Preface
Quaternary carbon stereocenters–carbon atoms to which four distinct carbon substituents are attached–are common features of molecules found in nature. However, prior to recent advances in chemical catalysis, there were few methods available for constructing single stereoisomers of this important structural motif. Here we discuss the many catalytic enantioselective reactions developed during the past decade for synthesizing organic molecules containing such carbon atoms. This progress now makes it possible to selectively incorporate quaternary stereocenters in many high-value organic molecules for use in medicine, agriculture, and other areas.
The properties of organic molecules are intimately tied to their shape. In many structurally complex organic molecules, shape is influenced–or dictated–by the three-dimensional orientation of substituents at stereogenic carbon centers (carbon atoms attached to four different substituents). During the last third of the 20th century, chemists succeeded in developing many powerful methods for directly forming a single three-dimensional orientation (configuration) of carbon centers of this type having one hydrogen substituent. In marked contrast, the construction of a single configuration of stereogenic carbon centers having four different carbon substituents (hereafter referred to as quaternary stereocenters) has until just recently been a daunting challenge for chemical synthesis. Remarkable advances have been recorded during the past decade in the stereocontrolled construction of quaternary stereocenters using chemical catalysis. No longer must the presence of a quaternary stereocenter in a molecule present a major hurdle for chemical synthesis. This exciting prospect prompts us to review the current status of this field of chemical synthesis.
Quaternary stereocenters are found in many biologically active small-molecule natural products, as exemplified by cortisone and morphine (Fig. 1a). One of the difficulties in constructing quaternary carbons is their congested nature, which is illustrated in the space-filling model of morphine wherein this carbon is barely visible at the end of the pointing arrow (Fig. 1b). Besides the challenge of steric hindrance, the stereoselective construction of quaternary stereocenters must involve the use of carbon–carbon bond-forming reactions that provide the desired three-dimensional orientation of the four attached substituents, i.e., the correct absolute configuration of the quaternary stereocenter. The structures of current pharmaceutical agents provide one indication of the substantial challenge in the chemical synthesis of quaternary stereocenters. Molecules containing this structural feature comprised 12% of the top 200 prescription drugs sold in the U.S. in 2011.1 However, all of these drugs are derived from naturally occurring compounds (steroids, opioids, or taxane diterpenoids), with a natural product precursor providing the quaternary stereocenters of the marketed drug in virtually every case.2 The near absence of approved drugs containing chemically synthesized quaternary carbon stereocenters reflects the situation that until recently few reliable methods for preparing such structures existed.3–5
Figure 1. Quaternary stereocenters are important structural features of many biologically active molecules as exemplified by the natural products cortisone and morphine.
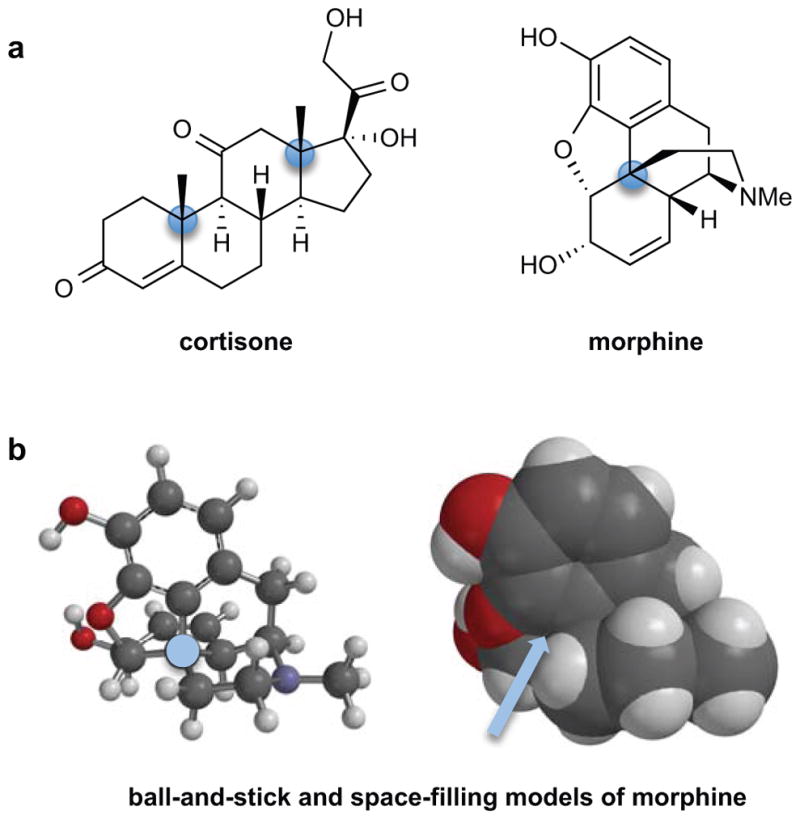
a, Structures of the steroid cortisone and opioid morphine with their quaternary stereocenters highlighted. Me, methyl. b, Steric congestion, which presents a formidable challenge for chemical synthesis of molecules containing quaternary stereocenters, is illustrated in the molecular models of morphine, particularly in the space-filling model on the right in which its sterically congested quaternary center is barely visible at the end of the pointing arrow.
In 2004, we surveyed the field of catalytic enantioselective synthesis of quaternary stereocenters and concluded that only four transformations—Diels–Alder reactions, reactions of chiral allylmetal intermediates with carbon nucleophiles, intramolecular Heck reactions, and reactions of chiral carbon nucleophiles with electrophiles—were well documented to be useful.5 In contrast, today a broad selection of methods is available for this purpose, prompting us to again review the status of this field. Our treatment will be organized by general reaction type in a fashion similar to our previous review.5 We will highlight methods for which some generality has been demonstrated, and wherever possible catalytic transformations whose utility has been validated by their use in the construction of complex chemical structures, typically natural products.
Cycloaddition reactions
The catalytic enantioselective construction of quaternary stereocenters by cycloaddition reactions has progressed significantly in the past decade. New catalytic paradigms have been introduced, and the type of cycloaddition that can be employed has been expanded beyond Diels–Alder reactions.
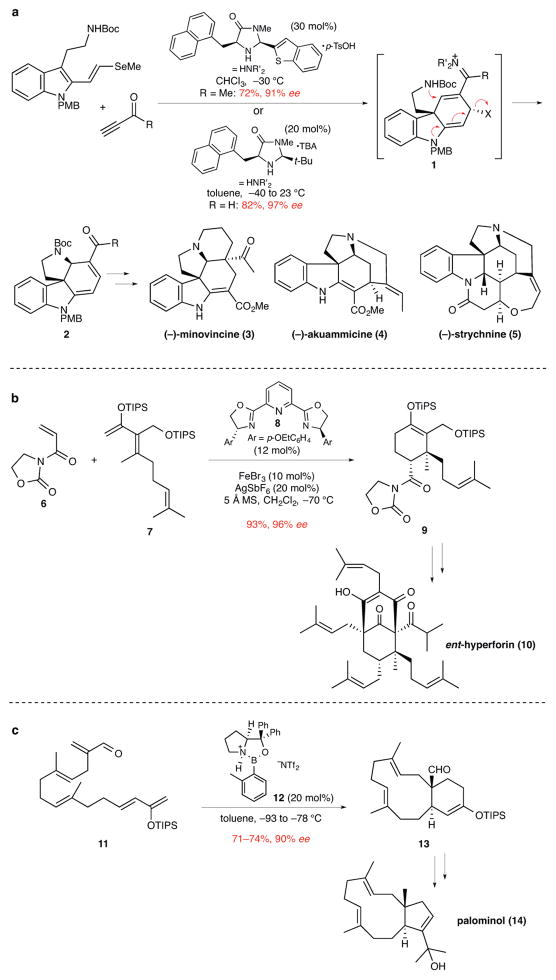
An important recent development in this area is the use of small organic molecules to activate the dienophile in Diels–Alder reactions. The MacMillan group has described a number of [4+2]-cycloaddition reactions that proceed via catalytically generated iminium ion intermediates.6 The utility of these reactions for the enantioselective synthesis of quaternary stereocenters was highlighted in concise total syntheses of various indole alkaloids.7,8 For example, the rapid construction of intermediate 2 was the central step in total syntheses of (−)-minovincine (3), (−)-akuammicine (4), and (−)-strychnine (5) (Fig. 2a). Tetracyclic product 2 is the result of a cascade sequence, the first step of which is a catalytic enantioselective [4+2]-cycloaddition generating tricyclic intermediate 1. Notable other recent reports of the construction of quaternary stereocenters using organocatalytic Diels–Alder reactions are the use of secondary amine9 and hydrogen-bonding thiourea catalysts10 to synthesize spirocyclic oxindoles and oxindole natural products.
Figure 2. The use of catalytic enantioselective Diels–Alder reactions to synthesize natural products containing quaternary stereocenters.
ee, enantiomeric excess. a, A bimolecular Diels–Alder reaction promoted by iminium ion activation forms intermediate 1 in the first step of a cascade sequence generating tetracyclic product 2. This product contains the quaternary stereocenter and four rings common to several groups of indole alkaloids and was employed to complete enantioselective total syntheses of various indole alkaloids, including (−)-minovincine (3), (−)-akuammicine (4), and (−)-strychnine (5).7 Boc, tert-butoxycarbonyl; Me, methyl; PMB, p-methoxybenzyl; p-TsOH, p-toluenesulfonic acid; t-Bu, tert-butyl; TBA, tribromoacetic acid. b, An iron-bisoxazoline catalyzed bimolecular Diels–Alder reaction forms product 9 whose quaternary stereocenter subsequently controlled the elaboration of the two additional quaternary stereocenters of ent-hyperforin (10).11 TIPS, triisopropylsilyl; Et, ethyl; MS, molecular sieves. c, Oxazaborolidinium-catalyzed intramolecular Diels–Alder reaction to form the 11-membered ring and quaternary stereocenter of palominol (14).12 Tf, trifluorosulfonyl; TIPS, triisopropylsilyl; Ph, phenyl.
The broad utility of catalytic enantioselective Diels–Alder reactions for constructing quaternary stereocenters is illustrated by several recent natural product total syntheses. For example, the total synthesis of ent-hyperforin (10) by the Shibasaki group featured a catalytic enantioselective Diels–Alder reaction between dienophile 6 and diene 7 in the presence of an iron complex generated from FeBr3 and the pyridine bisoxazoline (PyBOX) ligand 8 (Fig. 2b).11 The quaternary stereocenter of cycloadduct 9 subsequently played a decisive role in evolving the two additional quaternary stereocenters of ent-hyperforin. Catalytic enantioselective Diels–Alder reactions that form quaternary stereocenters have been orchestrated also in various intramolecular fashions, including macrobicyclization12 and transannular processes.13 The former construction is illustrated in the transformation of polyene aldehyde 11 in the presence of oxazaborolidinium catalyst 12 to form macrobicyclic product 13 in good yield and 90% ee. Snyder and Corey elaborated cycloadduct 13 to several natural products, including palominol (14) (Fig. 2c).
