Abstract
Background
Posttraumatic stress disorder (PTSD), depression, anxiety, and stress are significant problems among returning veterans and are associated with reduced quality of life.
Design
A correlational design was used to examine the impact of a polymorphism (5-HTTLPR) in the serotonin transporter promoter gene on post-deployment adjustment among returning veterans.
Methods
A total of 186 returning Iraq and Afghanistan veterans were genotyped for the 5-HTTLPR polymorphism. Symptoms of PTSD, depression, general stress, and anxiety were assessed along with quality of life.
Results
After controlling for combat exposure, age, sex of the participant, and race, 5-HTTLPR had a significant multivariate effect on post-deployment adjustment, such that S′ carriers reported more post-deployment adjustment problems and worse quality of life than veterans homozygous for the L′ allele. This effect was larger when the analyses were restricted to veterans of European ancestry.
Conclusions
Our findings suggest that veterans who carry the S′ allele of the 5-HTTLPR polymorphism may be at increased risk for adjustment problems and reduced quality of life following deployments to war zones.
Keywords: 5-HTTLPR, serotonin transporter, PTSD, trauma, depression, stress, anxiety
Returning Iraq and Afghanistan veterans are at increased risk for post-deployment adjustment problems, including posttraumatic stress disorder (PTSD), anxiety, and depression (e.g., Hoge et al., 2004, Smith et al., 2008). This increased risk appears to be at least partly due to exposure to stress and trauma during deployment. For example, Smith and colleagues (2008) reported a threefold increase in the incidence of PTSD among military personnel reporting combat exposure. Hoge et al. (2004) also reported a strong positive association between combat exposure and PTSD among Iraq and Afghanistan veterans. However, while the impact of combat exposure on post-deployment adjustment is evident, genetic factors also likely play a role. True and colleagues (1993) estimated that approximately 30% of susceptibility to PTSD among Vietnam veterans was due to genetic factors. There is also evidence that much of the comorbidity observed between PTSD and depression is due to shared genetic factors (Koenen et al., 2008).
The polymorphic region (5-HTTLPR) of the serotonin transporter promoter gene (SLC6A4) has been studied in relation to both PTSD and depression, as the serotonin transporter is responsible for terminating serotonin (5-HT) action in the synaptic cleft (Sangkuhl, Klein, & Altman, 2009). The short allele (S) of the 5-HTTLPR polymorphism appears to be less transcriptionally efficient than the long (L) allele (Lesch et al., 1996), resulting in decreased 5-HT expression and uptake. It is also associated with both functional (e.g., Hariri et al., 2002) and structural (e.g., Young et al., 2008) features of the brain. Thus, several lines of evidence suggest the 5-HTTLPR polymorphism may contribute to genetic susceptibility for PTSD and depression.
More recent research on 5-HTTLPR has determined additional variants that appear to affect CNS function (Hu et al., 2005). One of these is a functional polymorphism (rs25531) that codes for a relatively common A > G substitution. The combination of the L allele and the 25531 G polymorphism produces an “S” phenotype with respect to transporter gene expression in cell cultures. Consequently, LG alleles are often reclassified as S′ alleles. This reclassification (i.e., LA = L′; LG, SG and SA = S′) is commonly referred to as the triallelic classification.
Findings regarding 5-HTTLPR and PTSD have been mixed (e.g., Wang et al., 2011; Grabe et al., 2009). A recent meta-analysis by Gressier et al. (2013) did not find evidence for a direct association between 5-HTTLPR and PTSD, although the authors did note an association between 5-HTTLPR and PTSD among high trauma-exposed populations, such as veteran samples. For example, Wang et al. (2011) examined the influence of 5-HTTLPR on PTSD among combat veterans from the wars in Vietnam, Iraq, and Afghanistan and reported that the S′ allele was associated with increased risk for meeting diagnostic criteria for PTSD and greater PTSD symptom severity. To date, however, there has been only limited research on the potential role of the S′ allele in Iraq and Afghanistan war veterans’ post-deployment adjustment.
