Abstract
Background: MicroRNAs (miRNAs) are small, non-coding RNAs (18-25 nucleotides) that post-transcriptionally modulate gene expression by negatively regulating the stability or translational efficiency of their target mRNAs.The purpose of this study was to investigate the expression levels of miR-125b in human bladder cancer and its potential role in disease pathogenesis. Methods: The expression level of miR-125b was measured in 40 bladder cancer specimens and adjacent normal breast tissues by quantitative polymerase chain reaction (qPCR). MTT and colony formation assays, transwell, cell cycle assays were conducted to explore the potential function of miR-125b in human T24 bladder cancer cells. Luciferase reporter assays were performed to analyze the regulation of putative target of miR-125b. The effects of modulating miR-125b on endogenous levels of this target were subsequently confirmed via qRT-PCR and Western blot. Results: The expression of miR-125b in bladder cancer specimens was lower than adjacent normal tissues (P < 0.05). Overexpression of miR-125b inhibited cellular growth, suppressed cellular migration and caused an accumulation of cells in the G1 phase of the cell cycle, Luciferase assays revealed that miR-125b directly targeted the 3’UTR of SphK1. Overexpression of miR-125b led to the downregulation of SphK1 and protein level as assessed by qRT-PCR and Western blot. Targeted knockdown of SphK1 by siRNA significantly inhibited the proliferation of T24 bladder cancer cells. Conclusions: These findings suggest that miR-125b may act as a tumor suppressor gene in bladder cancer and that, in the future, targeting of this miRNA may provide a novel strategy for the diagnosis and treatment of patients with this lethal disease.
Keywords: MiR-125b, proliferation, SphK1, bladder cancer
Introduction
Bladder cancer is one of the most common worldwide malignancies. In developed countries, bladder cancer (BC) is the fifth most commonly diagnosed tumor and the second most common cause of death among genitourinary tumors [1], In the US, statistics illustrated that an estimated 74,690 cases were newly diagnosed bladder cancer, among which 15,580 were expected to die in 2014 [2]. It is well known that the pathogenesis of bladder cancer is complicated and is the result of the interaction of multiple factors, such as heredity, environment, and metabolism factors, play an important roles in the development of bladder cancer, The exact causes and pathogenesis of bladder cancer are poorly understood, So it is urgent to understand the molecular and cellular mechanisms of the development of bladder cancer.
MicroRNAs (miRNAs) are a small class of non-coding RNAs which regulate gene expression and may play pivotal roles in the physiological and pathological processes in a variety of eukaryotic organisms [3], During recent years, more and more studies have reported a functional contribution of specific miRNAs in diverse biological processes [4-6], including deregulation of miRNAs by acting on their targeted genes in the progression and tumorgenesis of human cancers [7-9]. Moreover, aberrant miRNA expression has been frequently observed in various types of human tumors. These reports suggest that miRNAs may function as either tumor-suppressor genes or oncogenes [10]. Recent studies indicate that miR-125b is downregulated in cervical cancer, acute myeloid leukemia, lung cancer, and colorectal neoplasia [11-14]. However, the mechanism by which miR-125b contributes to bladder cancer tumorigenesis is still relatively unclear.
SphK1 (Sphingosine kinase 1), a master kinase that regulates the balance between ceramide/sphingosine and S1P levels, mediates cellular behaviors and may determine cancer progression, including proliferation, migration, and invasion [15,16]. In addition, As an oncogenic kinase, SphK1 is up-regulated in several kinds of cancers and is correlated with the development and progression of the disease [17-19]. Furthermore, significant evidence indicates that overexpression of SphK1 in patients with bladder cancer is associated with a worse prognosis [20], thus, SphK1 has attracted increasing research interest and understanding the roles of miR-125b in bladder cancer and identifying relevant mRNA targets that mediate its tumor suppressor or oncogenic activities are essential in developing miR-125b as a therapeutic target.
In this study, we report that miR-125b expression is significantly decreased in human bladder cancer, and its overexpression inhibits the proliferation of T24 cells by targeting SphK1. These results indicate that miR-125b functions as a tumor suppressor, whose deregulation may be involved in the initiation and development of human bladder cancer.