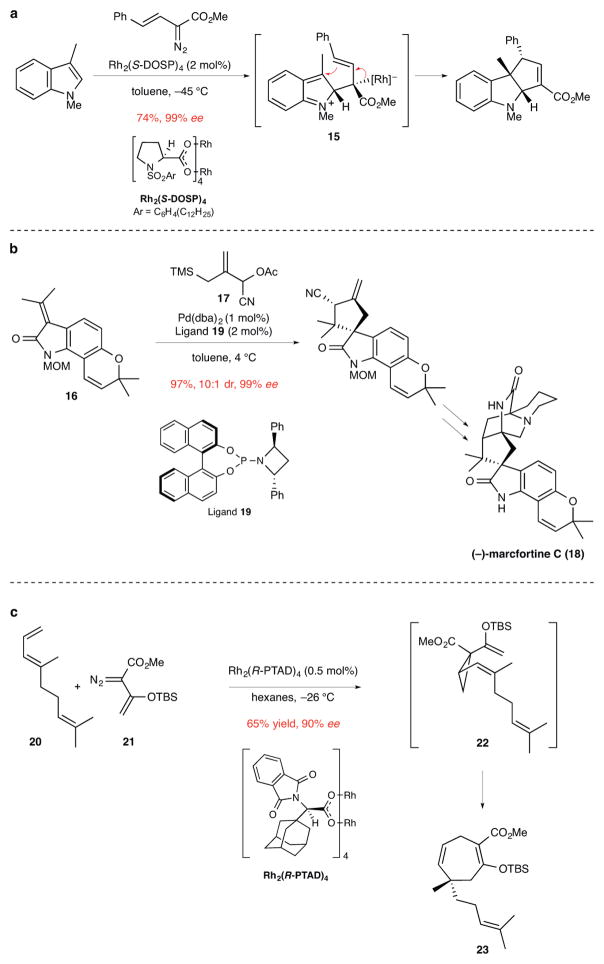
Catalytic enantioselective cycloaddition reactions of various types have now been used for forming quaternary stereocenters. In particular, constructions to form five-membered rings are widely developed. The Davies group described the reaction of indoles with Rh-carbenoids to produce cyclopentene-fused indolines in excellent yield and enantioselectivity (Fig. 3a).14 These reactions are believed to proceed in a stepwise fashion via a dipolar intermediate such as 15. Enantioselective cycloadditions of palladium-trimethylenemethane (Pd-TMM) complexes have been developed extensively over many years by Trost and co-workers.15 In this way, a variety of functionalized cyclopentene derivatives containing quaternary stereocenters can be accessed directly. For example, the catalytic-enantioselective cycloaddition of propylidene oxindole 16 and TMM donor 17 was the central step in the total synthesis of (−)-marcfortine C (18) (Fig. 3b).16 Several types of ligands were investigated for promoting this transformation, with phosphoramidite ligand 19 found to be optimal. Other notable examples of forming five-membered rings and quaternary stereocenters in cycloaddition reactions that employ organometallic17 or chiral phosphoric acid catalysts have been reported also.18,19
Figure 3. Examples of other catalytic enantioselective cycloaddition reactions used to prepare products containing quaternary stereocenters.
ee, enantiomeric excess. a, The synthesis of a cyclopentene-fused indoline by a formal [3+2]-cycloaddition of 1,3-dimethylindole and a vinyl diazoester using a rhodium catalyst. This reaction is suggested to take place in a stepwise fashion via dipolar intermediate 15.14 Me, methyl; Ph, phenyl. b, The [3+2]-cycloaddition of a Pd-trimethylenemethane intermediate generated from allylic acetate 17 to form a tetracyclic intermediate in the total synthesis of (−)-marcfortine C.16 MOM, methoxymethyl; TMS, trimethylsilyl; Ac, acetate; dba, dibenzylideneacetone; Ph, phenyl. c, Enantioselective synthesis of 1,4-cycloheptadiene 23 from triene 20 and vinyl diazoester 21. The first step in this sequence is Rh-catalyzed cyclopropanation of the terminal double bond of the acyclic triene to form divinyl cyclopropane 22, which upon in situ Cope rearrangement generates 23 and its quaternary stereocenter. Product 23 was employed in the total synthesis of the diterpenoid (−)-5-epi-vibsanin E.21 Me, methyl; TBS, tert-butyldimethylsilyl.
The formation of quaternary stereocenters by catalytic enantioselective cyclopropanation reactions was well established at the time of our previous review.5 Progress in this area continues at a rapid pace, with the scope of enantioselective Simmons–Smith cyclopropanations and transition-metal catalyzed decomposition of diazoalkanes being continually advanced.20 Catalytic enantioselective cyclopropanation reactions are also pivotal steps of cascade sequences developed to form larger rings. In their synthesis of (−)-5-epi-vibsanin E, the Davies group illustrates one variant: a cyclopropanation/Cope rearrangement sequence.21 In the example illustrated, cycloaddition of the vinylcarbenoid derived from vinyl diazoester 21 and Rh2(R-PTAD)4 with the terminal double bond of diene 20 delivered cis-divinylcyclopropane 22, which under the reaction conditions underwent Cope rearrangement to furnish cycloheptadiene 23 (Fig. 3c).
Polyene cyclizations
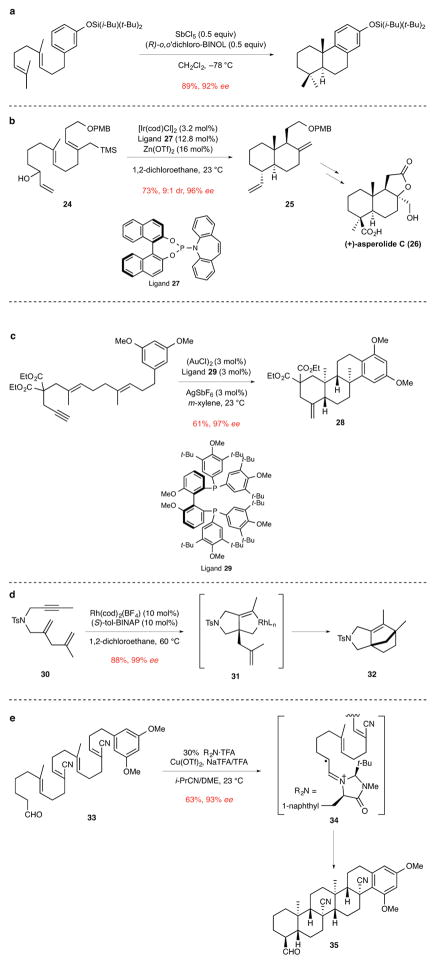
Enantioselective cyclization reactions of acyclic polyenes have been advanced considerably during the past decade. Yamamoto and co-workers described a number of enantioselective polyene cyclizations that proceed in the presence of stoichiometric amounts of protic acids generated upon complexation of SnCl4 with BINOL-derived ligands.22 Building on these disclosures, Corey and co-workers reported several concise total syntheses in which polyene cyclizations promoted by complexes formed from SbCl5 and (R)-o,o′-dichloro-BINOL were the central steps.23,24 Generally 1 equivalent of the complex was employed, although with structurally simpler substrates sub-stoichiometric amounts could be employed (Fig. 4a).
Figure 4. Catalytic enantioselective polyene cyclizations to construct polycyclic products having quaternary stereocenters.
ee, enantiomeric excess. a, The use of a protic acid catalyst for the cyclization of an aryl diene to form two rings and one quaternary stereocenter.24 i-Bu, isobutyl; t-Bu, tert-butyl; BINOL, 1,1′-bi-2-naphthol. b, The iridium-catalyzed cyclization of a triene alcohol to construct the trans-decalin core 25 of the labdane diterpenoid (+)-asperolide C (26). The first step in this cascade cyclization is the generation of a η3-allyliridium cation from the allylic alcohol fragment of 24.26 PMB, p-methoxybenzyl; TMS, trimethylsilyl; cod, 1,5-cyclooctadiene; Tf, trifluorosulfonyl. c, The gold-catalyzed cyclization of an aryl dienyne to form three rings and two quaternary stereocenters of tetracyclic product 28.27 Et, ethyl; Me, methyl; t-Bu, tert-butyl. d The rhodium-catalyzed cyclization of dienyne 30 to form bridged azatricyclic product 32. This reaction is suggested to take place via metallacyclic intermediate 31, which undergoes alkene insertion and reductive elimination to furnish product 32.30 Ts, p-toluenesulfonyl; tol-BINAP, 2,2′-bis(di-p-tolylphosphino)-1,1′-binaphthalene; cod, 1,5-cyclooctadiene; L, ligand. e, The cyclization of tetraene aldehyde 33 in the presence of an imidazolone catalyst and a Cu(II) oxidant to form five rings and four quaternary stereocenters of hexacyclic product 35. This novel reaction is suggested to proceed by single-electron oxidation of the initially formed iminium ion intermediate to generate 34, which undergoes a series of 6-endo radical cyclizations to eventually give product 35. The nitrile substituents are incorporated to disfavor 5-endo cyclizations in the formation of the second and fourth rings.32 Me, methyl; Tf, trifluorosulfonyl; TFA, trifluoroacetic acid; NaTFA, sodium trifluoroacetate; i-Pr, isopropyl; DME, 1,2-dimethoxyethane.
The use of transition-metal catalysts has been more successful in achieving good catalytic efficiency in enantioselective polyene cyclizations. Especially promising are iridium-catalyzed polyene cyclizations of allylic alcohol precursors developed by the Carreira group.25 A variety of functionalized decalins containing angular substituents can be obtained in this way in useful yields and high enantioselectivity. For example, the transformation of triene allylic alcohol 24 to decalin 25 in 73% yield and 96% ee was the central step of a short total synthesis of (+)-asperolide C (26) (Fig. 4b).26 Toste and co-workers have described several gold-catalyzed cyclizations that construct polycyclic products containing quaternary stereocenters, such as the dienyne polycyclization to form tetracyclic product 28 using BIPHEP ligand 29 (Fig. 4c).27 In addition, this group reported gold- and palladium-catalyzed cyclizations of silyloxyenynes28 and a palladium-catalyzed variant of the Conia-ene reaction29 to access functionalized cyclopentenes containing quaternary stereocenters. Rhodium catalysis has also been applied to the cyclization of dienynes to construct bicyclic, spirocyclic, and fused products depending upon the nature of the substrate. For example, the cyclization of acyclic dienyne 30 with a Rh/tol-BINAP catalyst led to the formation of bridged azatricyclic product 32 in 88% yield and 99% ee.30 This reaction presumably takes place via metallacyclic intermediate 31, which undergoes alkene insertion and reductive elimination to furnish product 32 (Fig. 4d).
The generation of chiral electrophiles to initiate polyene cyclizations using organic catalysts has also been developed in recent years. For example, the Jacobsen group reported the use of hydrogen-bonding thiourea catalysts to generate chiral N-acyliminium ion initiators of polyene cyclizations.31 A novel approach to the catalytic enantioselective cyclization of acyclic polyenes was reported by the MacMillan group in which iminium ion activation and Cu(II)-promoted single-electron oxidation were combined to promote polycyclizations of radical-cation intermediates, as illustrated in the cyclization of polyene 33 (Fig. 4e).32 Hexacyclic product 35 is produced stereoselectively in 63% yield and 93% ee in a remarkable pentacyclization that begins with the generation of radical-cation intermediate 34. The nitrile substituents were incorporated to favor 6-endo cyclizations of the radical intermediates, a requirement that likely limits the utility of this method for construction of natural terpenoids and steroids.