Objective and hypothesis
The primary objective of the present research was to replicate Wang et al.’s (2011) finding associating the S′ allele with veterans’ PTSD symptom severity scores after accounting for combat exposure, sex of the participant, age, and race. The current study also extended this work by examining the impact of 5-HTTLPR on a broader array of post-deployment outcomes, including depression, general stress/tension, anxiety, and quality of life, as veterans with mental health problems also frequently report low quality of life (e.g., Pittman, Goldsmith, Lemmer, Kilmber, & Baker, 2012). We chose to focus on Iraq and Afghanistan veterans in the current study because they represent the fastest growing group of U.S. veterans at the present time. They also present several advantages to researchers interested in the genetic basis of combat-related PTSD. For example, Iraq and Afghanistan veterans are likely to have more homogenous traumatic experiences compared with studying veterans from multiple eras in the same study. There is also less variability in the time between their traumatic experiences and the time of assessment, which represents an additional advantage of focusing on this particular group of veterans. We hypothesized that Iraq and Afghanistan veterans that carried the S′ allele would report significantly more post-deployment adjustment problems and significantly lower quality of life than Iraq and Afghanistan veterans homozygous for the 5-HTTLPR L′ allele after controlling for combat exposure, sex of the participant, age, and race.
Method
Participants
A total of 186 Iraq and Afghanistan war veterans were recruited through mailings, advertisements, and recruitment by VA health care providers. Recruitment efforts were targeted to oversample individuals with PTSD and depression. Participants were excluded if they: (a) were unwilling or unable to complete the assessments; (b) met criteria for a psychotic disorder or bipolar disorder on the Mini International Neuropsychiatric Interview (Sheehan et al., 1998); or (c) exhibited suicidal or homicidal risk warranting crisis intervention. If receiving mental health treatment at the time of the study, participants were required to be stable on psychiatric medications and in psychotherapy for at least three months to ensure reported symptoms were not reflective of recently starting or stopping treatment. The diagnostic screening assessments were conducted by either a clinical psychologist or a trained psychology technician working under the direct supervision of a clinical psychologist.
Procedures
The study was approved by the Central Texas Veterans Health Care System Institutional Review Board. All participants provided informed consent prior to completing study procedures. Psychiatric symptoms and combat exposure were assessed with validated self-report measures. Saliva samples were collected with Oragene DNA collection kits (DNA Genotek, Ottawa, Canada). Genomic DNA was extracted and purified according to the manufacturer’s specifications. 5-HTTLPR genotyping utilized the protocol from Wendland, Kruse, and Murphy (2006). Briefly, the 5-HTTLPR region was amplified as a ~500 bp fragment that was subjected to restriction endonuclease digestion with HpaII. Restriction fragments were electrophoretically separated in 3% agarose and visualized with ethidium bromide. Gel images were interpreted by an experienced molecular biologist (R. J.). Twenty-five percent of the DNA samples were run in duplicate to assess reliability, resulting in a concordance rate of 100%. Genotype frequencies for the total sample (N = 186) were as follows: LA/LA (36), LA/LG (24), LG/LG (5), SA/LA (66), SG/LA (1), SA/LG (15), SG/LG (1), SA/SA (38). Alleles were re-classified using the triallelic classification (Hu et al., 2005), resulting in 36 L′/L′, 91 S′/L′, and 59 S′/S′ for the total sample. The distribution did not vary from Hardy-Weinberg equilibrium (p = .40). Among veterans of European ancestry (EAs; n = 116), genotype frequencies were as follows: LA/LA (23), LA/LG (12), LG/LG (0), SA/LA (44), SG/LA (1), SA/LG (10), SG/LG (0), SA/SA (26), with triallelic reclassification resulting in 23 L′/L′, 57 S′/L′, and 36 S′/S′. This distribution was also in Hardy-Weinberg equilibrium (p = .62).
