Abstract
Objective: In this study, chronic compression of cervical spinal cord was introduced into twy/twy mice and the role of MK2 signaling pathway was investigated in this disease. Methods: twy/twy mice aged 6-24 weeks were used and the inflammatory response in the cervical spinal cord was observed. The Institute of Cancer Research (ICR) mice were used as controls. MK2 inhibitor (PF-3644022, 30 mg/kg) was administered intragastrically to twy/twy mice. The motor behavior was firstly observed in these three groups by Catwalk gait analysis. And the cervical spinal cord between C2 and C3 of vertebral segments was analyzed by MRI and Western blot assay. Results: The stride length of paws and interlimb coordination reduced in twy/twy mice. However, at 4 weeks after PF-3644022 treatment, a marked improvement was observed in the motor function. The expressions of inflammation related factors (such as IL-1β, NF-κB, TNF-α, MK2 and p-MK2) and apoptosis related proteins (such as cleaved caspase-8 and bax/bcl-2) in the spinal cord of twy/twy mice significantly increased as compared to controls, but 4-week treatment with PF-3644022 markedly reduced the expressions of these factors and apoptotic proteins in the cervical spinal cord. Conclusion: MK2 signaling pathway is involved in the chronic compression induced inflammation of the cervical spinal cord. Thus, to inhibit the MK2 pathway may used to improve the outcome and prevent the deterioration of neurological dysfunction.
Keywords: Cervical spinal cord, chronic compression, inflammation, mitogen-activated protein kinase-activated protein kinase 2
Introduction
Chronic compression of the cervical spinal cord is a common spinal cord disease. The compression induced injury can be caused by spinal tuberculosis, extramedullary tumor and other conditions. In fact, the most common causes of chronic cervical spinal cord compression are the herniated cervical discs and the ossification of the posterior longitudinal ligament (OPLL). Either herniated cervical discs or OPLL may cause compression of the spinal cord and induce ischemia and chronic inflammatory response. The clinical symptoms of chronic compression of the cervical spinal cord include limb numbness, weakness and even paralysis [1]. To date, surgical interventions (such as resection of the herniated disc or OPLL and expansion of the spinal canal) have been applied to treat this disease, but the outcome is still poor in some patients due to the severe inflammation as a result of long lasting compression induced injury. Currently, most investigations on the chronic compression of the cervical spinal cord focus on the disc, OPLL and others, but few studies have been conducted to investigate the molecular mechanisms underlying the chronic compression of the spinal cord [2,3].
The molecular mechanisms of chronic compression of the cervical spinal cord are complicated, in which the mechanical compression, micro-circulation disturbance of the spinal cord, neuroinflammation and others may be involved. Although the mechanical compression has been identified as a major mechanism, it cannot explain all the clinical symptoms. Increasing investigations indicate an important role of inflammatory response in the chronic compression of the cervical spinal cord [4]. The p38 mitogen-activated protein kinase signal pathway (p38a MAPK) [5] as well as its downstream kinase mitogen-activated protein kinase-activated protein kinase 2 (MK2) are key participants in the inflammation related signaling pathways. However, the role of MK2 signaling pathway in the pathogenesis of chronic compression of cervical spinal cord is still poorly understood [6,7].
In the present study, we hypothesized that MK2 signaling pathway was involved in the pathogenesis of chronic cervical spinal cord compression, and to inhibit the inflammation via regulating this signaling pathway was helpful for the therapy of this disease.
Materials and methods
Animals
Mutant twy/twy mice aged 6 weeks were purchased from the Experimental Animal Center (Kanagawa, Japan). The calcification of the ligamentum flavum between C2 and C3 deteriorates over age in these mice and thus they may develop motor paresis between 4 and 7 months. The typical symptom of homozygous hyperostotic mice is the tip-toe walking beginning at the age of 6-8 weeks. These mice can be used to investigate the chronic compression of the cervical spinal cord [8,9]. PF-3644022 [(10R)-10-methyl-3-(6-methylpyridin-3-yl)-9, 10,11,12-tetrahydro-8H-[1,4]diazepino [5,6,:4,5]thieno[3,2-f]quinolin-8-one] (Sigma Aldrich) is a potent benzothiophene MK2 inhibitor and used in chronic inflammation models [10]. The twy/twy mice aged 20 weeks were intragastrically treated with 30 mg/kg PF-3644022 once daily for 4 weeks. The Institute of Cancer Research (ICR) mice matched in age were used as controls and did not receive any treatment. The whole study protocol was approved by the Ethical Committee for Animal Research at Capital Medical University.
Magnetic resonance imaging (MRI)
Signal 7 Tesla MRI magnet (Varian In-vivo MR Imaging System) was employed in the present study. The twy/twy and ICR mice were anesthetized by a single inhalation of 2% isoflurane and 98% oxygen and then fixed at a prone position in the MR unit. The sagittal images of the spinal cord were obtained with the effective echo time (TE) of 72 ms, repetition time (TR) of 3000 ms, slice thickness of 1 mm, total number of averages (NEX) of 10, field of view (FOV) of sagittal view of 55 × 41 mm.