Transition metal-catalyzed insertions
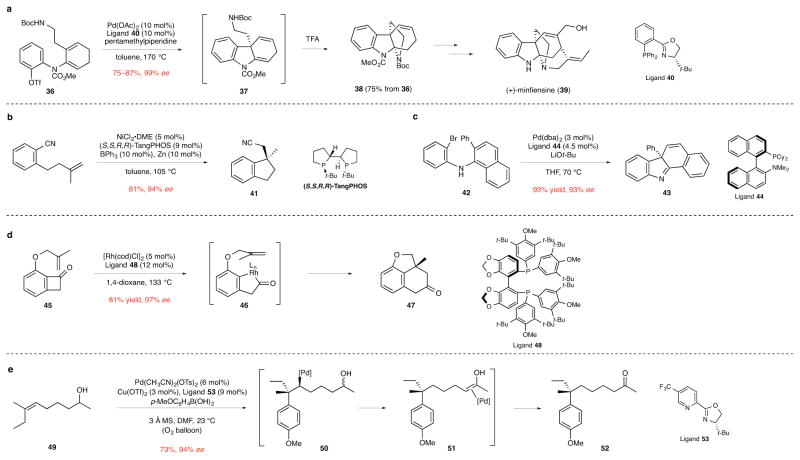
In our earlier review of catalytic enantioselective synthesis of quaternary stereocenters, intramolecular Heck reactions were suggested to have the broadest demonstrated scope.5 This method continues to be important. The total synthesis of (+)-minfiensine (39) reported by Overman and co-workers provides one recent illustration (Fig. 5a).33 In the Heck cyclization of dienyl triflate 36, the use of PHOX ligand 40 was critical in achieving both high stereoinduction and preventing isomerization of the 1,4-diene product 37 to the conjugated 1,3-diene; avoiding double-bond migration was essential in allowing the second azacyclic ring of tetracyclic intermediate 38 to be generated upon exposure of the crude Heck product 37 to excess trifluoroacetic acid (TFA).
Figure 5. Transition metal-catalyzed insertion reactions that form quaternary stereocenters.
ee, enantiomeric excess. a, The enantioselective intramolecular Heck cyclization of dienyl triflate 36 to form 1,4-diene intermediate 37, which upon exposure to excess trifluoroacetic acid provided tetracyclic product 38 in route to the indole alkaloid (+)-minfiensine (39). The use of PHOX ligand 40 was critical in achieving both high stereoinduction and preventing isomerization of the initially formed product 37 to the conjugated 1,3-diene regioisomer.33 Boc, tert-butoxycarbonyl; Me, methyl; Tf, trifluorosulfonyl; Ac, acetyl; TFA, trifluoroacetic acid. b, The intramolecular nickel-catalyzed arylcyanation of a tethered double bond to form indane 41.34 DME, 1,2-dimethoxyethane; Ph, phenyl; t-Bu, tert-butyl. c, The palladium-catalyzed cyclization/dearomatization of aryl(naphthyl)amine 42 to form tetracyclic product 43. This reaction is suggested to occur via a six-membered palladacyclic intermediate that undergoes reductive elimination to form generate product 43.36 Ph, phenyl; dba, dibenzylideneacetone; t-Bu, tert-butyl; THF, tetrahydrofuran; Me, methyl; Cy, cyclohexyl. d, The rhodium-catalyzed conversion of alkenyl benzocyclobutanone 45 to tricyclic ether 47. This transformation is believed to occur by initial insertion of rhodium into the C–C bond to form acylrhodium intermediate 46, which in the enantiodetermining step undergoes intramolecular carboacylation of the tethered alkene to form product 47.37 cod, 1,5-cyclooctadiene; L, ligand; Me, methyl; t-Bu, tert-butyl. e, The bimolecular Heck-type addition of an arylboronic acid to the trisubstituted double bond of 49 to form ketone product 52. This rare example of a bimolecular alkene insertion to form a quaternary stereocenter is suggested to occur by initial enantioselective carbopalladation of the alkene to generate intermediate 50, which undergoes sequential β-hydride eliminations/migratory insertions along the alkyl chain to form alkene complex 51 and then the ketone product.38 Ts, p-toluenesulfonyl; Tf, trifluorosulfonyl; Me, methyl; MS, molecular sieves; DMF, N,N-dimethylformamide; t-Bu, tert-butyl.
A variety of additional transition metal-catalyzed cyclization reactions have been developed recently for constructing polycyclic molecules containing quaternary stereocenters. Enantioselective nickel-catalyzed intramolecular arylcyanation reactions disclosed by the groups of Jacobsen34 and Nakao35 are notable examples. The synthesis of indane 41 illustrates this transformation (Fig. 5b). An attractive feature of these isomerization reactions is the avoidance of the waste that would be generated in more conventional Heck-type cyclizations of related halide or triflate substrates. In another approach, Buchwald and co-workers reported the construction of quaternary stereocenters by palladium-catalyzed cyclization/dearomatization of naphthalene derivatives. For example, tetracyclic amine 43 was obtained in high yield and enantioselectivity from bromodiarylamine precursor 42 using a catalyst generated from Pd(dba)2 and KenPhos (ligand 44) (Fig. 5c).36 Guangbin Dong and co-workers recently reported intramolecular carboacylation reactions of alkene-tethered benzocyclobutenones that construct various ring systems containing quaternary stereocenters, such as the formation of oxatricyclic ketone 47 from precursor 45 using a rhodium catalyst containing SEGPHOS ligand 48 (Fig. 5d).37 This transformation is suggested to proceed by initial formation of metallacyclic intermediate 46.
Apparent in our discussion to this point is the prevalence of intramolecular reactions that construct quaternary stereocenters during ring formation. In this context, the recent disclosure from the Sigman group of forming aryl-containing quaternary stereocenters in high enantioselectivity by bimolecular Heck-type reactions of arylboronic acids and acyclic trisubstituted alkenes containing alcohol substituents is particularly important.38 The enantioselective synthesis of ketone 52 from unsaturated alcohol 49 using a catalyst formed from Pd(CH3CN)2(OTs)2 and diamine ligand 53 is exemplary (Fig. 5e). This transformation is suggested to proceed by initial enantioselective carbopalladation of the alkene to form intermediate 50, followed by sequential β-hydride eliminations/migratory insertions along the alkyl chain to eventually yield alkene complex 51 and then ketone product 52. Considerable variation of the substituents on the alkene is tolerated, and depending upon the starting alcohol, either ketones or aldehydes containing remote quaternary stereocenters can be formed in high enantioselectivity in this way.
Coupling of chiral carbon nucleophiles
The enantioselective formation of quaternary stereocenters by the coupling of chiral carbon nucleophiles with achiral carbon electrophiles has progressed significantly over the past decade. Organocatalytic processes emerged to achieve such transformations, as well as numerous organometallic methods. Of particular note, high enantioselectivies can now be realized in copper-catalyzed additions of various organometallic nucleophiles to prochiral Michael acceptors.
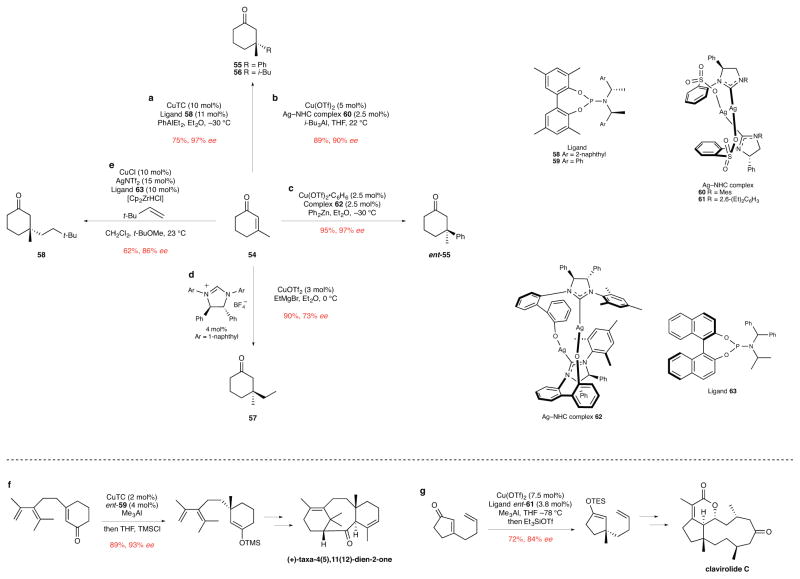
Ten years ago we noted that useful procedures for forming quaternary stereocenters by copper-catalyzed additions of carbon nucleophiles to prochiral β,β-disubstituted enones and related electrophiles were notably absent.5 This void is rapidly being filled, as many enantioselective copper-catalyzed 1,4-addition reactions have now been reported that proceed with high enantioselectivities. Some of the more important of these methods are illustrated in Fig. 6 for conjugate additions to 3-methyl-2-cyclohexen-1-one (54). Copper-catalyzed additions of various alkyl-,39 alkenyl-,40 and arylaluminum41 compounds to cyclic enones in the presence of phosphoramidite ligands such as 58 have been described by Alexakis and co-workers (e.g., 54→55, Fig. 6a). Hoveyda and coworkers reported the use of Cu/Ag–NHC catalyst for the conjugate addition of alkyl- and arylaluminum intermediates to conjugated enones (e.g., the synthesis of 56, Fig. 6b),42 as well as the addition of silicon-containing vinylaluminum intermediates.43 The addition of arylzinc reagents can also be accomplished using a structurally related Cu/Ag–NHC catalyst generated from silver complex 62, as exemplified in the formation of ent-55 (Fig. 6c).44 Catalytic enantioselective conjugate addition reactions of other organometallic intermediates have been disclosed also. Notable examples include enantioselective copper-catalyzed additions of Grignard reagents described by Alexakis and co-workers, as illustrated in the conversion of 54→57 (Fig. 6d),45 and an alkene hydrozirconation/conjugate addition sequence reported by Fletcher and co-workers to construct cyclohexanone 58 using a copper catalyst containing phosphoramidite ligand 63 (Fig. 6e).46
Figure 6. Enantioselective copper-catalyzed conjugate additions to construct quaternary stereocenters.
ee, enantiomeric excess. The upper segment of the Figure depicts several Cu-catalyzed conjugate additions to 3-methyl-2-cyclohexen-1-one (54) that form new quaternary stereocenters: a, The addition of an arylaluminum compound to 54 to form cyclohexanone 55.40 CuTC, copper(I) thiophene-2-carboxylate; Ph, phenyl; Et, ethyl. b, The addition of a trialkylaluminum compound to 54 to form cyclohexanone 56.42 Tf, trifluorosulfonyl; i-Bu, isobutyl; THF, tetrahydrofuran; Ph, phenyl; NHC, N-heterocyclic carbene. c, The addition of an arylzinc compound to 54 to form the enantiomer of cyclohexanone 55.44 Tf, trifluorosulfonyl; Ph, phenyl; Et, ethyl. d, The addition of an alkyl Grignard reagent to 54 to form 3,3-dialkylcyclohexanone 57.45 Tf, trifluorosulfonyl; Ph, phenyl; Et, ethyl. e, The addition of an alkylzirconium intermediate generated by hydrozirconation of 3,3-dimethyl-1-butene to 54 to form 3,3-dialkylcyclohexanone 58.46 Tf, trifluorosulfonyl; Cp, cyclopentadienyl; t-Bu, tert-butyl; Me, methyl. The lower segment of the Figure shows the use of two of these methods to form methyl-containing quaternary stereocenters in syntheses of a potential taxane terpenoid precursor and a dolabellane diterpenoid: f, The enantioselective copper-catalyzed conjugate addition/enolate trapping to introduce a quaternary methyl group in the construction of a taxadienone.51 CuTC, copper(I) thiophene-2-carboxylate; Me, methyl; THF, tetrahydrofuran; TMS, trimethylsilyl. g, The enantioselective copper-catalyzed conjugate addition/enolate trapping to introduce a quaternary methyl group in the total synthesis of clavirolide C.52 Tf, trifluorosulfonyl; Me, methyl; THF, tetrahydrofuran; TES, triethylsilyl.