Measures
The PTSD Checklist – Military Version (PCL-M; Weathers, Litz, Herman, Huska, & Keane, 1993) assessed PTSD symptom severity. The widely-used, 17-item PCL-M possesses excellent reliability and validity (e.g., Blanchard, Jones-Alexander, Buckley, & Forneris, 1996). Internal consistency was .97 in the current study. The Beck Depression Inventory – II (BDI-II; Beck, Steer, & Brown, 1996) assessed depressive symptoms. The 21-item BDI-II also has excellent reliability and validity (e.g., Beck, Steer, Ball, & Ranieri, 1996; Beck et al., 1996). Internal consistency was .94 in the current study. The Depression Anxiety Stress Scales – 21 (DASS-21; Lovibond & Lovibond, 1995) assessed depression, anxiety, and general stress/tension during the past week. An advantage of the DASS-21 over other self-report measures of depression and anxiety is that it was specifically designed to discriminate between anxiety and depression (to the degree possible) while still demonstrating excellent reliability and validity (Lovibond & Lovibond, 1995). The DASS contains three subscales (Depression, Anxiety, Stress), each of which exhibited good reliability in the current study (α’s from .88 – .94). The Quality of Life Scale (QOLS; Burckhardt, Woods, Schultz, & Ziebarth, 1989) assessed quality of life. The 16-item QOLS has demonstrated good reliability and validity in previous research (Burckhardt et al., 1993; Burckhardt & Anderson, 2003). Internal consistency was .94 in the current study. The 18-item Full Combat Exposure Scale (FCES; Hoge et al., 2004), one of the most comprehensive measures of combat available, was used to assess combat exposure. Internal consistency for the FCES .92 in the current study.
Results
Participant ages ranged from 22 to 63 years (M = 38.6 SD = 10.6). The sample was predominantly male (85%; n = 159) and white (62%; n = 116). Most participants (85%) had served in the Army. On average, participants had been discharged from the military for 3.1 years at the time of the assessment. Table 1 provides additional sample characteristics.
Table 1.
Participant characteristics
| Continuous Variables | Mean | SD | Range |
|---|---|---|---|
| PTSD Checklist - Military Version (PCL-M) | 41.1 | 19.2 | 17–83 |
| Beck Depression Inventory - II (BDI-II) | 15.3 | 12.7 | 0–56 |
| Quality of Life Scale (QOLS) | 75.6 | 18.5 | 24–109 |
| DASS-Depression | 5.6 | 5.8 | 0–21 |
| DASS-Anxiety | 4.5 | 5 | 0–21 |
| DASS-Stress | 7.2 | 5.8 | 0–21 |
| Full Combat Exposure Scale (FCES) | 20.8 | 14.5 | 0–64 |
| Years of Education | 13.9 | 2.1 | 11–21 |
| Age (years) | 38.6 | 10.6 | 22–63 |
| Years since military discharge | 3.1 | 2.2 | 0–10.8 |
|
| |||
| Categorical Variables | N | % | |
|
| |||
| 5-HTTLPR Genotype | |||
| L′/L′ | 36 | 19.4% | |
| S′/L′ | 91 | 48.9% | |
| S′/S′ | 59 | 31.7% | |
| Sex of the Participant | |||
| Male | 159 | 85.5% | |
| Female | 22 | 11.8% | |
| Military Branch | |||
| Army | 156 | 83.9% | |
| Marines | 12 | 6.5% | |
| Air Force | 12 | 6.5% | |
| Navy | 6 | 3.2% | |
| Race | |||
| European | 116 | 62.4% | |
| African-American | 44 | 23.7% | |
| American Indian | 7 | 3.8% | |
| Asian | 7 | 3.8% | |
| Hawaiian/Pacific Islander | 2 | 1.1% | |
| Other | 12 | 6.5% | |
Note: PTSD = posttraumatic stress disorder; DASS = Depression Anxiety Stress Scales.
Multivariate analysis of covariance (MANCOVA) was used to help control for Type I error and to account for correlations among the dependent variables (PCL-M, BDI-II, DASS-Depression, DASS-Anxiety, DASS-Stress, and QOLS scores). A Bonferroni correction (.05/6 = .0083) was also used because of the multiple comparisons. 5-HTTLPR genotype served as the independent variable and was dichotomized such that S′/S′ and S′/L′ carriers were grouped together (S′ carriers) and compared with L′/L′ homozygotes. Covariates included combat exposure (FCES scores), sex of the participant (female = 0; male = 1), age, and race (non-white = 0; white = 1).
