa-mediated signaling. Then, we identified whether PAR-2 was involved in FXa-induced signal transduction. To verify the specificity of FXa-mediated PAR-2 activation, we used 1 U/mL thrombin (high affinity ligand for PAR-1) and 200 nmol/L trypsin (high affinity ligand for PAR-2) to desensitize PAR-1 and PAR-2 prior to exposure to FXa. First, we verified the specificity of our desensitization experiments. As shown in Figure 5, PAR-1 desensitization with thrombin abolished subsequent stimulation by thrombin but not by trypsin. Reciprocally, PAR-2 desensitization with trypsin prevented subsequent ERK1/2 phosphorylation induced by trypsin but not by thrombin. Hence, we conclude that thrombin and trypsin can desensitize PAR-1 and PAR-2 specifically. Next, we assessed FXa-induced signal transduction in thrombin-desensitized or trypsin-desensitized cells. As shown in Figure 6, FXa still induced ERK1/2 phosphorylation in thrombin-desensitized cells. In contrast, trypsin-desensitized cells failed to respond to subsequent FXa stimulation, indicating that FXa and trypsin activate the same receptor, PAR-2. Based on above results, we conclude that PAR-2 mediates FXa-induced signal transduction in the RAW 264.7 macrophages.
Figure 4.
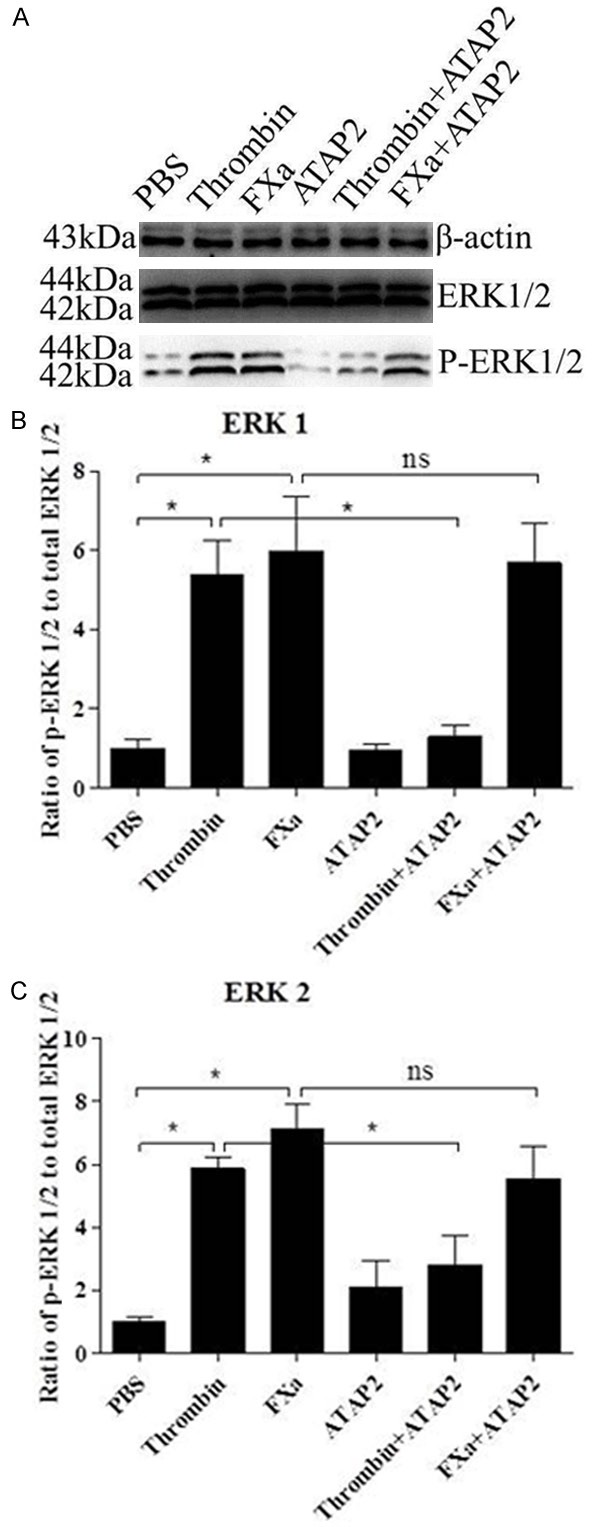
FXa-induced signal transduction is not mediated by PAR-1. Cells were incubated with the PAR-1 neutralizing antibody ATAP-2 (5 μg/mL) for 30 minutes prior to incubation with thrombin (1 U/mL) or FXa (0.75 U/mL) for 30 minutes. Western blot analysis of phospho-ERK1/2 indicates that both FXa and thrombin induced the phosphorylation of ERK1/2, moreover, PAR-1 neutralization didn’t impact the strong phosphorylation of ERK1/2 induced by FXa, but inhibited thrombin-induced ERK1/2 phosphorylation. Relative levels of ERK1/2 phosphorylation are expressed as a ratio of phospho-ERK1/2 to total ERK1/2. Protein loading was verified and normalized using β-actin. Data represent mean ± SEM. *P<0.05.