Gait analysis
Catwalk gait analysis was conducted according to previously reported [11]. Mice were allowed to walk freely in the Catwalk walkway system (Noldus Information Technology, Netherlands). When their paws contacted the glass plate in dark, the contact region was illuminated by a light emitting diode and recorded by a high-speed color video camera connected to a computer. The stride length and interlimb coordination measured as regularity index (percentage of normal step sequences) were analyzed with the Catwalk software.
Western blot assay
In brief, mice were anesthetized and sacrificed, and then 1-cm spinal cord was collected from the site of compression (n=3 or 4 per group). The spinal cord was homogenized in ice-cold buffer (50 mM tris-[hydroxymethyl]-aminomethane, pH7.4, 150 mM NaCl, 0.5% Triton X-100, 1 mM edetic acid, 1 M phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin and 60 μg/ml aprotinin), and centrifuged at 14,000 × g for 30 min at 4°C. Then, the supernatant was harvested for the detection of protein concentration. The proteins were subjected to 10-15% sodium dodecyl sulfate polyacrylamide gel (SDS-PAGE) and electrically transferred onto a nitrocellulose membrane. This membrane was then independently treated with primary antibodies including anti-MK2 (1:500; Abcam), anti-phosphorylated MK2 (1:1000; p-MK2) (Cell Signaling Tech-nology), anti-TNF-α (1:500; Abcam), anti-NF-κB (1:1000; Abcam), anti-IL-1β (1:200; Santa Cruz), anti- Caspase-8 (1:2000; Abcam), anti-Bax (1:500, Beyotime) and anti-Bcl-2 (1:200, Santa Cruz Biotechnology). The expression of a specific protein was normalized to that of β-actin [12].
Statistical analysis
All the data are represented as means ± standard error (SEM). Analysis of variance (ANOVA) followed by Scheffe or Turkey test was carried out with the Statistical Package for the Social Sciences software (SPSS 17.0 Statistical Software). A value of P < 0.05 was considered statistically significant.
Results
MRI scanning
Figure 1 displayed the representative T2-weighted sagittal MR images of twy/twy mice. The cervical ossification and spinal cord compression were identified at C2-C3.
Figure 1.
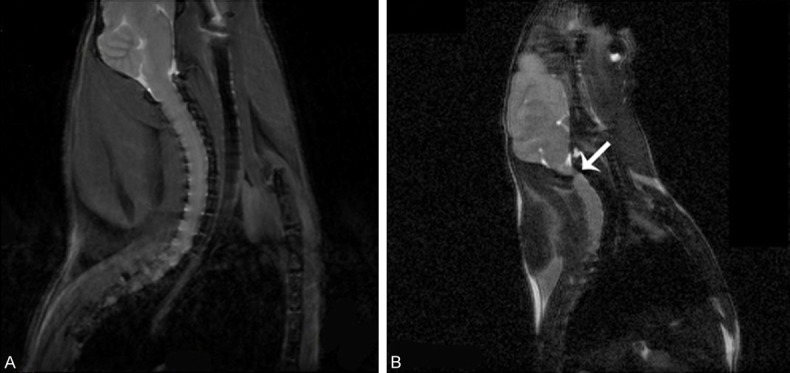
T2-weighted sagittal MR images. ICR mice aged 20 weeks (A) showed normal cervical spinal cord and aged-matched twy/twy mice showed obvious cervical spinal cord compression due to ectopic ossification (B, arrow).
Behavioral test
The catwalk gait was analyzed in ICR mice and twy/twy mice (Figure 2A). Results showed significant decreases in the stride length of four paws and the interlimb coordination in twy/twy mice as compared to ICR mice (P < 0.01) (Figure 2). However, after PF-3644022 treatment in twy/twy mice, the stride length of the right-fore, right-hind and left-fore paws and the interlimb coordination were significantly improved (P<0.05). This suggests that the inhibition of MK2 signaling pathway is able to improve the outcome and prevent the deterioration of neurological dysfunction of twy/twy mice.
Figure 2.
