Abstract
microRNAs (miRNAs) are endogenously expressed, conserved and small noncoding RNA that regulate gene expression by the post-transcriptional level. In this study, we aim to examine the role of miR-873 in lung adenocarcinoma. We found that the expression of miR-873 was upregulated in four lung adenocarcinoma cell lines and tissues. In addition, the expression levels of SRCIN1 were inversely correlated with the expression levels of miR-873 in lung adenocarcinoma tissues. Furthermore, SRCIN1 was confirmed asthe direct target of miR-873 by luciferase reporter assay and Western blotting. Overexpression of miR-873 promoted the proliferation and migration of lung adenocarcinoma cells, while SRCIN1 upregulation inhibited their proliferation and migration. Restoration of SRCIN1 could significantly reverse the proliferation and migration promotion imposed by miR-873. In summary, this study reveals for the first time that miR-873 increase the lung adenocarcinoma cell proliferation and migration through directly inhibiting SRCIN1 expression.
Keywords: Lung adenocarcinoma, microRNA, miR-873, SRCIN1
Introduction
Lung cancer is one of the leading types of cancer-related mortality worldwide, responsible for nearly 1.4 million deaths each year [1-4]. Among all types of lung cancers, non-small cell lung cancer (NSCLC) makes up the majority (about 80%) of lung cancer patients [5-7]. The predominant type of NSCLC is lung adenocarcinoma [8-10]. It is very difficult for early diagnosis of NSCLC with the majority patients diagnosed at the advanced stage [11-15]. Therefore, the underlying molecular mechanisms of lung adenocarcinoma mignt provide novel markers for the lung adenocarcinoma diagnosed.
MicroRNAs (miRNAs) are small (21-25 nucleotides), non-coding endogenous RNAs that post-transcriptionally regulate the expression messenger RNAs (mRNAs) [16-20]. MiRNAs play crucial roles in complicated cell processes including cell differentiation, development, metabolism, invasion, proliferation, stress, migration and other processes [21,22]. Accumulated studies have shown that deregulated expression of miRNAs is observed in many human cancers such as gastric cancer, oesophageal cancer, ovarian cancer, Ewing’s sarcoma, gallbladder cancer and cutaneous lymphomas [18,21,23-27]. Furthermore, miRNAs may be identified as biomarkers for diagnosis and treatment in cancers.
In the present study, the expression of miR-873 was upregulated while the expression of SRCIN1 was downregulated in four lung adenocarcinoma cell lines and tissues. Moreover, the expression level of SRCIN1 was inversely correlated with the expression level of miR-873 in lung adenocarcinoma tissues. Furthermore, SRCIN1 was a direct target of miR-873. Overexpression of miR-873 promoted the lung adenocarcinoma cell proliferation and migration, while SRCIN1 upregulation inhibited the lung adenocarcinoma cell proliferation and migration. Restoration of SRCIN1 significantly reversed the proliferation and migration promotion induced by miR-873. These results suggest that regulation of SRCIN1 by miR-873 may be a potential therapeutic target for lung adenocarcinoma patients.
Material and methods
Patients and cell lines and cell transfection
Thirty lung adenocarcinoma and matched non-tumor tissues were collected from our Department. The samples were then stored in liquid nitrogen. Human lung adenocarcinoma cell lines (H23, H1299, A549 and SPC-A1) were collected from the Cell bank of the Chinese Academy of Medical Sciences (Beijing, China), and were cultured in RPMI 1640 medium. miRNA mimic and scramble were obtained from Ribobio (Guangzhou, China). Transfection was done using the Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) following to the manufacturer’s description.
Quantitative RT-PCR
Total RNA was extracted from tissues or cells by using TRIzol. The expression levels of miR-873 were quantified by qRT-PCR using TaqMan microRNA assays (Applied Biosystems) on a LightCycler 480 System II (Roche). The expression of SRCIN1 was measured using SYBRGreen assays. SRCIN1 primer: forward 5’-AGCCCCGACAAAAGCAAAC-3’, reverse 5’-CCAAAGGAAGTCAATACAGGGATAG-3’; GAPDH primer: forward 5’-ATGTCGTGGAGTCTACTGGC-3’, reverse 5’-TGACCTTGCCCACAGCCTTG-3’. The small nuclear RNA U6 was used as control for miR-873. GAPDH was used as control for SRCIN1.
