Summary
Staining of molecules such as proteins and glycoconjugates allows for an analysis of their localization within the cell and provides insight into their functional status. Glycosyltransferases, a class of enzymes which are responsible for glycosylating host proteins, are mostly localized to the Golgi apparatus, and their localization is maintained in part by a protein vesicular tethering complex, the conserved oligomeric Golgi (COG) complex. Here we detail a combination of fluorescent lectin and immuno-staining in cells depleted of COG complex subunits to examine the status of Golgi glycosyltransferases. The combination of these techniques allows for a detailed characterization of the changes in function and localization of Golgi glycosyltransferases with respect to transient COG subunit depletion.
Keywords: Conserved oligomeric Golgi (COG) complex, Golgi, glycosyltransferases, immunofluorescence, lectin, siRNA knockdown
1. Introduction
The conserved oligomeric Golgi (COG) complex is a hetero-oligomeric protein complex that functions to tether intra-Golgi vesicles during vesicular trafficking. Vesicular trafficking, which occurs in both an anterograde (forward) and retrograde (reverse) direction(1), is responsible for maintaining the localization of resident Golgi proteins, like glycosyltransferases, to their correct Golgi cisternae. Maintaining the correct localization of glycosyltransferases is crucial for the accurate glycosylation of host proteins. (2)
The COG complex consists of eight proteins, named COG1-8 (3–6) which have been grouped into two lobes, COG1-4 in lobe A, and COG5-8 in lobe B (6–8). Mutations or depletions of COG complex subunits result in the improper glycosylation of a cells total glycoconjugates (2, 9–11). In humans, these defects in glycosylation manifest in multiple organ system pathologies referred to as congenital disorders of glycosylation (CDG) (12). Currently, patients with CDG’s stemming from defects in COG subunits COG1, COG4, COG5, COG6, COG7, and COG8 have been identified (13–21).
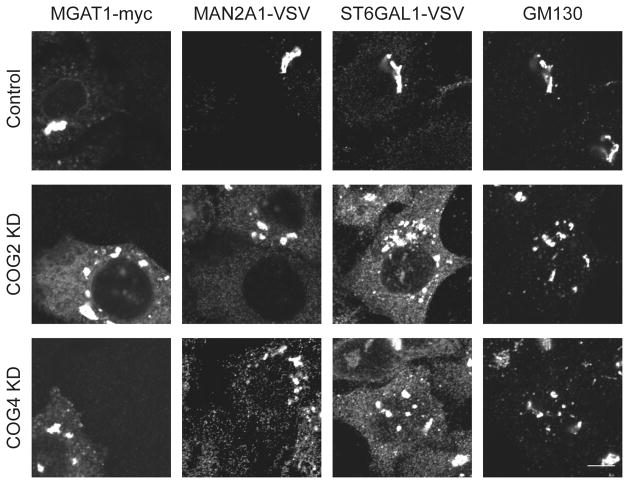
To properly assess the abnormalities of steady state conditions for Golgi glycosyltransferases that result from COG complex depletion, we have employed the use of fluorescent microscopy. The glycosyltransferases MAGT1 (α-1,3-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase), MAN2A1 (α-mannosidase II), and ST6GAL1-VSV (β-galactosidase α-2, 6-siayltransferase 1) are all localized to the Golgi apparatus under wild type conditions (MAGT1 localizes to cis-Golgi membranes, MAN2A1 localizes to medial-Golgi membranes, and ST6GAL1 localizes to trans-Golgi membranes). To determine the localization of these glycosyltransferases, we use stable cell lines expressing a tagged version of MAGT1-myc, MAN2A1-VSV, and ST6GAL1-VSV. Without a functional COG complex, the retrograde vesicles used to recycle the glycosyltransferases are not tethered to the Golgi cisternae where they function, and subsequently the protein is not recycled. Upon depletion of the COG complex the enzymes are now partially mislocalized, being found on both small vesicle like membranes and fragmented Golgi mini stacks, (Figure 1) (2). The mislocalization of these enzymes results in the incomplete processing of glycoconjugates. In particular, reduced activity of MAGT1 and MAN2A1 will increase a population of glycoconjugates with the immature terminal mannose residues, while the reduced activity of ST6GAL1 will increase a population of glycoconjugates with terminal nonreducing N-acetyl-D-glucosaminyl residues. The majority of cell synthetized glycoconjugates are destined for the plasma membrane and therefore are detectable by the lectin staining.