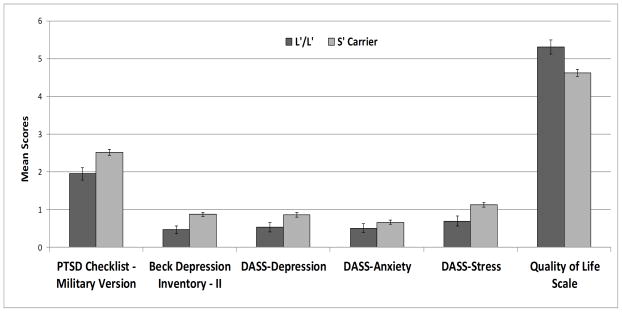
As can be seen in Table 2, after controlling for combat exposure and demographic variables, the MANCOVA revealed a significant multivariate effect for the 5-HTTLPR polymorphism on the dependent variables (medium effect). Consistent with our hypothesis, S′ carriers reported more post-deployment problems and worse quality of life than veterans homozygous for the L′ allele. Higher combat exposure was also associated with post-deployment problems (large effect). No other variables in the model reached statistical significance. Significant (p < .0083) univariate effects for the 5-HTTLPR polymorphism were observed on four of the six dependent variables (see Table 3 and Figure 1), including the PCL-M (small effect), BDI-II (medium effect), DASS-Stress (small effect), and QOLS (medium effect). The 5-HTTLPR polymorphism also had a nominally significant effect on DASS-Depression (small effect); however, it had no effect on DASS-Anxiety, p = .22.
Table 2.
Summary of the MANCOVA model examining the influence of the 5-HTTLPR polymorphism on PTSD, depression, anxiety, and quality of life among Iraq and Afghanistan veterans.
| Total Sample (N = 186) | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variable | Wilk’s Λ | F | df | Error df | p | η2 |
| Intercept* | .224 | 93.27 | 6 | 162 | < .001 | .78 |
| Combat exposure* | .725 | 10.26 | 6 | 162 | < .001 | .28 |
| Sex of the Participant | .951 | 1.389 | 6 | 162 | .222 | .05 |
| Age | .952 | 1.369 | 6 | 162 | .23 | .05 |
| Race | .956 | 1.231 | 6 | 162 | .293 | .04 |
| 5-HTTLPR* | .887 | 3.446 | 6 | 162 | .003 | .11 |
|
| ||||||
| Veterans of European Ancestry only (n = 116) | ||||||
|
| ||||||
| Variable | Wilk’s Λ | F | df | Error df | p | η2 |
|
| ||||||
| Intercept* | .194 | 72.55 | 6 | 105 | < .001 | .81 |
| Combat exposure* | .717 | 6.892 | 6 | 105 | < .001 | .28 |
| Sex of the Participant | .964 | .651 | 6 | 105 | .689 | .04 |
| Age | .875 | 2.499 | 6 | 105 | .027 | .13 |
| 5-HTTLPR* | .816 | 3.957 | 6 | 105 | .001 | .18 |
Note:
< .0083;
MANCOVA = multivariate analysis of covariance; PTSD = posttraumatic stress disorder.
Table 3.
Summary of the univariate effects for the 5-HTTLPR polymorphism on PTSD, depression, anxiety, and quality of life.
| Total sample (N = 186) | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Dependent Variable | Type III Sum of Squares | df | Error df | F | p | η2 |
| PTSD Checklist - Military Version (PCL-M)* | 8.511 | 1 | 167 | 9.448 | .002 | .05 |
| Beck Depression Inventory - II (BDI-II)* | 4.303 | 1 | 167 | 12.49 | .001 | .07 |
| DASS-Depression | 2.825 | 1 | 167 | 5.097 | .025 | .03 |
| DASS-Anxiety | .626 | 1 | 167 | 1.507 | .221 | .01 |
| DASS-Stress* | 4.918 | 1 | 167 | 8.801 | .003 | .05 |
| Quality of Life Scale (QOLS)* | 12.392 | 1 | 167 | 10.446 | .001 | .06 |
|
| ||||||
| Veterans of European Ancestry only (n = 116) | ||||||
|
| ||||||
| Dependent Variable | Type III Sum of Squares | df | Error df | F | p | η2 |
|
| ||||||
| PTSD Checklist - Military Version (PCL-M)* | 11.888 | 1 | 110 | 13.511 | < .001 | .11 |
| Beck Depression Inventory - II (BDI-II)* | 5.61 | 1 | 110 | 17.346 | < .001 | .14 |
| DASS-Depression* | 6.255 | 1 | 110 | 11.537 | .001 | .10 |
| DASS-Anxiety | 2.149 | 1 | 110 | 5.459 | .021 | .05 |
| DASS-Stress* | 8.678 | 1 | 110 | 15.768 | < .001 | .13 |
| Quality of Life Scale (QOLS)* | 16.218 | 1 | 110 | 14.691 | < .001 | .12 |
Note:
< .0083;
PTSD = posttraumatic stress disorder; DASS = Depression Anxiety Stress Scales.