Materials and methods
Patients and ethics
Bladder tumor tissues and adjacent nontumor bladder tissues were selected from the archives of the Department of Urology, Tongji Hospital of Tongji University, Shanghai, China. This study was approved by the Institutional Review Board of Tongji Hospital, Tongji University School of Medicine. The samples were immediately snap-frozen in liquid nitrogen. All samples were confirmed as bladder cancer by trained pathologists. No patients received chemotherapy or radiotherapy prior to surgery. All clinical investigations were conducted according to the principles expressed in the Declaration of Helsinki.
Cell culture
The T24 bladder cancer cell lines and human embryonic kidney 293T cells were obtained from the Chinese Science Institute and cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, USA) supplemented with 10% fetal bovine serum (FBS, Gibco), as well as 100 units of penicillin/ml and 100 μg of streptomycin/ml (Enpromise, China). Cells were incubated at 37°C in a humidified chamber containing 5% CO2.
Analysis of miRNA expression using TaqMan RT-PCR
Total RNA from tissue samples and cell lines was harvested using the miRNA Isolation Kit (Ambion, USA). Expression of mature miRNAs was assayed using Taqman MicroRNA Assay (Applied Biosystems) specific for hsa-miR-125b. Briefly, 5 ng of total RNA were reverse transcribed to cDNA with specific stem-loop RT primers. Quantitative real-time PCR was performed by using an Applied Biosystems 7300 Real-time PCR System and a TaqMan Universal PCR Master Mix. All the primers were obtained from the TaqMan miRNA Assays. Small nuclear U6 snRNA (Applied Biosystems) was used as an internal control.
Cell proliferation assay (MTT assay)
Cells (2 × 103) were plated in 96-well plates (BD Biosciences, USA) and incubated at 37°C until the cells reached 30-40% confluence, followed by transfection with 50 nM or 100 nM miR-125b or NC mimics. Cell proliferation was assessed at 24, 48, 72, 96 h as follows: 20 μl (5 mg/ml) MTT solution (Sigma, USA) was added in each well, and after 4 h of incubation at 37°C, the supernatant was discarded and 150 μl of dimethyl sulfoxide (DMSO) were added. After 10 min of low speed shaking (100 rpm) and incubation, the OD at 490 nm was read by a microplate spectrophotometer. Each sample was tested with six replicates. All experiments were performed in biological triplicate.
Colony formation assay
After transfection with 100 nmol/l miR-125b or NC, cells were trypsinized, counted, and seeded for colony formation assay in 6-well plates at 300/well. During colony growth, the culture medium was replaced every 3 days. On the 8th day after seeding, the cells were fixed and then stained with crystal violet, and the number of colonies was counted. The colony was counted only if it contained more than 50 cells. Each treatment was carried out in triplicate.
Transwell experiment
In accordance with Transwell chamber instructions, DMEM/high glucose medium containing 10% FBS was added to the lower chamber while T24 cell suspension transfected with miR-125b, NC for 48 h was added to the upper chamber. After incubation at 37°C, 5% CO2 for 12-18 h, the lower chamber was observed using inverted microscope. Incubation was terminated when cells passed into lower chamber. Inside of the upper chamber was cleaned with a cotton swab. Lower chamber was immersed and washed with PBS, fixed with 4% paraformaldehyde, stained with 0.1% crystal violet, washed for three times with running water, and then photographed. Membrane-binding crystal violet was dissolved with 300 μl 33% glacial acetic acid, and then absorbance at 573 nm was measured using microplate reader.
Cell cycle assay
miR-125b (100 nM), NC cells were harvested at 48 h after transfection, centrifuged at 1,200 rpm for 10 min and washed three times with cold PBS. Ice-cold 70% ethanol was subsequently added dropwise, and the cells were fixed at 4°C overnight. After a 30-min digestion in RNase (0.1 g/l), a total of 250 μl (0.05 g/l) propidium iodide (PI) staining solution was added to each sample which was then incubated for 30 min at room temperature (RT) in the dark. The cell cycle was then analyzed by a flow cytometer (FACSCanto™ II; BD Biosciences).
Western blotting
Protein samples were separated by 12% SDS-polyacrylamide gel (SDS-PAGE) and transferred onto PVDF membranes (Beyotime, China). Immune complexes were formed by incubation of membranes with primary antibody (BioVision, USA) overnight at 4°C. Blots were washed and incubated for 1 h with HRP-conjugated anti-rabbit secondary antibody. Immunoreactive protein bands were detected using an Odyssey Scanning system.
