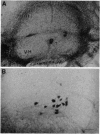
Abstract
Defects in neurotransmitter glutamate transport may be an important component of chronic neurotoxicity in diseases such as amyotrophic lateral sclerosis. There are no reliable models of slow glutamate neurotoxicity. Most previous in vitro systems have studied the rapid neurotoxic effects of direct-acting glutamate agonists. Therefore, we developed a model of slow toxicity in cultured organotypic spinal cord slices. The model was based on selective inhibition of glutamate transport, which continuously raised the concentration of glutamate in the culture medium. This resulted in the slow degeneration of motor neurons over several weeks. Motor neuron toxicity was selectively prevented by non-N-methyl-D-aspartate glutamate receptor antagonists and glutamate synthesis or release inhibitors but not by N-methyl-D-aspartate receptor antagonists. Thus, selective inhibition of glutamate transport produces a model of clinically relevant slow neurotoxicity and appears to be mediated by the action of non-N-methyl-D-aspartate receptors. This data supports the hypothesis that the slow loss of motor neurons in amyotrophic lateral sclerosis could be due, in part, to defective glutamate transport.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong D. M., Brady R., Hersh L. B., Hayes R. C., Wiley R. G. Expression of choline acetyltransferase and nerve growth factor receptor within hypoglossal motoneurons following nerve injury. J Comp Neurol. 1991 Feb 22;304(4):596–607. doi: 10.1002/cne.903040407. [DOI] [PubMed] [Google Scholar]
- Bradford H. F., Young A. M., Crowder J. M. Continuous glutamate leakage from brain cells is balanced by compensatory high-affinity reuptake transport. Neurosci Lett. 1987 Oct 29;81(3):296–302. doi: 10.1016/0304-3940(87)90399-5. [DOI] [PubMed] [Google Scholar]
- Bridges R. J., Stanley M. S., Anderson M. W., Cotman C. W., Chamberlin A. R. Conformationally defined neurotransmitter analogues. Selective inhibition of glutamate uptake by one pyrrolidine-2,4-dicarboxylate diastereomer. J Med Chem. 1991 Feb;34(2):717–725. doi: 10.1021/jm00106a037. [DOI] [PubMed] [Google Scholar]
- Delfs J., Friend J., Ishimoto S., Saroff D. Ventral and dorsal horn acetylcholinesterase neurons are maintained in organotypic cultures of postnatal rat spinal cord explants. Brain Res. 1989 May 29;488(1-2):31–42. doi: 10.1016/0006-8993(89)90690-2. [DOI] [PubMed] [Google Scholar]
- Dykens J. A., Stern A., Trenkner E. Mechanism of kainate toxicity to cerebellar neurons in vitro is analogous to reperfusion tissue injury. J Neurochem. 1987 Oct;49(4):1222–1228. doi: 10.1111/j.1471-4159.1987.tb10014.x. [DOI] [PubMed] [Google Scholar]
- Fonnum F. A rapid radiochemical method for the determination of choline acetyltransferase. J Neurochem. 1975 Feb;24(2):407–409. doi: 10.1111/j.1471-4159.1975.tb11895.x. [DOI] [PubMed] [Google Scholar]
- Hertz L. Functional interactions between neurons and astrocytes I. Turnover and metabolism of putative amino acid transmitters. Prog Neurobiol. 1979;13(3):277–323. doi: 10.1016/0301-0082(79)90018-2. [DOI] [PubMed] [Google Scholar]
- Kalb R. G., Lidow M. S., Halsted M. J., Hockfield S. N-methyl-D-aspartate receptors are transiently expressed in the developing spinal cord ventral horn. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8502–8506. doi: 10.1073/pnas.89.18.8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyama H., Emson P. C. Distribution of somatostatin mRNA in the rat nervous system as visualized by a novel non-radioactive in situ hybridization histochemistry procedure. Neuroscience. 1990;38(1):223–244. doi: 10.1016/0306-4522(90)90388-k. [DOI] [PubMed] [Google Scholar]
- Levey A. I., Hallanger A. E., Wainer B. H. Choline acetyltransferase immunoreactivity in the rat thalamus. J Comp Neurol. 1987 Mar 15;257(3):317–332. doi: 10.1002/cne.902570302. [DOI] [PubMed] [Google Scholar]
- Martin L. J., Blackstone C. D., Levey A. I., Huganir R. L., Price D. L. AMPA glutamate receptor subunits are differentially distributed in rat brain. Neuroscience. 1993 Mar;53(2):327–358. doi: 10.1016/0306-4522(93)90199-p. [DOI] [PubMed] [Google Scholar]