Figure 1.
Effect of the 5-HTTLPR polymorphism on post-deployment adjustment among Iraq and Afghanistan veterans (N = 186). Marginal means for the scale means were estimated from the MANCOVA model with covariates appearing in the model at the following values: Full Combat Exposure Scale mean = 1.19; sex of the participant = .87; age = 38.59; race = .66. Error bars reflect standard errors. DASS = Depression Anxiety Stress Scales.
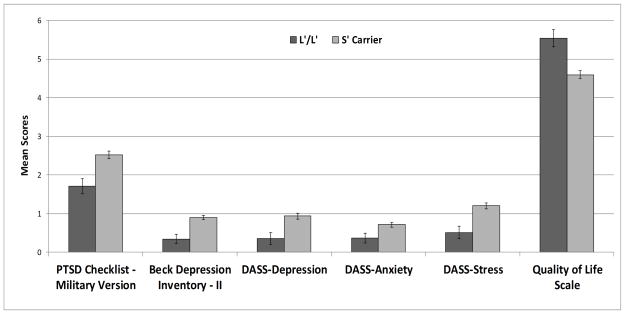
To address concerns related to population stratification, analyses were repeated among the subset of veterans who self-reported European ancestry (n = 116). A similar pattern of findings emerged, with combat exposure (large effect), and 5-HTTLPR (large effect size), continuing to exhibit significant multivariate effects on post-deployment adjustment after accounting for demographic variables (Table 2). Notably, the effect of 5-HTTLPR polymorphism on post-deployment adjustment among individuals of European ancestry was larger than the effect observed in the overall sample. In addition, age was inversely related to post-deployment adjustment (medium effect). No other variables in the model reached statistical significance. As can be seen in Table 3, 5-HTTLPR had significant (p < .0083) univariate effects on five of the six dependent variables in the restricted sample (Figure 2), including PCL-M (medium effect), BDI-II (large effect), DASS-Depression (medium effect), DASS-Stress (medium effect), and QOLS (medium effect). The 5-HTTLPR polymorphism also had a nominally significant effect on DASS-Anxiety (small effect).
Figure 2.
Effect of the 5-HTTLPR polymorphism on post-deployment adjustment among Iraq and Afghanistan eterans of European ancestry (n = 116). Marginal means for the scale means were estimated from the MANCOVA model with covariates appearing in the model at the following values: Full Combat Exposure Scale mean = 1.27; sex of the participant = .90; age = 38.15. Error bars reflect standard errors. DASS = Depression Anxiety Stress Scales.
Discussion
The present study increases our understanding of the impact of 5-HTTLPR on veterans’ post-deployment adjustment in several ways. First, our findings suggest that variation in the serotonin transporter polymorphism is associated with PTSD symptom severity among returning veterans over and above the effects of combat exposure. After accounting for combat exposure, age, sex of the participant, and race, symptoms of PTSD symptoms (PCL-M scores) were elevated by 29% among S′ carriers in the overall sample and by 47% in the sample restricted to veterans of European ancestry. Thus, our findings are consistent with those of Wang and colleagues (2011), who provided the first report of an association between the S′ allele and PTSD in combat veterans. The present study also extended Wang and colleagues’ findings by demonstrating that the S′ allele was associated with higher levels of depression and stress and lower quality of life among returning veterans. In the overall sample, depression symptoms were 61–86% higher (BDI-II and DASS-D scores, respectively) among S′ carriers, and general stress and tension symptoms (DASS-S scores) were 62% higher. In contrast, veterans homozygous for the L′ allele reported significantly better (15% higher) quality of life (QOLS scores) compared to S′ carriers. These differences were even more pronounced among veterans of European ancestry.
