Abstract
Objective
To determine the effect of exercise on chemerin in relation to changes in fat loss, insulin action, and dyslipidemia in older adults.
Participants
Thirty older (65.9±0.9yr) obese adults (BMI:34.5±0.7kg/m2).
Setting
Single-center, Cleveland Clinic.
Design
Prospective clinical trial.
Intervention
Twelve-weeks of exercise training (60minutes/day, 5day/week at ~85% HRmax). Subjects were instructed to maintain habitual nutrient intake.
Measurements
Plasma chemerin was analyzed using an enzyme-linked immunosorbent assay. Peripheral and hepatic insulin sensitivity was assessed using a euglycemic-hyperinsulinic clamp with glucose kinetics. First-phase and total glucose-stimulated insulin secretion (GSIS) was calculated from an oral glucose tolerance test. Fasting blood lipids (cholesterol, triglycerides), total/visceral fat (dual-x-ray absorptiometry and computerized tomography) and cardiorespiratory fitness (treadmill test) were also tested pre and post intervention.
Results
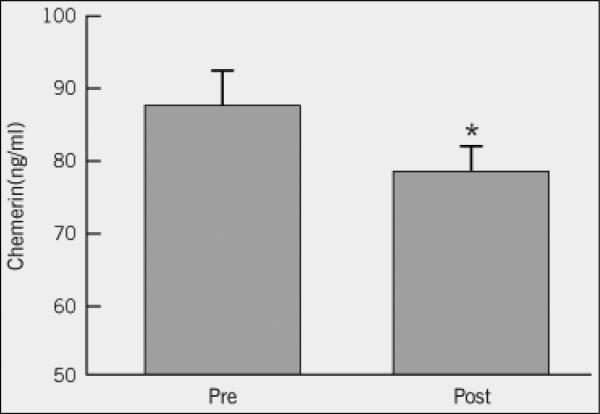
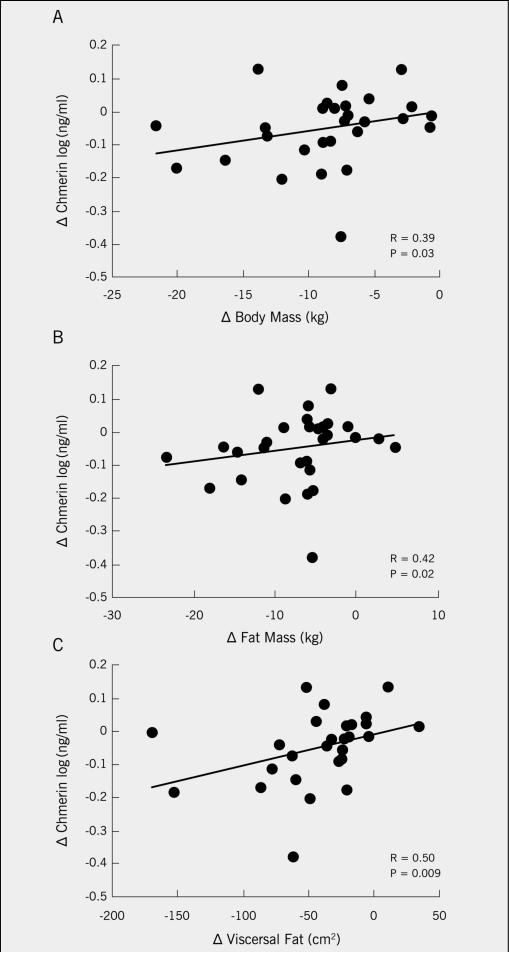
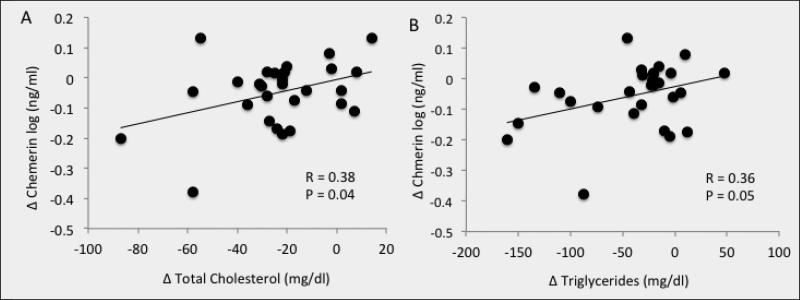
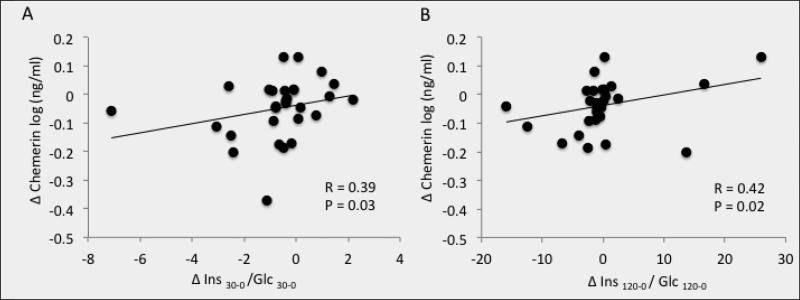
Exercise increased fitness and reduced total/visceral fat, blood lipids, and first-phase GSIS (P<0.05). Training also increased peripheral insulin sensitivity and lowered basal/insulin-related hepatic glucose production (P<0.01). The intervention reduced chemerin (87.1±6.0 vs. 78.1±5.8ng/ml; P=0.02), and the reduction correlated with decreased visceral fat (r=0.50, P=0.009), total body fat (r=0.42, P=0.02), cholesterol (r=0.38, P=0.04), triglycerides (r=0.36, P=0.05), and first-phase and total GSIS (r=0.39, P=0.03 and r=0.43, P=0.02, respectively).
Conclusions
Lower chemerin appears to be an important hormone involved in cardiometabolic risk and GSIS reduction following exercise in older adults.