Good success has been realized also in conjugate addition reactions that form quaternary stereocenters using rhodium and palladium catalysts. For example, Hayashi described the use of Rh(I) complexes of chiral dienes to catalyze the addition of arylboronic acids or tetraaryl boronates to maleimides47 and enones.48 Exemplary is the synthesis of cyclohexanone 55 in this fashion in 85% yield and 98% ee. Rhodium-catalyzed additions of arylaluminum reagents to β-substituted cyclic enones were reported also by the Alexakis group, including the synthesis of 55 in 71% yield and 98% ee using an Rh-BINAP catalyst.49 In addition, Stoltz and co-workers reported palladium-catalyzed variants of the 1,4-addition of boronic acids to enones for the enantioselective formation of chiral 3,3-disubstituted cyclohexanones.50
As prochiral β,β-disubstituted α,β-unsaturated carbonyl compounds can be constructed in many ways and are common intermediates in retrosynthetic analysis, the recent development of versatile catalytic methods to transform these intermediates into products containing new quaternary stereocenters is certain to find broad application. The transformations depicted at the bottom of Fig. 6, which were pivotal steps in enantioselective total syntheses of (+)-taxa-4(5),11(12)-dien-2-one51 and clavirolide C,52 are two recent examples.
When we discussed this approach for constructing quaternary stereocenters in our earlier review,5 organic catalysts—typically phase-transfer catalysts—had been employed with considerable success to join enolate intermediates with carbon electrophiles. The notable utility of cinchona alkaloid derivatives in such constructions has been further illustrated by Deng and co-workers in catalytic enantioselective additions of 1,3-dicarbonyl and related compounds to nitroalkenes and α,β-unsaturated ketones,53 and by Jørgensen in similar additions to allenic esters and ketones and for enantioselective alkynylations of 1,3-dicarbonyl compounds.54 In addition, the use of enamine catalysis in the enantioselective construction of quaternary stereocenters from α-branched aldehydes—a reaction with broad potential utility for introducing quaternary stereocenters in a diversity of molecules55—was reported first by Barbas in 2004.56 Organocatalysis has been utilized also to construct quaternary stereocenters by enantioselective intramolecular Stetter reactions of aromatic or aliphatic aldehydes (e.g., 64→65) using triazolium catalysts such as 66 (Fig. 7a).57 Other promising methods reported recently to exploit catalytically generated nucleophiles in the construction of quaternary stereocenters include the enantioselective insertion of diazoesters into the carbon–carbon bond of aryl aldehydes using an oxazaborolidinium catalyst,58 and the enantioselective alkylation of acyclic tributyltin enolates in the presence of a Cr(salen) catalyst.59
Figure 7. Enantioselective intramolecular Stetter reaction and allylic alkylation reactions to construct quaternary stereocenters.
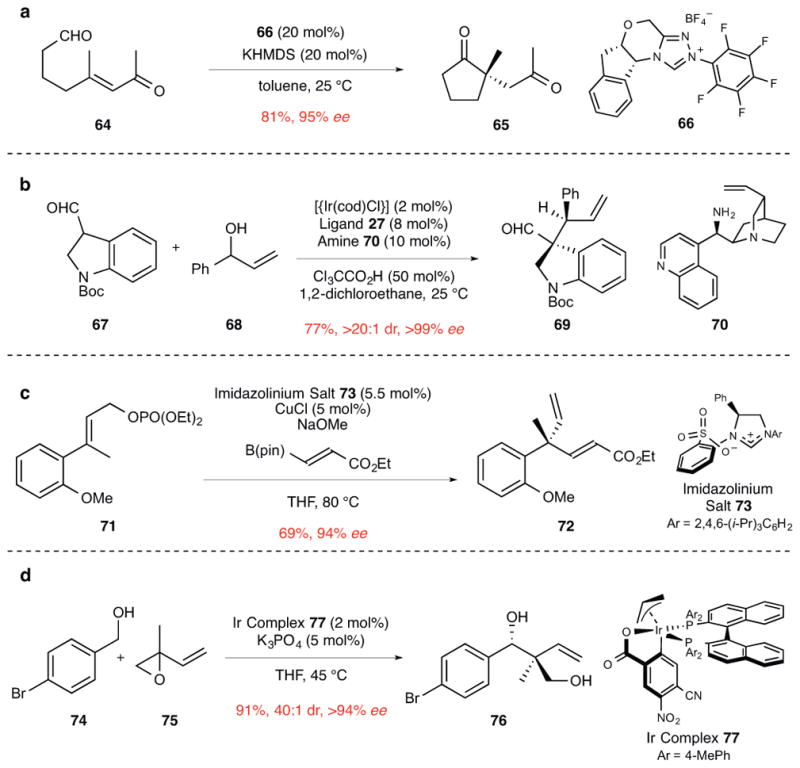
ee, enantiomeric excess. a, The intramolecular Stetter reaction of enone aldehyde 64 catalyzed by the carbene generated from triazolium salt 66 to form 2,2-disubstituted cyclopentanone 65.57 KHMDS, potassium bis(trimethylsilyl)amide. b, The α-allylation of indoline aldehyde 67 with allylic alcohol 68 using the dual activity of iridium and amine catalysts. This reaction constructs the quaternary and adjacent secondary stereocenter of product 69.60 Boc, tert-butoxycarbonyl; cod, 1,5-cyclooctadiene; Ph, phenyl. c, The formation of 4,4-disubstituted 2,5-hexadienoic ester 72 by allylic displacement of phosphate triester 71. In this reaction, an alkenylcopper carbene complex is generated from a vinylboronate precursor.61 Me, methyl; pin, pinacolato; THF, tetrahydrofuran; Ph, phenyl. d, The anti-diastereoselective coupling of benzyl alcohol 74 with vinyl epoxide 75 using an iridium catalyst to give the product 76 of carbonyl tert-(hydroxy)prenylation. This reaction proceeds by the coupling of aldehyde and (E)-σ-allyliridium intermediates respectively generated in situ from the alcohol and vinyl epoxide precursors by an iridium-catalyzed redox process.64 Ph, phenyl.
A variety of enantioselective allylic substitution reactions have been reported in recent years that provide many opportunities for incorporating quaternary stereocenters in complex molecules. For example, a procedure developed by the Carreira group utilizes an Ir-cinchona alkaloid derivative dual-catalyst for the allylation of aldehydes. As exemplified in Fig. 7b, 3,3-disubstituted indoline 69 was constructed in this way with excellent enantio- and diastereoselectivity from indoline aldehyde 67 and allylic alcohol 68.60 Of most significance, catalytic enantioselective allylic alkylation reactions now allow quaternary stereocenters to be incorporated into many acyclic molecules or acyclic molecular fragments. The Hoveyda group has pioneered in this area by introducing a variety of enantioselective copper-catalyzed allylic substitution reactions.61–63 In particular, this group has shown that a diverse array of carbon nucleophiles—such as dialkylzinc, vinylboron, vinylaluminum, and alkynylaluminum reagents—can be employed in allylic substitution reactions that form new quaternary stereocenters. The efficient and highly enantioselective alkylation of allylic phosphate 71 with an ester-containing vinylboron nucleophile to form product 72 in the presence of a copper-NHC catalyst is exemplary (Fig. 7c).61 As a final example, the enantio- and anti-diastereoselective allylic coupling of benzyl alcohol 74 with vinyl epoxide 75 to yield 1,3-diol 76 using Ir catalyst 77 disclosed by Krische and co-workers even allows a benzyl alcohol to be employed as the pro-nucleophile in the construction of quaternary stereocenters (Fig. 7d).64 This reaction, which results in appending a 1-(hydroxymethyl)-1-methylallyl unit to the alcohol fragment, should find use in the synthesis of terpenoid natural products that incorporate this (hydroxy)prenyl motif. A notable feature of redox-triggered couplings of this type pioneered by the Krische group is the absence of stoichiometric byproducts.
Coupling of chiral carbon electrophiles
Reactions of chiral carbon electrophiles with carbon nucleophiles encompass a range of transformations that can be used to form quaternary stereocenters in structurally complex molecules. During the past decade, palladium-catalyzed enantioselective allylic alkylation reactions have been applied widely to achieve this aim, and a number of other promising methods employing transition metal or organic catalysts have been introduced.
The use of enantioselective palladium-catalyzed allylic alkylation reactions to form quaternary stereocenters adjacent to ketone carbonyl groups was initially reported by the Stoltz65 and Trost groups.66 Since these initial disclosures, this method has been featured in several natural product total syntheses.4 For example, a variety of chiral 3,3-disubstituted oxindoles have been prepared in this fashion with good enantioselectivity,67,68 as exemplified by the enantioselective and regioselective prenylation (78→79) utilized in the synthesis of ent-flustramines A (80) and B (Fig. 8a).69 In a strategically incisive example, Stoltz and co-workers employed an enantioselective double allylation of racemic bis-β-ketoester 82 to form C2-symmetric diketone 83 in route to (−)-cyanthiwigin F (84) (Fig. 8b).70 Other significant recent developments in this area include the use of a vinyl epoxide as a coupling partner in the total syntheses of (−)-biyouyanagin A and hyperolactone C,71 and the application of molybdenum72 and iridium73 catalysts in enantioselective allylic alkylation reactions. In addition, the enantioselective C-3 allylation of an indole derivative using allyl alcohol in combination with a trialkylborane as the alkylating reagent was featured in a synthesis of (−)-esermethole.74
Figure 8. Use of palladium-catalyzed asymmetric allylic alkylation reactions for constructing quaternary centers in alkaloid and terpenoid natural products.