Luciferase assay
The 3’ untranslated region (3’UTR) of SphK1 containing the predicted miR-125b binding site was amplified by PCR in a total volume of 50 μl using the Primer star kit (Takara) in accordance with the manufacturer’s instructions. The mutant constructs were generated by mutation.Fragments were subcloned into the Xho I site in the 3’-UTR of Renilla luciferase of the psiCHECK-2 reporter vector. psiCHECK-2/SphK1 3’-UTR or psiCHECK-2/SphK1 3’-UTR mutant reporter plasmids (200 ng) were co-transfected with miR-125b mimics or miR-NC (100 nM) into T24 cells (60% confluence) using Lipofectamine 2000 (Invitrogen), in accordance with the manufacturer’s instructions. After 48 h, cells were lysed and reporter activity was assessed using the Dual-luciferase reporter assay system (Promega, USA) in accordance with the manufacturer’s protocols. Renilla luciferase activity was normalized to firefly luciferase activity.
Statistical methods
Experimental data was showed as mean ± SD. Two groups were compared using t-test comparison and multi-groups were compared using variance analysis by SPSS17.0 statistical software. P<0.05 indicates significant difference.
Results
Lower expression of miR-125b in bladder cancer specimens
Firstly, To determine the expression level of miR-125b in bladder cancer. we analyzed levels of miR-125b in 40 paired invasive bladder cancer pecimens and pair-matched adjacent noncancerous bladder tissues by quantitative reverse transcription polymerase chain reaction (qRT-PCR). Our results demonstrated that the expression of miR-125b was lower in bladder cancer specimens (1.51±0.031) than adjacent noncancerous bladder tissues (9.58±0.53) (P<0.05) (Figure 1).
Figure 1.
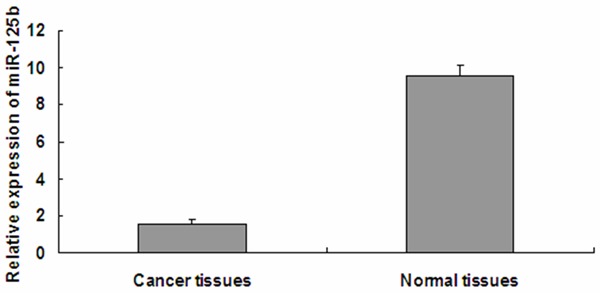
miR-125b levels are significantly decreased in bladder cancer specimens. The graph represents the 2-ΔΔCt values ± SEM; *P<0.05. SEM, standard error of the mean.
Overexpression of miR-125b in T24 cells inhibits cell proliferation and colony formation ability
To explore the effect of miR-125b on bladder cancer cell proliferation, miR-125b mimics were transfected into the human bladder cancer cell line, T24 and proliferation was assessed by MTT assay. As shown in Figure 2, Compared with the NC group, miR-125b significantly repressed the growth of bladder cancer cells cellular proliferation gradually declined following transfection with miR-125b, in a time- and dose-dependent manner. As shown in Figure 3, Proliferation was also assessed by colony formation assay. Colonies of the miR-125b group were 28.3±1.75, which was significantly less than that of the NC group (76.78±2.17) (p<0.05). Together these results indicated that Overexpression of miR-125b in T24 cells inhibits cell proliferation and colony formation ability.
Figure 2.
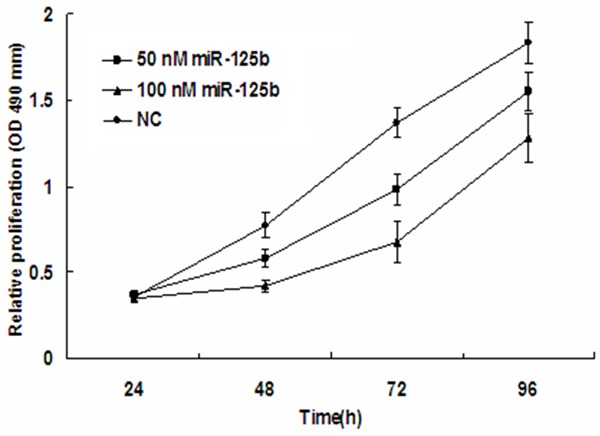
MiR-125b expression inhibits T24 proliferation. MTT cell proliferation assays were performed with miR-125b and NC expressing cells. The proliferation of T24 cells transfected with miR-125b was inhibited in a dose- and time-dependent manner compared with the NC group. The graph represents OD values ± SEM, *P < 0.05. NC, negative control; SEM, standard error of the mean.