Keywords: Glucose tolerance, insulin sensitivity, obesity, adipokine, inflammation
Introduction
Adipose tissue is regarded as an active endocrine organ secreting cytokines that regulate fat mass, inflammation, as well as glucose and lipid metabolism (1). Chemerin is a novel adipokine secreted from both visceral and subcutaneous adipose tissue and is associated with metabolic syndrome, cardiovascular disease and type 2 diabetes (2-6). Chemerin was originally described as a chemoattractant protein that modulates chemotaxis and activation of macrophages through the G protein-coupled receptors (e.g. CMKLR1, GPR1, and CCRLs) (7). More recently, chemerin was shown to play an important role in adipocyte differentiation as well as glucose homeostasis. The exact mechanism by which chemerin contributes to cardiometabolic disease is unclear, but chemerin is associated with inflammation, elevated triglycerides, and arterial stiffness (3, 4, 8, 9). Chemerin also induces insulin resistance in adipocytes, hepatocytes and primary human skeletal muscle cells in vitro by impairing glucose uptake (10-13), and this is how it may contribute to glucose intolerance (4, 6). However, controversy exists regarding chemerin as a pro- or anti-inflammatory hormone in regards to the regulation of glucose metabolism (14). Thus, further work in humans is needed to clarify whether chemerin is a leading adipokine involved in the progression and/or reversal of cardiometabolic risk.
Exercise reduces cardiometabolic risk factors in part by lowering adipokines related to insulin resistance and glucose-stimulated insulin secretion (15). As a result, exercise is recommended as a first-line therapy in weight management and glycemic control. We have shown that exercise, with or without weight loss, decreases metabolic syndrome severity, inflammation, and insulin resistance (16-18). Thus, it would seem that chemerin should decrease after an exercise intervention. However, data on the effect of exercise on chemerin is limited (19-23), and there are no data determining the effect of an exercise intervention on chemerin in older, obese insulin resistant adults. Moreover, no study to our knowledge has examined the effect of exercise-induced improvement in peripheral or hepatic insulin sensitivity via the euglycemic-clamp with glucose isotopes or glucose-stimulated insulin secretion in relation to changes in circulating chemerin to gain mechanistic insight into the role of chemerin in regulating cardiometabolic risk. In order to address this knowledge gap, we tested the hypothesis that exercise training would decrease chemerin, and the change in chemerin would correlate with improvements in body fat, dyslipidemia, and insulin sensitivity. Understanding if chemerin is reduced after exercise in an older cohort is clinically relevant since older men and women are at high risk of developing type 2 diabetes and cardiovascular disease (24).
Methods
Subjects
Thirty older (65.9 ± 0.9 yr) obese adults (Table 1) volunteered for this study, and some of the glucose kinetic data were previously reported (17). All subjects were weight stable (<2 kg in the previous 6 months), sedentary (<60 minutes/week), and free of chronic disease (i.e. hematological, renal, hepatic, cardiovascular, type 2 diabetes). All women were post-menopausal for at least 1 year, and subjects were excluded if they smoked or took medications (e.g. metformin, statins, etc.) known to affect the study outcomes. Subjects were verbally briefed about the study and signed written informed consent documents approved by our Institutional Review Board.
Table 1.
Demographics and cardiometabolic risk factors before and after a 12-week exercise training intervention
| Variable | Pre-intervention | Post-intervention | % Change | P-value |
|---|---|---|---|---|
| Age (years) | 65.9 (0.9) | - | ||
| Sex (M/F) | 16/14 | - | ||
| Body weight (kg) | 98.8(2.4) | 90.5(2.1) | −8.3(0.7) | <0.001 |
| WC (cm) | 106.6(2.1) | 101.6(1.8) | −4.7(0.4) | <0.001 |
| Body fat (%) | 43.8(1.3) | 39.2(1.3) | −10.7(0.8) | <0.001 |
| Fat mass (kg) | 42.7(1.8) | 35.6(1.5) | −16.4(1.7) | <0.001 |
| Fat free mass (kg) | 55.6(1.6) | 54.9(1.6) | −0.7(0.6) | 0.30 |
| Visceral fat (cm2) | 144.57(22.3) | 101.7(15.4) | −30.6(4.0) | <0.001 |
| Subcutaneous fat (cm2) | 453.3(24.0) | 368.8(25.7) | −19.6(2.8) | <0.001 |
| VO2max (L/min) | 2.1(0.1) | 2.5(0.1) | 14.2(1.7) | <0.001 |
| Fasting glucose (mg/dl)^ | 105.6(1.3) | 94.8(1.2) | −9.4(1.2) | <0.001 |
| Glucose AUC (mg/dl*2hr)^ | 19507.7(536.4) | 17369.9(434.3) | −7.8(2.0) | 0.04 |
| Fasting insulin (μU/ml) | 22.8(2.0) | 11.2(0.6) | −34.5(4.6) | <0.001 |
| Insulin AUC (μU/ml*2hr) | 14817.7(1336.5) | 8340.8(841.2) | −31.5(6.8) | <0.001 |
| Total cholesterol (mg/dl) | 197.4(6.3) | 173.8(5.6) | −11.6(1.4) | <0.001 |
| Triglycerides (mg/dl) | 142.9(12.5) | 102.8(6.3) | −22.5(3.3) | <0.001 |
| LDL cholesterol (mg/dl) | 121.9(4.7) | 108.6(5.1) | −9.8(2.2) | <0.001 |
| HDL cholesterol (mg/dl) | 45.8(3.1) | 43.5(3.0) | −3.7(2.5) | 0.15 |
| TNF-α (pg/ml)^ | 2.11(0.1) | 2.4(0.2) | 5.8(15.4) | 0.88 |
| Systolic BP (mm Hg) | 133.9(2.7) | 123.1(2.1) | −7.5(0.8) | <0.001 |
| Diastolic BP (mm Hg)^ | 80.0(2.3) | 74.3(1.7) | −5.9(2.8) | 0.02 |
Data reported as Mean (SEM).
Log-transformed for statistical analysis. WC = waist circumference. BP = blood pressure. HDL = high-density lipoprotein. LDL = low-density lipoprotein. AUC = total area under the curve.
Exercise Training Intervention
Subjects were screened with a resting ECG as well as an incremental maximally graded exercise stress test before participation in the intervention to exclude individuals with underlying cardiovascular dysfunction. Participants underwent a supervised treadmill-walking and cycle ergometer exercise intervention 5 days/week for 60 minutes/day at approximately 85% of heart rate max (HRmax) for 12 weeks. Appropriate exercise intensity was managed using heart rate monitors (Polar Electro, Inc. Woodbury, NY). Subjects met with a dietitian once per week to review 3-day diet logs and were advised to maintain their pre-intervention macronutrient intake. Food records were averaged over a 3-day period for analysis before and after the intervention to assess dietary intake.
Control Period
Pre- and post-intervention metabolic assessments were conducted during a 3-day inpatient stay at the Clinical Research Unit (CRU) as described before (16-18). At this time, subjects were provided weight-maintenance meals (resting metabolic rate × 1.2 activity factor; 55% CHO, 30% fat, 15% protein). Resting metabolic rate was determined after subjects rested in the supine position for 30 minutes. Expired air (i.e. VO2 and VCO2) was collected using a ventilated hood and indirect calorimetry (Vmax Encore, Viasys, Yorba Linda, CA). Post-intervention outcome measures were obtained 16-18 hours after the last exercise session.