To our knowledge, the present study is the first to study the effects of 5-HTTLPR in a cohort strictly limited to Iraq and Afghanistan veterans. Our findings indicate that in this group, and especially among those of European ancestry, the 5-HTTLPR S′ allele had surprisingly robust effects on post-deployment adjustment. One possible explanation for the effect sizes observed in the present study may be that the long periods of stress and high levels of trauma that Iraq and Afghanistan veterans frequently encounter during deployments may magnify the effects of 5-HTTLPR on PTSD, depression, and general stress/tension. That is, it may be the case that 5-HTTLPR exerts more influence when traumatic stressors are prolonged and highly distressing compared to more time-limited traumatic experiences (e.g., natural disasters or motor-vehicle accidents); however, larger samples and more detailed measures of trauma exposure are needed to fully address this hypothesis, as the current study was not sufficiently well-powered to test for gene x environment effects.
An additional strength of the study was the multivariate data analytic approach employed. The results obtained from the MANCOVA models suggest that 5-HTTLPR genetic variation could help to explain some of the genetic overlap between PTSD and depression (e.g., Koenen et al., 2008). Support for this interpretation comes from examination of the univariate effects of the S′ allele on the DASS subscales. In particular, our findings suggest that the S′ allele was most strongly associated with depression and the general negative affect component of emotional disorders that is proposed to be shared by both depression and the anxiety disorders (Clark & Watson, 1991; Lovibond & Lovibond, 1995). In contrast, 5-HTTLPR had no effect on the more unique aspects of anxiety tapped by the DASS-Anxiety scale in the overall sample and only a small effect among veterans of European ancestry, which was only nominally significant. Such findings are particularly interesting in light of recent findings demonstrating that PTSD and depression appear to have a high rate of comorbidity among veterans and to comprise a “distress” factor (e.g., Miller, Fogler, Wolf, Kaloupek, & Keane, 2008; Kimbrel et al., in press). Future research should consider other types of multivariate approaches to the study of the relationship between 5-HTTLPR, PTSD, and depression, such as structural equation modeling, to determine if 5-HTTLPR is uniquely associated with the higher-order “distress” latent factor of psychiatric comorbidity. Finally, to our knowledge, the present study is the first to demonstrate that 5-HTTLPR genetic variation is associated with lower quality of life following deployment to a war zone. This finding is consistent with prior research demonstrating a positive correlation between serotonin transporter availability and quality of life in healthy civilians (Tsai et al., 2009); however, additional research aimed at replicating this finding is warranted.
Clinical implications
With respect to clinical implications, the findings from the present study suggest that Iraq and Afghanistan veterans who carry the S′ allele may be at increased risk for a variety of psychiatric problems in the years following their warzone service. Even more notable is the fact that the association observed between 5-HTTLPR and post-deployment adjustment problems was observed even after many of the veterans had already been enrolled in VA for several years and were likely to be receiving pharmacological treatment, psychosocial treatment, or both. While the correlational design of the current study prevents us from drawing any direct inferences about the association between the S′ allele and treatment outcome, the findings from this study are consistent with other recent studies suggesting that individuals with PTSD that carry the S′ allele may be more treatment resistant and more likely to drop out of treatment compared to individuals with PTSD who are homozygous for the L′ allele (Bryant et al., 2010; Mushtaq, Ali, Margoob, Murtaza, & Andrade, 2012).
Study limitations and future directions
Findings should be interpreted within the context of several limitations. First, the sample was relatively small for a genetic study and a replication sample was not included. Thus, these findings should be considered preliminary until they are replicated in an independent study. Second, our genotyping panel did not contain ancestral informative markers (AIMs). We attempted to overcome this limitation by including race as a covariate in the analyses and by reanalyzing the data using only participants of European ancestry. Importantly, race was not associated with any of the outcomes, and our findings were even stronger in the subset of participants of European ancestry. As a result, we believe that it is unlikely that our findings are due to population stratification; however, given evidence that 5-HTTLPR may operate differently in African Americans than it does in European Americans (e.g., Walsh, Uddin, Soliven, Wildman, & Bradley, 2014), there remains a clear need for additional research on the relationship between 5-HTTLPR, PTSD, and other psychiatric outcomes among African American veterans. A third limitation was our reliance on self-report measures. Future studies that include diagnostic measures of PTSD and depression are needed to validate our results. A final limitation was that our genotyping panel was restricted to examining 5-HTTLPR. Additional research into the impact of other genes and other SLC6A4 variants on returning veteran’s post-deployment outcomes is warranted.