Figure 5.
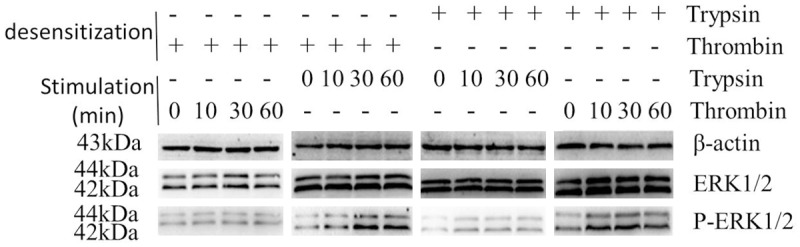
PAR-1 and PAR-2 desensitization. Cells were exposed to thrombin (1 U/mL, PAR-1 desensitization) or trypsin (200 nmol/L, PAR-2 desensitization) for 150 minutes, subsequently stimulated with 1 U/mL thrombin or 200 nmol/L trypsin for the indicated time points. Western blot analysis of phospho-ERK1/2 indicates that thrombin and trypsin can desensitize PAR-1 and PAR-2 specifically. Protein loading was verified and normalized using β-actin.
Figure 6.
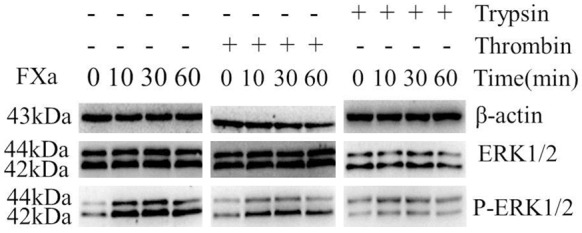
FXa-induced signal transduction is mediated by PAR-2. Cells were exposed to PBS, thrombin (1 U/mL, PAR-1 desensitization) or trypsin (200 nmol/L, PAR-2 desensitization) for 150 minutes prior to stimulation with 0.75 U/mL FXa for the indicated time points. Western blot analysis of phospho-ERK1/2 indicates that FXa induced ERK1/2 phosphorylation in PAR-1 desensitization cells, but not PAR-2 desensitization cells. Protein loading was verified and normalized using β-actin.
FXa enhances the expression of pro-inflammatory mediators
To determine the potential pro-inflammatory effect of FXa, the gene expression of TNF-α, IFN-γ, IL-6 and IL-8 in RAW 264.7 macrophages stimulated with FXa were determined. As shown in Figure 7, FXa enhanced the gene expression of TNF-α, IFN-γ, IL-6 and IL-8 at a concentration of 0.75 U/mL. To verify that FXa-induced pro-inflammatory effect is specific, cells were pre-incubated with TAP for 2 hours and subsequently incubated with FXa for 24 hours. As shown in Figure 7, TAP pretreatment almost completely inhibited the FXa-induced expression of pro-inflammatory mediators. Furthermore, we verified the involvement of ERK1/2 in FXa-induced pro-inflammatory effect by pretreatment with the ERK1/2 inhibitor U0126. As shown in Figure 7, ERK1/2 inhibition abolished FXa-induced expression of pro-inflammatory mediators. To assess the role of PAR-2 in FXa-induced pro-inflammatory effect, cells were pre-treated with the PAR-2-blocking antibody SAM11 for 30 minutes and subsequently incubated with FXa for 24 hours. As shown in Figure 7, SAM11 blocked FXa-induced expression of pro-inflammatory mediators.
Figure 7.