Luciferase assays
Cells were cultured at 3×105 cells/wells in 24 cell plates. Luciferase assays were performed according to previous studies. Cells were co-transfected with miR-873 mimic or scramble and pGL3-SRCIN1-3’UTR, pGL3-SRCIN1-3’UTR Mut plasmid and phRL-SV40 control vector (Promega, Beijing, China) by using the Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). Relative luciferase data was normalized to Renilla luciferase activity.
Cell proliferation and migration
CCK8 (Dojindo, Kumamoto, Japan) was used to measure the cell proliferation. Cells were seeded in 96 cell plates and cultured for 0 h, 24 h, 48 h and 72 h respectively. The 450 nm absorbance was read on a microplate reader. For migration assay, wound-healing was performed. An artificial wound was performed using 200-ll pipette tip and mitomyclin C was added to cells. Images of migrated cells were taken on a phase-contrast microscope.
Western blots
Proteins were isolated and detected by using the BCA kit (Thermo Scientific, Rockford, IL). Proteins was separated by 12% SDS-PAGE and then transferred to membranes (PVDF, Millipore, and Danvers, MA). Membranes were probed with antibodies: SRCIN1 or GAPDH (Cell Signaling Technology, Danvers, MA). GAPDH were used for the loading control.
Statistical analysis
The statistical significance between two groups was performed using Student’s t-tests and between more than two groups was performed using ANOVA. Data was shown as mean ± standard deviation. p<0.05 was considered as statistically significant.
Results
The expression of SRCIN1 was downregulated while miR-873 expression was upregulated in lung adenocarcinoma cell lines
We showed that the expression of SRCIN1 was downregulated in four lung adenocarcinoma cell lines (H23, H1299, A549 and SPC-A1) and one lung adenocarcinoma sample compared to in one adjacent nonneoplastic tissue (Figure 1A). However, the expression of miR-873 was upregulated in four lung adenocarcinoma cell lines (H23, H1299, A549 and SPC-A1) and one lung adenocarcinoma sample compared with that in one adjacent nonneoplastic tissue (Figure 1B).
Figure 1.
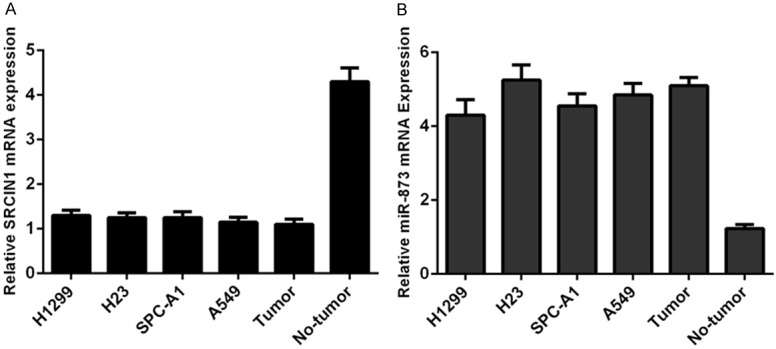
The expression of SRCIN1 was downregulated and miR-873 expression was upregulated in lung adenocarcinoma cell lines. A. The expression of SRCIN1 in four lung adenocarcinoma cell lines (H23, H1299, A549 and SPC-A1) and one lung adenocarcinoma sample and adjacent nonneoplastic tissue was examined by qRT-PCR. B. The expression of miR-873 in four lung adenocarcinoma cell lines (H23, H1299, A549 and SPC-A1) and one lung adenocarcinoma sample and adjacent nonneoplastic tissue was examined by qRT-PCR.
The expression of SRCIN1 was downregulated and miR-873 expression was upregulated in lung adenocarcinoma
The expression of SRCIN1 was lower in lung adenocarcinoma tissues compared with that in adjacent no-tumor tissues (Figure 2A). The expression of SRCIN1 was downregulated in 23 cases (23/30, 77%) in comparison with the adjacent tissues among the 30 lung adenocarcinoma samples measured (Figure 2B). The expression of miR-873 was higher in lung adenocarcinoma tissues compared with adjacent no-tumor tissues (Figure 2C). The expression of miR-873 was upregulated in 22 cases (22/30, 73%) in comparison with the adjacent tissues among the 30 lung adenocarcinoma samples measured (Figure 2D). Moreover, the expression level of SRCIN1 was inversely correlated with the expression level of miR-873 in lung adenocarcinoma tissues (Figure 3).