Figure 1. Localization of Golgi enzymes in COG KD cells.
HeLa cells that stably express VSV or myc-tagged Golgi enzymes were mock-transfected or transfected with siRNA to COG2 or COG4. 96 hours after transfection cells were fixed, stained with antibodies as indicated and analyzed by laser confocal fluorescent microscopy. In control cells MGAT1-myc (cis-Golgi localized glycosyltransferase), MAN2A-VSV (medial-Golgi localized glycosyltransferase), and ST6GAL1-VSV (trans-Golgi localized glycosyltransferase) localized to the perinuclear area and co-localized with the Golgi marker GM130. In COG2 and COG4 depleted cells the glycosyltransferases were severely mis-localized, now being found in the periphery on vesicle like structures as well as fragmented Golgi mini-stacks (Golgi marker GM130 positive membranes). These results indicate a severe mislocalization of glycosyltransferases upon depletion of COG complex subunits.
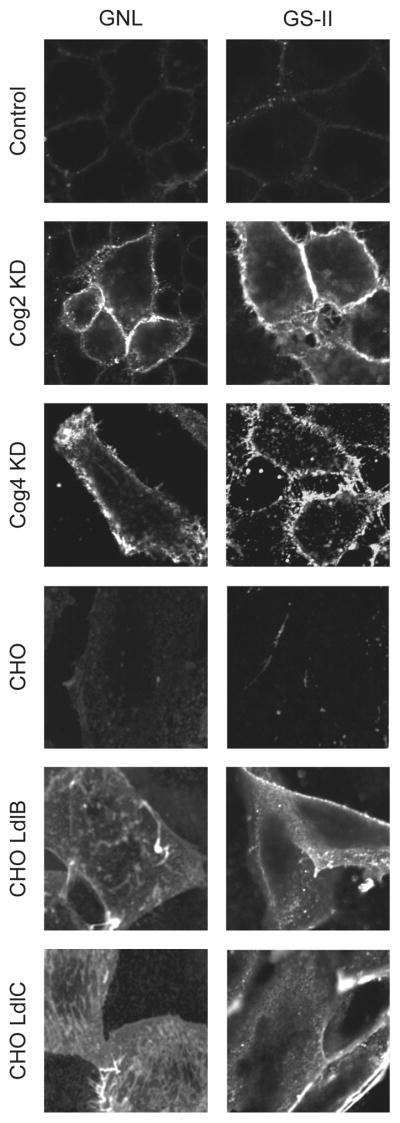
In this study we have combined siRNA induced knockdown of individual COG subunits with differential lectin staining techniques to determine the effect of COG subunit depletion on the cells plasma membrane population of glycoconjugates. Through our studies we found two lectins, Griffonia simplicifolia lectin-II (GSII; binds with high selectivity to terminal, nonreducing α- and β-N-acetyl-D-glucosaminyl (GlcNAc) residues of glycoconjugates) and Galanthus nivalus lectin (GNL; binds to terminal mannose residues of glycoconjugates) that specifically bind to immature glycoconjugates localized on plasma membrane in COG depleted cells (Figure 2). HeLa cells treated with a scrambled siRNA were used as a negative control. The ldlB (COG1 KO) and ldlC (COG2 KO) CHO cells were used as positive controls. Control CHO cells were not capable to bind PNA-rhodamine, while both ldlB and ldlC cells were intensively decorated with this lectin, validating our staining procedure (data not shown). The plasma membrane of all tested COG KD cells was specifically stained with GS-II and GNL. Likewise, the plasma membrane of both ldlB and ldlC cells was also distinctly stained with GS-II and GNL. No staining was observed for either control HeLa or CHO cells. This indicated that the COG subunit knockdown cells express immature plasma membrane-localized galactosylated N-glycans and glycoconjugates with an increased amount of terminal mannose residues.
Figure 2. Lectin staining of COG complex depleted HeLa cells reveals altered glycosylation of plasma membrane glycoconjugates.
HeLa cells were mock-transfected or transfected with siRNA to COG2 or COG4. 96 hours after transfection, HeLa cells, and CHO cells for control staining, were fixed, stained with GS-II-Alexa 594 or GNL-Alexa 647 lectins for 30 minutes and analyzed by wide-field fluorescent microscopy. The control HeLa cells and control CHO cells were negative for both lectins, indicating that plasma membrane localized glycoconjugates’ polysaccharide chains are completely mature. In cells depleted of either COG2 or COG4, the plasma membrane was extensively labelled with both GNL and GS-II lectins. Likewise, the plasma membranes of both ldlB and ldlC cells were also distinctly stained with GNL and GS-II. This indicates that the COG subunit knockdown cells express plasma membrane-localized glycoconjugates with an increased amount of terminal mannoses and GlcNAc.