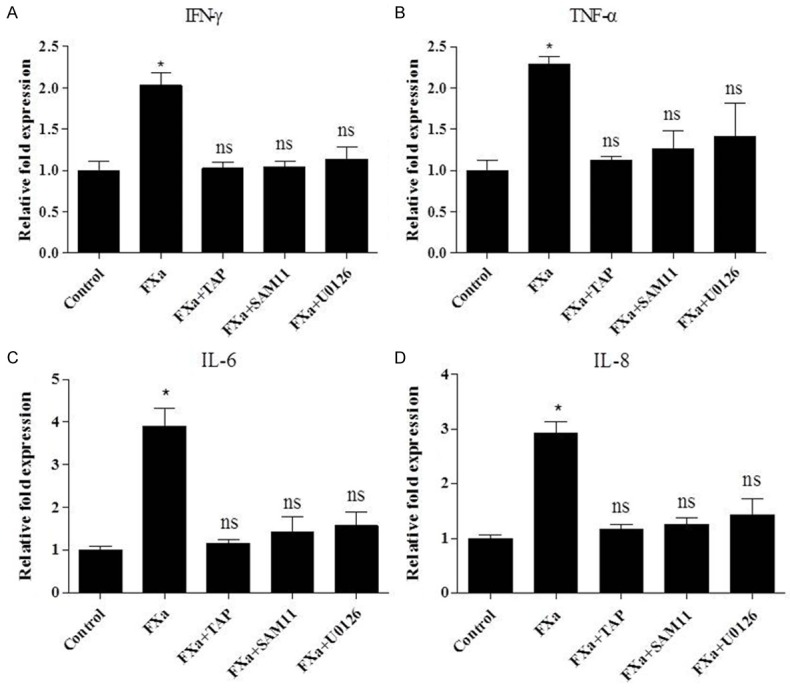
FXa enhances the expression of pro-inflammatory mediators in RAW 264.7 macrophages. Cells were pretreated with PBS, TAP (200 nmol/L, 2 hours), SAM11 (25 μg/mL, 30 minutes), U0126 (10 μmol/L, 2 hours), then stimulated with 0.75 U/mL FXa for 24 hours. Total RNA of cells was then extracted and analyzed for IFN-γ (A), TNF-α (B), IL-6 (C) and IL-8 (D) expression by relative quantitative PCR. Data represent relative fold of mRNA expression as compared to the control group. Data represent mean ± SEM. *P <0.05.
Discussion
It is generally recognized that activation of coagulation is closely linked to immune and inflammatory responses. It has become evident that, in addition to their critical role in coagulation and fibrinolytic processes, plasma serine proteases trigger a diversity of cellular responses, including inflammatory reactions. For instance, FXa, increasing evidence has shown that FXa induces inflammatory responses, it increases the expression of adhesion molecules and pro-inflammatory cytokines in human endothelial cells [7], smooth muscle cells [23] and atrial tissue [24]. In mouse fibroblasts, FXa also can induce the secretion of pro-inflammatory cytokines [25]. Moreover, the inhibition of FXa has already been proved to have anti-inflammatory effect independent of the antithrombotic actions [26,27]. Such as rivaroxaban, the direct FXa inhibitor, it can reduce the expression of inflammatory mediators [24,28]. Fondaparinux, the indirect FXa inhibitor, also has showed the anti-inflammatory potency in our previous study. In the present study, we have shown that FXa enhanced the expression of pro-inflammatory cytokines (IL-6, IL-8, TNF-α, and IFN-γ) in RAW 264.7 macrophages.
Increasing evidence shows that FXa acts as a signaling molecule mediating cellular responses by activating PARs [22,29], in addition to its central role in the coagulation cascade linking the extrinsic and intrinsic pathway. FXa mediates cellular signaling via PAR-1, or PAR-2, or both, depending on cell type and cofactor expression. In fact, studies on dermal fibroblasts and endothelial cells have demonstrated that FXa induced secretion of cytokines via activation of both receptors [7,15]. However, in lung fibroblasts, PAR-1 played a dominant role in FXa-induced responses [23]. In contrast, in human vascular smooth muscle cells, FXa increased only transcriptional expression of PAR-2 [30]. Moreover, recent studies have shown that multiple serine proteases exert pro-inflammatory actions by signaling via PAR-2 [31,32].