Figure 2.
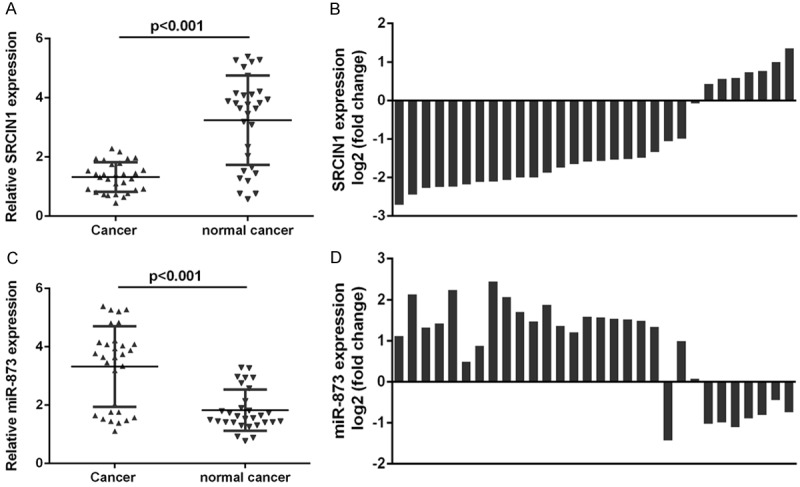
The expression of SRCIN1 was downregulated and miR-873 expression was upregulated in lung adenocarcinoma. A. The expression of SRCIN was detected by qRT-PCR. B. The expression of SRCIN1 was down-regulated in 23 cases (23/30, 77%) in comparison with the adjacent tissues. C. The expression of miR-873 was detected by qRT-PCR. D. The expression of miR-873 was up-regulated in 22 cases (22/30, 73%) in comparison with the adjacent tissues.
Figure 3.
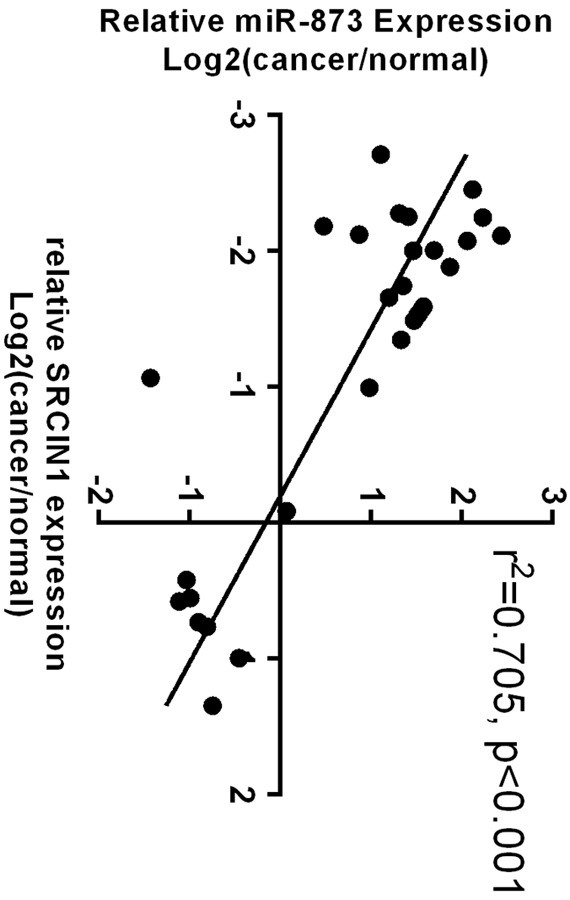
The expression of SRCIN1 was inversely correlated with the expression of miR-873 in lung adenocarcinoma tissues.
