Abstract
The development of endemic Burkitt's lymphoma (eBL) is closely associated with EBV infection and holoendemic malaria infections. The role of EBV in the development of malignancy has been studied in depth, but there is still little known about the mechanisms by which malaria affects Burkitt's lymphomagenesis. Activation induced cytidine deaminase (AID) expression is necessary for the introduction of c-myc translocations that are characteristic of BL, but a link between AID and EBV or malaria is unclear. To determine if frequency of malaria exposure leads to increased AID expression in peripheral blood mononuclear cells (PBMC) we examined two cohorts of children in western Kenya with endemic and sporadic malaria transmission dynamics. High frequency of malaria exposure led to increased expression of AID, which coincided with decreases in the IgM+ memory B cells. In the children from the malaria endemic region, the presence of a detectible EBV viral load was associated with higher AID expression compared to children with undetectable EBV, but this effect was not seen in children with sporadic exposure to malaria. This study demonstrates that intensity of malaria transmission correlates with AID expression levels in the presence of EBV suggesting that malaria and EBV infection have a synergistic effect on the development of c-myc translocations and BL.
Introduction
Endemic Burkitt's lymphoma (eBL) is a rapidly dividing B cell malignancy that is fatal if untreated and it is found primarily in children in sub-Saharan Africa1. The etiology of eBL is closely linked to infection with Epstein-Barr virus (EBV) and holoendemic exposure to malaria2,3. EBV is a ubiquitous virus that is found in >90% of people worldwide, and is present in nearly all cases of endemic African BL4. A common feature of eBL is the translocation of the oncogene c-myc to the control of the immunoglobulin promoter leading to constitutive expression of c-myc5,6. Translocations of c-myc in the presence of EBV are sufficient to produce transformed cells6, but the etiology of the c-myc translocations and whether malaria plays a role in inducing these translocations is unknown.
The induction of c-myc translocations is likely an early event in the development of eBL7. Studies in humans and mice have shown that there is a direct link between the activity and expression of activation induced cytidine deaminase (AID) and c-myc translocations8. AID is a necessary enzyme for somatic hypermutation (SHM) and class-switch recombination (CSR)9. AID expression is generally restricted to germinal center B cells within the spleen and lymph nodes9. AID is rarely expressed in the peripheral blood of healthy individuals, however it has been shown that people infected with HIV have AID expression in circulating lymphocytes10. Additionally, HIV infected patients were more likely to have high levels of AID expression in their blood during the years preceding diagnosis with non-Hodgkin's lymphoma than patients that did not develop lymphoma10. HIV-associated BL is similar to African endemic BL because both diseases are initiated by chronic infection, and both have c-myc translocations.
EBV has several reported effects on AID expression in B cells by different mechanisms. For example, the EBV latent membrane protein (LMP)-1 is capable of acting in place of CD40 to activate infected B cells and lead to AID mediated class-switch recombination11. In contrast, another EBV latent protein, Epstein-Barr nuclear antigen (EBNA)-2, was shown to inhibit AID expression during proliferation of infected B cells. In addition to direct effects on AID expression, infection with EBV could lead to the rescue of cells from apoptosis that are over-expressing c-myc6. High EBV viral loads such as those observed in children from malaria holoendemic regions12, could increase the likelihood of a B cell with a c-myc translocation getting rescued from cell death3.
P. falciparum malaria has several components that act as B cell activators during the course of infection. Plasmodium falciparum erythrocyte membrane protein-1 (PfEMP-1) is a polyclonal B cell activator leading to increased cytokine and Ig secretion and activation marker expression13. P. falciparum is also capable of activating B cells through Toll-like receptors (TLR). The hemoglobin metabolism breakdown product, hemozoin, bound to DNA of Plasmodium parasites is capable of stimulating B cells through TLR914. Of note, AID expression and activity can be induced through TLR9 activation15. In addition, Plasmodium glycosylphosphatidylinositol (GPI) anchors are capable of stimulating TLR216. The effects of these B cell activators could be even greater in a population that is chronically exposed to malaria.