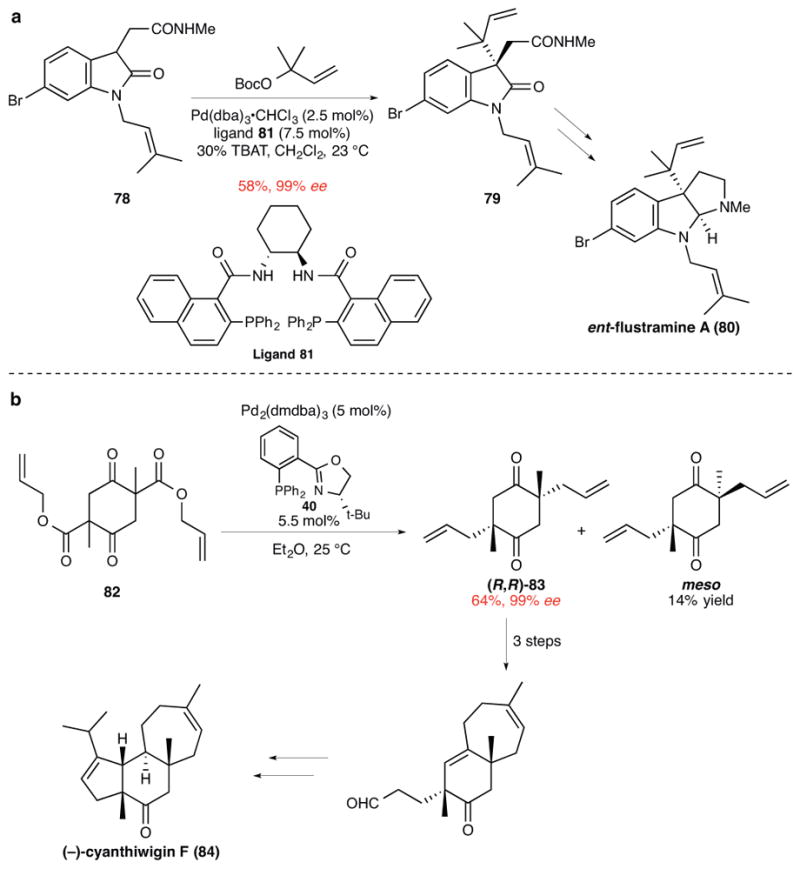
a, The regioselective prenylation of oxindole 78 upon base-promoted reaction with the η3-allylpalladium electrophile generated from a prenyl carbonate to form 79. This product was a late-stage intermediate in the enantioselective total synthesis of ent-flustramine A (80).69 Me, methyl; Boc, tert-butoxycarbonyl; dba, dibenzylideneacetone; TBAT, tetrabutylammonium difluorotriphenylsilicate; Ph, phenyl. b, The syn-diastereoselective diallylation of β-ketoester 82 (a mixture of racemic diastereomers) to give (R,R)-83, a pivotal intermediate in the enantioselective total synthesis of (−)-cyanthiwigin F.70 dmdba, bis(3,5-dimethoxybenzylidene)acetone; Ph, phenyl; t-Bu, tert-butyl.
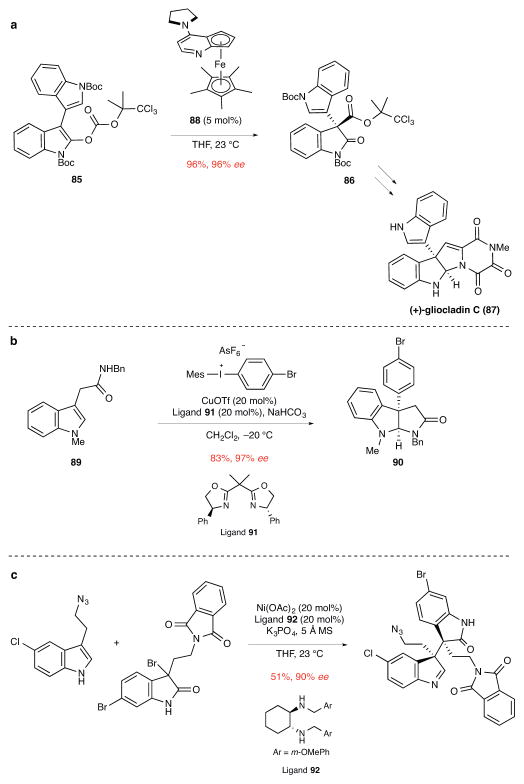
Organocatalytic reactions can be employed also to generate chiral electrophiles for constructing quaternary stereocenters. Particularly well developed is the use of catalytic enantioselective Steglich rearrangements. Fu75 and Vedejs76 developed chiral-enantiopure variants of 4-(dimethylamino)pyridine (DMAP) to accomplish enantioselective rearrangements of enoxycarbonate derivatives, including those derived from oxindoles and furanones. The Fu group also described related transformations involving the acylation of silyl ketene imines and employed this method as the central step in a synthesis of (S)-verapamil.77 In a concise second-generation total synthesis of (+)-gliocladin C (87), Overman and co-workers exploited the planar-chiral DMAP variant 8875 to catalyze the enantioselective Steglich rearrangement of enoxycarbonate 85 to yield oxindole 86 (Fig. 9a).78 In this study, the practicality of Fu’s method was highlighted by the formation of 86 in 96% yield and 96% ee on multigram scales. In a quite different approach to generating chiral carbon electrophiles, iminium activation developed by the MacMillan group has been used for the enantioselective construction of 3a-substituted pyrrolidinoindolines and featured in the synthesis of (−)-flustramine B.79
Figure 9. Miscellaneous methods involving the union of a catalytically generated chiral carbon electrophile with a carbon nucleophile.
ee, enantiomeric excess. a, The Steglich rearrangement of indole carbonate 85 in the presence of Fu’s planar-chiral catalyst 88 to give 3,3-disubstituted oxindole 86 in route to (+)-gliocladin C.78 Boc, tert-butoxycarbonyl; Me, methyl; THF, tetrahydrofuran. b, The copper-catalyzed β-arylation of indole 89 and concomitant cyclization to form 3a-arylpyrrolidinoindolinone 90.80 Me, methyl; Bn, benzyl; Tf, trifluorosulfonyl; Mes, 1,3,5-trimethylbenzene; Ph, phenyl. c, The Ni-catalyzed coupling of an indole with a 3-bromooxindole in route to (+)-perophoramidine. This reaction sets the two contiguous quaternary stereocenters of (+)-perophoramidine.85 OAc, acetoxy; MS, molecular sieves; THF, tetrahydrofuran; Me, methyl; Ph, phenyl.
Although their scope is less well defined at this point than enantioselective palladium-catalyzed allylation reactions or Steglich rearrangements, enantioselective transition metal-catalyzed arylations, vinylations, and alkylations of prochiral nucleophiles have been described recently for the enantioselective construction of quaternary stereocenters. One example is the enantioselective copper-catalyzed indole arylation/cyclization sequence reported by the MacMillan group.80 In this transformation, tryptophan amides undergo efficient indole arylation in the presence of a diaryliodonium salt, CuOTf, and enantiopure bisoxazoline ligand 91, followed by intramolecular trapping of the pendant amide to form 3a-arylpyrrolidinoindolines (89→90) (Fig. 9b). Other notable examples of copper-catalyzed arylation and vinylation reactions to construct quaternary stereocenters are copper-catalyzed arylations of prochiral β-ketoesters with 2-iodotrifluoroacetanilides described by Ma and co-workers,81 and palladium-catalyzed enantioselective α-arylations of α-branched aldehydes82 and the C-3 arylations or vinylations of oxindoles reported by the Buchwald group.83 Catalytic enantioselective alkylations of prochiral nucleophiles can be achieved as well. A double alkylation of a 3,3′-dioxindole with nitroethylene was reported the Shibasaki group in route to (+)-chimonanthine, (+)-folicanthine, and (−)-calycanthine.84 In a mechanistically intriguing variant, catalytic enantioselective alkylations of 3-bromooxindoles with 3-substituted indoles were reported by the Wang group for the construction of vicinal quaternary stereocenters using a catalyst formed from Ni(OAc)2 and diamine ligand 92. This step in the total synthesis of (+)-perophoramidine is illustrated in Fig. 9c.85 This reaction is suggested to occur by loss of HBr from the 3-bromooxindole to generate an electrophilic indol-2one intermediate, which couples with the indole nucleophile. How the Ni-diamine catalyst organizes this coupling to achieve high enantio- and diastereoselction is unclear at present.
Desymmetrization reactions
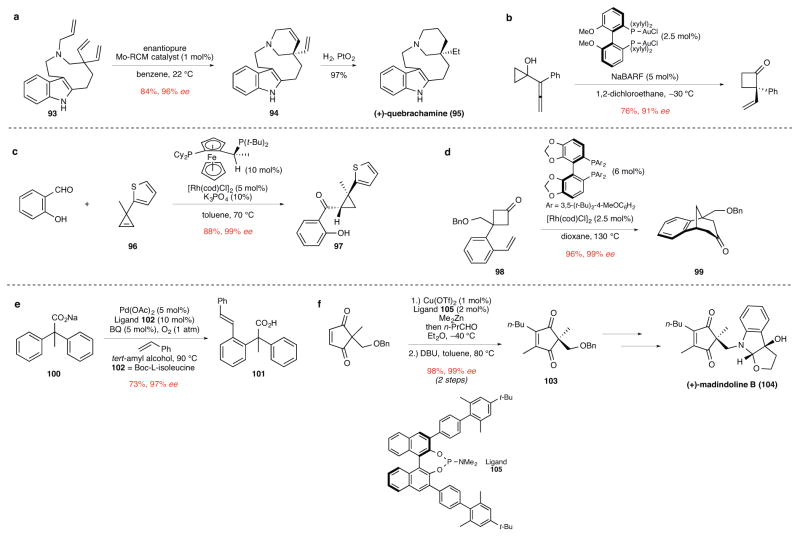
In principle, any catalytic enantioselective reaction could be employed to construct a product containing a quaternary stereocenter by desymmetrization of an appropriately constituted prochiral precursor. Since our earlier review,5 numerous additional examples of using group-selective catalytic enantioselective reactions for this purpose have been described. For instance in their synthesis of (+)-quebrachamine (95), Hoveyda and Schrock reported the use of a chiral molybdenum metathesis catalyst to fashion the tetrahydropyridine ring of intermediate 94 from triene precursor 93 in excellent yield and enantioselectivity (Fig. 10a).86 Enantioselective ring-opening/cross-metathesis has been described also by the Hoveyda group to construct acyclic products bearing quaternary centers.87 In a quite different approach reported by the Toste group, cyclobutanones containing α-quaternary stereocenters can be prepared by enantioselective gold-catalyzed ring expansion of prochiral allenylcyclopropanols (Fig. 10b).88 Applications of two recently developed rhodium-catalyzed C–C-bond constructions for enantioselective desymmetrization are exemplified in Figures 10c and 10d. In the first example from the laboratory of Vy Dong, intermolecular hydroacylation of the prochiral cyclopropene 96 with salicylaldehyde delivers the highly substituted cyclopropane product 97 in high yield and enantiomeric purity (Fig. 10c).89 The second example from the Cramer group, illustrates the use of C–C bond activation in the efficient and enantioselective formation of bridged tricyclic ketone 99 from the prochiral cyclobutanone precursor 98 (Fig. 10d).90 In an example exploiting enantioselective C–H activation, the Yu group reported palladium(II)-catalyzed group-selective functionalizations of diphenylacetic acid derivatives.91 Utilizing a palladium catalyst containing protected-amino acid ligands, various diphenylacetic derivatives underwent selective alkenylation with acrylates or styrenes exemplified by the conversion of 100→101 (Fig. 10e). In a final example, a number of prochiral cyclopentene-1,3-diones have been desymmetrized by copper-catalyzed enantioselective additions of dialkylzinc or organoaluminum reagents.92 The use of a phosphoramidite ligand such as 105 proved optimal in this method, as illustrated in the enantioselective synthesis of cyclopentene-1,3-dione 103, a key step in the synthesis of (+)-madindoline B (104) (Fig. 10f). Organocatalytic methods have also proven useful for constructing quaternary stereocenters by desymmetrization. Protic-acid catalyzed vinylogous α-ketol rearrangements to yield spirocyclic diones,93 and the preparation of five-membered rings from 1,3-diketone precursors using chiral NHC-catalysts are two important examples.94
Figure 10. Enantioselective desymmetrization reactions of precursors containing prochiral quaternary carbons.