Summary and conclusions
In summary, 5-HTTLPR genetic variation was found to influence symptoms of PTSD, depression, general stress, and quality of life among returning Iraq and Afghanistan veterans after accounting for combat exposure, sex of the participant, age, and race. These findings suggest that returning veterans who carry the 5-HTTLPR S′ allele may be at increased risk for both mental health problems and reduced quality of life following their warzone deployments.
Acknowledgments
This work was supported by the Department of Veterans Affairs (VA) through a Career Development Award-2 (IK2 CX000525) to Dr. Kimbrel from the Clinical Science Research and Development Service of the VA Office of Research and Development (ORD), a VA VISN 17 New Investigator Award to Dr. Kimbrel, and a Merit Award (I01RX000304) to Dr. Morissette from the Rehabilitation Research and Development Service of VA ORD. This work was also supported by the VA VISN 17 Center of Excellence for Research on Returning War Veterans, Central Texas Veterans Health Care System, the Mental Health and Research Services of the Durham VA Medical Center, and the VA Mid-Atlantic Mental Illness Research, Education, and Clinical Center.
Footnotes
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the VA or the United States government.
The authors have no conflicts of interest to declare.
References
- Beck AT, Steer RA, Ball R, Ranieri WF. Comparison of Beck Depression Inventories–IA and –II in psychiatric outpatients. Journal of Personality Assessment. 1996;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory manual. 2. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Burckhardt CS, Woods SL, Schultz AA, Ziebarth DM. Quality of life of adults with chronic illness: A psychometric study. Research in Nursing & Health. 1989;12:347–354. doi: 10.1002/nur.4770120604. [DOI] [PubMed] [Google Scholar]
- Burckhardt CS, Anderson KL. The Quality of Life Scale (QOLS): Reliability, validity, and utilization. Health and Quality of Life Outcomes. 2003;1:60–67. doi: 10.1186/1477-7525-1-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard EB, Jones-Alexander J, Buckley TC, Forneris CA. Psychometric properties of the PTSD Checklist (PCL) Behavior Research & Therapy. 1996;34(8):669–673. doi: 10.1016/0005-7967(96)00033-2. [DOI] [PubMed] [Google Scholar]
- Bryant RA, Felmingham KL, Falconer EM, Pe Benito L, Dobson-Stone C, Pierce KD, Schofield PR. Preliminary evidence of the short allele of the serotonin transporter gene predicting poor response to cognitive behavior therapy in posttraumatic stress disorder. Biological Psychiatry. 2010;67:1217–1219. doi: 10.1016/j.biopsych.2010.03.016. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D. Tripartite model of anxiety and depression: Psychometric evidence and taxonomic implications. Journal of Abnormal Psychology. 1991;100(3):316–336. doi: 10.1037/0021-843X.100.3.316. [DOI] [PubMed] [Google Scholar]
- Grabe H, Spitzer C, Schwann C, Marcinek A, Frahnow A, Barnow S, et al. Serotonin transporter gene (SLC6A4) promoter polymorphisms and the susceptibility to posttraumatic stress disorder in the general population. American Journal of Psychiatry. 2009;166(8):926–933. doi: 10.1176/appi.ajp.2009.08101542. [DOI] [PubMed] [Google Scholar]
- Gressier F, Calati R, Balestri M, Marsano A, Alberti S, Antypa N, Serretti A. The 5-HTTLPR polymorphism and posttraumatic stress disorder: A meta-analysis. Journal of Traumatic Stress. 2013;26:645–653. doi: 10.1002/jts.21855. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Hoge CW, Castro CA, Messer SC, McGurk D, Cotting DI, Coffman RL. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. The New England Journal of Medicine. 2004;351:13–22. doi: 10.1056/NEJMoa040603. [DOI] [PubMed] [Google Scholar]