Figure 3.
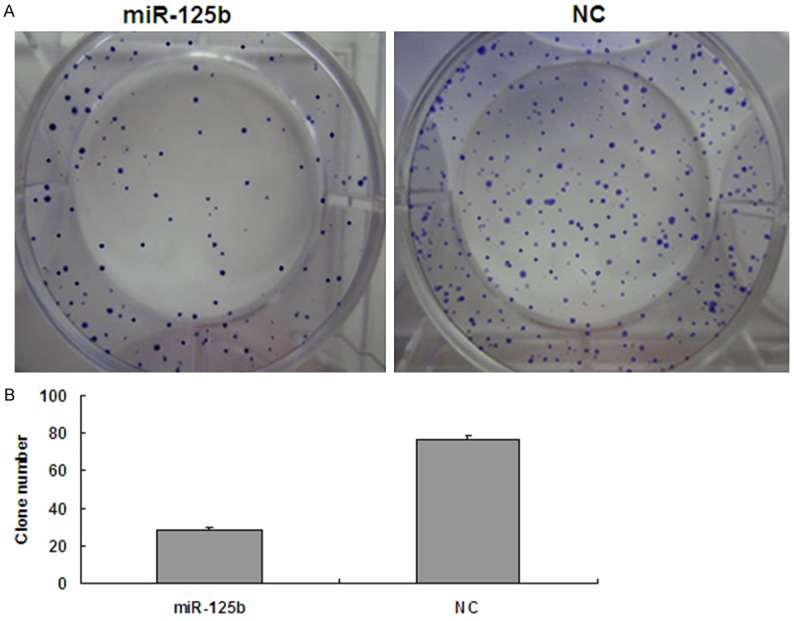
Colony formation assay. A. Representative images of crystal violet stained colonies in T24 cells. The miR-125b group exhibited fewer colonies than the NC group. B. Quantification of the clone numbers, *p<0.05. NC, negative control.
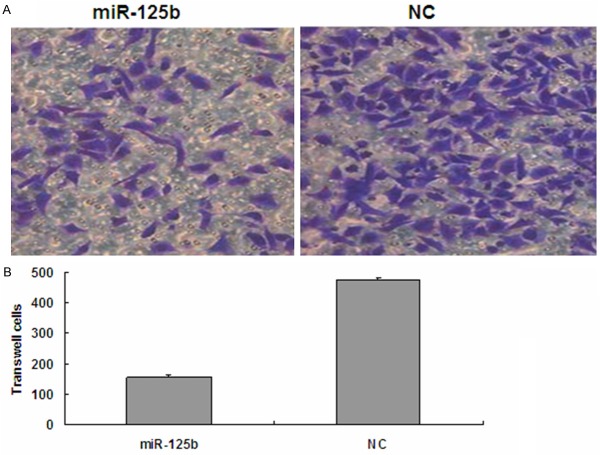
Overexpression of miR-125b in T24 cells inhibits cell migratory ability
The transwell migration assay is a useful method to investigate migratory ability. Our results showed that 20 h after transfection, the number of invaded cells stained with Crystal Violet was significantly decreased in the miR-125b (100 nM) group (Figure 4). Invasion rates of both groups were determined by counting the number of cells that invaded through matrigel and confirmed the results observed by inverted microscope. These data indicate that the migratory ability of T24 cells may be inhibited by miR-125b.
Figure 4.
Overexpression of miR-125b in T24 cells inhibits cell migratory ability. Cell migration ability was analyzed by transwell chamber assay 20 h after miR-125b and NC transfection. A. Representative images of crystal violet stained T24 migratory cells transfected with miR-125b and NC. B. Quantification of the number of crystal violet-stained cells. Data represent means ± SEM; *P<0.05. NC, negative control; SEM, standard error of the mean.