Aerobic Fitness
Maximum oxygen consumption (VO2max) was determined using a continuous incremental treadmill exercise test, (Jaeger Oxygcon Pro; Viasys, Yorba Linda, CA). The HRmax obtained during this test was used during exercise training. VO2max was repeated at weeks 4 and 8 so that the appropriate exercise intensity could be adjusted accordingly throughout training.
Body Composition and Cardiometabolic Risk
After an overnight fast, weight was recorded on a digital platform scale in a hospital gown, and height was measured without shoes using a wall-mounted stadiometer. Dual-x-ray absorptiometry (DXA; Lunar Prodigy, Madison, WI) was used to quantify total fat mass, and waist circumference was measured with a plastic tape measure 2 cm above the umbilicus. Computerized tomography, with a SOMOTOM Sensation 16 Scanner (Siemens Medical Solutions, Malvern, PA) was used to determine visceral and subcutaneous adipose tissue mass as discussed before (24). Systolic and diastolic blood pressure was recorded in the seated position after approximately 10 minutes of rest. Fasting glucose, triglyceride, total cholesterol, high-density lipoprotein (HDL), and low-density lipoprotein (LDL) were obtained from an antecubital vein. A standard 75 gram oral glucose tolerance test (OGTT) was performed to measure post-prandial insulin and glucose. Blood samples were obtained every 30 minutes up to 120 minutes, and total area under the curve (AUC) was calculated using the trapezoidal model. First phase and total glucose-stimulated insulin secretion (GSIS), i.e. the insulinogenic index, was calculated by dividing the increment in plasma insulin at 30 and 120 minutes by the increment in plasma glucose at 30 and 120 minutes of the OGTT, respectively (i.e. Ins30-0/Glc30-0 and Ins120-0/Glc120-0).
Insulin Sensitivity with Glucose Kinetics
After an overnight fast, a euglycemic-hyperinsulinemic clamp was performed. A primed (3.28 mg/kg) continuous (0.036 mg·kg−1·min−1) infusion of [6,6-2H]-glucose was started at t = −120 minutes. At t = 0, a constant infusion (40 mU/m2·min−1) of insulin was administered via an indwelling catheter placed in an antecubital vein. Glucose was infused for 120 minutes at a variable rate to maintain plasma glucose at 90 mg/dl. A retrograde hand catheter was also placed and the hand was warmed to 60°C for collection of arterialized blood samples. Peripheral insulin sensitivity, which mostly reflects skeletal muscle glucose uptake, was averaged during the last 30 minutes of the clamp and defined as the rates of glucose disposal (Rd) divided by the ambient insulin concentrations [i.e. Rd/I]. Basal rates of glucose appearance (Ra), which primarily comprises of hepatic glucose production, was averaged during minutes t = −30 to t = 0. Endogenous hepatic glucose production (HGP) during the clamp was defined as the difference between HGPclamp and the exogenous glucose infusion rate. The suppression of HGP was defined as [1-(HGPclamp/HGPfast)*100%]) and used to characterize hepatic insulin sensitivity. Standard equations were used to calculate steady-state rates of glucose metabolism (26). Insulin-stimulated suppression of free fatty acids (FFA) was defined as: [1-(FFAclamp/FFAfast)*100%] and used as a surrogate for adipose insulin sensitivity. Carbohydrate oxidation was determined by indirect calorimetry using standard equations (27). Non-oxidative glucose disposal (NOGD) was calculated during the final 30 minutes of the clamp (NOGD = Rd – total carbohydrate oxidation).
Biochemical Analysis
Blood samples were centrifuged at 4°C for 10 minutes at 1000 rpm, and then stored at −80°C until subsequent analysis. Plasma chemerin and TNF-α concentrations were measured using an enzyme-linked immunosorbent assay (Roche Modular Diagnostics, Indianapolis, IN). Plasma glucose was determined by a glucose oxidase assay (YSI 2300 STAT Plus, Yellow Springs, OH). Plasma insulin was measured using a radioimmunoassay (Millipore, Billerica, MA). Plasma triglycerides and cholesterol were analyzed using enzymatic methods with an automated platform (Roche Modular Diagnostics, Indianapolis, IN). Plasma FFA concentrations were analyzed by a colorimetric assay (Wako Chemicals, Richmond, VA). Plasma samples for isotopic enrichment were deproteinized, extracted, and then derivatized before analysis by gas chromatography-mass spectrometry as described previously (24).
Statistical Analysis
Pre and post group means were compared using the statistical program R (Leopard build 64-bit, The R Foundation, Vienna, Austria, 2011). Non-normally distributed data were log-transformed for statistical analysis. All outcomes were assessed using paired t-tests. Bivariate correlation analysis was used to determine associations between variables, and significance was accepted as P<0.05. Data are expressed as mean ± standard error of the mean (SEM).
Results
Fitness and Body Composition
Overall adherence to our exercise program was excellent (94.4 ± 1.2%), and all subjects completed the intervention. Based on 3-day food logs, subjects tended to report less food intake (Pre = 1873.0 ± 69.5 vs. Post = 1771.7 ± 86.8 kcal/d, P=0.06). Since carbohydrate (Pre = 55.8 ± 0.7 vs. Post = 56.2 ± 0.6%, P=0.57) and protein (Pre = 17.2 ± 0.6 vs. Post = 17.5 ± 0.6%, P=0.25) intake did not change during the intervention, the majority of caloric deficit came from dietary fat (Pre = 29.9 ± 0.7 vs. Post = 27.7 ± 0.7%, P=0.01). Exercise training increased VO2max by 14.2 ± 1.7% (P<0.001). Exercise significantly reduced body weight by −8.3 ± 0.7% (BMI: 34.5 ± 0.7 vs. 31.6 0.6 kg/m2, P<0.001), and this was predominately fat mass (−16.4 ± 1.7%, P<0.001) since fat-free mass was unchanged at the end of the intervention (−0.7 ± 0.6%, P=0.30; Table 1). Visceral and subcutaneous fat mass was also significantly reduced after training (Table 1).
Cardiometabolic Risk Factors
The intervention significantly lowered blood pressure, triglycerides, total cholesterol, fasting glucose and insulin concentrations (Table 1). Although plasma TNF-α was unchanged after training (P=0.88) the intervention did significantly lower glucose and insulin AUC after training (Table 1).