ee, enantiomeric excess. a, The ring-closing metathesis of triene 93 to give tetrahydropyridine 94 using a molybdenum catalyst. Catalytic hydrogenation of product 94 then completes a novel construction of (+)-quebrachamine (95).86 RCM, ring-closing metathesis; Et, ethyl. b, The gold-catalyzed ring expansion of an allenylcyclopropanol to form (R)-2-ethenyl-2-phenylcyclobutanone.88 Ph, phenyl; Me, methyl, xylyl, 3,5-dimethylphenyl; NaBARF, sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate. c, The rhodium-catalyzed hydroacylation of cyclopropene 96 with salicyaldehyde to form cyclopropane 97. Coordination of the phenolic oxygen of salicyaldehyde and the ring strain of the cyclopropene promotes this bimolecular hydroacylation reaction. The observed diastereoselectivity is suggested to result from Rh-hydride insertion and subsequent C–C bond reductive elimination taking place preferentially from the cycloproprne face opposite the larger substituent.89 Me, methyl; Cy, cyclohexyl; t-Bu, tert-butyl; cod, 1,5-cyclooctadiene. d, The enantiotopic rhodium-catalyzed insertion into a C–C bond of cyclobutanone 98, followed by intramolecular insertion of the rhodium-acyl intermediate to give bridged-tricyclic ketone 99.90 Bn, benzyl; Me, methyl; cod, 1,5-cyclooctadiene; t-Bu, tert-butyl. e, The palladium(II)-catalyzed enantiotopic C–H activation of sodium diphenylacetate 100 templated by the carboxylate group, followed by bimolecular Heck coupling with styrene to give product 101.91 OAc, acetoxy; BQ, benzoquinone; Ph, phenyl; Boc, tert-butoxycarbonyl. f, The desymmetrization of a prochiral 1,4-cyclopentenone by copper-catalyzed conjugate addition of a methyl group to give chiral product 103 was the key step in the total synthesis of (+)-madindoline B.92 Bn, benzyl; Tf, trifluorosulfonyl; Pr, propyl; Me, methyl; Bu, butyl; DBU, 1,8-diazabicyclo[5.4.0]undec-7-ene; t-Bu, tert-butyl.
Looking forward
The research highlighted in this brief survey shows that a variety of chemical transformations are now available to synthetic chemists for incorporating quaternary stereocenters with high enantioselectivity in organic molecules. When the catalytic transformations that are the focus of our analysis are combined with non-catalytic methods, a diversity of chemical transformations are now available for meeting this formidable challenge. Nonetheless, the scope of the majority of the methods discussed in this review is only partially defined, and limitations are certain to be uncovered. One area where the development of methods is still in its early stages is the introduction of quaternary stereocenters in acyclic molecules or acyclic molecular fragments.95 Even in areas where substantial progress has been recorded recently in fashioning quaternary stereocenters in cyclic molecules—for example, by conjugate additions to cyclohexenones—enantioselectivities realized in identical reactions with cyclic enones of other ring sizes or acyclic enones can be inferior. It is instructive to note that almost all the methods exemplified in this review involve the functionalization of π-bonds. With the intense attention currently being paid to the direct functionalization of Csp3–H σ-bonds, one can anticipate that catalytic C–H insertions will play a much larger role in the future in the enantioselective synthesis of quaternary stereocenters. For example, the scope of such transformations for desymmetrizing prochiral quaternary carbons is certain to expand,96 and new methods exploiting selective C–H functionalizations will likely be developed for transforming chiral tertiary carbons (enantiopure or racemic) and prochiral secondary carbons to new quaternary stereocenters. As nearly half of the transformations we exemplified involve the use of catalysts containing rare and/or expensive metals, the development of alternate catalytic methods based on readily available and less expensive catalysts remains a critical future challenge in this area.
The methods now available for fashioning quaternary stereocenters enantioselectively remove much of the previous barrier to incorporating such functionality in organic molecules for use in medicine, agriculture, and other areas where high-value organic molecules play an important role. One can already see this impact in small-molecules currently undergoing clinical evaluation such as anamorelin.97 With several recent studies suggesting that drug candidates that contain a larger fraction of sp3 carbons and chiral centers have lower attrition in the clinic,98 we anticipate seeing an ever increasing number of drug candidates containing quaternary stereocenters being designed, synthesized, and evaluated. In this context, the unrivaled stability of quaternary stereocenters toward metabolic modification is a unique attraction.
Acknowledgments
Our research in this area was supported by the National Institutes of Health (R01 GM030859 and GM098601).
References
- 1.Bartholow M. Top 200 drugs of 2011. Pharmacy Times. 2012;(July):48–51. [Google Scholar]
- 2.Ding HX, Liu KK-C, Sakya SM, Flick AC, O’Donnell CJ. Synthetic approaches to the 2011 new drugs. Bioorg Med Chem. 2013;21:2795–2825. doi: 10.1016/j.bmc.2013.02.061. and previous reviews in this series. [DOI] [PubMed] [Google Scholar]
- 3.Christoffers J, Baro A, editors. Quaternary Stereocenters–Challenges and Solutions for Organic Synthesis. Wiley–VCH; Weinheim: 2005. [Google Scholar]
- 4.Hong AY, Stoltz BM. The construction of all-carbon quaternary stereocenters by use of Pd-catalyzed asymmetric allylic alkylation reactions in total synthesis. Eur J Org Chem. 2013:2745–2759. doi: 10.1002/ejoc.201201761. and previous reviews of enantioselective synthesis of quaternary stereocenters cited in footnote 2 therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Douglas CJ, Overman LE. Catalytic asymmetric synthesis of all-carbon quaternary stereocenters. Proc Natl Acad Sci USA. 2004;101:5363–5367. doi: 10.1073/pnas.0307113101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson RM, Jen WS, MacMillan DWC. Enantioselective organocatalytic intramolecular Diels–Alder reactions. The asymmetric synthesis of solanapyrone D. J Am Chem Soc. 2005;127:11616–11617. doi: 10.1021/ja054008q. [DOI] [PubMed] [Google Scholar]
- 7.Jones SB, Simmons B, Mastracchio A, MacMillan DWC. Collective synthesis of natural products by means of organocascade catalysis. Nature. 2011;475:183–188. doi: 10.1038/nature10232. A highlight of the use of iminium activation and cascade catalysis in the synthesis of alkaloid natural products. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laforteza BN, Pickworth M, MacMillan DWC. Enantioselective total synthesis of (−)-minovincine in nine chemical steps: An approach to ketone activation in cascade catalysis. Angew Chem Int Ed. 2013;52:11269–11272. doi: 10.1002/anie.201305171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y, Nappi M, Arceo E, Vera S, Melchiorre P. Asymmetric catalysis of Diels–Alder reactions with in situ generated heterocyclic ortho-quinodimethanes. J Am Chem Soc. 2011;133:15212–15218. doi: 10.1021/ja206517s. [DOI] [PubMed] [Google Scholar]
- 10.Tan B, Hernández-Torres G, Barbas CF., III Highly efficient hydrogen-bonding catalysis of the Diels–Alder reaction of 3-vinylindoles and methyleneindolinones provides carbazolespirooxindole skeletons. J Am Chem Soc. 2011;133:12354–12357. doi: 10.1021/ja203812h. [DOI] [PubMed] [Google Scholar]
- 11.Shimizu Y, Shi SL, Usuda H, Kanai M, Shibasaki M. Catalytic asymmetric total synthesis of ent-hyperforin. Angew Chem Int Ed. 2010;49:1103–1106. doi: 10.1002/anie.200906678. [DOI] [PubMed] [Google Scholar]
- 12.Snyder SA, Corey EJ. Concise total syntheses of palominol, dolabellatrienone, β-araneosene, and isoedunol via an enantioselective Diels–Alder macrobicyclization. J Am Chem Soc. 2006;128:740–742. doi: 10.1021/ja0576379. [DOI] [PubMed] [Google Scholar]
- 13.Balskus EP, Jacobsen EN. Asymmetric catalysis of the transannular Diels–Alder reaction. Science. 2007;317:1736–1740. doi: 10.1126/science.1146939. [DOI] [PubMed] [Google Scholar]
- 14.Lian Y, Davies HML. Rhodium-catalyzed [3 + 2] annulation of indoles. J Am Chem Soc. 2010;132:440–441. doi: 10.1021/ja9078094. [DOI] [PubMed] [Google Scholar]
- 15.Trost BM, Silverman SM, Stambuli JP. Development of an asymmetric trimethylenemethane cycloaddition reaction: Application in the enantioselective synthesis of highly substituted carbocycles. J Am Chem Soc. 2011;133:19483–19497. doi: 10.1021/ja207550t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trost BM, Bringley DA, Zhang T, Cramer N. Rapid access to spirocyclic oxindole alkaloids: Application of the asymmetric palladium-catalyzed [3 + 2] trimethylenemethane cycloaddition. J Am Chem Soc. 2013;135:16720–16735. doi: 10.1021/ja409013m. An informative discussion of development of the TMM reaction for the synthesis of spirocyclic oxindoles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohmatsu K, Imagawa N, Ooi T. Ligand-enabled multiple absolute stereocontrol in metal-catalysed cycloaddition for construction of contiguous all-carbon quaternary stereocentres. Nature Chem. 2014;6:47–51. doi: 10.1038/nchem.1796. [DOI] [PubMed] [Google Scholar]
- 18.Chen XH, Wei Q, Luo SW, Xiao H, Gong LZ. Organocatalytic synthesis of spiro[pyrrolidin-3,3′-oxindoles] with high enantiopurity and structural diversity. J Am Chem Soc. 2009;131:13819–13825. doi: 10.1021/ja905302f. [DOI] [PubMed] [Google Scholar]
- 19.Martínez A, Webber MJ, Müller S, List B. Versatile access to chiral indolines by catalytic asymmetric Fischer indolization. Angew Chem Int Ed. 2013;52:9486–9490. doi: 10.1002/anie.201301618. [DOI] [PubMed] [Google Scholar]
- 20.Pellissier H. Recent developments in asymmetric cyclopropanation. Tetrahedron. 2008;64:7041–7095. [Google Scholar]
- 21.Schwartz BD, Denton JR, Lian Y, Davies HML, Williams CM. Asymmetric [4 + 3] cycloadditions between vinylcarbenoids and dienes: Application to the total synthesis of the natural product (−)-5-epi-vibsanin E. J Am Chem Soc. 2009;131:8329–8332. doi: 10.1021/ja9019484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishibashi H, Ishihara K, Yamamoto H. A new artificial cyclase for polyprenoids: Enantioselective total synthesis of (−)-chromazonarol, (+)-8-epi-puupehedione, and (−)-11′-deoxytaondiol methyl ether. J Am Chem Soc. 2004;126:11122–11123. doi: 10.1021/ja0472026. [DOI] [PubMed] [Google Scholar]