2. Materials
2.1. siRNA induced knockdown of COG subunits components
Coverslips: #1.5 12 mm, 0.17 mm thickness, round glass coverslips (Warner Instruments; Hamden, CT) (maximum of 5 coverslips per well on a 6 well plate).
Culture dishes: 6 well tissue culture plates (TPP).
HeLa cells, wild type or stably expressing tagged glycosyltransferase (MAGT1-myc (α-1,3-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase) (22) grown on coverslips to 30% confluency on a 6 well plate.
HeLa cells, wild type or stably expressing tagged glycosyltransferase MAN2A1-VSV (α-mannosidase II) (23) grown on coverslips to 30% confluency on a 6 well plate.
HeLa cells, wild type or stably expressing tagged glycosyltransferase ST6GAL1-VSV (β-galactosidase α-2, 6-sialyltransferase 1) (23) grown on coverslips to 30% confluency on a 6 well plate.
Dulbecco’s Phosphate Buffered Saline (dPBS 1X) without calcium and magnesium (Thermo Fisher Scientific Inc; Waltham, MA).
Growth Media: dilute 50 mL of heat inactivated Fetal Bovine Serum (FBS) (Atlas Biological; Fort Collins, CO) in 450 mL of in DMEM/F-12 50/50 medium supplemented with 15 mM HEPES, 2.5 mM L-glutamine (Invitrogen; Carlsbad, CA). Filter solution in .45 μm PES Corning (Lowell, MA) filtration system.
Transfection media: Opti-MEM® I Reduced Serum Media buffered with HEPES and sodium bicarbonate and supplemented with hypoxanthine, thymidine, sodium pyruvate, L-glutamine, trace elements and growth factors (Invitrogen; Carlsbad, CA).
Gibco® 0.25% Trypsin-EDTA (1x) phenol red (Invitrogen; Carlsbad, CA).
Lipofectamine RNAiMAX siRNA Transfection Reagent (Invitrogen; Carlsbad, CA).
SiRNA: siGENOME siRNA - Human COG2 (target sequence: GGGCAGTTGATGAACGAAT), ON-TARGETplus siRNA - Human COG4 (target sequence: GTGCTGAAATCCACCTTTA), and control scrambled ON-TARGETplus siRNA) (Dharmacon; Chicago, IL).
Primers: hCOG2 Forward: GGACACGCTCTGCTTCGACA; hCOG2 Reverse: ACAGAAAGCTGGTTGAGGGC ; and hCOG4 Forward: TCTGCAGGTGGAATGTGACAGACA; hCOG4 Reverse: CTGTGCATGATGTTCACGGCACTT (Invitrogen; Carlsbad, CA).
2.2. Lectin-staining of intact cell components
HeLa cells transfected with siRNAs grown on coverslips to 70% confluency on a 6 well plate.
CHO cells, CHO ldl (low density lipoprotein) B cells (stable COG1 knockout), and CHO ldlC cells (stable COG2 knockout) (24–26) grown on coverslips to 70% confluency.
Coverslips: #1.5 12 mm, 0.17 mm thickness, round glass coverslips (Warner Instruments; Hamden, CT).
Lectins: Griffonia simplicifolia lectin II (GSII)-Alexa 594 (100 μg/ml, Invitrogen; Carlsbad, California), Galanthus nivalus lectin (GNL)-Fluorescin (20 μg/ml, Vector laboratories; Burlingame, CA) (see Note 1).
Dulbecco’s Phosphate Buffered Saline (dPBS 1X) without calcium and magnesium (Thermo Fisher Scientific Inc; Waltham, MA).
Cell fixative solution: 1% solution paraformaldehyde solution in dPBS prepared by diluting 16% stock solution (Electron Microscopy Sciences; Hatfield, PA) (see Note 2).
Quenching solution: 50 mM NH4Cl prepared by dissolving 134 mg of NH4Cl (Sigma-Aldrich; St. Louis, Missouri) in 50 ml of dPBS. Store at 4°C.
Blocking A solution: 1% BSA prepared by dissolving 1 g of Bovine serum albumin (BSA, Fraction V) (Research Products International Corporation; Mount Prospect, IL) in 100 ml dPBS. Filter completely dissolved solution in Corning (Lowell, MA) 250 mL .22 μm PES filer system. Store at 4°C.