Insulin Sensitivity and GSIS
Exercise training increased peripheral insulin sensitivity (P<0.001; Table 2), which was explained by a rise in both non-oxidative (Pre = 2.1 ± 0.5 vs. Post = 2.8 ± 0.3 mg/kg/min; P<0.02) and oxidative carbohydrate metabolism (Pre = 1.1 ± 0.2 vs. Post = 1.6 ± 0.1 mg/kg/min; P<0.01). The intervention lowered basal HGP (P=0.01) and insulin-related HGP (P<0.001; Table 2). Exercise training had no statistical effect on FFA suppression (P=0.38; Table 2). Exercise training also reduced first-phase GSIS (Pre = 1.9 ± 0.2 vs. Post = 1.2 ± 0.15, P=0.04), but not total GSIS (Pre = 4.1 ± 2.1 vs. Post = 2.9 ± 1.2, P=0.54).
Table 2.
Changes in insulin resistance and FFA suppression after a 12-week exercise training intervention
| Variable | Pre-intervention | Post-intervention | % Change | P-value |
|---|---|---|---|---|
| Peripheral insulin sensitivity | ||||
| Rd (mg/kg/min) | 3.09(0.3) | 4.2(0.2) | 61.8(15.2) | <0.001 |
| Rd/I (mg/kg/min/μU/ml) | 3.03(0.4) | 4.9(0.5) | 91.2(19.3) | <0.001 |
| Hepatic Glucose Production | ||||
| Basal Ra (mg/kg/min) | 2.7 (0.2) | 2.0 (0.3) | −22.9(6.7) | 0.01 |
| Clamp Ra (mg/kg/min) | 1.5(0.2) | 0.83(0.2) | −42.9(12.3) | <0.001 |
| % suppression | 46.3(6.0) | 57.6(9.1) | 27.3(16.1) | 0.07 |
| FFA suppression | ||||
| 0 min | 0.62(0.02) | 0.55(0.02) | −4.0(5.4) | 0.15 |
| 120 min^ | 0.17(0.03) | 0.13(0.02) | −18.8(8.1) | 0.45 |
| % suppression^ | 74.3(6.2) | 82.6(3.6) | 11.8(12.0) | 0.31 |
Data reported as Mean(SEM).
Log-transformed for statistical analysis. Rd = Rate of glucose disposal. I = ambient insulin concentration. Ra = Rate of glucose appearance. FFA = free fatty acid.
Chemerin and Correlations
Exercise training significantly decreased plasma chemerin (P=0.02; Figure 1). Baseline chemerin levels were associated with HDL (r=−0.39, P=0.03) and visceral fat mass (r=0.38, P=0.05), but not VO2max (r=0.03, P=0.87). Lower chemerin levels after training were associated with reductions in body weight (r=0.39, P=0.03, Figure 2a), total body fat (r=0.42, P=0.02; Figure 2b) and visceral fat mass (r=0.50, P=0.009, Figure 2c). Lower chemerin concentrations after training were also associated with decreased total cholesterol (r=0.38, P=0.04, Figure 3a) and triglyceride concentrations (r=0.36, P=0.05, Figure 3b). Reduced insulin AUC from the OGTT was not correlated with lower chemerin levels (r=0.32, P=0.09), however, first-phase and total GSIS were significantly associated with decreased chemerin after training (r=0.39, P=0.03 and r=0.42, P=0.02, Figure 4a and 4b, respectively). However, lower chemerin levels after training did not correlate with VO2max (r=0.14, P=0.46), TNF-α (r=0.13, P=0.59), dietary fat (r=−0.17, P=0.37), peripheral insulin sensitivity (r=−0.22, P=0.26) or insulin-related HGP (r=0.19, P=0.35). Weight loss was associated with increased peripheral (r=−0.47, P=0.04) and reduced clamp-derived HGP (r=0.48, P=0.04). Increased VO2max tended to correlate with improved peripheral (r=0.35, P=0.06) and insulin related HGP (r=−0.50, P=0.04).
Figure 1.
Effect of a 12-week exercise intervention on plasma chemerin concentrations. *Significant compared to Pre-test (P=0.02). Data were log-transformed for statistical analysis
Figure 2.
Correlation between the change (Δ) in chemerin chemerin and weight loss (A), total fat mass reduction (B), and decreased visceral adiposity (C) after a 12-week exercise intervention
Figure 3.
Correlation between the change (Δ) in chemerin and the Δ in total cholesterol (A) and triglycerides (B) after a 12-week exercise intervention
Figure 4.
Correlation between the change (Δ) in chemerin and the Δ in first phase glucose-stimulated insulin secretion (GSIS; A) and total GSIS (B) after a 12-week exercise intervention
Discussion
Exercise is widely encouraged in older adults in order to reduce body fat, improve insulin sensitivity, and decrease cardiometabolic risk factors (16, 28). However, there are limited data regarding the effect of exercise training on chemerin (19-21), and the relationship between changes in chemerin and exercise-induced changes in cardiometabolic risk factors has not been examined in older adults. Therefore, this is the first clinical intervention to determine the effects of a fully supervised exercise training program on chemerin in previously sedentary, older, obese men and women. Despite the absence of a control group in this study, prior work that did include a control group has shown no change or slight increases in chemerin after 12 weeks (20, 21), thus supporting the view that physical activity and/or a weight maintenance diet is important for sustaining low chemerin levels (19). The main finding from this study is that exercise training decreased plasma chemerin concentrations (Figure 1), and this reduction in chemerin was significantly correlated with lower body weight/fat (Figure 2) as well as decreased blood lipids (Figure 3). The exercise-induced findings seen herein are consistent with the few recent studies reporting that aerobic and/or resistance exercise programs ranging between 3-5 days/week at 55-85% HRmax are effective at lowering chemerin levels in middle-aged adults at risk for type 2 diabetes (19-21). Importantly, unlike previous work (19-21), our subjects underwent rigorous dietary control 3 days before metabolic testing and exercised with full supervision (adherence ~94%) at approximately 85% of HRmax for 60 minutes per session 5 days/week for 12 weeks. Although changes in caloric intake were not statistically significant, we acknowledge that some of the weight loss induced by our exercise training intervention may be due to reductions in dietary fat intake. However, the change in fat intake was not related to changes in chemerin. Thus, our findings strengthen the current body of literature and show for the first time that moderate to vigorous intensity exercise training effectively regulates chemerin in older men and women. Future work should consider determining the effects of a single-bout of exercise on plasma chemerin to tease out the cumulative effect of exercise training on chemerin.