- McNamara J. O., Fridovich I. Human genetics. Did radicals strike Lou Gehrig? Nature. 1993 Mar 4;362(6415):20–21. doi: 10.1038/362020a0. [DOI] [PubMed] [Google Scholar]
- Meldrum B., Garthwaite J. Excitatory amino acid neurotoxicity and neurodegenerative disease. Trends Pharmacol Sci. 1990 Sep;11(9):379–387. doi: 10.1016/0165-6147(90)90184-a. [DOI] [PubMed] [Google Scholar]
- Olney J. W. Glutamate-induced neuronal necrosis in the infant mouse hypothalamus. An electron microscopic study. J Neuropathol Exp Neurol. 1971 Jan;30(1):75–90. doi: 10.1097/00005072-197101000-00008. [DOI] [PubMed] [Google Scholar]
- Ouardouz M., Durand J. GYKI 52466 antagonizes glutamate responses but not NMDA and kainate responses in rat abducens motoneurones. Neurosci Lett. 1991 Apr 15;125(1):5–8. doi: 10.1016/0304-3940(91)90115-a. [DOI] [PubMed] [Google Scholar]
- Paulsen R. E., Odden E., Fonnum F. Importance of glutamine for gamma-aminobutyric acid synthesis in rat neostriatum in vivo. J Neurochem. 1988 Oct;51(4):1294–1299. doi: 10.1111/j.1471-4159.1988.tb03099.x. [DOI] [PubMed] [Google Scholar]
- Phelps P. E., Barber R. P., Houser C. R., Crawford G. D., Salvaterra P. M., Vaughn J. E. Postnatal development of neurons containing choline acetyltransferase in rat spinal cord: an immunocytochemical study. J Comp Neurol. 1984 Nov 1;229(3):347–361. doi: 10.1002/cne.902290306. [DOI] [PubMed] [Google Scholar]
- Plaitakis A., Constantakakis E., Smith J. The neuroexcitotoxic amino acids glutamate and aspartate are altered in the spinal cord and brain in amyotrophic lateral sclerosis. Ann Neurol. 1988 Sep;24(3):446–449. doi: 10.1002/ana.410240314. [DOI] [PubMed] [Google Scholar]
- Rosen D. R., Siddique T., Patterson D., Figlewicz D. A., Sapp P., Hentati A., Donaldson D., Goto J., O'Regan J. P., Deng H. X. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993 Mar 4;362(6415):59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Rothstein J. D., Kuncl R., Chaudhry V., Clawson L., Cornblath D. R., Coyle J. T., Drachman D. B. Excitatory amino acids in amyotrophic lateral sclerosis: an update. Ann Neurol. 1991 Aug;30(2):224–225. doi: 10.1002/ana.410300223. [DOI] [PubMed] [Google Scholar]
- Rothstein J. D., Martin L. J., Kuncl R. W. Decreased glutamate transport by the brain and spinal cord in amyotrophic lateral sclerosis. N Engl J Med. 1992 May 28;326(22):1464–1468. doi: 10.1056/NEJM199205283262204. [DOI] [PubMed] [Google Scholar]
- Rothstein J. D., Tabakoff B. Alteration of striatal glutamate release after glutamine synthetase inhibition. J Neurochem. 1984 Nov;43(5):1438–1446. doi: 10.1111/j.1471-4159.1984.tb05406.x. [DOI] [PubMed] [Google Scholar]
- Rothstein J. D., Tsai G., Kuncl R. W., Clawson L., Cornblath D. R., Drachman D. B., Pestronk A., Stauch B. L., Coyle J. T. Abnormal excitatory amino acid metabolism in amyotrophic lateral sclerosis. Ann Neurol. 1990 Jul;28(1):18–25. doi: 10.1002/ana.410280106. [DOI] [PubMed] [Google Scholar]
- Stewart G. R., Olney J. W., Pathikonda M., Snider W. D. Excitotoxicity in the embryonic chick spinal cord. Ann Neurol. 1991 Dec;30(6):758–766. doi: 10.1002/ana.410300604. [DOI] [PubMed] [Google Scholar]
- Stoppini L., Buchs P. A., Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991 Apr;37(2):173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Tasker R. C., Coyle J. T., Vornov J. J. The regional vulnerability to hypoglycemia-induced neurotoxicity in organotypic hippocampal culture: protection by early tetrodotoxin or delayed MK-801. J Neurosci. 1992 Nov;12(11):4298–4308. doi: 10.1523/JNEUROSCI.12-11-04298.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooten G. F., Park D. H., Joh T. H., Reis D. J. Immunochemical demonstration of reversible reduction in choline acetyltransferase concentration in rat hypoglossal nucleus after hypoglossal nerve transection. Nature. 1978 Sep 28;275(5678):324–325. doi: 10.1038/275324a0. [DOI] [PubMed] [Google Scholar]
- Ziskind-Conhaim L. NMDA receptors mediate poly- and monosynaptic potentials in motoneurons of rat embryos. J Neurosci. 1990 Jan;10(1):125–135. doi: 10.1523/JNEUROSCI.10-01-00125.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]