In the present study, to examine whether FXa induces the expression of pro-inflammatory cytokines in RAW 264.7 macrophages through the cleavage and subsequent activation of PARs, we used antibodies to specifically block PAR-1 and PAR-2 cleavage sites. We found that incubation with the PAR-1 neutralizing antibody (ATAP-2) had no significant impact on the FXa response, suggesting that PAR-1 was not involved in FXa-mediated cellular effects. To further verify the specificity of FXa-mediated PAR-2 activation, we showed that desensitization of PAR-2 with trypsin decreased the level of ERK1/2 phosphorylation elicited by subsequent application of FXa markedly, while desensitization of PAR-1 with thrombin didn’t affect FXa-mediated cellular effects, suggesting that PAR-2 activation mediated FXa-induced signaling. Finally, to assess definitely the role of PAR-2, we used PAR-2 neutralizing antibody (SAM11) and showed that SAM11 completely suppressed the FXa-induced expression of pro-inflammatory cytokines. We demonstrated that FXa induced the expression of pro-inflammatory cytokines in RAW 264.7 macrophages via activation of PAR-2.
FXa induces ERK1/2 phosphorylation in several cell lines, the phosphorylation of ERK1/2 is widely used as a surrogate marker for PAR-1 and PAR-2 activation [22]. Indeed, FXa induced PAR-2 cleavage results in phosphorylation of ERK1/2 also in our study. The activation of ERK1/2 is closely associated with a wide range of PAR-mediated cellular processes including hypertrophy [33], cellular proliferation, fibrosis and inflammatory signaling [25]. ERK1/2 phosphorylation is accompanied by the secretion of pro-inflammatory cytokines in fibroblasts after stimulation with FXa [4], which reveals that ERK1/2 pathway is critically involved in mediating the pro-inflammatory response of FXa. In fact, in the present study, the ERK1/2 inhibitor, U0126, completely suppressed the FXa-induced mRNA expression of pro-inflammatory cytokines strongly suggests that the pro-inflammatory response induced by FXa is ERK1/2-dependent.
In conclusion, our findings provide evidence that FXa is capable of inducing pro-inflammatory cytokine expression in RAW 264.7 macrophages through the activation of PAR-2 and the ERK1/2 pathway is essential to this process. These findings could be important for improving our understanding of the inflammation induced by FXa, targeting FXa may be a potential therapeutic strategy to reduce PAR-2-mediated inflammation.
Acknowledgements
This work was supported by the Natural Science Foundation of Jiangsu Province, China (grant number 7790000048). We are grateful to Dr. Meng Zhou for his valuable technical assistance.
Disclosure of conflict of interest
None.
References
- 1.Dung NT, Bajpai VK, Yoon JI, Kang SC. Antiinflammatory effects of essential oil isolated from the buds of Cleistocalyx operculatus (Roxb.) Merr and Perry. Food Chem Toxicol. 2009;47:449–453. doi: 10.1016/j.fct.2008.11.033. [DOI] [PubMed] [Google Scholar]
- 2.Lee MY, Lee JA, Seo CS, Ha H, Lee H, Son JK, Shin HK. Anti-inflammatory activity of Angelica dahurica ethanolic extract on RAW 264.7 cells via upregulation of heme oxygenase-1. Food Chem Toxicol. 2011;49:1047–1055. doi: 10.1016/j.fct.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 4.Coker RK, Laurent GJ. Pulmonary fibrosis: cytokines in the balance. Eur Respir J. 1998;11:1218–1221. doi: 10.1183/09031936.98.11061218. [DOI] [PubMed] [Google Scholar]