The mechanism by which exercise training reduces chemerin has yet to be fully elucidated. Physically active individuals typically have more favorable adipokine profiles than less active people (15), suggesting that high cardiorespiratory fitness may reflect “healthy” adipocytes. However, we did not observe a direct correlation between increased VO2max and decreased chemerin in this study. Based on this association, an exercise training induced increase in VO2max does not appear to be required for lowering chemerin. Alternatively, chemerin increases with adipocyte differentiation (29-31) and chemerin is associated with both subcutaneous and visceral fat accumulation (9, 32). This latter observation is in line with the direct correlation between the change in chemerin and visceral fat loss after exercise training in the current study (Figure 2), as well as previous weight loss research (22). Interestingly, lowering chemerin in cell models abrogates adipogenesis (33), and use of rosiglitazone and metformin decreased chemerin expression in adipocytes by activating peroxisome proliferator-activated receptor-γ (PPAR-γ) and reducing endoplasmic reticulum stress, respectively (29, 34). It is worth noting though that chemerin may be more directly related to circulating inflammatory adipokines (e.g. TNF-α) rather than fat mass per se (8). Although exercise training did not reduce plasma TNF-α concentrations in our subjects, circulating and monocyte-derived TNF-α is a well-established cytokine that induces insulin resistance and generally responds to lifestyle interventions (15, 18). Because resident adipose macrophages secrete additional cytokines that contribute to both local and systemic inflammation, it remains possible that paracrine or autocrine inflammatory secretion, rather than fat mass, contributed to the change in chemerin levels seen in this study (7, 35-37). In fact, recent work in patients with rheumatoid arthritis demonstrated that anti-TNF therapy reduced chemerin concentrations (37). Taken together, it would seem reasonable that at least some of the reductions in chemerin are due to exercise-induced declines in inflammation and obesity.
Habitual exercise has beneficial effects on glucose tolerance and insulin sensitivity. Chemerin has been shown in vitro to decrease glucose uptake via insulin signaling (i.e. insulin receptor substrate-1 (IRS-1) serine phosphorylation, Akt) and glycogen synthase in adipocytes and primary human skeletal muscle cells (10, 12, 36). Chemerin treatment also reduced hepatic glucose uptake in obese/diabetic (db/db) mice and attenuated glucose intolerance (13). As such, it would seem likely that improvements in peripheral and/or hepatic insulin sensitivity would be associated with reductions in plasma chemerin concentrations after exercise in older adults. Our results indicate, however, that changes in chemerin after exercise training were not associated with improvements in clamp-derived peripheral or hepatic insulin sensitivity or OGTT measured insulin AUC. A possible explanation for this observation may be related to the disparate effects of chemerin on glucose metabolism in adipocytes. While some have shown chemerin to impair insulin-stimulated glucose uptake (36), Takahashi et al. reported that chemerin enhanced IRS-1 tyrosine phosphorylation and promoted glucose uptake in adipocytes (11). Thus, these opposing in vitro data in adipocytes (11, 36) may help explain why in vivo correlations between chemerin and insulin sensitivity are observed in some (19, 21, 38) but not all human studies (4, 35, 39). It is likely that weight loss and/or enhanced aerobic fitness in the current study induced alternative mechanisms (e.g. mitochondrial function or reduced cytokines vis-a-vi IL-6, CRP, etc.) that had more pronounced effects on increasing insulin sensitivity (40-43).
Plasma chemerin levels are positively associated with aspects of cardiovascular disease, including metabolic syndrome and pro-atherogenic dyslipidemia (4, 35, 44). In fact, high fat feeding in mouse models has been shown to promote chemerin expression via elevated FFA concentrations (31). In the current study, although we observed a reduction in dietary fat after the intervention, there was no statistical difference in FFA concentrations, suggesting that dietary fat and FFAs per se are not essential for influencing chemerin. However, we did observe a significant inverse correlation between baseline chemerin and HDL levels. In addition, the reduction in chemerin after exercise training was positively correlated with decreased total cholesterol and triglyceride concentrations (Figure 3a and 3b), suggesting that chemerin may have a direct role in lipoprotein metabolism. In obesity, sterol regulatory element-binding protein 2 (SREBP2), which activates genes in cholesterol metabolism like 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) reductase and LDL-receptor, is elevated compared to lean controls (31). Chemerin has been suggested to increase cholesterol uptake in human monocyte derived macrophages and play a role in the development of atherosclerosis and foam cell formation (3). Since one of the major cardio-protective effects of habitual exercise is to manage cholesterol levels, our data suggest that some of the exercise effect to reduce cardiometabolic risk in older adults may be associated with lower chemerin concentrations. We acknowledge that associations do not equate to causation, and further investigation is required to elucidate the mechanism(s) by which chemerin is linked to blood lipids in older, obese, insulin resistant men and women.
Exercise training is known to decrease glucose-stimulated insulin secretion (45-47). This reduction in compensatory hyperinsulinemia to glucose loads in insulin resistant adults is considered beneficial for preserving pancreatic beta-cell function and improving long-term glycemic control (48). Recent work has demonstrated that chemerin plays a physiologic role in stimulating first phase GSIS in mice through a MafA dependent pathway (49). We extend upon this previous work by calculating both first phase and total GSIS to gain mechanistic insight into the relationship between chemerin and the regulatory steps of insulin secretion. In this study, exercise training reduced GSIS and this change in GSIS was significantly correlated with reductions in chemerin. Consistent with previous work in mice (49), our novel human data suggest that chemerin may be involved in the secretion of previously primed insulin secretory granules as well as maturation and/or docking of new insulin secretory granules that occur in response to oral glucose ingestion (50). Whether chemerin influences the ability of incretins to potentiate GSIS awaits further investigation. Taken together, our findings suggest that chemerin has a role in glucose homeostasis through a multi-organ dependent manner.
In conclusion, 12 weeks of aerobic exercise training reduced chemerin in previously sedentary, older, obese adults. The decrease in chemerin concentrations was associated with reductions in body fat, cholesterol/triglycerides, and insulin secretion. The degree to which treatments improve insulin action and regulate body fat likely involves cross-talk between liver, skeletal muscle, pancreas and adipose tissue. Our findings suggest that chemerin is an important hormone connecting obesity with cardiometabolic risk. Further work is required to elucidate the mechanism by which chemerin regulates glucose and lipid metabolism to gain insight into how exercise with weight loss contributes to the prevention of type 2 diabetes and cardiovascular disease.