- 23.Surendra K, Corey EJ. Highly enantioselective proton-initiated polycyclization of polyenes. J Am Chem Soc. 2012;134:11992–11994. doi: 10.1021/ja305851h. [DOI] [PubMed] [Google Scholar]
- 24.Surendra K, Rajendar G, Corey EJ. Useful catalytic enantioselective cationic double annulation reactions initiated at an internal π-bond: Method and applications. J Am Chem Soc. 2014;136:642–645. doi: 10.1021/ja4125093. [DOI] [PubMed] [Google Scholar]
- 25.Schafroth MA, Sarlah D, Krautwald S, Carreira EM. Iridium-catalyzed enantioselective polyene cyclization. J Am Chem Soc. 2012;134:20276–20278. doi: 10.1021/ja310386m. A potentially broadly applicable approach for orchestrating enantioselective polyene cyclizations. [DOI] [PubMed] [Google Scholar]
- 26.Jeker OF, Kravina AG, Carreira EM. Total synthesis of (+)-asperolide C by iridium-catalyzed enantioselective polyene cyclization. Angew Chem Int Ed. 2013;52:12166–12169. doi: 10.1002/anie.201307187. [DOI] [PubMed] [Google Scholar]
- 27.Sethofer SG, Mayer T, Toste FD. Gold(I)-catalyzed enantioselective polycyclization reactions. J Am Chem Soc. 2010;132:8276–8277. doi: 10.1021/ja103544p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brazeau JF, Zhang S, Colomer I, Corkey BK, Toste FD. Enantioselective cyclizations of silyloxyenynes catalyzed by cationic metal phosphine complexes. J Am Chem Soc. 2012;134:2742–2749. doi: 10.1021/ja210388g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corkey BK, Toste FD. Catalytic enantioselective Conia-ene reaction. J Am Chem Soc. 2005;127:17168–17169. doi: 10.1021/ja055059q. [DOI] [PubMed] [Google Scholar]
- 30.Shibata T, Tahara YK, Tamura K, Endo K. Enantioselective syntheses of various chiral multicyclic compounds with quaternary carbon stereocenters by catalytic intramolecular cycloaddition. J Am Chem Soc. 2008;130:3451–3457. doi: 10.1021/ja0762083. [DOI] [PubMed] [Google Scholar]
- 31.Knowles RR, Lin S, Jacobsen EN. Enantioselective thiourea-catalyzed cationic polycyclizations. J Am Chem Soc. 2010;132:5030–5032. doi: 10.1021/ja101256v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rendler S, MacMillan DWC. Enantioselective polyene cyclization via organo-SOMO catalysis. J Am Chem Soc. 2010;132:5027–5029. doi: 10.1021/ja100185p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dounay AB, Humphreys PG, Overman LE, Wrobleski AD. Total synthesis of the strychnos alkaloid (+)-minfiensine: Tandem enantioselective intramolecular Heck–iminium ion cyclization. J Am Chem Soc. 2008;130:5368–5377. doi: 10.1021/ja800163v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watson MP, Jacobsen EN. Asymmetric intramolecular arylcyanation of unactivated olefins via C–CN bond activation. J Am Chem Soc. 2008;130:12594–12595. doi: 10.1021/ja805094j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakao Y, Ebata S, Yada A, Hiyama T, Ikawa M, Ogoshi S. Intramolecular arylcyanation of alkenes catalyzed by nickel/AlMe2Cl. J Am Chem Soc. 2008;130:12874–12875. doi: 10.1021/ja805088r. [DOI] [PubMed] [Google Scholar]
- 36.García-Fortanet J, Kessler F, Buchwald SL. Palladium-catalyzed asymmetric dearomatization of naphthalene derivatives. J Am Chem Soc. 2009;131:6676–6677. doi: 10.1021/ja9025193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu T, Ko HM, Savage NA, Dong G. Highly enantioselective Rh-catalyzed carboacylation of olefins: Efficient syntheses of chiral poly-fused rings. J Am Chem Soc. 2012;134:20005–20008. doi: 10.1021/ja309978c. [DOI] [PubMed] [Google Scholar]
- 38.Mei T-S, Patel HH, Sigman MS. Enantioselective construction of remote quaternary stereocenters. Nature. 2014;508:340–344. doi: 10.1038/nature13231. A pioneering method for constructing remote stereocenters in acyclic substrates by enantioselective Heck reactions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vuagnoux-d’Augustin M, Alexakis A. Copper-catalyzed asymmetric conjugate addition of trialkylaluminium reagents to trisubstituted enones: Construction of chiral quaternary centers. Chem Eur J. 2007;13:9647–9662. doi: 10.1002/chem.200701001. [DOI] [PubMed] [Google Scholar]
- 40.Müller D, Alexakis A. Formation of quaternary stereogenic centers by copper-catalyzed asymmetric conjugate addition reactions of alkenylaluminums to trisubstituted enones. Chem Eur J. 2013;19:15226–15239. doi: 10.1002/chem.201302856. [DOI] [PubMed] [Google Scholar]
- 41.Hawner C, Li K, Cirriez V, Alexakis A. Copper-catalyzed asymmetric conjugate addition of aryl aluminum reagents to trisubstituted enones: Construction of aryl-substituted quaternary centers. Angew Chem Int Ed. 2008;47:8211–8214. doi: 10.1002/anie.200803436. [DOI] [PubMed] [Google Scholar]
- 42.May TL, Brown MK, Hoveyda AH. Enantioselective synthesis of all-carbon quaternary stereogenic centers by catalytic asymmetric conjugate additions of alkyl and aryl aluminum reagents to five-, six-, and seven-membered-ring β-substituted cyclic enones. Angew Chem Int Ed. 2008;47:7358–7362. doi: 10.1002/anie.200802910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.May TL, Dabrowski JA, Hoveyda AH. Formation of vinyl-, vinylhalide- or acyl-substituted quaternary carbon stereogenic centers through NHC–Cu-catalyzed enantioselective conjugate additions of Si-containing vinylaluminums to β-substituted cyclic enones. J Am Chem Soc. 2011;133:736–739. doi: 10.1021/ja110054q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee KS, Brown MK, Hird AW, Hoveyda AH. A practical method for enantioselective synthesis of all-carbon quaternary stereogenic centers through NHC-Cu-catalyzed conjugate additions of alkyl and arylzinc reagents to β-substituted cyclic enones. J Am Chem Soc. 2006;128:7182–7184. doi: 10.1021/ja062061o. [DOI] [PubMed] [Google Scholar]
- 45.Martin D, Kehrli S, d’Augustin M, Clavier H, Mauduit M, Alexakis A. Copper-catalyzed asymmetric conjugate addition of Grignard reagents to trisubstituted enones. Construction of all-carbon quaternary chiral centers. J Am Chem Soc. 2006;128:8416–8417. doi: 10.1021/ja0629920. [DOI] [PubMed] [Google Scholar]
- 46.Sidera M, Roth PMC, Maksymowicz RM, Fletcher SP. Formation of quaternary carbon centers by copper-catalyzed asymmetric conjugate addition of alkylzirconium reagents. Angew Chem Int Ed. 2013;52:7995–7999. doi: 10.1002/anie.201303202. [DOI] [PubMed] [Google Scholar]
- 47.Shintani R, Duan WL, Hayashi T. Rhodium-catalyzed asymmetric construction of quaternary stereocenters: Ligand-dependent regiocontrol in the 1,4-addition to substituted maleimides. J Am Chem Soc. 2006;128:5628–5629. doi: 10.1021/ja061430d. [DOI] [PubMed] [Google Scholar]
- 48.Shintani R, Tsutsumi Y, Nagaosa M, Nishimura T, Hayashi T. Sodium tetraarylborates as effective nucleophiles in rhodium/diene-catalyzed 1,4-addition to β,β-disubstituted α,β-unsaturated ketones: Catalytic asymmetric construction of quaternary carbon stereocenters. J Am Chem Soc. 2009;131:13588–13589. doi: 10.1021/ja905432x. [DOI] [PubMed] [Google Scholar]
- 49.Hawner C, Müller D, Gremaud L, Felouat A, Woodward S, Alexakis A. Rhodium-catalyzed asymmetric 1,4-addition of aryl alanes to trisubstituted enones: Binap as an effective ligand in the formation of quaternary stereocenters. Angew Chem Int Ed. 2010;49:7769–7772. doi: 10.1002/anie.201003300. [DOI] [PubMed] [Google Scholar]
- 50.Holder JC, Zou L, Marziale AN, Liu P, Lan Y, Gatti M, Kikushima K, Houk KN, Stoltz BM. Mechanism and enantioselectivity in palladium-catalyzed conjugate addition of arylboronic acids to β-substituted cyclic enones: Insights from computation and experiment. J Am Chem Soc. 2013;135:14996–15007. doi: 10.1021/ja401713g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mendoza A, Ishihara Y, Baran PS. Scalable enantioselective total synthesis of taxanes. Nature Chem. 2012;4:21–25. doi: 10.1038/nchem.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brown MK, Hoveyda AH. Enantioselective total synthesis of clavirolide C. Applications of Cu-catalyzed asymmetric conjugate additions and Ru-catalyzed ring-closing metathesis. J Am Chem Soc. 2008;130:12904–12906. doi: 10.1021/ja8058414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu F, Li H, Hong R, Deng L. Construction of quaternary stereocenters by efficient and practical conjugate additions to α,β-unsaturated ketones with a chiral organic catalyst. Angew Chem Int Ed. 2006;45:947–950. doi: 10.1002/anie.200502658. [DOI] [PubMed] [Google Scholar]
- 54.Poulsen TB, Bernardi L, Alemán J, Overgaard J, Jørgensen KA. Organocatalytic asymmetric direct α-alkynylation of cyclic β-ketoesters. J Am Chem Soc. 2007;129:441–449. doi: 10.1021/ja067289q. [DOI] [PubMed] [Google Scholar]
- 55.Penon O, Carlone A, Mazzanti A, Locatelli M, Sambri L, Bartoli G, Melchiorre P. Quaternary stereogenic carbon atoms in complex molecules by an asymmetric, organocatalytic, triple-cascade reaction. Chem Eur J. 2008;14:4788–4791. doi: 10.1002/chem.200800440. [DOI] [PubMed] [Google Scholar]
- 56.Mase N, Thayumanavan R, Tanaka F, Barbas CF., III Direct asymmetric organocatalytic Michael reactions of α,α-disubstituted aldehydes with β-nitrostyrenes for the synthesis of quaternary carbon-containing products. Org Lett. 2004;6:2527–2530. doi: 10.1021/ol049196o. [DOI] [PubMed] [Google Scholar]
- 57.Kerr MS, Rovis T. Enantioselective synthesis of quaternary stereocenters via a catalytic asymmetric Stetter reaction. J Am Chem Soc. 2004;126:8876–8877. doi: 10.1021/ja047644h. [DOI] [PubMed] [Google Scholar]
- 58.Gao L, Kang BC, Ryu DH. Catalytic asymmetric insertion of diazoesters into aryl-CHO bonds: Highly enantioselective construction of chiral all-carbon quaternary centers. J Am Chem Soc. 2013;135:14556–14559. doi: 10.1021/ja408196g. [DOI] [PubMed] [Google Scholar]
- 59.Doyle AG, Jacobsen EN. Enantioselective alkylation of acyclic α,α-disubstituted tributyltin enolates catalyzed by a {Cr(salen)} complex. Angew Chem Int Ed. 2007;46:3701–3705. doi: 10.1002/anie.200604901. [DOI] [PubMed] [Google Scholar]