SRCIN1 was a direct target of miR-873
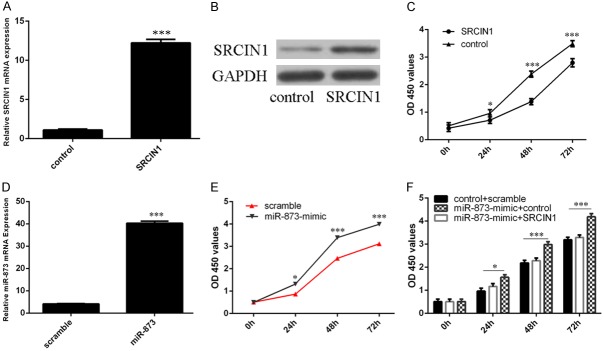
The predicted interactions between miR-873 and its target sites in the SRCIN1 3’-UTR are showed in Figure 4A. MiR-873 overexpression inhibited the luciferase activity of the reporter gene with the wild-type construct but not the mutant type (Figure 4B). Overexpression of miR-873 repressed the expression of SRCIN1 in the A549 cell.
Figure 4.
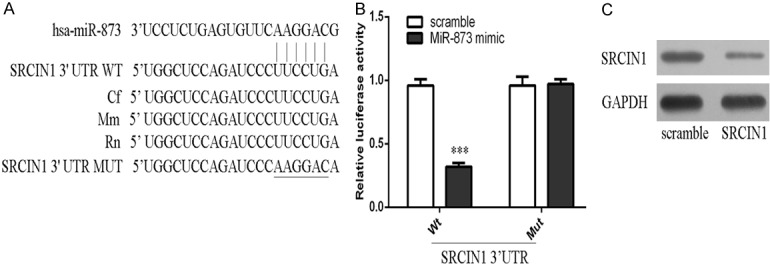
SRCIN1 was a direct target of miR-873. A. The predicted interactions between miR-873 and its target sites in the SRCIN1 3’-UTR are showed. B. miR-873 overexpression inhibited the luciferase activity of the reporter gene with the wild-type construct but not the mutant SRCIN1 3’UTR construct. C. The protein expression of SRCIN1 was measured by western blot. ***p<0.001.
MiR-873 promoted lung adenocarcinoma cell proliferation
The expression of SRCIN1 was increased after treated by SRCIN1 vector (Figure 5A and 5B). Overexpression of SRCIN1 inhibited the A549 cell proliferation (Figure 5C). The expression level of miR-873 was increased after treated by miR-873 mimic (Figure 5D). Overexpression of miR-873 promoted the A549 cell proliferation (Figure 5E). Restoration of SRCIN1 could significantly reverse the proliferation promotion imposed by miR-873 (Figure 5F).
Figure 5.
miR-873 promoted lung adenocarcinoma cell proliferation. A. The mRNA expression of SRCIN1 was measured by qRT-PCR. B. The protein expression of SRCIN1 was measured by western blot. C. CCK-8 was performed to measure the cell proliferation. D. The mRNA expression of miR-873 was measured by qRT-PCR. E. CCK-8 was performed to measure the cell proliferation. F. Restoration of SRCIN1 could significantly reverse the proliferation promotion imposed by miR-873. *p<0.05, **p<0.01 and ***p<0.001.
MiR-873 promoted lung adenocarcinoma cell migration
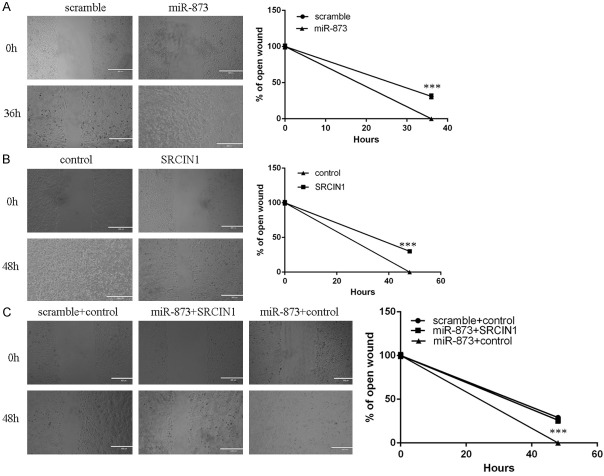
Overexpression of SRCIN1 inhibited the A549 cell migration (Figure 6A). Overexpression of miR-873 promoted the A549 cell migration (Figure 6B). Restoration of SRCIN1 could significantly reverse the migration promotion imposed by miR-873 (Figure 5F).