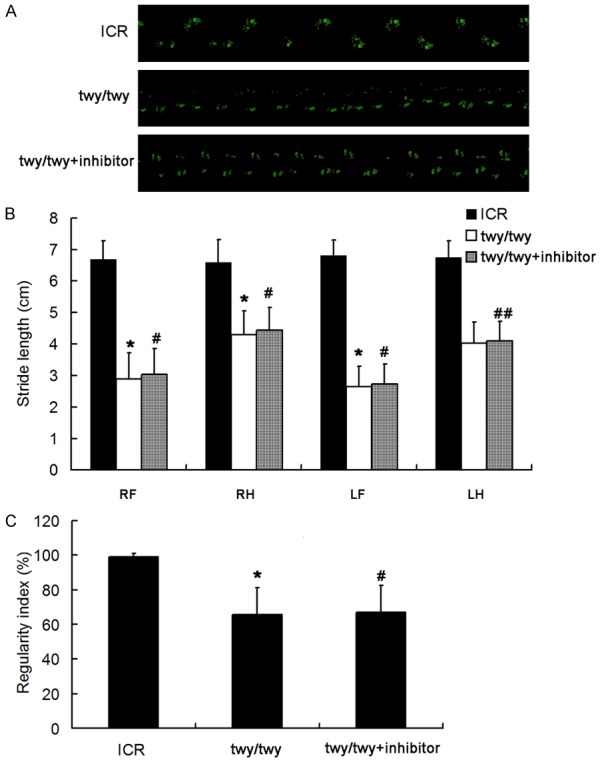
Inhibition of MK2 signaling pathway improves the neurological dysfunction in twy/twy mice. (A) Representative images of gait in three groups. (B) Effect of MK2 inhibitor on the stride length of four limbs of twy/twy mice. (C) Effect of MK2 inhibitor on the regularity index of twy/twy mice (n = 8 per group). Data are expressed as means ± SEM. *P < 0.01 vs ICR group; #P < 0.05 vs twy/twy group; ##P > 0.05 vs twy/twy group.
Western blot assay for MK2 and p-MK2
To investigate the role of MK2 signaling pathway in the pa-thogenesis of chronic compression of the cervical spinal cord, the expressions of total MK2 and p-MK2 were detected by Western blot assay. As shown in Figure 3, MK2 expression (49 kDa) increased in twy/twy mice as compared to controls. MK2 inhibitor PF-3644022 down-regulated MK2 expression in twy/twy mice (Figure 3). In addition, p-MK2 expression was up-regulated in twy/twy mice, which was reversed by the MK2 inhibitor (Figure 4).
Figure 3.
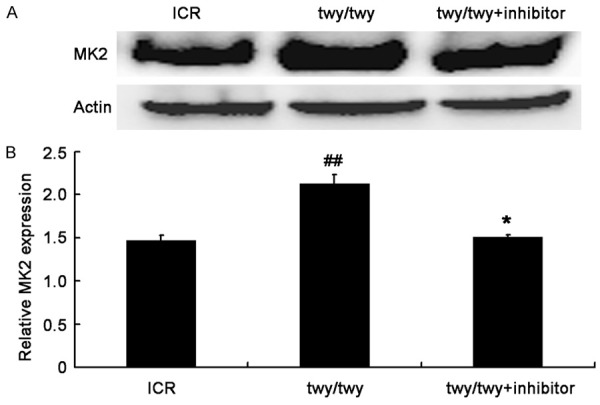
PF-3644022 (a MK2 inhibitor) down-regulated MK2 expression in twy/twy mice. A. Western blot assay of MK2 (49 kDa). B. Quantification of MK2 expression (n=4 for control and twy/twy groups; n=3 for MK2 inhibitor group). Data are expressed as means ± SEM. ##P<0.01 vs control group; *P<0.05 vs twy/twy mice.
Figure 4.
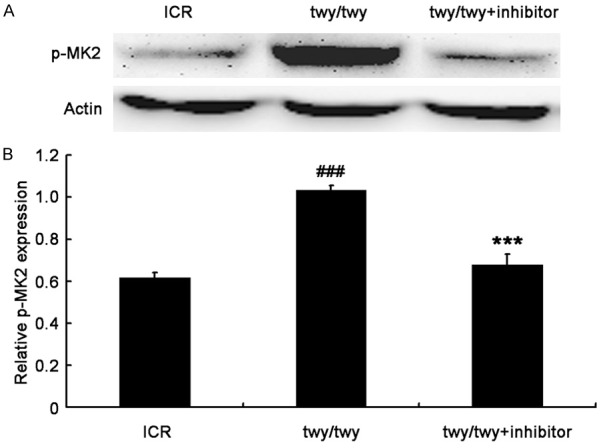
PF-3644022 (a MK2 inhibitor) significantly decreased p-MK2 expression in twy/twy mice. A. Western blot assay of p-MK2 expression (49 kDa) in the spinal cord of controls and twy/twy mice. B. Quantification of p-MK2 expression (n=4 for control and twy/twy groups; n=3 for MK2 inhibitor group). Data are expressed as mean ± SEM. ###P<0.001 vs control group; ***P<0.001 vs twy/twy mice.
Western blot assay for inflammatory cytokines
The IL-1β expression was significantly up-regulated in the spinal cord following chronic compression in twy/twy mice as compared to ICR mice (P<0.01, Figure 5). However, MK2 inhibitor reduced the IL-1β expression in twy/twy mice (P<0.05). Similar findings were also observed in the expressions of NF-κB and TNF-α, two important inflammatory cytokines (Figures 6 and 7). All these findings indicate that the inflammatory response was present in the cervical spinal cord undergoing chronic compression in twy/twy mice, and MK2 inhibitor is able to attenuate this inflammation.