Cell cycle was affected following overexpression of miR-125b in T24 cells
The cell cycle distributions of the miR-125b (100 nM) group and NC group were analyzed by flow cytometry as shown in Figure 5. All these findings demonstrate that miR-125b inhibits the proliferation of T24 bladder cancer cells. Flow cytometer analysis revealed that the percentage of G1 phase T24 cells (58.53±1.62%) dramatically increased in the 100 nmol/L miR-125b group, which was higher than those in NC group (49.42±0.64%, P<0.05), while the proportion of G2 and S phase cells decreased in the miR-125b group compared with those of the NC group (P<0.05). These results suggest that the overexpression of miR-125b could impact cell cycle progression in T24 cells.
Figure 5.
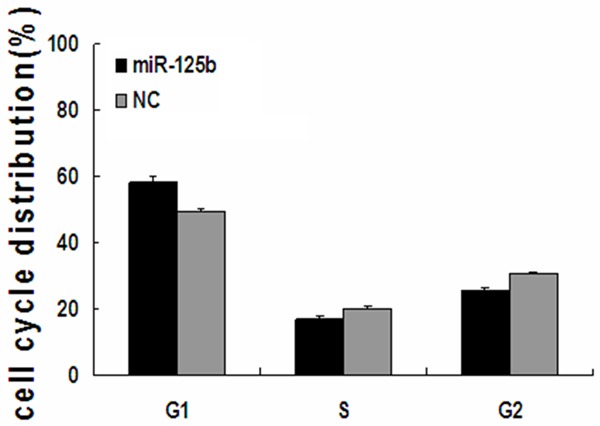
miR-125b disrupts the cell cycle of T24 cells. T24 cells transfected with miR-125b or NC mimics were analyzed by flow cytometry. The respective proportion of G1 phase, S phase and G2 phase cells of miR-125b and NC groups are indicated. *P<0.05. NC, negative control.
MiR-125b regulates SphK1 expression in bladder cancer cells
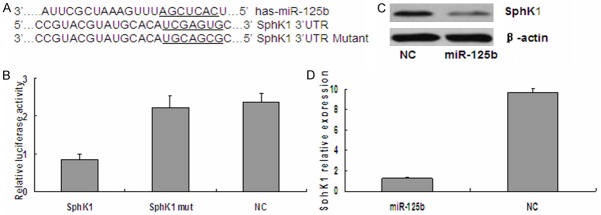
To identify possible miR-125b target genes, we performed a computational screen for genes with complementary sites of miR-125b in their 3’UTR using open-access software. The software included TargetScan, Sanger microRNA target, and Miranda. We focused our attention on SphK1.
To investigate the downstream targets of miR-125b that may the putative miR-125b binding site into a luciferase reporter construct, in addition to a mutated SphK1 3’-UTR (Figure 6A). Luciferase activity was significantly decreased following co-transfection of psiCHECK-2/SphK1 3’-UTR with miR-125b, compared with the NC group (Figure 6B). Furthermore, luciferase activity was also decreased following co-transfection of psiCHECK-2/SphK1 3’-UTR mutant and miR-125b (Figure 6B). These results suggest that miR-125b specifically binds to the 3’-UTR of SphK1. The effect of miR-125b transfection on endogenous SphK1 mRNA and protein expression was subsequently evaluated in T24 cells by Western blot and qRT-PCR. As shown in Figure 6C and 6D, the expression of SphK1 mRNA and protein was decreased in T24 cells transfected with 100 nM miR-125b mimics compared with the control. Collectively, our results demonstrated that SphK1 is a direct target of miR-125b in bladder cancer cells.
Figure 6.
SphK1 is a direct target of miR-125b in bladder cancer. A. The binding site for miR-125b in the 3’-UTR of SphK1 mRNA. B. The relative luciferase activity (Renilla/firefly) was measured in T24 cells after co-transfection of the SphK1 3’-UTR or SphK1 3’-UTR mutant luciferase construct with either miR-125b mimics or NC. C. Western blot analysis was used to detect SphK1 protein expression levels. D. qRT-PCR analysis was used to detect SphK1 mRNA expression levels.