Figure 6.
miR-873 promoted lung adenocarcinoma cell migration. A. Wound-healing analysis has been performed to detect the A549 cell migration. B. Overexpression of miR-873 promoted the A549 cell migration. C. Restoration of SRCIN1 could significantly reverse the migration promotion imposed by miR-873. ***p<0.001.
Discussion
Lung adenocarcinoma carcinogenesis is a complicated process with many changes in physiological structure and gene expression [12,13,28]. Increasing evidences have demonstrated that miRNA are involved in a variety of biological processes including transcriptional cell differentiation, cell regulation and carcinogenesis [15,29,30]. In the present study, the expression of miR-873 was upregulated while the expression of SRCIN1 was downregulated in four lung adenocarcinoma cell lines and tissues. Moreover, the expression level of SRCIN1 was inversely correlated with the expression level of miR-873 in lung adenocarcinoma tissues. Furthermore, SRCIN1 was a direct target of miR-873. Overexpression of miR-873 promoted the lung adenocarcinoma cell proliferation and migration, while SRCIN1 inhibited its proliferation and migration. Restoration of SRCIN1 could significantly reverse the proliferation and migration promotion imposed by miR-873. These results suggest that regulation of SRCIN1 by miR-873 may be a potential therapeutic target for lung adenocarcinoma therapy.
Previous studies demonstrated that miR-873 was downregulated in glioblastoma and colorectal cancer [31,32]. For example, Wang et al. demonstrated that miR-873 was downregulated in glioblastoma multiforme and its overexpression inhibited the GBM cell proliferation, migration, and invasion [32]. Cui et al. showed that miR-873 was downregulated in breast tumor and overexpression of miR-873 inhibited the transcriptional activity of estrogen receptor-α [33]. However, the role of miR-873 in lung adenocarcinoma is still unknown. In our study, the expression of miR-873 was upregulated in four lung adenocarcinoma cell lines and tissues. Furthermore, overexpression of miR-873 suppressed the lung adenocarcinoma cell proliferation and migration. These results proved that miR-873 might act as an oncogene in lung adenocarcinoma.
SRCIN1, previously known as p140 cas-associated protein (p140CAP), contains two amino acids, two proline-rich regions and two coiled-coil domains [34-36]. Previous studies showed that SRCIN1 was involved in tumor development and progression [37,38]. For example, overexpression of SRCIN1 inhibited the breast cancer cells spreading, migration and invasion [39]. Moreover, Xu et al. showed that miR-374a acted as an oncogene in gastric cancer by directly inhibiting SRCIN1 [40]. In our study, the expression of SRCIN1 was inversely correlated with the expression of miR-873 in lung adenocarcinoma tissues. Luciferase reporter and western blot assay demonstrated that SRCIN1 was a direct target of miR-873. Restoration of SRCIN1 could significantly reverse the proliferation and migration promotion imposed by miR-873.
In conclusion, this study revealed for the first time that miR-873 increased the lung adenocarcinoma cell proliferation and migration through directly inhibiting SRCIN1 expression. This study also indicated a potential diagnostic and therapeutic target for lung adenocarcinoma treatment.