4′,6-Diamidino-2-phenylindole dihydrochloride (DAPI) (Sigma-Aldrich; St. Louis, Missouri).
Mounting media: Prolong® Gold antifade mounting media from Invitrogen (Carlsbad, CA).
Glass slides: Fisherbrand frosted microscope slides (precleaned).
Parafilm.
Vacuum apparatus for collecting waste: Büchner flask, with extended intake tubing, connected to a vacuum source.
Zeiss Axiovert 200M fluorescent microscope.
2.3. Immunofluorescence staining components
HeLa cells stably expressing tagged glycosyltransferases (MAGT1-myc (α-1,3-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase), MAN2A1-VSV (α-mannosidase II), ST6GAL1-VSV (β-galactosidase α-2, 6-siayltransferase 1)) transfected with siRNAs grown on coverslips to 70% confluency on a 6 well plate.
Coverslips: #1.5 12 mm, 0.17 mm thickness, round glass coverslips (Warner Instruments; Hamden, CT).
Dulbecco’s Phosphate Buffered Saline (dPBS 1X) without calcium and magnesium (Thermo Fisher Scientific Inc; Waltham, MA).
20% (w/v) Triton X-100 solution prepared by weighing out 10 g of Triton X-100 (Sigma-Aldrich; St. Louis, Missouri) and diluting to a total volume of 50 mL with Milli-Q water (see Note 3).
Cell fixative solution: 4% paraformaldehyde solution in dPBS prepared by diluting 250 μL of 16% stock solution (Electron Microscopy Sciences; Hatfield, PA) in 750 μL of dPBS (see Note 2).
Cell permeabilizing solution: 0.1% Triton solution prepared by diluting 250 uL of 20% Triton X-100 stock in 50 mL of dPBS. Store at 4°C.
Quenching solution: 50 mM NH4Cl prepared by dissolving 134 mg of NH4Cl (Sigma-Aldrich; St. Louis, Missouri) in 50 mL of dPBS. Store at 4°C.
Blocking B solution: 1% BSA, 0.1% saponin prepared by dissolving 1 g of Bovine serum albumin (BSA, Fraction V) (Research Products International Corporation; Mount Prospect, IL) and 100 mg saponin (Sigma-Aldrich; St. Louis, Missouri) in 100 mL dPBS. Filter completely dissolved solution in Corning (Lowell, MA) 250 mL .22 μm PES filer system. Store at 4°C.
Diluent solution: 1% fish gelatin, 0.1% saponin prepared by dissolving 1 g gelatin from cold water fish skin (Sigma-Aldrich; St. Louis, Missouri) and 100 mg saponin (Sigma-Aldrich; St. Louis, Missouri) in 100 mL dPBS. Filter completely dissolved solution in Corning (Lowell, MA) 250 mL .22 μm PES filter system. Store at 4°C.
Primary antibodies: Anti-myc tag rabbit polyclonal antibodies (Bethyl Laboratories; Montgomery, TX) 1:3000 dilution in diluent solution. Anti-VSV tag rabbit polyclonal antibodies (Bethyl Laboratories; Montgomery, TX) 1:400 dilution in diluent solution. Anti-GM130 mouse monoclonal antibodies (BD Bioscienecs; San Jose, CA) 1:400 in diluent solution.
Secondary antibodies: Anti-rabbit HiLyte 488 1:400 in diluent solution, anti-mouse HiLyte 555 (for GM130) 1:1000 in diluent solution (AnaSpec, Inc, San Jose, CA).
Mounting media: Prolong® Gold antifade mounting media with Dapi (Invitrogen; Carlsbad, California).
Glass slides: Fisherbrand frosted microscope slides (precleaned).
Parafilm.
Vacuum apparatus for collecting waste: Büchner flask, with extended intake tubing, connected to a vacuum source.
Zeiss LSM510 laser inverted microscope outfitted with confocal optics, 63X oil 1.4 numerical aperture (NA) objective Image acquisition is controlled with LSM510 software (Release Version 4.0 SP1).
3. Methods
3.1. siRNA induced knockdown of COG subunits
All steps are performed under a sterile hood. Gloves are worn at all times to prevent contamination. 2 wells for each transfection were used, one well with coverslips, and one well without coverslips for knockdown efficiency analysis.