Figure 5.
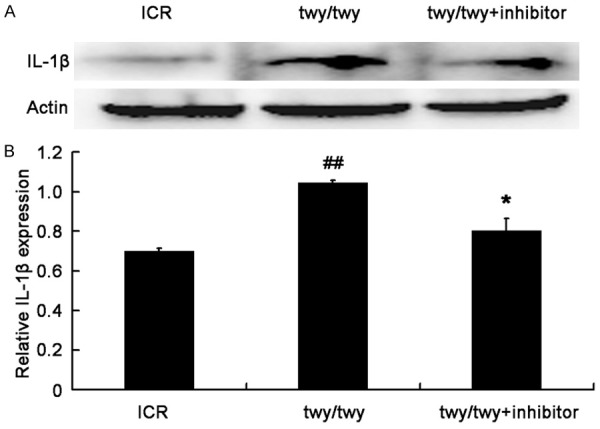
PF-3644022 (a MK2 inhibitor) decreased the IL-1β expression in twy/twy mice. A. Western blot assay of IL-1β expression in three groups: ICR mice, twy/twy mice, and twy/twy mice treated with MK2 inhibitor. B. Quantification of IL-1β expression (n=3 per group). Data are expressed as mean ± SEM. ##P<0.01 vs control mice; *P<0.05 vs twy/twy mice.
Figure 6.
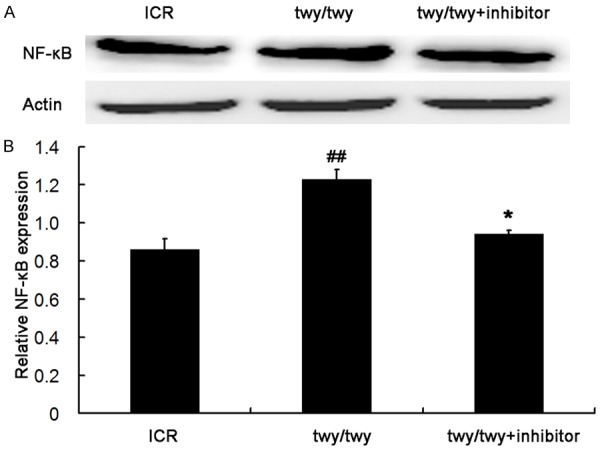
PF-3644022 (a MK2 inhibitor) down-regulated the NF-κB expression in twy/twy mice. A. Western blot assay of NF-κB expression in three groups: ICR mice, twy/twy mice, and twy/twy mice treated with MK2 inhibitor. B. Quantification of NF-κB expression (n=3 per group). Data are expressed as mean ± SEM. ##P<0.01 vs ICR mice; *P<0.05 vs twy/twy mice.
Figure 7.
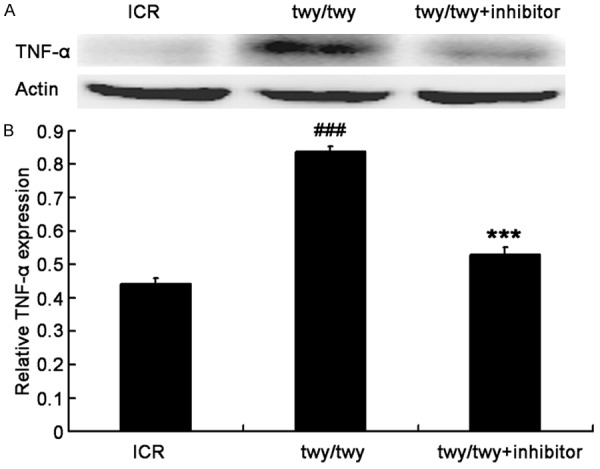
PF-3644022 (a MK2 inhibitor) down-regulated the TNF-α expression in twy/twy mice. A. Western blot assay of TNF-α expression in the spinal cord. B. Quantification of TNF-α expression (n=4 for control and twy/twy groups; n=3 for MK2 inhibitor group). Data are expressed as mean ± SEM. ###P<0.001 vs the control mice; ***P<0.001 vs twy/twy mice.
Western blot assay for apoptosis related proteins
A remarkable increase was observed in the cleavaged caspase-8 (Figure 8) in twy/twy mice as compared to ICR mice. However, MK2 inhibitor PF-3644022 reduced the cleavaged caspase-8 expression. In addition, the quantification of Bax and Bcl-2 expressions showed that the Bax/Bcl-2 ratio increased by 2.1 folds in twy/twy mice as compared to ICR mice, whereas this increase was attenuated by MK-2 inhibitor (Figure 9).
Figure 8.