MiR-125b inhibits the proliferation of bladder cancer cells via regulation of SphK1
Since overexpression of miR-125b suppressed the proliferation of T24 bladder cancer cells, and given that SphK1 is a direct target of miR-125b, We hypothesized that miR-125b has its inhibitory effect on bladder cancer cell through its target gene SphK1. We next determined to knockdown SphK1 and evaluated its expression by western blot in T24 cells. The knockdown of SphK1 was confirmed by western blot (Figure 7A). We assessed the effect of targeted knockdown of SphK1 on T24 cell growth by MTT assay. Treatment of cells with 100 nmol/L SphK1 siRNA markedly suppressed cell viability by 23%, 35% and 45% at 48 h, 72 h and 96 h, respectively, compared with control siRNA (P<0.01) (Figure 7B). This suggests that SphK1 promotes the proliferation of bladder cancer cells in vitro. These results demonstrate that down regulation of SphK1 expression by miR-125b contributes, at least in part, to the suppression of the growth of bladder cancer cells.
Figure 7.
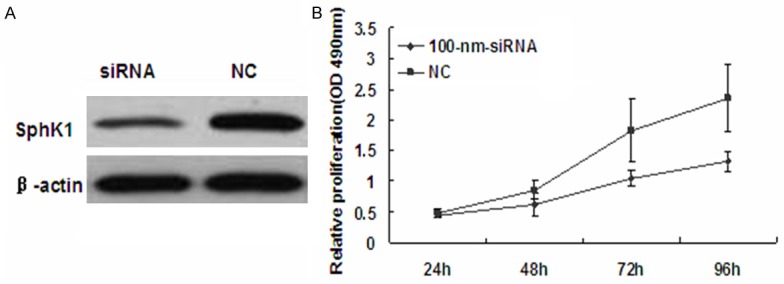
knockdown of SphK1 significantly supresses the proliferation of breast cancer cells. A. Western blot analysis was used to detect SphK1 protein levels in T24 cells after transfection with SphK1 siRNA or siRNA-NC. B. The MTT assay was performed to monitor the proliferation level of T24 cells at 24, 48, 72 and 96 h.
Discussion
Bladder cancer is one of the most common malignancies worldwide. Besides surgery, chemotherapy is the major therapeutic method. However, there are still patients that exhibit resistance to chemotherapy, as demonstrated through early recurrence and metastasis, leading to poor prognosis. Therefore, to successfully relieve drug resistance and overcome bladder cancer, It is a very urgent task to explore new treatments. Recently in bladder cancer research, the search for novel therapeutic agents for bladder cancer is one of the hot topics of bladder cancer research.
In our study, we examined the expression levels of miR-125b in bladder cancer specimens and adjacent normal tissues. Compared to the normal bladder tissues, miR-125b expression was significantly down-regulated in the bladder cancer specimens. The abnormal expression of miRNAs in bladder cancer has been previously evaluated. such as acute myeloid leukemia [21], chronic lymphocytic leukemia [22], and some solid tumors [23,24]. In agreement with our results. Thus, we hypothesized that miR-125b may function as a tumor suppressor. Next, we transfected miR-125b mimics into T24 cells to induce it overexpression. Exogenous overexpression of miR-125b significantly inhibited the cell growth and colony formation ability of T24 cells as indicated by MTT and colony formation assays. Using the transwell migration assay, we found that the overexpression of miR-125b in bladder cancer cells could suppress their migratory ability. Furthermore, by flow cytometry we found that miR-125b distinctly arrests cancer cells at the G1 phase when compared with the cell cycle of NC groups.
To ascertain why miR-125b exhibited these effects on the cell function, we investigated putative targets of miR-125b and identified SphK1, which drives cells from the G1 to the S phase. Based on three databases. Including targetscan, miRanda, and miRGen. The interaction between miR-125b and Sphk1 mRNA has not been previously reported. To confirm whether SphK1 was a real target of miR-125b, we integrated a fragment of the SphK1 3’-UTR containing the target sequence, or a fragment whose target site was mutated, into a luciferase reporter vector. Luciferase activity was significantly repressed in cells transfected with the construct harboring the miR-125b target sequence compared with the mutated control vector. Both SphK1 mRNA and protein levels decreased after transfection of T24 cells with miR-125b. Together, these data indicate that miR-125b directly interacts with SphK1 mRNA and suppresses SphK1 protein expression. Additionally, silencing of SphK1 expression by siRNA in T24 cells significantly inhibited cellular proliferation. These findings support the hypothesis that SphK1 is a new target of miR-125b.