Plate wild type HeLa cells, or HeLa cells stably expressing tagged glycosyltransferases, on 6 well culture dishes with coverslips one day prior to transfection in 10% FBS DMEM/F-12 media that does not contain any antibiotics so that the day of the transfection the cells are 30% confluent and evenly spread (see Note 4). Grow cells at 37°C and 5% CO2 in a 90% humidified incubator.
Prepare transfection solutions as detailed by manufacturers protocol. For a 6 well plate: in a 1.5 mL microcentrifuge tube dilute 5 μL of Lipofectamine™ RNAiMAX in 245 μL of Opti-MEM®, set aside and let incubate for 5–10 minutes. In a separate tube, combine 10 μL of 20μM hCOG2 or hCOG4 siRNA stock with 240 μL of Opti-MEM® gently mixing the solution. After 10 minutes, combine the diluted siRNA with the diluted Lipofectamine™ RNAiMAX and incubate for 20 minutes (see Note 5).
While solution is incubating, wash cells two times with sterile dPBS. Remove residual PBS and incubate cells in 2 mL of Opti-MEM®.
After 20 minutes of incubation, add in drop wise manner the siRNA- Lipofectamine™ RNAiMAX complexes to their corresponding wells. Mix gently by rocking the plate back and forth.
Incubate the cells for 12 hours at 37°C, 5% CO2, and 90% humidity, then remove transfection solution and replace with 10% FBS DMEM/F-12 growth media and allow cells to recover for an additional 12 hours.
24 hours after the transfection, repeat the siRNA knockdown as described above (2–5).
Incubate cells for total of 96 hours after the first transfection, and then proceed to harvesting and staining.
Knockdown efficiency is determined 48 h after first transfection by qRT-PCR using primers for COG2 and COG4 (see Note 6).
3.2. Lectin staining of COG subunit depleted cells
Lectin staining was performed with both fixed and unfixed cells (see Note 7 and Note 8). All steps are performed at room temperature. Solutions are stored at 4°C until use. Method corresponds to Figure 1.
Untreated (control) and siRNA treated cells were grown on 12 mm glass coverslips at 70% of confluency. 96 hrs after the siRNA-induced transfection cells were rinsed with dPBS to remove the broken cell material and growth media.
Coverslips were taken out and placed on parafilm sheet at room temperature with the cell covered surface facing up (do not let cells to dry at any step!).
Incubate coverslips in 100 μL freshly made 1% paraformaldehyde in dPBS solution for 10 minutes.
Remove the residual 1% paraformaldehyde by pipette (see Note 9) and rinse coverslips 2 times with 200 μL of dPBS
Incubate coverslips in 100 μL of 50 mM NH4Cl in PBS for 5 minutes.
Remove 50 mM NH4Cl and wash cover slips with 100 μL of dPBS.
Incubate cover slips in 100 μL of 0.1% BSA in dPBS for 10 minutes.
Stain with lectins (100–150 μL) diluted to proper concentration (see 2.2) for 1 hour at room temperature. Place a small box over surface that coverslips are being stained on to protect from light.
Remove lectin solution by vacuum and wash coverslips 4 times with 200 μL of 1X dPBS, incubating for 2 minutes for each wash.
Incubate coverslips with DAPI (1 μL diluted in 5 mL dPBS) for 30 seconds (see Note 10).
Pick up coverslip with forceps and immerse them into beaker of dPBS 10 times, followed by dipping in beaker with Milli-Q water 10 times.
Gently remove excess liquid by tapping a Kim wipe to edge of coverslip.
Place coverslip cell side down on glass side with a small drop (5 μL) of mounting media.
Once all coverslips are mounted to glass slide (no more than 6 per slide) remove excess solution with vacuum.
Store slides on a flat, dry surface protected from light (slide book), and let cure overnight.
Image coverslips with the 63X oil 1.4 numerical aperture (NA) objective of a Zeiss Axiovert 200M fluorescent microscope.
3.3. Immunofluorescence staining of glycosyltransferases in COG subunit depleted cells
All steps are performed at room temperature. Solutions are stored at 4°C until use. (see Note 8) Method corresponds to Figure 2.
Coverslips with control or siRNA treated cells grown to near confluency (70%) are removed from culture dishes and placed on parafilm covered surface with the cell covered surface facing up (do not let cells to dry at any step!).
Wash coverslips rapidly three times by adding 200 μL 1X dPBS to each coverslip, removing the used solution.
Remove the residual 1X dPBS and incubate coverslips in 100 μL of 4% paraformaldehyde for 15 minutes.