Several studies have examined the effects of P. falciparum infection on B cell homeostasis. Kassa et al found that adults with acute malaria infections had decreased percentages of CD19+ B cells17. Similarly, we found that children in Western Kenya with acute malaria had decreased CD19+ B cells and also decreased classical memory B cells (MBC) compared to a four-week recovery sample18. While examining the B cell responses to acute infection is important19, it is also necessary to understand how repeated infections and transmission intensity affect B cell homeostasis. We characterized the peripheral B cell subsets in a longitudinal cohort of infants enrolled from two regions of Western Kenya that experience different patterns of malaria transmission intensity throughout the year: Kisumu district, where malaria transmission and endemic Burkitt lymphoma (eBL) is high, and Nandi district, where transmission and risk for eBL is low and episodic20. No differences were observed between the infants from the two sites in frequencies of naive B cells or classical MBC. However, the levels of non-class switched IgD+CD27+ MBC were significantly lower in Kisumu infants relative to Nandi at 12, 18, and 24 months of age. These data suggest that even early in life, malaria transmission is altering the development of MBC subsets.
Further evidence for effects of malaria on alterations in B cell homeostasis is the increase in atypical MBC expanded in children and adults in Mali, which has high malaria transmission21,22. They were defined as ‘exhausted’ B cells based on their poor proliferative response in vitro and it was proposed that they may contribute to poor antibody responses21. As eBL is thought to derive from MBC23, understanding whether there are alterations in MBC subsets in children living in areas with high malaria transmission and eBL risk is important.
In this study, we analyzed peripheral blood mononuclear cells (PBMC) isolated from children at 36 months of age from Kisumu and Nandi regions and performed a detailed B cell phenotype analysis. The B cell subsets were then correlated with AID expression and EBV viral load.
Materials and Methods
Study Population
Two cohorts of children were established in predominantly rural areas of western Kenya that have previously been described20,24,25. The cohorts were from populations with divergent levels of malaria transmission due to their geographical location. The cohort with endemic/high malaria transmission is in Kisumu District (n=54) within Nyanza Province and sporadic/low malaria transmission is characteristic of the cohort from Nandi District (n=34) of the Rift Valley Province. The cohorts are part of a larger study and have been previously described26,27. The Kenya Medical Research Institute Ethical Review Committee and the Institutional Review Board at State University of New York Upstate Medical University gave ethical approval for this study. Infants were enrolled at 1 month old during a 3-month period between April 2006-June 2006. All children were born to HIV-seronegative mothers and their health was closely monitored and treated for illness as per Kenya Ministry of Health guidelines. Sample collection began at 1 month old and continued monthly until 12 months of age and then blood was drawn at 18, 24, 30, and 36 months old. The present study is focused on the blood drawn at 36 months old.
Blood Collection
Study participants were examined and children that were not febrile had 1-3ml of blood drawn by venipuncture into heparinized vacutainer tubes. Within 1 hour of collection PBMC were isolated by ficoll density gradient centrifugation. The cells were frozen in freezing media (RPMI, 20% Fetal Bovine Serum, 10% DMSO) and stored in liquid nitrogen until analysis.