- Hu X, Oroszi G, Chun J, Smith TL, Goldman D, Schuckit MA. An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcoholism: Clinical and Experimental Research. 2005;29:8–16. doi: 10.1097/01.ALC.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- Kimbrel NA, Calhoun PS, Elbogen EB, Brancu M, Beckham JC VA Mid-Atlantic MIRECC Registry Workgroup. The factor structure of psychiatric comorbidity among Iraq/Afghanistan-era veterans and its relationship to violence, incarceration, suicide attempts, and suicidality. Psychiatry Research. doi: 10.1016/j.psychres.2014.07.064. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen KC, Fu QJ, Ertel K, Lyons MJ, Eisen SA, True WR, et al. Common genetic liability to major depression and posttraumatic stress disorder in men. Journal of Affective Disorders. 2008;105:109–115. doi: 10.1016/j.jad.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Lovibond PF, Lovibond SH. The structure of negative emotional states: comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behavior Research and Therapy. 1995;33(3):335–343. doi: 10.1016/0005-7967(94)00075-U. [DOI] [PubMed] [Google Scholar]
- Mushtaq D, Ali A, Margoob MA, Murtaza I, Andrade C. Association between serotonin transporter gene promoter-region polymorphism and 4- and 12-week treatment response to sertraline in posttraumatic stress disorder. Journal of Affective Disorders. 2012;136(3):955–62. doi: 10.1016/j.jad.2011.08.033. [DOI] [PubMed] [Google Scholar]
- Miller MW, Fogler JM, Wolf EJ, Kaloupek DG, Keane TM. The internalizing and externalizing structure of psychiatric comorbidity in combat veterans. Journal of Traumatic Stress. 2008;21(1):58–65. doi: 10.1002/jts.20303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman JOE, Goldsmith AA, Lemmer JA, Kilmer MT, Baker DG. Post-traumatic stress disorder, depression, and health-related quality of life in OEF/OIF veterans. Quality of Life Research. 2012;21:99–103. doi: 10.1007/s11136-011-9918-3. [DOI] [PubMed] [Google Scholar]
- Sangkuhl K, Klein T, Altman R. Selective serotonin reuptake inhibitors (SSRI) pathway. Pharmacogenetics and Genomics. 2009;19(11):907–909. doi: 10.1097/FPC.0b013e32833132cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan K, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Smith TC, Ryan MAK, Wingard DL, Slymen DJ, Sallis JF, Kritz-Silverstein D. New onset and persistent symptoms of posttraumatic stress disorder self-reported after deployment and combat exposures: Prospective population based US military cohort study. British Medical Journal. 2008;336:366–371. doi: 10.1136/bmj.39430.638241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HC, Yeh TL, Hsieh MH, Lee IH, Chen KC, Chen PS, et al. Association between serotonin transporter availability and overall rating scores of quality of life in healthy volunteers. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2009;33(4):711–714. doi: 10.1016/j.pnpbp.2009.03.018. [DOI] [PubMed] [Google Scholar]
- True WR, Rice J, Eisen SA, Heath AC, Goldberg J, Lyons MJ, et al. A twin study of genetic and environmental contributions to liability for posttraumatic stress symptoms. Archives of General Psychiatry. 1993;50(4):257–264. doi: 10.1001/archpsyc.1993.01820160019002. [DOI] [PubMed] [Google Scholar]
- Walsh K, Uddin M, Soliven R, Wildman DE, Bradley B. Associations between the SS variant of 5-HTTLPR and PTSD among adults with histories of childhood emotional abuse: Results from two African American independent samples. Journal of Affective Disorders. 2014;161:91–96. doi: 10.1016/j.jad.2014.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Baker DG, Harrer J, Hamner M, Price M, Amstadter A. The relationship between combat-related posttraumatic stress disorder and the 5-HTTLPR/rs25531 polymorphism. Depression & Anxiety. 2011;28(2):1067–1073. doi: 10.1002/da.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers FW, Litz BT, Herman DS, Huska JA, Keane TM. The PTSD Checklist: Reliability, validity, and diagnostic utility. Paper presented at the Annual Meeting of the International Society for Traumatic Stress Studies; San Antonio, TX. 1993. Oct, [Google Scholar]
- Wendland J, Kruse M, Murphy D. Functional SLITRK1 var321, varCDfs and SLC6A4 G56A variants and susceptibility to obsessive-compulsive disorder. Molecular Psychiatry. 2006;11:802–804. doi: 10.1038/sj.mp.4001848. [DOI] [PubMed] [Google Scholar]
- Young KA, Bonkale W, Holcomb LA, Hicks PB, German DC. Major depression, 5HTTLPR genotype, suicide and antidepressant influence on thalamic volume. British Journal of Psychiatry. 2008;192(4):285–289. doi: 10.1192/bjp.bp.107.039180. [DOI] [PubMed] [Google Scholar]