Conclusion
In summary, our study demonstrated that miR-125b is downregulated in bladder cancer specimens compared with normal tissue Overexpression of miR-125b inhibited the proliferation and colony formation ability, suppressed migration and caused G1 phase arrest by targeting SphK1 in T24 bladder cancer cells. These data indicate that miR-125b may serve as a tumor suppressor gene involved in bladder cancer pathogenesis. Therefore; miR-125b may be a potential diagnostic and therapeutic target for bladder cancer.
Acknowledgements
Foundation of China and the Shanghai Education Commission Research and Innovation projects (No. 12ZZ034).
Disclosure of conflict of interest
None.
References
- 1.Parkin DM. The global burden of urinary bladder cancer. Scand J Urol Nephrol Suppl. 2008;218:12–20. doi: 10.1080/03008880802285032. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, Somerfield MR, Hayes DF, Bast RC Jr. Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J. Clin. Oncol. 2007;25:5287–5312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 4.Vecchione A, Belletti B, Lovat F, Volinia S, Chiappetta G, Giglio S, Sonego M, Cirombella R, Onesti EC, Pellegrini P, Califano D, Pignata S, Losito S, Canzonieri V, Sorio R, Alder H, Wernicke D, Stoppacciaro A, Baldassarre G, Croce CM. A microRNA signature defines chemoresistance in ovarian cancer through modulation of angiogenesis. Proc Natl Acad Sci U S A. 2013;110:9845–9850. doi: 10.1073/pnas.1305472110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aigner A. MicroRNAs (miRNAs) in cancer invasion and metastasis: therapeutic approaches based on metastasis-related miRNAs. J Mol Med (Berl) 2011;89:445–457. doi: 10.1007/s00109-010-0716-0. [DOI] [PubMed] [Google Scholar]
- 6.Furuta M, Kozaki KI, Tanaka S, Arii S, Imoto I, Inazawa J. miR-124 and miR-203 are epigenetically silenced tumor-suppressive microRNAs in hepatocellular carcinoma. Carcinogenesis. 2010;31:766–776. doi: 10.1093/carcin/bgp250. [DOI] [PubMed] [Google Scholar]
- 7.Farazi TA, Hoell JI, Morozov P, Tuschl T. MicroRNAs in human cancer. Adv Exp Med Biol. 2013;774:1–20. doi: 10.1007/978-94-007-5590-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaman MS, Maher DM, Khan S, Jaggi M, Chauhan SC. Current status and implications of microRNAs in ovarian cancer diagnosis and therapy. J Ovarian Res. 2012;5:44. doi: 10.1186/1757-2215-5-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Leva G, Croce CM. The Role of microRNAs in the Tumorigenesis of Ovarian Cancer. Front Oncol. 2013;3:153. doi: 10.3389/fonc.2013.00153. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Ryan BM, Robles AI, Harris CC. Genetic variation in microRNA networks: the implications for cancer research. Nat Rev Cancer. 2010;10:389–402. doi: 10.1038/nrc2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ribeiro J, Marinho-Dias J, Monteiro P, Loureiro J, Baldaque I, Medeiros R, Sousa H. miR-34a and miR-125b expression in HPV infection and cervical cancer development. Biomed Res Int. 2015;2015:304584. doi: 10.1155/2015/304584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao Q, Cao J, Wu YC, Liu X, Han J, Huang XC, Jiang LH, Hou XX, Mao WM, Ling ZQ. Circulating miRNAs is a potential marker for gefitinib sensitivity and correlation with EGFR mutational status in human lung cancers. Am J Cancer Res. 2015;5:1692–1705. [PMC free article] [PubMed] [Google Scholar]