Remove 4% paraformaldehyde solution by pipette and incubate coverslips in 100 μL of 1% Triton X-100 for 1 minute.
Remove 1% Triton X-100 solution and incubate coverslips in 100 μL of 50 mM NH4Cl for 5 minutes.
Remove 50 mM NH4Cl solution and incubate coverslips with 100 μL of 1% BSA, 0.1% saponin 10 minutes, removed this solution and then repeat incubation with the same solution for another 10 minutes.
Remove 1% BSA, 0.1% saponin solution and add 100 μL of diluted primary antibody solution to coverslips and incubate for 40 minutes. For Myc tagged cells, the primary antibody solution is 1 mL of diluted anti-myc antibodies (1 μL antibodies in 3mL 1% fish gelatin, 0.1% saponin) and 2.5 μL of anti-GM130 antibodies. For VSV tagged cells, the primary antibody solution is 2.5 μL anti-VSV antibodies and 2.5 μL anti-GM130 antibodies diluted in 1 mL 1% fish gelatin, 0.1% saponin (see Note 11).
Remove primary antibody solution and wash coverslips 4 times with 200 μL of 1X dPBS, incubating for 2 minutes for each wash.
Remove dPBS and add 100 μL of diluted secondary antibodies to coverslips and incubate for 30 minutes. The secondary antibody solution is 1 μL anti-rabbit HiLyte 488 and 1 μL anti-mouse HiLyte 555 in 1 mL 1% fish gelatin, 0.1% saponin (see Note 11). Place a small box over surface that coverslips are being stained on to protect samples from light.
Remove secondary antibody solution and wash coverslips 5 times with 1X dPBS, incubating for 2 minutes for each wash.
Pick up coverslip with forceps and immerse them into beaker of dPBS 10 times, followed by dipping in beaker with Milli-Q water 10 times.
Gently remove excess liquid by tapping a Kim wipe to edge of coverslip.
Place coverslip cell side down on glass side with a small drop (5 μL) of mounting media.
Once all coverslips are mounted to glass slide (no more than 6 per slide) remove excess of mounting media with vacuum.
Store slides on a flat, dry surface protected from light (slide book), and let cure overnight.
Image coverslips with the 63X oil 1.4 numerical aperture (NA) objective of a LSM510 Zeiss Laser inverted microscope outfitted with confocal optics. Image acquisition is controlled with LSM510 software (Release Version 4.0 SP1).
Footnotes
Lectins are stored in dark at 4°C. In addition to commercially-available fluorescent-labelled lectins, unlabelled lectins can be purchased from Vector laboratories (Vector laboratories; Burlingame, CA) and labelled with Alexa-647 protein-labelling kit (Invitrogen; Carlsbad, CA).
Fixative solution works best when diluted fresh before start of experiment. Seal unused 16% paraformaldehyde with parafilm and store away from light.
Rock at 4°C overnight and store at 4°C until use. Do not store for more than 1 month.
Actual number of cells to achieve desired confluency varies depending on cell line. Differences in size and growth rates should be considered when plating cells for transfection.
siRNA is extremely fragile and should be handled very delicately. When diluting siRNA only gently mix it with the Opti-MEM®. For best results, do not use stock aliquot more than 2 times because continually thawing will destroy siRNA.
qRT-PCR was used to validate the efficiency knockdowns because reliable commercial antibodies against COG2 and COG4 are not available. Actin qRT-PCR was used as a control
For lectin staining of unfixed cells, all steps are performed in cold room at 4°C. Protocol was done as follows: Rinse cover slips in dPBS (chilled to 4°C) and incubate for 15 minutes to reduce endocytosis of lectin. After cells have been chilled, incubate with lectins for 20 minutes (lectin solutions should be chilled to 4°C as well). Wash cover slips 4 times with cold dPBS and then fix with 4% paraformaldehyde (prepared fresh and chilled to 4°C). Mount cover slips to slides as indicated above. While both methods will yield the same result, we have found that the aforementioned protocol (staining then fixing) is simpler and more reproducible.
It is important to never let the coverslips dry during the staining procedure.
With the exception of the paraformaldehyde, the removal of all of the staining solutions can be done either by pipette, or with a vacuum (see Materials). We prefer to use the vacuum because it allows for a faster removal of solutions, thereby increasing the accuracy of incubation time.
DAPI staining is optional.
Diluted antibodies should be mix thoroughly and then centrifuged at 20,000 g for 1min. This step removes any large particles that could provide a high background during visualization.