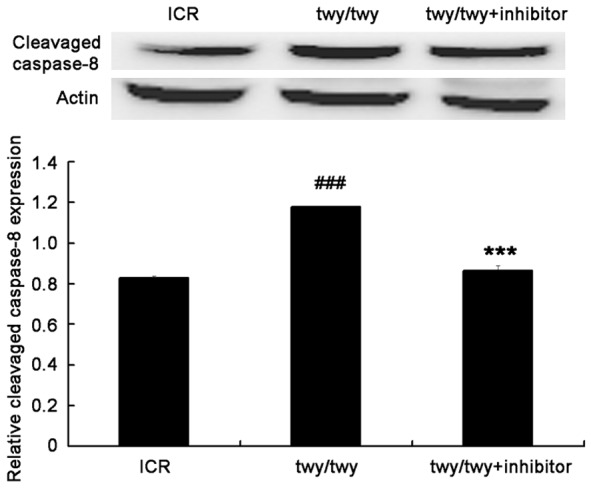
PF-3644022 (a MK2 inhibitor) down-regulated the cleavaged Caspase-8 expression in twy/twy mice. A. Western blot assay of cleavaged caspase-8 expression (18 kDa). B. Quantification of cleavaged caspase-8 expression (n=3 per group). Data are expressed as mean ± SEM. ##P<0.01 vs ICR mice; *P<0.05 vs twy/twy mice.
Figure 9.
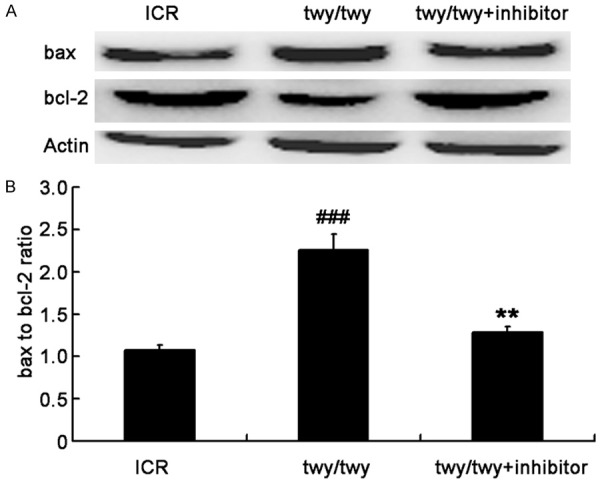
PF-3644022 (MK2 inhibitor) reduced the Bax/Bcl-2 ratio in twy/twy mice. The expressions of Bax (21 kDa) and Bcl-2 (26 kDa) in the spinal cord undergoing chronic compression were detected by Western blot assay. A. Western blot assay of Bax and Bcl-2 expressions. B. Quantification of Bax and Bcl-2 expressions and determination of Bax/Bcl-2 ratio (n=4 for control and twy/twy groups; n=3 for MK2 inhibitor group). Data are expressed as mean ± SEM. ###P<0.001 vs ICR mice; **P<0.01 vs twy/twy mice.
Discussion
Inflammation in the pathogenesis of chronic cervical spinal cord compression
To date, several methods have been applied to induce chronic compression of the cervical spinal cord (implantation of screws, induction of ectopic ossification and use of aromatic polyether) [13-15]. However, there are limitations in the application of these methods: implantation of screws needs screwing repeatedly, which may result in a high rate of infection or even high mortality; the ectopic ossification varies between individuals; application of aromatic polyether is still in its infancy. Mutant twy/twy mice with natural OPLL are available and suitable for the animal model. Extremity paresis in these mice is present as early as 3 weeks after birth and progresses gradually between 4 and 7 months. Currently, twy/twy mice have been used to investigate the chronic compression of the cervical spinal cord although they are expensive.
Studies on the pathology of acute spinal injury have indicated that inflammation is involved in its pathogenesis. However, few studies concern the pathology of chronic compression of the cervical spinal cord [16]. Several studies reveal that inflammation in the chronically oppressed spinal cord is characterized by significant increases in MMP-9, NF-κB and u-PA in a rabbit model. Nevertheless, the roles of these inflammatory cytokines in the chronic spinal cord compression are needed to be clarified further [1,17,18].
Among numerous inflammatory cytokines, TNF-α and IL-1β are regarded as key ones that are able to induce the inflammatory injury [19]. In apoptosis triggered by Fas, a subset of caspases is activated. Among them, caspase 8 activation is one of up-stream events during the cell apoptosis induced by Fas. Upon activation, cleavaged caspase 8 activates downstream caspase 3, thereby promoting cells to undergo apoptosis [20-22]. Bcl-2 is a key member of anti-apoptotic Bcl-2 family, but Bax is a pro-apoptotic member. The ratio of Bax to Bcl-2 has been found to be correlated with apoptosis [23-25]. In the present study, as compared to ICR mice, the expressions of TNF-α, IL-1β and NF-κB in the oppressed cervical spinal cord of twy/twy mice significantly increased, accompanied by increases in cleavaged caspase-8 expression and bax/bcl-2 ratio. Catwalk gait analysis indicated a remarkable decrease in the stride length of right-fore, right-hind and left-fore paw, and the interlimb coordination was also significantly compromised in twy/twy mice. These findings suggest that inflammation is involved in the pathology of chronic compression of the cervical spinal cord, and it may result in apoptosis and neurological dysfunction.