- 60.Krautwald S, Sarlah D, Schafroth MA, Carreira EM. Enantio- and diastereodivergent dual catalysis: α-allylation of branched aldehydes. Science. 2013;340:1065–1068. doi: 10.1126/science.1237068. [DOI] [PubMed] [Google Scholar]
- 61.Gao F, Carr JL, Hoveyda AH. Copper-catalyzed enantioselective allylic substitution with readily accessible carbonyl- and acetal-containing vinylboron reagents. Angew Chem Int Ed. 2012;51:6613–6617. doi: 10.1002/anie.201202856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van Veldhuizen JJ, Campbell JE, Giudici RE, Hoveyda AH. A readily available chiral Ag-based N-heterocyclic carbene complex for use in efficient and highly enantioselective Ru-catalyzed olefin metathesis and Cu-catalyzed allylic alkylation reactions. J Am Chem Soc. 2005;127:6877–6882. doi: 10.1021/ja050179j. [DOI] [PubMed] [Google Scholar]
- 63.Dabrowski JA, Gao F, Hoveyda AH. Enantioselective synthesis of alkyne-substituted quaternary carbon stereogenic centers through NHC-Cu-catalyzed allylic substitution reactions with (i-Bu)2(alkynylaluminum reagents. J Am Chem Soc. 2011;133:4778–4781. doi: 10.1021/ja2010829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Feng J, Garza VJ, Krische MJ. Redox-triggered C–C coupling of alcohols and vinyl epoxides: Diastereo- and enantioselective formation of all-carbon quaternary centers via tert-(hydroxy)-prenylation. J Am Chem Soc. 2014;136:8911–8914. doi: 10.1021/ja504625m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Behenna DC, Stoltz BM. The enantioselective Tsuji allylation. J Am Chem Soc. 2004;126:15044–15045. doi: 10.1021/ja044812x. [DOI] [PubMed] [Google Scholar]
- 66.Trost BM, Xu J. Regio- and enantioselective Pd-catalyzed allylic alkylation of ketones through allyl enol carbonates. J Am Chem Soc. 2005;127:2846–2847. doi: 10.1021/ja043472c. An excellent synopsis of the development of the asymmetric allylic alkylation reaction. [DOI] [PubMed] [Google Scholar]
- 67.Trost BM, Frederiksen MU. Palladium-catalyzed asymmetric allylation of prochiral nucleophiles: Synthesis of 3-allyl-3-aryl oxindoles. Angew Chem Int Ed. 2005;44:308–310. doi: 10.1002/anie.200460335. [DOI] [PubMed] [Google Scholar]
- 68.Trost BM, Xie J, Sieber JD. The palladium catalyzed asymmetric addition of oxindoles and allenes: An atom-economical versatile method for the construction of chiral indole alkaloids. J Am Chem Soc. 2011;133:20611–20622. doi: 10.1021/ja209244m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Trost BM, Malhotra S, Chan WH. Exercising regiocontrol in palladium-catalyzed asymmetric prenylations and geranylation: Unifying strategy toward flustramines A and B. J Am Chem Soc. 2011;133:7328–7331. doi: 10.1021/ja2020873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Enquist JA, Jr, Stoltz BM. The total synthesis of (−)-cyanthiwigin F by means of double catalytic enantioselective alkylation. Nature. 2008;453:1228–1231. doi: 10.1038/nature07046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Du C, Li L, Li Y, Xie Z. Construction of two vicinal quaternary carbons by asymmetric allylic alkylation: Total synthesis of hyperolactone C and (−)-biyouyanagin A. Angew Chem Int Ed. 2009;48:7853–7856. doi: 10.1002/anie.200902908. [DOI] [PubMed] [Google Scholar]
- 72.Trost BM, Miller JR, Hoffman CM., Jr A highly enantio- and diastereoselective molybdenum-catalyzed asymmetric allylic alkylation of cyanoesters. J Am Chem Soc. 2011;133:8165–8167. doi: 10.1021/ja2029602. [DOI] [PubMed] [Google Scholar]
- 73.Liu WB, Reeves CM, Virgil SC, Stoltz BM. Construction of vicinal tertiary and all-carbon quaternary stereocenters via Ir-catalyzed regio-, diastereo-, and enantioselective allylic alkylation and applications in sequential Pd catalysis. J Am Chem Soc. 2013;135:10626–10629. doi: 10.1021/ja4052075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Trost BM, Quancard J. Palladium-catalyzed enantioselective C-3 allylation of 3-substituted-1H-indoles using trialkylboranes. J Am Chem Soc. 2006;128:6314–6315. doi: 10.1021/ja0608139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hills ID, Fu GC. Catalytic enantioselective synthesis of oxindoles and benzofuranones that bear a quaternary stereocenter. Angew Chem Int Ed. 2003;42:3921–3924. doi: 10.1002/anie.200351666. [DOI] [PubMed] [Google Scholar]
- 76.Shaw SA, Aleman P, Christy J, Kampf JW, Va P, Vedejs E. Enantioselective TADMAP-catalyzed carboxyl migration reactions for the synthesis of stereogenic quaternary carbon. J Am Chem Soc. 2006;128:925–934. doi: 10.1021/ja056150x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mermerian AH, Fu GC. Nucleophile-catalyzed asymmetric acylations of silyl ketene imines: Application to the enantioselective synthesis of verapamil. Angew Chem Int Ed. 2005;44:949–952. doi: 10.1002/anie.200461886. [DOI] [PubMed] [Google Scholar]
- 78.DeLorbe JE, Jabri SY, Mennen SM, Overman LE, Zhang FL. Enantioselective total synthesis of (+)-gliocladine C: Convergent construction of cyclotryptamine-fused polyoxopiperazines and a general approach for preparing epidithiodioxopiperazines from trioxopiperazine precursors. J Am Chem Soc. 2011;133:6549–6552. doi: 10.1021/ja201789v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Austin JF, Kim SG, Sinz CJ, Xiao WJ, MacMillan DWC. Enantioselective organocatalytic construction of pyrroloindolines by a cascade addition–cyclization strategy: Synthesis of (−) flustramine B. Proc Natl Acad Sci USA. 2004;101:5482–5487. doi: 10.1073/pnas.0308177101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhu S, MacMillan DWC. Enantioselective copper-catalyzed construction of aryl pyrroloindolines via an arylation–cyclization cascade. J Am Chem Soc. 2012;134:10815–10818. doi: 10.1021/ja305100g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xie X, Chen Y, Ma D. Enantioselective arylation of 2-methylacetoacetates catalyzed by CuI/trans-4-hydroxy-L-proline at low reaction temperatures. J Am Chem Soc. 2006;128:16050–16051. doi: 10.1021/ja066991j. [DOI] [PubMed] [Google Scholar]
- 82.García-Fortanet J, Buchwald SL. Asymmetric palladium-catalyzed intramolecular α-arylation of aldehydes. Angew Chem Int Ed. 2008;47:8108–8111. doi: 10.1002/anie.200803809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Taylor AM, Altman RA, Buchwald SL. Palladium-catalyzed enantioselective α-arylation and α-vinylation of oxindoles facilitated by an axially chiral P-stereogenic ligand. J Am Chem Soc. 2009;131:9900–9901. doi: 10.1021/ja903880q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mitsunuma H, Shibasaki M, Kanai M, Matsunaga S. Catalytic asymmetric total synthesis of chimonanthine, folicanthine, and calycanthine through double Michael reaction of bisoxindole. Angew Chem Int Ed. 2012;51:5217–5221. doi: 10.1002/anie.201201132. [DOI] [PubMed] [Google Scholar]
- 85.Zhang H, Hong L, Kang H, Wang R. Construction of vicinal all-carbon quaternary stereocenters by catalytic asymmetric alkylation reaction of 3-bromooxindoles with 3-substituted indoles: Total synthesis of (+)-perophoramidine. J Am Chem Soc. 2013;135:14098–14101. doi: 10.1021/ja408336v. [DOI] [PubMed] [Google Scholar]
- 86.Malcolmson SJ, Meek SJ, Sattely ES, Schrock RR, Hoveyda AH. Highly efficient molybdenum-based catalysts for enantioselective alkene metathesis. Nature. 2008;456:933–937. doi: 10.1038/nature07594. An excellent example of using desymmetrization to install a key quaternary stereocenter in a structurally complex natural product. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Giudici RE, Hoveyda AH. Directed catalytic asymmetric olefin metathesis. Selectivity control by enoate and ynoate groups in Ru-catalyzed asymmetric ring-opening/cross-metathesis. J Am Chem Soc. 2007;129:3824–3825. doi: 10.1021/ja070187v. [DOI] [PubMed] [Google Scholar]
- 88.Kleinbeck F, Toste FD. Gold(I)-catalyzed enantioselective ring expansion of allenylcyclopropanols. J Am Chem Soc. 2009;131:9178–9179. doi: 10.1021/ja904055z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Phan DHT, Kou KGM, Dong VM. Enantioselective desymmetrization of cyclopropenes by hydroacylation. J Am Chem Soc. 2010;132:16354–16355. doi: 10.1021/ja107738a. [DOI] [PubMed] [Google Scholar]
- 90.Souillart L, Parker E, Cramer N. Highly enantioselective rhodium(I)-catalyzed activation of enantiotopic cyclobutanone C–C bonds. Angew Chem Int Ed. 2014;53:3001–3005. doi: 10.1002/anie.201311009. [DOI] [PubMed] [Google Scholar]
- 91.Shi B-F, Zhang Y-H, Lam JK, Wang D-H, Yu J-Q. Pd(II)-catalyzed enantioselective C-H olefination of diphenylacetic acids. J Am Chem Soc. 2010;132:460–461. doi: 10.1021/ja909571z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aikawa K, Okamoto T, Mikami K. Copper(I)-catalyzed asymmetric desymmetrization: Synthesis of five-membered-ring compounds containing all-carbon quaternary stereocenters. J Am Chem Soc. 2012;134:10329–10332. doi: 10.1021/ja3032345. [DOI] [PubMed] [Google Scholar]
- 93.Zhang E, Fan CA, Tu YQ, Zhang FM, Song Y-L. Organocatalytic asymmetric vinylogous α-ketol rearrangement: Enantioselective construction of chiral all-carbon quaternary stereocenters in spirocyclic diketones via semipinacol-type 1,2-carbon migration. J Am Chem Soc. 2009;131:14626–14627. doi: 10.1021/ja906291n. [DOI] [PubMed] [Google Scholar]
- 94.Wadamoto M, Phillips EM, Reynolds TE, Scheidt KA. Enantioselective synthesis of α,α-disubstituted cyclopentenes by an N-heterocyclic carbene-catalyzed desymmetrization of 1,3-diketones. J Am Chem Soc. 2007;129:10098–10099. doi: 10.1021/ja073987e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Das JP, Marek I. Enantioselective synthesis of all-carbon quaternary stereogenic centers in acyclic systems. Chem Commun. 2011;47:4593–4623. doi: 10.1039/c0cc05222a. [DOI] [PubMed] [Google Scholar]
- 96.Xiao KJ, Lin DW, Miura M, Zhu RY, Gong W, Wasa M, Yu JQ. Palladium(II)-catalyzed enantioselective C(sp3)–H activation using a chiral hydroxamic acid ligand. J Am Chem Soc. 2014;136:8138–8142. doi: 10.1021/ja504196j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Notte GT. New chemical entities entering phase III trials in 2012. Annu Rep Med Chem. 2014;48:451–469. [Google Scholar]
- 98.Lovering F. Escape from flatland 2: complexity and promiscuity. Med Chem Comm. 2013;4:515–519. [Google Scholar]