References
- 1.Shorter J, Warren G. Golgi architecture and inheritance. Annu Rev Cell Dev Biol. 2002;18:379–420. doi: 10.1146/annurev.cellbio.18.030602.133733. [DOI] [PubMed] [Google Scholar]
- 2.Pokrovskaya ID, Willett R, Smith RD, Morelle W, Kudlyk T, Lupashin VV. COG complex specifically regulates the maintenance of Golgi glycosylation machinery. Glycobiology. 2011;21(12):1554–69. doi: 10.1093/glycob/cwr028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suvorova ES, Kurten RC, Lupashin VV. Identification of a human orthologue of Sec34p as a component of the cis-Golgi vesicle tethering machinery. Journal of Biological Chemistry. 2001;276:22810–22818. doi: 10.1074/jbc.M011624200. [DOI] [PubMed] [Google Scholar]
- 4.Suvorova ES, Duden R, Lupashin VV. The Sec34/Sec35p complex, a Ypt1p effector required for retrograde intra-Golgi trafficking, interacts with Golgi SNAREs and COPI vesicle coat proteins. Journal of Cell Biology. 2002;157:631–643. doi: 10.1083/jcb.200111081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whyte JR, Munro S. The Sec34/35 Golgi Transport Complex Is Related to the Exocyst, Defining a Family of Complexes Involved in Multiple Steps of Membrane Traffic. Developmental Cell. 2001;1:527–537. doi: 10.1016/s1534-5807(01)00063-6. [DOI] [PubMed] [Google Scholar]
- 6.Ungar D, Oka T, Brittle EE, Vasile E, Lupashin VV, Chatterton JE, Heuser JE, Krieger M, Waters MG. Characterization of a mammalian Golgi-localized protein complex, COG, that is required for normal Golgi morphology and function. Journal of Cell Biology. 2002;157:405–415. doi: 10.1083/jcb.200202016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ungar D, Oka T, Vasile E, Krieger M, Hughson FM. Subunit architecture of the conserved oligomeric golgi complex. Journal of Biological Chemistry. 2005;280:32729–32735. doi: 10.1074/jbc.M504590200. [DOI] [PubMed] [Google Scholar]
- 8.Fotso P, Koryakina Y, Pavliv O, Tsiomenko AB, Lupashin VV. Cog1p plays a central role in the organization of the yeast conserved oligomeric golgi complex. Journal of Biological Chemistry. 2005;280:27613–27623. doi: 10.1074/jbc.M504597200. [DOI] [PubMed] [Google Scholar]
- 9.Bruinsma P, Spelbrink RG, Nothwehr SF. Retrograde transport of the mannosyltransferase Och1p to the early Golgi requires a component of the COG transport complex. J Biol Chem. 2004;279:39814–23. doi: 10.1074/jbc.M405500200. [DOI] [PubMed] [Google Scholar]
- 10.Shestakova A, Zolov S, Lupashin V. COG complex-mediated recycling of Golgi glycosyltransferases is essential for normal protein glycosylation. Traffic. 2006;7:191–204. doi: 10.1111/j.1600-0854.2005.00376.x. [DOI] [PubMed] [Google Scholar]
- 11.Kingsley DM, Kozarsky KF, Segal M, Krieger M. Three types of low density lipoprotein receptor-deficient mutant have pleiotropic defects in the synthesis of N-linked, O-linked, and lipid-linked carbohydrate chains. J Cell Biol. 1986;102:1576–85. doi: 10.1083/jcb.102.5.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reynders E, Foulquier F, Annaert W, Matthijs G. How Golgi glycosylation meets and needs trafficking: the case of the COG complex. Glycobiology. 2011;21:853–863. doi: 10.1093/glycob/cwq179. [DOI] [PubMed] [Google Scholar]
- 13.Wu X, Steet RA, Bohorov O, Bakker J, Newell J, Krieger M, Spaapen L, Kornfeld S, Freeze HH. Mutation of the COG complex subunit gene COG7 causes a lethal congenital disorder. Nat Med. 2004;10:518–23. doi: 10.1038/nm1041. [DOI] [PubMed] [Google Scholar]
- 14.Foulquier F, Ungar D, Reynders E, Zeevaert R, Mills P, Garcia-Silva MT, Briones P, Winchester B, Morelle W, Krieger M, Annaert W, Matthijs G. A new inborn error of glycosylation due to a Cog8 deficiency reveals a critical role for the Cog1-Cog8 interaction in COG complex formation. Hum Mol Genet. 2007;16:717–30. doi: 10.1093/hmg/ddl476. [DOI] [PubMed] [Google Scholar]