Flow Cytometry
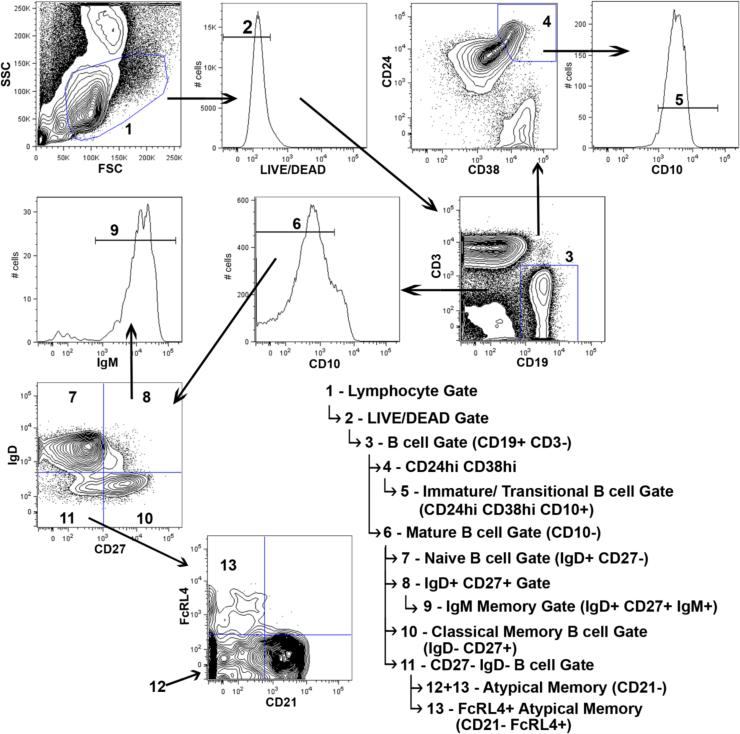
PBMC were thawed and stained with the LIVE/DEAD Aqua (Life Technologies, Grand Island, NY) viability dye according to the manufacturer protocol. Before fixation, cells were incubated with Human Fc Receptor Binding Inhibitor (eBioscience, San Diego, CA) then stained with the following monoclonal antibodies: CD38-PE-TexasRed (Life Technologies), IgD-FITC (BD Biosciences, San Jose, CA), IgM-Brilliant Violet 421, CD307d (FcRL4)-PE, CD3-Alexa Fluor 700, CD10-PE/Cy7, Streptavidin-APC/Cy7, CD19-PerCP/C5.5 (BioLegend, San Diego, CA), CD24-Biotin, CD21-APC, and CD27-650NC (eBioscience). Cells were then fixed in 2% formaldehyde and analyzed within 1-2 hours on an LSR Fortessa (BD Biosciences). An average of 8×105 events were collected (range of 2×105 to 2×106 events), which resulted in an average of 6.7×104 total B cells analyzed (range of 5×103 to 2×105 B cells). All flow cytometry data was processed using FlowJo Software (Tree Star Inc., San Carlos, CA). Percentages presented for total CD19+ B cell population (CD19+, CD3−) are derived from the percentage of cells within the live gate based on LIVE/DEAD Aqua staining that is within the lymphocyte gate. All B cell subset percentages are the frequency of the total B cell gate (CD19+, CD3−) described above, with the exception of the IgM+ memory B cell subset, which is presented as frequency of CD19+, CD3−, CD10−, CD27+, and IgD−.
Quantitative PCR
EBV viral load was determined by extracting DNA from up to 200 μl of blood using the Qiagen DNeasy kit (Qiagen, Valencia, CA) according to the manufacturer protocol. DNA was eluted in water and stored at −20°C until analysis. The detection of the EBV BALF5 and human β-actin genes was performed using primers and probes previously described12,28. RNA was isolated from PBMC that were not stained for flow cytometric analysis using the Qiagen RNeasy kit (Qiagen) according to the manufacturer protocol. The volume of RNA put into the reverse transcription reaction was based upon the cell number and was performed using the High Capacity cDNA Reverse Transcription kit (Life Technologies) according to the manufacturer protocol. AID mRNA levels were determined by using the TaqMan Primers and Probe set Hs00757808_m1 (Life Technologies) and the HPRT gene was used as a control (Integrated DNA Technologies, Coralville, IA). AID qPCR was performed with a standard curve generated by inserting the PCR fragment into a TOPO vector (Life Technologies) according to the manufacturer protocol.