Role of MK2 signaling pathway in the chronic cervical spinal cord compression
MAPK is one of the most important signal transduction systems. It is involved in the regulations of cell reproduction, differentiation, apoptosis and other physiological and pathological processes. Three major pathways related to MAPK are extra-cellular signal regulated protein kinase (ERK), p38 MAPK and C-Jun amino-terminal kinase (JNK), among which p38a MAPK is an important pathway in the regulation of inflammatory reaction. The inhibitors of p38a MAPK have been applied in the treatment of many diseases, such as stroke and Alzheimer disease (AD). However, it may cause severe adverse events due to their by-products. Moreover, the target of such treatment is not clearly defined yet [26].
Recent studies have indicated that, after microglia activation, the downstream kinase MK2 of p38a MPAK pathway may increase the expressions of TNF-α, IL-1β and IL-6. These pro-inflammatory cytokines then activate p38a MAPK pathway via a positive feedback, and amplify the inflammatory cascade. In addition, there are no unwanted adverse events after MK2 inhibition and the target of MK2 inhibitor has been clearly defined. Thus, MK2 is an ideal target for the attenuation of inflammatory response [6,7].
In central nervous system, MK2 is mainly expressed in neurons, astrocytes and microglia. However, as p38a, MK2 expression in the microglia is 5-10 folds higher than in the neurons and astrocytes [27]. There is evidence showing that the inflammatory response is significantly improved in MK2 knockout animals with AD, indicating the pro-inflammatory effect of MK2 [7,28-30].
In the present study, the expressions of total MK2 and p-MK2 increased significantly in the cervical spinal cord undergoing chronic compression in twy/twy mice, which may enhance the inflammatory cytokines, induce the cell apoptosis in the spinal cord and cause neurological dysfunction. The enhanced inflammatory cytokines were able to stimulate the p38-MK2 signaling pathway. This positive feedback may aggravate the inflammation in the spinal cord. However, MK2 inhibitor reduced the MK2 and p-MK2 expressions as well as the expressions of inflammatory cytokines such as TNF-α, IL-1β and NF-κB and further decreased cell apoptosis in the spinal cord. These attributed to the improvement of the stride length and interlimb coordination. These findings demonstrate that MK2 signaling pathway is involved in the inflammation of the chronic compression induced spinal cord injury, and MK2 inhibitor PF-3644022 is able to attenuate this inflammation and facilitate the recovery of chronic spinal cord compression.
The p38a inhibitors, the pyridinyl imidazole class of compounds, were firstly reported in 1994 [31]. Of note, the small molecule inhibitor of MK2, aminocyanopyridine, has not been approved until 2005 [32]. Although MK2 seems to be an appropriate target in the treatment of central nervous system diseases, the MK2 inhibitors are in the stage of development currently, and further investigations are required to confirm our findings.
Conclusion
The present study for the first time finds that MK2 signaling pathway is involved in the inflammation of the chronic compression induced cervical spinal cord injury. And to inhibit MK2 is able to attenuate the inflammation and inhibit cell apoptosis in the cervical spinal cord, leading to significant improvement of neurological dysfunction. Our findings will be helpful to understand the molecular mechanisms in the pathogenesis of chronic cervical spinal cord compression and provide a new target for its therapy.
Acknowledgements
This study was supported by National Natural Science Foundation of China (No. 81470103).
Disclosure of conflict of interest
None.