References
- 1.Ohdaira H, Sekiguchi M, Miyata K, Yoshida K. MicroRNA-494 suppresses cell proliferation and induces senescence in A549 lung cancer cells. Cell Prolif. 2012;45:32–38. doi: 10.1111/j.1365-2184.2011.00798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu T, Li J, Yan M, Liu L, Lin H, Zhao F, Sun L, Zhang Y, Cui Y, Zhang F, Li J, He X, Yao M. MicroRNA-193a-3p and -5p suppress the metastasis of human non-small-cell lung cancer by downregulating the ERBB4/PIK3R3/mTOR/S6K2 signaling pathway. Oncogene. 2015;34:413–423. doi: 10.1038/onc.2013.574. [DOI] [PubMed] [Google Scholar]
- 3.Yu SH, Zhang CL, Dong FS, Zhang YM. miR-99a suppresses the metastasis of human nonsmall cell lung cancer cells by targeting AKT1 signaling pathway. J Cell Biochem. 2015;116:268–276. doi: 10.1002/jcb.24965. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J, Xu L, Yang Z, Lu H, Hu D, Li W, Zhang Z, Liu B, Ma S. MicroRNA-10b indicates a poor prognosis of non-small cell lung cancer and targets E-cadherin. Clin Transl Oncol. 2015;17:209–214. doi: 10.1007/s12094-014-1213-7. [DOI] [PubMed] [Google Scholar]
- 5.Liu C, Yang H, Xu Z, Li D, Zhou M, Xiao K, Shi Z, Zhu L, Yang L, Zhou R. microRNA-548l is involved in the migration and invasion of nonsmall cell lung cancer by targeting the AKT1 signaling pathway. J Cancer Res Clin Oncol. 2015;141:431–441. doi: 10.1007/s00432-014-1836-7. [DOI] [PubMed] [Google Scholar]
- 6.Ma Y, Li X, Cheng S, Wei W, Li Y. MicroRNA-106a confers cisplatin resistance in non-small cell lung cancer A549 cells by targeting adenosine triphosphatase-binding cassette A1. Mol Med Rep. 2015;11:625–632. doi: 10.3892/mmr.2014.2688. [DOI] [PubMed] [Google Scholar]
- 7.Lee HY, Han SS, Rhee H, Park JH, Lee JS, Oh YM, Choi SS, Shin SH, Kim WJ. Differential expression of microRNAs and their target genes in non-small-cell lung cancer. Mol Med Rep. 2015;11:2034–2040. doi: 10.3892/mmr.2014.2890. [DOI] [PubMed] [Google Scholar]
- 8.Meng W, Ye Z, Cui R, Perry J, Dedousi-Huebner V, Huebner A, Wang Y, Li B, Volinia S, Nakanishi H, Kim T, Suh SS, Ayers LW, Ross P, Croce CM, Chakravarti A, Jin VX, Lautenschlaeger T. MicroRNA-31 predicts the presence of lymph node metastases and survival in patients with lung adenocarcinoma. Clin Cancer Res. 2013;19:5423–5433. doi: 10.1158/1078-0432.CCR-13-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu YJ, Lin YF, Chen YF, Luo EC, Sher YP, Tsai MH, Chuang EY, Lai LC. MicroRNA-449a enhances radiosensitivity in CL1-0 lung adenocarcinoma cells. PLoS One. 2013;8:e62383. doi: 10.1371/journal.pone.0062383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang H, Su Y, Xu F, Kong J, Yu H, Qian B. Circulating microRNAs in relation to EGFR status and survival of lung adenocarcinoma in female non-smokers. PLoS One. 2013;8:e81408. doi: 10.1371/journal.pone.0081408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin S, Sun JG, Wu JB, Long HX, Zhu CH, Xiang T, Ma H, Zhao ZQ, Yao Q, Zhang AM, Zhu B, Chen ZT. Aberrant microRNAs expression in CD133(+)/CD326(+) human lung adenocarcinoma initiating cells from A549. Mol Cells. 2012;33:277–283. doi: 10.1007/s10059-012-2252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rothschild SI, Tschan MP, Federzoni EA, Jaggi R, Fey MF, Gugger M, Gautschi O. MicroRNA-29b is involved in the Src-ID1 signaling pathway and is dysregulated in human lung adenocarcinoma. Oncogene. 2012;31:4221–4232. doi: 10.1038/onc.2011.578. [DOI] [PubMed] [Google Scholar]
- 13.Feng B, Wang R, Song HZ, Chen LB. MicroRNA-200b reverses chemoresistance of docetaxelresistant human lung adenocarcinoma cells by targeting E2F3. Cancer. 2012;118:3365–3376. doi: 10.1002/cncr.26560. [DOI] [PubMed] [Google Scholar]