Acknowledgments
S.K.M, S.N., and J.P.K contributed to the study design. S.K.M was primarily responsible for data analysis and statistical integrity. S.K.M wrote the manuscript. S.N. A.M., H.H. and J.P.K contributed to discussion, interpretation, and edited the manuscript. We thank all participants for their efforts and the nursing staff, particularly Julianne Filion, BSN, RN for outstanding technical assistance and study organization. The authors report no conflict of interest. This research was supported by RO1 AG12834 (to J.P.K.) and was supported in part by the National Institutes of Health, National Center for Research Resources, CTSA 1UL1 RR-024989, Cleveland, Ohio. S.K.M was supported by T32 DK007319.
References
- 1.Rabe K, Lehrke M, Parhofer KG, Broedl UC. Adipokines and insulin resistance. Molecular medicine. 2008;14:741–51. doi: 10.2119/2008-00058.Rabe. doi: 10.2119/2008-00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yilmaz Y, Yonal O, Kurt R, Alahdab Y, Eren F, Ozdogan O, Celikel C, Imeryuz N, Kalayci C, Avsar E. Serum levels of omentin, chemerin and adipsin in patients with biopsy-proven nonalcoholic fatty liver disease. Scand J Gastroenterol. 2011;46:91–7. doi: 10.3109/00365521.2010.516452. doi: 10.3109/00365521.2010.516452. [DOI] [PubMed] [Google Scholar]
- 3.Yan Q, Zhang Y, Hong J, Gu W, Dai M, Shi J, Zhai Y, Wang W, Li X, Ning G. The association of serum chemerin level with risk of coronary artery disease in Chinese adults. Endocrine. 2012;41:281–8. doi: 10.1007/s12020-011-9550-6. doi: 10.1007/s12020-011-9550-6. [DOI] [PubMed] [Google Scholar]
- 4.Bozaoglu K, Bolton K, McMillan J, Zimmet P, Jowett J, Collier G, Walder K, Segal D. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology. 2007;148:4687–94. doi: 10.1210/en.2007-0175. doi: 10.1210/en.2007-0175. [DOI] [PubMed] [Google Scholar]
- 5.Tan BK, Chen J, Farhatullah S, Adya R, Kaur J, Heutling D, Lewandowski KC, O'Hare JP, Lehnert H, Randeva HS. Insulin and metformin regulate circulating and adipose tissue chemerin. Diabetes. 2009;58:1971–7. doi: 10.2337/db08-1528. doi: 10.2337/db08-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tonjes A, Fasshauer M, Kratzsch J, Stumvoll M, Blher M. Adipokine pattern in subjects with impaired fasting glucose and impaired glucose tolerance in comparison to normal glucose tolerance and diabetes. PLoS ONE. 2010;5:e13911. doi: 10.1371/journal.pone.0013911. doi: 10.1371/journal.pone.0013911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cash JL, Hart R, Russ A, Dixon JP, Colledge WH, Doran J, Hendrick AG, Carlton MB, Greaves DR. Synthetic chemerin-derived peptides suppress inflammation through ChemR23. J Exp Med. 2008;205:767–75. doi: 10.1084/jem.20071601. doi: 10.1084/jem.20071601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weigert J, Neumeier M, Wanninger J, Filarsky M, Bauer S, Wiest R, Farkas S, Scherer M, Schaffler A, Aslanidis C, Scholmerich J, Buechler C. Systemic chemerin is related to inflammation rather than obesity in type 2 diabetes. Clin Endocrinol (Oxf) 2010;72:342–8. doi: 10.1111/j.1365-2265.2009.03664.x. doi: 10.1111/j.1365-2265.2009.03664.x. [DOI] [PubMed] [Google Scholar]
- 9.Shin HY, Lee DC, Chu SH, Jeon JY, Lee MK, Im J, Lee JW. Chemerin levels are positively correlated with abdominal visceral fat accumulation. Clin Endocrinol (Oxf) 2012;77:47–50. doi: 10.1111/j.1365-2265.2011.04217.x. doi: 10.1111/j.1365-2265.2011.04217.x. [DOI] [PubMed] [Google Scholar]
- 10.Sell H, Laurencikiene J, Taube A, Eckardt K, Cramer A, Horrighs A, Arner P, Eckel J. Chemerin is a novel adipocyte-derived factor inducing insulin resistance in primary human skeletal muscle cells. Diabetes. 2009;58:2731–40. doi: 10.2337/db09-0277. doi: 10.2337/db09-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takahashi M, Takahashi Y, Takahashi K, Zolotaryov FN, Hong KS, Kitazawa R, Iida K, Okimura Y, Kaji H, Kitazawa S, Kasuga M, Chihara K. Chemerin enhances insulin signaling and potentiates insulin-stimulated glucose uptake in 3T3-L1 adipocytes. FEBS Lett. 2008;582:573–8. doi: 10.1016/j.febslet.2008.01.023. doi: 10.1016/j.febslet.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 12.Becker M, Rabe K, Lebherz C, Zugwurst J, Goke B, Parhofer KG, Lehrke M, Broedl UC. Expression of human chemerin induces insulin resistance in the skeletal muscle but does not affect weight, lipid levels, and atherosclerosis in LDL receptor knockout mice on high-fat diet. Diabetes. 2010;59:2898–903. doi: 10.2337/db10-0362. doi: 10.2337/db10-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ernst MC, Issa M, Goralski KB, Sinal CJ. Chemerin exacerbates glucose intolerance in mouse models of obesity and diabetes. Endocrinology. 2010;151:1998–2007. doi: 10.1210/en.2009-1098. doi: 10.1210/en.2009-1098. [DOI] [PubMed] [Google Scholar]
- 14.Ernst MC, Sinal CJ. Chemerin: at the crossroads of inflammation and obesity. Trends Endocrinol Metab. 2010;21:660–7. doi: 10.1016/j.tem.2010.08.001. doi: 10.1016/j.tem.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 15.You T, Nicklas BJ. Effects of exercise on adipokines and the metabolic syndrome. Curr Diab. Rep. 2008;8:7–11. doi: 10.1007/s11892-008-0003-4. doi: 10.1007/s11892-008-0003-4. [DOI] [PubMed] [Google Scholar]