References
- 1.Inukai T, Uchida K, Nakajima H, Yayama T, Kobayashi S, Mwaka ES, Guerrero AR, Baba H. Tumor necrosis factor-alpha and its receptors contribute to apoptosis of oligodendrocytes in the spinal cord of spinal hyperostotic mouse (twy/twy) sustaining chronic mechanical compression. Spine (Phila Pa 1976) 2009;34:2848–2857. doi: 10.1097/BRS.0b013e3181b0d078. [DOI] [PubMed] [Google Scholar]
- 2.Matsunaga S, Sakou T. Ossification of the posterior longitudinal ligament of the cervical spine: etiology and natural history. Spine (Phila Pa 1976) 2012;37:E309–314. doi: 10.1097/BRS.0b013e318241ad33. [DOI] [PubMed] [Google Scholar]
- 3.Song HX, Scarpatetti M, Kreil W, Shen HL, Bodo K, Ebner B, Schrottner H, Mokry M. Quantitative analysis of cyclooxygenase 2 in the posterior longitudinal ligament of cervical spondylotic myelopathy. Chin Med J (Engl) 2011;124:2480–2484. [PubMed] [Google Scholar]
- 4.Yu WR, Liu T, Kiehl TR, Fehlings MG. Human neuropathological and animal model evidence supporting a role for Fas-mediated apoptosis and inflammation in cervical spondylotic myelopathy. Brain. 2011;134:1277–1292. doi: 10.1093/brain/awr054. [DOI] [PubMed] [Google Scholar]
- 5.Xing B, Bachstetter AD, Van Eldik LJ. Microglial p38alpha MAPK is critical for LPSinduced neuron degeneration, through a mechanism involving TNFalpha. Mol Neurodegener. 2011;6:84. doi: 10.1186/1750-1326-6-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bachstetter AD, Van Eldik LJ. The p38 MAP Kinase Family as Regulators of Proinflammatory Cytokine Production in Degenerative Diseases of the CNS. Aging Dis. 2010;1:199–211. [PMC free article] [PubMed] [Google Scholar]
- 7.Arthur JS, Ley SC. Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol. 2013;13:679–692. doi: 10.1038/nri3495. [DOI] [PubMed] [Google Scholar]
- 8.Uchida K, Nakajima H, Watanabe S, Yayama T, Guerrero AR, Inukai T, Hirai T, Sugita D, Johnson WE, Baba H. Apoptosis of neurons and oligodendrocytes in the spinal cord of spinal hyperostotic mouse (twy/twy): possible pathomechanism of human cervical compressive myelopathy. Eur Spine J. 2012;21:490–497. doi: 10.1007/s00586-011-2025-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu WR, Baptiste DC, Liu T, Odrobina E, Stanisz GJ, Fehlings MG. Molecular mechanisms of spinal cord dysfunction and cell death in the spinal hyperostotic mouse: implications for the pathophysiology of human cervical spondylotic myelopathy. Neurobiol Dis. 2009;33:149–163. doi: 10.1016/j.nbd.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 10.Mourey RJ, Burnette BL, Brustkern SJ, Daniels JS, Hirsch JL, Hood WF, Meyers MJ, Mnich SJ, Pierce BS, Saabye MJ, Schindler JF, South SA, Webb EG, Zhang J, Anderson DR. A benzothiophene inhibitor of mitogen-activated protein kinase-activated protein kinase 2 inhibits tumor necrosis factor alpha production and has oral anti-inflammatory efficacy in acute and chronic models of inflammation. J Pharmacol Exp Ther. 2010;333:797–807. doi: 10.1124/jpet.110.166173. [DOI] [PubMed] [Google Scholar]
- 11.Masocha W, Parvathy SS. Assessment of weight bearing changes and pharmacological antinociception in mice with LPS-induced monoarthritis using the Catwalk gait analysis system. Life Sci. 2009;85:462–469. doi: 10.1016/j.lfs.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 12.Zhang YH, Wang Y, Yusufali AH, Ashby F, Zhang D, Yin ZF, Aslanidi GV, Srivastava A, Ling CQ, Ling C. Cytotoxic genes from traditional Chinese medicine inhibit tumor growth both in vitro and in vivo. J Integr Med. 2014;12:483–494. doi: 10.1016/s2095-4964(14)60057-1. [DOI] [PubMed] [Google Scholar]
- 13.Kanchiku T, Taguchi T, Kaneko K, Yonemura H, Kawai S, Gondo T. A new rabbit model for the study on cervical compressive myelopathy. J Orthop Res. 2001;19:605–613. doi: 10.1016/S0736-0266(00)00058-9. [DOI] [PubMed] [Google Scholar]
- 14.Mimatsu K, Kishi S, Hashizume Y. Experimental chronic compression on the spinal cord of the rabbit by ectopic bone formation in the ligamentum flavum with bone morphogenetic protein. Spinal Cord. 1997;35:740–746. doi: 10.1038/sj.sc.3100533. [DOI] [PubMed] [Google Scholar]
- 15.Klironomos G, Karadimas S, Mavrakis A, Mirilas P, Savvas I, Papadaki E, Papachristou DJ, Gatzounis G. New experimental rabbit animal model for cervical spondylotic myelopathy. Spinal Cord. 2011;49:1097–1102. doi: 10.1038/sc.2011.71. [DOI] [PubMed] [Google Scholar]