- 14.Han HS, Yun J, Lim SN, Han JH, Lee KH, Kim ST, Kang MH, Son SM, Lee YM, Choi SY, Yun SJ, Kim WJ, Lee OJ. Downregulation of cell-free miR-198 as a diagnostic biomarker for lung adenocarcinoma-associated malignant pleural effusion. Int J Cancer. 2013;133:645–652. doi: 10.1002/ijc.28054. [DOI] [PubMed] [Google Scholar]
- 15.Song Q, Xu Y, Yang C, Chen Z, Jia C, Chen J, Zhang Y, Lai P, Fan X, Zhou X, Lin J, Li M, Ma W, Luo S, Bai X. miR-483-5p promotes invasion and metastasis of lung adenocarcinoma by targeting RhoGDI1 and ALCAM. Cancer Res. 2014;74:3031–3042. doi: 10.1158/0008-5472.CAN-13-2193. [DOI] [PubMed] [Google Scholar]
- 16.Tejero R, Navarro A, Campayo M, Viñolas N, Marrades RM, Cordeiro A, Ruíz-Martínez M, Santasusagna S, Molins L, Ramirez J, Monzó M. miR-141 and miR-200c as markers of overall survival in early stage non-small cell lung cancer adenocarcinoma. PLoS One. 2014;9:e101899. doi: 10.1371/journal.pone.0101899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu L, Ding GF, He C, Sun L, Jiang Y, Zhu L. MicroRNA-424 is down-regulated in hepatocellular carcinoma and suppresses cell migration and invasion through c-Myb. PLoS One. 2014;9:e91661. doi: 10.1371/journal.pone.0091661. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Yu X, Li Z, Liu J. MiRNAs in primary cutaneous lymphomas. Cell Prolif. 2015;48:271–277. doi: 10.1111/cpr.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Z, Yu X, Shen J, Chan MT, Wu WK. MicroRNA in intervertebral disc degeneration. Cell Prolif. 2015;48:278–283. doi: 10.1111/cpr.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li M, Yu M, Liu C, Zhu H, He X, Peng S, Hua J. miR-34c works downstream of p53 leading to dairy goat male germline stem-cell (mGSCs) apoptosis. Cell Prolif. 2013;46:223–231. doi: 10.1111/cpr.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Z, Yu X, Shen J, Wu WK, Chan MT. MicroRNA expression and its clinical implications in Ewing’s sarcoma. Cell Prolif. 2015;48:1–6. doi: 10.1111/cpr.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu X, Li Z, Shen J, Wu WK, Liang J, Weng X, Qiu G. MicroRNA-10b Promotes Nucleus Pulposus Cell Proliferation through RhoC-Akt Pathway by Targeting HOXD10 in Intervetebral Disc Degeneration. PLoS One. 2013;8:e83080. doi: 10.1371/journal.pone.0083080. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Li J, You T, Jing J. MiR-125b inhibits cell biological progression of Ewing’s sarcoma by suppressing the PI3K/Akt signalling pathway. Cell Prolif. 2014;47:152–160. doi: 10.1111/cpr.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang J, Zhang SY, Gao YM, Liu YF, Liu YB, Zhao ZG, Yang K. MicroRNAs as oncogenes or tumour suppressors in oesophageal cancer: potential biomarkers and therapeutic targets. Cell Prolif. 2014;47:277–286. doi: 10.1111/cpr.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo X, Dong Z, Chen Y, Yang L, Lai D. Enrichment of ovarian cancer stem-like cells is associated with epithelial to mesenchymal transition through an miRNA-activated AKT pathway. Cell Prolif. 2013;46:436–446. doi: 10.1111/cpr.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Z, Yu X, Wang Y, Shen J, Wu WK, Liang J, Feng F. By downregulating TIAM1 expression, microRNA-329 suppresses gastric cancer invasion and growth. Oncotarget. 2015;6:17559–69. doi: 10.18632/oncotarget.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Z, Yu X, Shen J, Law PT, Chan MT, Wu WK. MicroRNA expression and its implications for diagnosis and therapy of gallbladder cancer. Oncotarget. 2015;6:13914–13924. doi: 10.18632/oncotarget.4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho WC, Chow AS, Au JS. Restoration of tumour suppressor hsa-miR-145 inhibits cancer cell growth in lung adenocarcinoma patients with epidermal growth factor receptor mutation. Eur J Cancer. 2009;45:2197–2206. doi: 10.1016/j.ejca.2009.04.039. [DOI] [PubMed] [Google Scholar]