Statistical Analysis
All statistical analysis was performed using GraphPad Prism software (GraphPad Software Inc, La Jolla, CA). Differences between the two cohorts were compared using Mann-Whitney U-test. Correlation analysis was performed with a Spearman rank correlation test. Statistical analysis was considered significant if P values were ≤0.05.
Results
Study population and clinical characteristics
Two cohorts of children were recruited and enrolled at 1 month of age from two rural study sites in western Kenya. These cohorts have been the focus of several previously published studies on the effects of malaria transmission on EBV infection25–27. The first study site was in Nyanza Province of western Kenya in the Kisumu District near Lake Victoria. This area has holoendemic malaria transmission and an increased risk of eBL, and will be referred to as Kisumu24. The second study site was in the Rift Valley Province within the Nandi District. This site has a low malaria transmission rate with only sporadic outbreaks and will be referred to as Nandi24. Each cohort was monitored until 3 years of age at regularly scheduled intervals. Venous blood was obtained at 3 years of age for this study. There were a significantly higher number of children in Kisumu with detectible P. falciparum parasitemia by blood smear or qPCR analysis at nearly every time tested compared to Nandi, confirming differences in malaria transmission26.
Children from areas with high malaria transmission have differences in memory B cell subsets compared to children from a low malaria transmission region
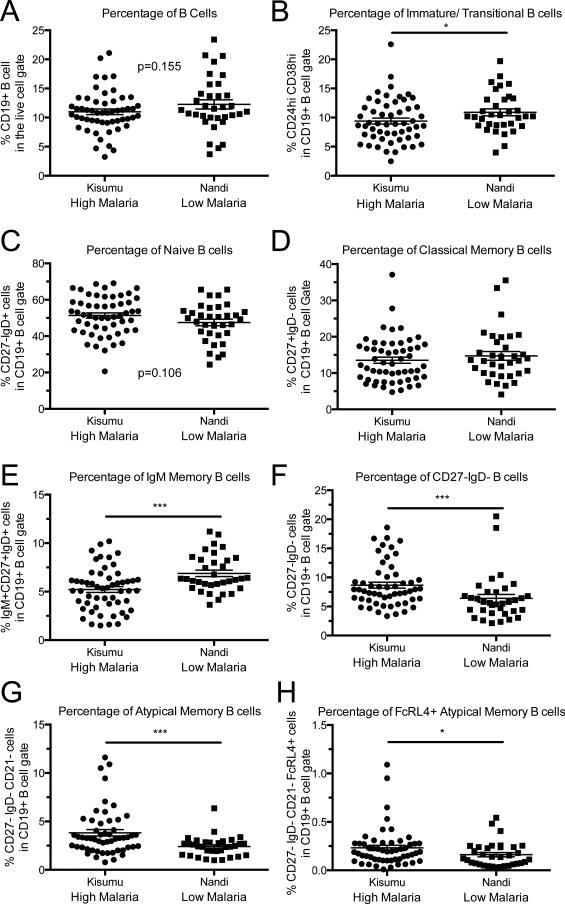
To determine the impact of malaria infection on the distribution of peripheral B cell subsets in 3-year-old children, we performed a comprehensive 11-color flow cytometric analysis on PBMCs isolated from children at each study site. Samples were analyzed for the presence of CD19, CD3, CD10, CD24, CD38, CD27, CD21, IgM, IgD, FcRL4, and binding to an amine reactive viability dye. The staining strategy allowed us to perform detailed analysis on B cell subsets, as demonstrated in Figure 1. We analyzed the total CD19+ B cell population, as well as the immature/transitional, naïve, and memory B cell subsets (summarized in Table 1). The memory B cell subsets included classical CD27+ IgD−, IgM+ classical, atypical CD27− IgD−, FcRL4+ atypical, and MZ-like CD27+IgD+ MBCs. There was no statistically significant difference in the total CD19+ B cell percentages between the two study cohorts (Figure 2A). The percentage of immature B cells of the total CD19+ B cell population was significantly decreased in Kisumu compared to Nandi (Figure 2B).