- 5.Kim HG, Yoon DH, Lee WH, Han SK, Shrestha B, Kim CH, Lim MH, Chang W, Lim S, Choi S, Song WO, Sung JM, Hwang KC, Kim TW. Phellinus linteus inhibits inflammatory mediators by suppressing redox-based NF-kappaB and MAPKs activation in lipopolysaccharideinduced RAW 264.7 macrophage. J Ethnopharmacol. 2007;114:307–315. doi: 10.1016/j.jep.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Schroder K, Sweet MJ, Hume DA. Signal integration between IFN gamma and TLR signalling pathways in macrophages. Immunobiology. 2006;211:511–524. doi: 10.1016/j.imbio.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Senden NH, Jeunhomme TM, Heemskerk JW, Wagenvoord R, van’t Veer C, Hemker HC, Buurman WA. Factor Xa induces cytokine production and expression of adhesion molecules by human umbilical vein endothelial cells. J Immunol. 1998;161:4318–4324. [PubMed] [Google Scholar]
- 8.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 9.Riewald M, Kravchenko VV, Petrovan RJ, O’Brien PJ, Brass LF, Ulevitch RJ, Ruf W. Gene induction by coagulation factor Xa is mediated by activation of protease-activated receptor 1. Blood. 2001;97:3109–3116. doi: 10.1182/blood.v97.10.3109. [DOI] [PubMed] [Google Scholar]
- 10.Camerer E, Huang W, Coughlin SR. Tissue factor- and factor X-dependent activation of protease-activated receptor 2 by factor VIIa. Proc Natl Acad Sci U S A. 2000;97:5255–5260. doi: 10.1073/pnas.97.10.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rao LV, Pendurthi UR. Tissue factor-factor VIIa signaling. Arterioscler Thromb Vasc Biol. 2005;25:47–56. doi: 10.1161/01.ATV.0000151624.45775.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leger AJ, Covic L, Kuliopulos A. Proteaseactivated receptors in cardiovascular diseases. Circulation. 2006;114:1070–1077. doi: 10.1161/CIRCULATIONAHA.105.574830. [DOI] [PubMed] [Google Scholar]
- 13.Mackman N, Tilley RE, Key NS. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler Thromb Vasc Biol. 2007;27:1687–1693. doi: 10.1161/ATVBAHA.107.141911. [DOI] [PubMed] [Google Scholar]
- 14.Rothmeier AS, Ruf W. Protease-activated receptor 2 signaling in inflammation. Semin Immunopathol. 2012;34:133–149. doi: 10.1007/s00281-011-0289-1. [DOI] [PubMed] [Google Scholar]
- 15.Bachli EB, Pech CM, Johnson KM, Johnson DJ, Tuddenham EG, McVey JH. Factor Xa and thrombin, but not factor VIIa, elicit specific cellular responses in dermal fibroblasts. J Thromb Haemost. 2003;1:1935–1944. doi: 10.1046/j.1538-7836.2003.00363.x. [DOI] [PubMed] [Google Scholar]
- 16.Steinhoff M, Buddenkotte J, Shpacovitch V, Rattenholl A, Moormann C, Vergnolle N, Luger TA, Hollenberg MD. Proteinase-activated receptors: transducers of proteinase-mediated signaling in inflammation and immune response. Endocr Rev. 2005;26:1–43. doi: 10.1210/er.2003-0025. [DOI] [PubMed] [Google Scholar]
- 17.al-Ani B, Saifeddine M, Hollenberg MD. Detection of functional receptors for the proteinase-activated-receptor-2-activating polypeptide, SLIGRL-NH2, in rat vascular and gastric smooth muscle. Can J Physiol Pharmacol. 1995;73:1203–1207. doi: 10.1139/y95-172. [DOI] [PubMed] [Google Scholar]
- 18.Ikawa K, Nishioka T, Yu Z, Sugawara Y, Kawagoe J, Takizawa T, Primo V, Nikolic B, Kuroishi T, Sasano T, Shimauchi H, Takada H, Endo Y, Sugawara S. Involvement of neutrophil recruitment and protease-activated receptor 2 activation in the induction of IL-18 in mice. J Leukoc Biol. 2005;78:1118–1126. doi: 10.1189/jlb.0305151. [DOI] [PubMed] [Google Scholar]
- 19.Moraes TJ, Martin R, Plumb JD, Vachon E, Cameron CM, Danesh A, Kelvin DJ, Ruf W, Downey GP. Role of PAR2 in murine pulmonary pseudomonal infection. Am J Physiol Lung Cell Mol Physiol. 2008;294:L368–377. doi: 10.1152/ajplung.00036.2007. [DOI] [PubMed] [Google Scholar]
- 20.Noorbakhsh F, Tsutsui S, Vergnolle N, Boven LA, Shariat N, Vodjgani M, Warren KG, Andrade-Gordon P, Hollenberg MD, Power C. Proteinase-activated receptor 2 modulates neuroinflammation in experimental autoimmune encephalomyelitis and multiple sclerosis. J Exp Med. 2006;203:425–435. doi: 10.1084/jem.20052148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Afkhami-Goli A, Noorbakhsh F, Keller AJ, Vergnolle N, Westaway D, Jhamandas JH, Andrade-Gordon P, Hollenberg MD, Arab H, Dyck RH, Power C. Proteinase-activated receptor-2 exerts protective and pathogenic cell type-specific effects in Alzheimer’s disease. J Immunol. 2007;179:5493–5503. doi: 10.4049/jimmunol.179.8.5493. [DOI] [PubMed] [Google Scholar]