- 15.Foulquier F, Vasile E, Schollen E, Callewaert N, Raemaekers T, Quelhas D, Jaeken J, Mills P, Winchester B, Krieger M, Annaert W, Matthijs G. Conserved oligomeric Golgi complex subunit 1 deficiency reveals a previously uncharacterized congenital disorder of glycosylation type II. Proc Natl Acad Sci U S A. 2006;103:3764–9. doi: 10.1073/pnas.0507685103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kranz C, Ng BG, Sun L, Sharma V, Eklund EA, Miura Y, Ungar D, Lupashin V, Winkel RD, Cipollo JF, Costello CE, Loh E, Hong W, Freeze HH. COG8 deficiency causes new congenital disorder of glycosylation type IIh. Hum Mol Genet. 2007;16:731–41. doi: 10.1093/hmg/ddm028. [DOI] [PubMed] [Google Scholar]
- 17.Ng BG, Kranz C, Hagebeuk EE, Duran M, Abeling NG, Wuyts B, Ungar D, Lupashin V, Hartdorff CM, Poll-The BT, Freeze HH. Molecular and clinical characterization of a Moroccan Cog7 deficient patient. Mol Genet Metab. 2007;91:201–4. doi: 10.1016/j.ymgme.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeevaert R, Foulquier F, Jaeken J, Matthijs G. Deficiencies in subunits of the Conserved Oligomeric Golgi (COG) complex define a novel group of Congenital Disorders of Glycosylation. Mol Genet Metab. 2008;93:15–21. doi: 10.1016/j.ymgme.2007.08.118. [DOI] [PubMed] [Google Scholar]
- 19.Reynders E, Foulquier F, Leao Teles E, Quelhas D, Morelle W, Rabouille C, Annaert W, Matthijs G. Golgi function and dysfunction in the first COG4-deficient CDG type II patient. Hum Mol Genet. 2009;18:3244–56. doi: 10.1093/hmg/ddp262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paesold Burda P, Maag C, Troxler H, Foulquier F, Kleinert P, Schnabel S, Baumgartner M, Hennet T. Deficiency in COG5 causes a moderate form of congenital disorders of glycosylation. Hum Mol Genet. 2009;18(22):4350–6. doi: 10.1093/hmg/ddp389. [DOI] [PubMed] [Google Scholar]
- 21.Lubbehusen J, Thiel C, Rind N, Ungar D, Prinsen BH, de Koning TJ, van Hasselt PM, Korner C. Fatal outcome due to deficiency of subunit 6 of the conserved oligomeric Golgi complex leading to a new type of congenital disorders of glycosylation. Hum Mol Genet. 2010;19:3623–33. doi: 10.1093/hmg/ddq278. [DOI] [PubMed] [Google Scholar]
- 22.Nilsson T, Hoe MH, Slusarewicz P, Rabouille C, Watson R, Hunte F, Watzele G, Berger EG, Warren G. Kin recognition between medial Golgi enzymes in HeLa cells. Embo J. 1994;13:562–74. doi: 10.1002/j.1460-2075.1994.tb06294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miles S, McManus H, Forsten KE, Storrie B. Evidence that the entire Golgi apparatus cycles in interphase HeLa cells: sensitivity of Golgi matrix proteins to an ER exit block. J Cell Biol. 2001;155:543–55. doi: 10.1083/jcb.200103104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krieger M, Brown M, Goldstein J. Isolation of Chinese hamster cell mutants defective in the receptor-mediated endocytosis of low density lipoprotein. Journal of Molecular Biology. 1981;150:167–84. doi: 10.1016/0022-2836(81)90447-2. [DOI] [PubMed] [Google Scholar]
- 25.Podos SD, Reddy P, Ashkenas J, Krieger M. LDLC encodes a brefeldin A-sensitive, peripheral Golgi protein required for normal Golgi function. J Cell Biol. 1994;127:679–91. doi: 10.1083/jcb.127.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chatterton JE, Hirsch D, Schwartz JJ, Bickel PE, Rosenberg RD, Lodish HF, Krieger M. Expression cloning of LDLB, a gene essential for normal Golgi function and assembly of the ldlCp complex. Proc Natl Acad Sci U S A. 1999;96:915–20. doi: 10.1073/pnas.96.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]