- 16.Malin SK, Niemi N, Solomon TPJ, Haus JM, Kelly KR, Filion J, Rocco M, Kashyap SR, Barkoukis H, Kirwan JP. Exercise training with weight loss and either a high- or low-glycemic index diet reduces metabolic syndrome severity in older Adults. Ann Nutr Metab. 2012;61:135–41. doi: 10.1159/000342084. doi: 10.1159/000342084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haus JM, Solomon TPJ, Marchetti CM, Edmison JM, Gonzlez F, Kirwan JP. Free fatty acid-induced hepatic insulin resistance is attenuated following lifestyle intervention in obese individuals with impaired glucose tolerance. J Clin Endocrinol Metab. 95:323–7. doi: 10.1210/jc.2009-1101. doi: 2010;10.1210/jc.2009-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelly KR, Haus JM, Solomon TPJ, Patrick Melin AJ, Cook M, Rocco M, Barkoukis H, Kirwan JP. A low-glycemic index diet and exercise intervention reduces TNF(alpha) in isolated mononuclear cells of older, obese adults. J Nutr. 2011;141:1089–94. doi: 10.3945/jn.111.139964. doi: 10.3945/jn.111.139964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chakaroun R, Raschpichler M, Kloting N, Oberbach A, Flehmig G, Kern M, Schron, Shang E, Lohmann T, Drebler M, Fasshauer M, Stumvoll M, Bluher M. Effects of weight loss and exercise on chemerin serum concentrations and adipose tissue expression in human obesity. Metabolism. 2012;61:706–14. doi: 10.1016/j.metabol.2011.10.008. doi: 10.1016/j.metabol.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Venojarvi M, Wasenius N, Manderoos S, Heinonen O, Hernelahti M, Lindholm H, Surakka J, Lindholm J, Aunola S, Atalay M, Eriksson JG. Nordic walking decreased circulating chemerin and leptin concentrations in middle-aged men with impaired glucose regulation. Ann Med. 2012;45(2):162–170. doi: 10.3109/07853890.2012.727020. doi: 10.3109/07853890.2012.727020. [DOI] [PubMed] [Google Scholar]
- 21.Saremi A, Shavandi N, Parastesh M, Daneshmand H. Twelve-week aerobic training decreases chemerin level and improves cardiometabolic risk factors in overweight and obese men. Asian J Sports Med. 2010;1(3):151–158. doi: 10.5812/asjsm.34860. PMID: 22375203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee MK, Chu SH, Lee DC, An KY, Park JH, Kim DI, Kim J, Hong S, Im JA, Lee JW, Jeon JY. The association between chemerin and homeostasis assessment of insulin resistance at baseline and after weight reduction via lifestyle modifications in young obese adults. Clin Chim Acta. 2013;421:109–15. doi: 10.1016/j.cca.2013.02.017. doi: 10.1016/j.cca.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 23.Kim SH, Lee SH, Ahn KY, Lee DH, Suh YJ, Cho SG, Choi YJ, Hong SB, Kim Y, Jeon JY, Nam M. Effect of lifestyle modification on serum chemerin concentration and its association with insulin sensitivity in overweight and obese adults with type 2 diabetes. Clin Endocrinol (Oxf). (Epub ahead of print) 2013 doi: 10.1111/cen.12249. doi: 10.1111/cen.12249. [DOI] [PubMed] [Google Scholar]
- 24.Prior SJ, Joseph LF, Brandauer J, Katzel LI, Hagberg JM, Ryan AS. Reduction in midthigh low-density muscle with aerobic exercise training and weight loss impacts glucose tolerance in older men. J Clin Endocrinol Metab. 2007;92:880–6. doi: 10.1210/jc.2006-2113. doi:10.1210/jc.2006-2113. [DOI] [PubMed] [Google Scholar]
- 25.O'Leary VB, Marchetti CM, Krishnan RK, Stetzer BP, Gonzalez F, Kirwan JP. Exercise-induced reversal of insulin resistance in obese elderly is associated with reduced visceral fat. J Appl Physiol. 2006;100:1584–9. doi: 10.1152/japplphysiol.01336.2005. doi: 10:1152/japplphysiol.01336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolfe RR. Radioactive and stable isotope tracers in biomedicine. Wiley-Liss; New York: 1992. [Google Scholar]
- 27.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol. 1983;55(2):628–34. doi: 10.1152/jappl.1983.55.2.628. [DOI] [PubMed] [Google Scholar]
- 28.Bateman LA, Slentz CA, Willis LH, Shields AT, Piner WL, Bales CW, Houmard JA, Kraus WE. Comparison of aerobic versus resistance exercise training effects on metabolic syndrome (from the studies of a targeted risk reduction intervention through defined Exercise - STRRIDE-AT/RT). Am J Cardiol. 2011;108:838–44. doi: 10.1016/j.amjcard.2011.04.037. Doi:10.1016/j.amjcard.2011.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muruganandan S, Parlee SD, Rourke JL, Ernst MC, Goralski KB, Sinal CJ. Chemerin, a novel peroxisome proliferator-activated receptor gamma (PPARgamma) target gene that promotes mesenchymal stem cell adipogenesis. J Biol Chem. 2011;286:23982–95. doi: 10.1074/jbc.M111.220491. doi: 10.1074/jbc.M111.220491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muruganandan S, Roman A, Sinal CJ. Role of chemerin/CMKLR1 signaling in adipogenesis and osteoblastogenesis of bone marrow stem cells. J Bone Miner Res. 2010;25:222–34. doi: 10.1359/jbmr.091106. doi: 10.1359/jbmr.091106. [DOI] [PubMed] [Google Scholar]
- 31.Bauer S, Wanninger J, Schmidhofer S, Weigert J, Neumeier M, Dorn C, Hellerbrand C, Zimara N, Schmidhoffler A, Aslanidis C, Buechler C. Sterol regulatory element-binding protein 2 (SREBP2) activation after excess triglyceride storage induces chemerin in hypertrophic adipocytes. Endocrinology. 2011;152:26–35. doi: 10.1210/en.2010-1157. doi: 10.1210/en.2010-1157. [DOI] [PubMed] [Google Scholar]
- 32.Jialal I, Devaraj S, Kaur H, Adams Huet B, Bremer AA. Increased chemerin and decreased omentin-1 in both adipose tissue and plasma in nascent metabolic Syndrome. J. Clin Endocrinol Metab. 2013;98(3):E514–517. doi: 10.1210/jc.2012-3673. doi: 10.1210/jc.2012-3673. [DOI] [PubMed] [Google Scholar]
- 33.Goralski KB, McCarthy TC, Hanniman EA, Zabel BA, Butcher EC, Parlee SD, Muruganandan S, Sinal CJ. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J Biol Chem. 2007;282:28175–88. doi: 10.1074/jbc.M700793200. doi: 10.1074/jbc.M700793200. [DOI] [PubMed] [Google Scholar]