- 13.Yamada A, Horimatsu T, Okugawa Y, Nishida N, Honjo H, Ida H, Kou T, Kusaka T, Sasaki Y, Yagi M, Higurashi T, Yukawa N, Amanuma Y, Kikuchi O, Muto M, Ueno Y, Nakajima A, Chiba T, Boland CR, Goel A. Serum miR-21, miR-29a, and miR-125b Are Promising Biomarkers for the Early Detection of Colorectal Neoplasia. Clin Cancer Res. 2015;21:4234–4242. doi: 10.1158/1078-0432.CCR-14-2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vargas Romero P, Cialfi S, Palermo R, De Blasio C, Checquolo S, Bellavia D, Chiaretti S, Foà R, Amadori A, Gulino A, Zardo G, Talora C, Screpanti I. The deregulated expression of miR-125b in acute myeloid leukemia is dependent on the transcription factor C/EBPα. Leukemia. 2015;29:2442–5. doi: 10.1038/leu.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Milstien S, Spiegel S. Targeting sphingosine-1-phosphate: a novel avenue for cancer therapeutics. Cancer Cell. 2006;9:148–150. doi: 10.1016/j.ccr.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 16.Fuereder T, Hoeflmayer D, Jaeger-Lansky A, Rasin-Streden D, Strommer S, Fisker N, Hansen B, Crevenna R, Wacheck V. Sphingosine kinase 1 is a relevant molecular target in gastric cancer. Anticancer Drugs. 2011;22:245–252. doi: 10.1097/cad.0b013e328340bd95. [DOI] [PubMed] [Google Scholar]
- 17.Pan J, Tao YF, Zhou Z, Cao BR, Wu SY, Zhang YL, Hu SY, Zhao WL, Wang J, Lou GL, Li Z, Feng X, Ni J. An novel role of sphingosine kinase-1 (SPHK1) in the invasion and metastasis of esophageal carcinoma. J Transl Med. 2011;9:157. doi: 10.1186/1479-5876-9-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarkar S, Maceyka M, Hait NC, Paugh SW, Sankala H, Milstien S, Spiegel S. Sphingosine kinase 1 is required for migration, proliferation and survival of MCF-7 human breast cancer cells. FEBS Lett. 2005;579:5313–5317. doi: 10.1016/j.febslet.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 19.Bao M, Chen Z, Xu Y, Zhao Y, Zha R, Huang S, Liu L, Chen T, Li J, Tu H, He X. Sphingosine kinase 1 promotes tumour cell migration and invasion via the S1P/EDG1 axis in hepatocellular carcinoma. Liver Int. 2012;32:331–338. doi: 10.1111/j.1478-3231.2011.02666.x. [DOI] [PubMed] [Google Scholar]
- 20.Meng XD, Zhou ZS, Qiu JH. Increased SPHK1 expression is associated with poor prognosis in bladder cancer. Tumour Biol. 2014;5:2075–80. doi: 10.1007/s13277-013-1275-0. [DOI] [PubMed] [Google Scholar]
- 21.Garzon R, Liu S, Fabbri M, Liu Z, Heaphy CE, Callegari E, Schwind S, Pang J, Yu J, Muthusamy N, Havelange V, Volinia S, Blum W, Rush LJ, Perrotti D, Andreeff M, Bloomfield CD, Byrd JC, Chan K, Wu LC, Croce CM, Marcucci G. MicroRNA-29b induces global DNA hypomethylation and tumor suppressor gene reexpression in acute myeloid leukemia by targeting directly DNMT3A and 3B and indirectly DNMT1. Blood. 2009;113:6411–6418. doi: 10.1182/blood-2008-07-170589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santanam U, Zanesi N, Efanov A, Costinean S, Palamarchuk A, Hagan JP, Volinia S, Alder H, Rassenti L, Kipps T, Croce CM, Pekarsky Y. Chronic lymphocytic leukemia modeled in mouse by targeted miR-29 expression. Proc Natl Acad Sci U S A. 2010;107:12210–12215. doi: 10.1073/pnas.1007186107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sengupta S, den Boon JA, Chen IH, Newton MA, Stanhope SA, Cheng YJ, Chen CJ, Hildesheim A, Sugden B, Ahlquist P. MicroRNA 29c is down-regulated in nasopharyngeal carcinomas, up-regulating mRNAs encoding extracellular matrix proteins. Proc Natl Acad Sci U S A. 2008;105:5874–5878. doi: 10.1073/pnas.0801130105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iorio MV, Ferracin M, Liu CG, Veronese A, Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M, Ménard S, Palazzo JP, Rosenberg A, Musiani P, Volinia S, Nenci I, Calin GA, Querzoli P, Negrini M, Croce CM. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65:7065–7070. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]