- 22.Ossovskaya VS, Bunnett NW. Proteaseactivated receptors: contribution to physiology and disease. Physiol Rev. 2004;84:579–621. doi: 10.1152/physrev.00028.2003. [DOI] [PubMed] [Google Scholar]
- 23.Scotton CJ, Krupiczojc MA, Konigshoff M, Mercer PF, Lee YC, Kaminski N, Morser J, Post JM, Maher TM, Nicholson AG, Moffatt JD, Laurent GJ, Derian CK, Eickelberg O, Chambers RC. Increased local expression of coagulation factor X contributes to the fibrotic response in human and murine lung injury. J Clin Invest. 2009;119:2550–2563. doi: 10.1172/JCI33288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bukowska A, Zacharias I, Weinert S, Skopp K, Hartmann C, Huth C, Goette A. Coagulation factor Xa induces an inflammatory signalling by activation of protease-activated receptors in human atrial tissue. Eur J Pharmacol. 2013;718:114–123. doi: 10.1016/j.ejphar.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Borensztajn K, Aberson H, Peppelenbosch MP, Spek CA. FXa-induced intracellular signaling links coagulation to neoangiogenesis: potential implications for fibrosis. Biochim Biophys Acta. 2009;1793:798–805. doi: 10.1016/j.bbamcr.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 26.McLean K, Schirm S, Johns A, Morser J, Light DR. FXa-induced responses in vascular wall cells are PAR-mediated and inhibited by ZK-807834. Thromb Res. 2001;103:281–297. doi: 10.1016/s0049-3848(01)00330-9. [DOI] [PubMed] [Google Scholar]
- 27.Hoberg JE, Yeung F, Mayo MW. SMRT derepression by the IkappaB kinase alpha: a prerequisite to NF-kappaB transcription and survival. Mol Cell. 2004;16:245–255. doi: 10.1016/j.molcel.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Q, Bea F, Preusch M, Wang H, Isermann B, Shahzad K, Katus HA, Blessing E. Evaluation of plaque stability of advanced atherosclerotic lesions in apo E-deficient mice after treatment with the oral factor Xa inhibitor rivaroxaban. Mediators Inflamm. 2011;2011:432080. doi: 10.1155/2011/432080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steinberg SF. The cardiovascular actions of protease-activated receptors. Mol Pharmacol. 2005;67:2–11. doi: 10.1124/mol.104.003103. [DOI] [PubMed] [Google Scholar]
- 30.Jobi K, Rauch BH, Dangwal S, Freidel K, Doller A, Eberhardt W, Fischer JW, Schror K, Rosenkranz AC. Redox regulation of human protease-activated receptor-2 by activated factor X. Free Radic Biol Med. 2011;51:1758–1764. doi: 10.1016/j.freeradbiomed.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Borensztajn K, Stiekema J, Nijmeijer S, Reitsma PH, Peppelenbosch MP, Spek CA. Factor Xa stimulates proinflammatory and profibrotic responses in fibroblasts via proteaseactivated receptor-2 activation. Am J Pathol. 2008;172:309–320. doi: 10.2353/ajpath.2008.070347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lohman RJ, Cotterell AJ, Barry GD, Liu L, Suen JY, Vesey DA, Fairlie DP. An antagonist of human protease activated receptor-2 attenuates PAR2 signaling, macrophage activation, mast cell degranulation, and collagen-induced arthritis in rats. FASEB J. 2012;26:2877–2887. doi: 10.1096/fj.11-201004. [DOI] [PubMed] [Google Scholar]
- 33.Pawlinski R, Tencati M, Hampton CR, Shishido T, Bullard TA, Casey LM, Andrade-Gordon P, Kotzsch M, Spring D, Luther T, Abe J, Pohlman TH, Verrier ED, Blaxall BC, Mackman N. Protease-activated receptor-1 contributes to cardiac remodeling and hypertrophy. Circulation. 2007;116:2298–2306. doi: 10.1161/CIRCULATIONAHA.107.692764. [DOI] [PMC free article] [PubMed] [Google Scholar]