- 16.Yu WR, Fehlings MG. Fas/FasL-mediated apoptosis and inflammation are key features of acute human spinal cord injury: implications for translational, clinical application. Acta Neuropathol. 2011;122:747–761. doi: 10.1007/s00401-011-0882-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beattie MS, Manley GT. Tight squeeze, slow burn: inflammation and the aetiology of cervical myelopathy. Brain. 2011;134:1259–1261. doi: 10.1093/brain/awr088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karadimas SK, Klironomos G, Papachristou DJ, Papanikolaou S, Papadaki E, Gatzounis G. Immunohistochemical profile of NF-kappaB/p50, NF-kappaB/p65, MMP-9, MMP-2, and u-PA in experimental cervical spondylotic myelopathy. Spine (Phila Pa 1976) 2013;38:4–10. doi: 10.1097/BRS.0b013e318261ea6f. [DOI] [PubMed] [Google Scholar]
- 19.Basu A, Krady JK, Levison SW. Interleukin-1: a master regulator of neuroinflammation. J Neurosci Res. 2004;78:151–156. doi: 10.1002/jnr.20266. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Zhang DS, Ye JC, Li CM, Qi M, Liang DD, Xu XR, Xu L, Liu Y, Zhang H, Zhang YY, Deng FF, Feng J, Shi D, Chen JJ, Li L, Chen G, Sun YF, Peng LY, Chen YH. Dynamin-2 mediates heart failure by modulating Ca2+ -dependent cardiomyocyte apoptosis. Int J Cardiol. 2013;168:2109–2119. doi: 10.1016/j.ijcard.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Zhang C, Zhao J, Deng H. 17beta-estradiol up-regulates miR-155 expression and reduces TP53INP1 expression in MCF-7 breast cancer cells. Mol Cell Biochem. 2013;379:201–211. doi: 10.1007/s11010-013-1642-6. [DOI] [PubMed] [Google Scholar]
- 22.Harrington HA, Ho KL, Ghosh S, Tung KC. Construction and analysis of a modular model of caspase activation in apoptosis. Theor Biol Med Model. 2008;5:26. doi: 10.1186/1742-4682-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korsmeyer SJ, Shutter JR, Veis DJ, Merry DE, Oltvai ZN. Bcl-2/Bax: a rheostat that regulates an anti-oxidant pathway and cell death. Semin Cancer Biol. 1993;4:327–332. [PubMed] [Google Scholar]
- 24.Zhang L, Yu H, Sun Y, Lin X, Chen B, Tan C, Cao G, Wang Z. Protective effects of salidroside on hydrogen peroxide-induced apoptosis in SH-SY5Y human neuroblastoma cells. Eur J Pharmacol. 2007;564:18–25. doi: 10.1016/j.ejphar.2007.01.089. [DOI] [PubMed] [Google Scholar]
- 25.Zhao DL, Zou LB, Lin S, Shi JG, Zhu HB. 6,7-di-O-glucopyranosyl-esculetin protects SHSY5Y cells from dopamine-induced cytotoxicity. Eur J Pharmacol. 2008;580:329–338. doi: 10.1016/j.ejphar.2007.11.057. [DOI] [PubMed] [Google Scholar]
- 26.Duraisamy S, Bajpai M, Bughani U, Dastidar SG, Ray A, Chopra P. MK2: a novel molecular target for anti-inflammatory therapy. Expert Opin Ther Targets. 2008;12:921–936. doi: 10.1517/14728222.12.8.921. [DOI] [PubMed] [Google Scholar]
- 27.Culbert AA, Skaper SD, Howlett DR, Evans NA, Facci L, Soden PE, Seymour ZM, Guillot F, Gaestel M, Richardson JC. MAPK-activated protein kinase 2 deficiency in microglia inhibits pro-inflammatory mediator release and resultant neurotoxicity. Relevance to neuroinflammation in a transgenic mouse model of Alzheimer disease. J Biol Chem. 2006;281:23658–23667. doi: 10.1074/jbc.M513646200. [DOI] [PubMed] [Google Scholar]
- 28.MacKenzie KF, Van Den Bosch MW, Naqvi S, Elcombe SE, McGuire VA, Reith AD, Blackshear PJ, Dean JL, Arthur JS. MSK1 and MSK2 inhibit lipopolysaccharide-induced prostaglandin production via an interleukin-10 feedback loop. Mol Cell Biol. 2013;33:1456–1467. doi: 10.1128/MCB.01690-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naqvi S, Macdonald A, McCoy CE, Darragh J, Reith AD, Arthur JS. Characterization of the cellular action of the MSK inhibitor SB-747651A. Biochem J. 2012;441:347–357. doi: 10.1042/BJ20110970. [DOI] [PubMed] [Google Scholar]
- 30.Liu X, Wu T, Chi P. Inhibition of MK2 shows promise for preventing postoperative ileus in mice. J Surg Res. 2013;185:102–112. doi: 10.1016/j.jss.2013.05.028. [DOI] [PubMed] [Google Scholar]
- 31.Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D, McNulty D, Blumenthal MJ, Heys JR, Landvatter SW, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 32.Anderson DR, Hegde S, Reinhard E, Gomez L, Vernier WF, Lee L, Liu S, Sambandam A, Snider PA, Masih L. Aminocyanopyridine inhibitors of mitogen activated protein kinase-activated protein kinase 2 (MK-2) Bioorg Med Chem Lett. 2005;15:1587–1590. doi: 10.1016/j.bmcl.2005.01.067. [DOI] [PubMed] [Google Scholar]