- 29.Zhao C, Xu Y, Zhang Y, Tan W, Xue J, Yang Z, Zhang Y, Lu Y, Hu X. Downregulation of miR-145 contributes to lung adenocarcinoma cell growth to form brain metastases. Oncol Rep. 2013;30:2027–2034. doi: 10.3892/or.2013.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baglio SR, Devescovi V, Granchi D, Baldini N. MicroRNA expression profiling of human bone marrow mesenchymal stem cells during osteogenic differentiation reveals Osterix regulation by miR-31. Gene. 2013;527:321–331. doi: 10.1016/j.gene.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki H, Takatsuka S, Akashi H, Yamamoto E, Nojima M, Maruyama R, Kai M, Yamano HO, Sasaki Y, Tokino T, Shinomura Y, Imai K, Toyota M. Genome-wide profiling of chromatin signatures reveals epigenetic regulation of MicroRNA genes in colorectal cancer. Cancer Res. 2011;71:5646–5658. doi: 10.1158/0008-5472.CAN-11-1076. [DOI] [PubMed] [Google Scholar]
- 32.Wang RJ, Li JW, Bao BH, Wu HC, Du ZH, Su JL, Zhang MH, Liang HQ. MicroRNA-873 (miRNA-873) inhibits glioblastoma tumorigenesis and metastasis by suppressing the expression of IGF2BP1. J Biol Chem. 2015;290:8938–8948. doi: 10.1074/jbc.M114.624700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cui J, Yang Y, Li H, Leng Y, Qian K, Huang Q, Zhang C, Lu Z, Chen J, Sun T, Wu R, Sun Y, Song H, Wei X, Jing P, Yang X, Zhang C. MiR-873 regulates ERalpha transcriptional activity and tamoxifen resistance via targeting CDK3 in breast cancer cells. Oncogene. 2015;34:4018. doi: 10.1038/onc.2015.201. [DOI] [PubMed] [Google Scholar]
- 34.Cao M, Hou D, Liang H, Gong F, Wang Y, Yan X, Jiang X, Wang C, Zhang J, Zen K, Zhang CY, Chen X. miR-150 promotes the proliferation and migration of lung cancer cells by targeting SRC kinase signalling inhibitor 1. Eur J Cancer. 2014;50:1013–1024. doi: 10.1016/j.ejca.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 35.Ito H, Atsuzawa K, Sudo K, Di Stefano P, Iwamoto I, Morishita R, Takei S, Semba R, Defilippi P, Asano T, Usuda N, Nagata K. Characterization of a multidomain adaptor protein, p140Cap, as part of a pre-synaptic complex. J Neurochem. 2008;107:61–72. doi: 10.1111/j.1471-4159.2008.05585.x. [DOI] [PubMed] [Google Scholar]
- 36.Damiano L, Di Stefano P, Camacho Leal MP, Barba M, Mainiero F, Cabodi S, Tordella L, Sapino A, Castellano I, Canel M, Frame M, Turco E, Defilippi P. p140Cap dual regulation of E-cadherin/EGFR cross-talk and Ras signalling in tumour cell scatter and proliferation. Oncogene. 2010;29:3677–3690. doi: 10.1038/onc.2010.128. [DOI] [PubMed] [Google Scholar]
- 37.Di Stefano P, Leal MP, Tornillo G, Bisaro B, Repetto D, Pincini A, Santopietro E, Sharma N, Turco E, Cabodi S, Defilippi P. The adaptor proteins p140CAP and p130CAS as molecular hubs in cell migration and invasion of cancer cells. Am J Cancer Res. 2011;1:663–673. [PMC free article] [PubMed] [Google Scholar]
- 38.Sharma N, Repetto D, Aramu S, Grasso S, Russo I, Fiorentino A, Mello-Grand M, Cabodi S, Singh V, Chiorino G, Turco E, Stefano PD, Defilippi P. Identification of two regions in the p140Cap adaptor protein that retain the ability to suppress tumor cell properties. Am J Cancer Res. 2013;3:290–301. [PMC free article] [PubMed] [Google Scholar]
- 39.Di Stefano P, Damiano L, Cabodi S, Aramu S, Tordella L, Praduroux A, Piva R, Cavallo F, Forni G, Silengo L, Tarone G, Turco E, Defilippi P. p140Cap protein suppresses tumour cell properties, regulating Csk and Src kinase activity. EMBO J. 2007;26:2843–2855. doi: 10.1038/sj.emboj.7601724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu X, Wang W, Su N, Zhu X, Yao J, Gao W, Hu Z, Sun Y. miR-374a promotes cell proliferation, migration and invasion by targeting SRCIN1 in gastric cancer. FEBS Lett. 2015;589:407–413. doi: 10.1016/j.febslet.2014.12.027. [DOI] [PubMed] [Google Scholar]