- 34.Pei L, Yang J, Du J, Liu H, Ao N, Zhang Y. Downregulation of chemerin and alleviation of endoplasmic reticulum stress by metformin in adipose tissue of rats. Diabetes Res Clin Pract. 2012;97:267–75. doi: 10.1016/j.diabres.2012.02.023. doi: 10.1016/j.diabres.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 35.Chu SH, Lee MK, Ahn KY, Im JA, Park MS, Lee DC, Jeon JY, Lee JW. Chemerin and adiponectin contribute reciprocally to metabolic syndrome. PLoS ONE. 2012;7:e34710–e34710. doi: 10.1371/journal.pone.0034710. doi: 10.1371/journal.pone.0034710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kralisch S, Weise S, Sommer G, Lipfert J, Lossner U, Bluher M, Stumvoll M, Fasshauer M. Interleukin-1beta induces the novel adipokine chemerin in adipocytes in vitro. Regul Pept. 2009;154:102–6. doi: 10.1016/j.regpep.2009.02.010. doi: 10.1016/j.regpep.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 37.Herenius MM, Oliveira AS, Wijbrandts CA, Gerlag DM, Tak PP, Lebre MC. Anti-TNF therapy reduces serum levels of chemerin in rheumatoid arthritis: a new mechanism by which anti-TNF might reduce inflammation. PLoS One. 2013;8(2):e57802. doi: 10.1371/journal.pone.0057802. doi: 10.1371/journal.prone.0057802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ouwens DM, Bekaert M, Lapauw B, Van Nieuwenhove Y, Lehr S, Hartwig S, Calders P, Kaufman JM, Sell H, Eckel J, Ruige JB. Chemerin as biomarker for insulin sensitivity in males without typical characteristics of metabolic syndrome. Arch Physiol Biochem. 2012;118:135–8. doi: 10.3109/13813455.2012.654800. doi: 10.3109/13813455.2012.654800. [DOI] [PubMed] [Google Scholar]
- 39.Bauer S, Bala M, Kopp A, Eisinger K, Schmid A, Schneider S, Neumeier M, Buechler C. Adipocyte chemerin release is induced by insulin without being translated to higher levels in vivo. Eur J Clin Invest. 2012;42:1213–20. doi: 10.1111/j.1365-2362.2012.02713.x. doi: 10.1111/j.1365-2362.2012.02713.x. [DOI] [PubMed] [Google Scholar]
- 40.Kalyani RR, Varadhan R, Weis CO, Fried LP, Cappola AR. Frailty status and altered dynamics of circulating energy metabolism hormones after oral glucose in older women. J Nutr Health Aging. 2012;16(8):679–86. doi: 10.1007/s12603-012-0066-4. Doi: 10.1007/s12603-012-0369-5. [DOI] [PubMed] [Google Scholar]
- 41.Moreira PF, Dalboni MA, Cendorogio M, Santos GM, Cendorogio MS. Postprandial interleukin-6 response in elderly with abdominal obesity and metabolic syndrome. J Nutr Health Aging. 2013;17(3):206–10. doi: 10.1007/s12603-012-0400-x. Doi: 10.1007/s12603-012-0400-x. [DOI] [PubMed] [Google Scholar]
- 42.Little JP, Safdar A, Benton CR, Wright DC. Skeletal muscle and beyond: the role of exercise as a mediator of systemic mitochondrial biogenesis. Appl Physiol Nutr Metab. 2011;36(5):598–607. doi: 10.1139/h11-076. doi: 10.1139/h11-076. [DOI] [PubMed] [Google Scholar]
- 43.Ryan AS, Nicklas BJ. Exercise with calorie restriction improves insulin sensitivity and glycogen synthase activity in obese postmenopausal women with impaired glucose tolerance. Am J Physiol Endocrinol Metab. 2004;302(1):E145–52. doi: 10.1152/ajpendo.00618.2010. doi: 10.1152/ajpendo.00618. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roman AA, Parlee SD, Sinal CJ. Chemerin: a potential endocrine link between obesity and type 2 diabetes. Endocrine. 2012;42:243–51. doi: 10.1007/s12020-012-9698-8. doi: 10.1007/s12020-012-9698-8. [DOI] [PubMed] [Google Scholar]
- 45.Bloem CJ, Chang AM. Short-term exercise improves beta-cell function and insulin resistance in older people with impaired glucose tolerance. J.Clin.Endocrinol.Metab. 2008;932:387–392. doi: 10.1210/jc.2007-1734. doi: 10.1210/jc.2007-1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Solomon TPJ, Haus JM, Kelly KR, Rocco M, Kashyap SR, Kirwan JP. Improved pancreatic beta-cell function in type 2 diabetic patients after lifestyle-induced weight loss is related to glucose-dependent insulinotropic polypeptide. Diabetes Care. 2010;33(7):1561–1566. doi: 10.2337/dc09-2021. doi: 10.2337/dc09-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kelly KR, Brooks LM, Solomon TP, Kashyap SR, O'Leary VB, Kirwan JP. The glucose-dependent insulinotropic polypeptide and glucose-stimulated insulin response to exercise training and diet in obesity. Am J Physiol Endocrinol Metab. 2009;296(6):E1269–74. doi: 10.1152/ajpendo.00112.2009. doi: 10.1152/ajpendo.00112.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DeFronzo RA, Abdul Ghani MA. Preservation of ß-cell function: the key to diabetes prevention. J.Clin.Endocrinol.Metab. 2011;96(8):2354–2366. doi: 10.1210/jc.2011-0246. doi: 10.1210/jc.2011-0246. [DOI] [PubMed] [Google Scholar]
- 49.Takahashi M, Okimura Y, Iguchi G, Nishizawa H, Yamamoto M, Suda K, Kitazawa R, Fujimoto W, Takahashi K, Zolotaryov FN, Hong KS, Kiyonari H, Abe T, Kaji H, Kitazawa S, Kasuga M, Chihara K, Takahashi Y. Chemerin regulates β-cell function in mice. Sci Rep. 2011;1:123. doi: 10.1038/srep00123. doi: 10.1038/srep00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cobelli C, Toffolo GM, Dalla Man C, Campioni M, Denti P, Caumo A, Butler P, Rizza R. Assessment of beta-cell function in humans, simultaneously with insulin sensitivity and hepatic extraction, from intravenous and oral glucose tests. Am J Physiol Endocrinol Metab. 2007;293(1):E1–E15. doi: 10.1152/ajpendo.00421.2006. doi:10.1152/ajpendo.00421.2006. [DOI] [PubMed] [Google Scholar]