Figure 1. Representative flow cytometry demonstrating the gating strategy used for analysis.
Flow cytometry images shown are representative of study participants from Kisumu, which suffer a high frequency of malaria transmission. Beginning with the upper left panel, samples were gated as shown, with parent gating denoted by indented arrows in the legend.
Table 1.
Summary of B cell phenotyping in Nandi and Kisumu children.
| Kisumu Mean | Kisumu Range | Nandi Mean | Nandi Range | P value | |
|---|---|---|---|---|---|
| Total B cells | 10.98 | 3.23-21.1 | 12.26 | 3.7-23.4 | 0.1548 |
| Immature/Transitional | 9.39 | 2.49-22.6 | 10.90 | 3.99-19.7 | 0.0436 |
| Naïve | 51.34 | 20.6-69.1 | 47.52 | 24.4-65.6 | 0.1062 |
| Classical Memory | 13.52 | 4.75-37.1 | 14.72 | 4.08-35.5 | 0.4769 |
| MZ-like Memory | 5.22 | 1.51-10.2 | 6.89 | 3.64-11.2 | 0.001 |
| IgM+ Classical | 11.06 | 3.11-28.5 | 18.41 | 6.97-46.2 | < 0.0001 |
| Atypical Memory | 3.82 | 0.786-11.6 | 2.40 | 0.995-6.36 | 0.0008 |
| FcRL4+ Atypical Memory | 0.46 | 0.071-1.96 | 0.32 | 0.081-0.91 | 0.0374 |
| CD27- IgD- B cells | 8.67 | 3.35-18.6 | 6.40 | 2.14-20.5 | 0.0007 |
Mean percentage and range are shown for each B cell subset as shown in Figure 2.
Figure 2. Atypical, FcRL4+, and IgM Memory B cell subsets were significantly different in children living in the presence of high malaria transmission regions.
Comparison of the different B cell populations in children from high and low malaria transmission regions based on the gating strategy shown in Figure 1. The numbers in (#) represent the line number in the flow chart from Figure 1 demonstrating the hierarchy of populations. A) Percentage of B cells in the live cell gate (3). B) Transitional/Immature B cells (4), C) Naïve B cells (7), D) Classical Memory B cells (10), E) MZ-like Memory B cells (9), F) IgM+ Classical memory B cells (11), G) Atypical Memory B cells (13), H) FcRL4+ Atypical Memory B cells (13+14), and I) CD27− IgD− B cells (12). Kisumu n=54, Nandi n=34. *p<0.05, **p<0.001, ***p<0.0001. P-value determined by a Mann-Whitney U-test.
The distributions of memory B cell subsets are altered in adults from malaria endemic regions22. To determine if a high frequency of malaria transmission affects children by altering the balance between B cell memory subsets we analyzed the cell surface expression of CD27 and IgD on mature (CD19+, CD10−) B cells. There was no statistical difference in the percentages naïve B cells (CD19+, CD10−, CD27−, IgD+) (Figure 2C) or of classical memory B cells (CD19+, CD10−, CD27+, IgD−) between both sites (Figure 2D). This suggests the development and maintenance of the naïve and classical memory B cell subsets is not affected by differential exposure to malaria transmission consistent with our earlier observations using a more limited panel of markers27. However, there was a significant increase in the frequency of IgM+ B cells in the classical memory pool of children in the low malaria transmission region.
Previous studies of the Kisumu and Nandi cohorts at 1-2 years of age have shown an increase in atypical memory B cells in children from Kisumu, but were unable to determine if these populations included immature B cells, due to technical limitations27. Our panel allowed us to define the atypical memory B phenotype, by the excluding CD10+ cells. The percentage of CD27− IgD− B cells was significantly higher in children from Kisumu, compared to Nandi, at 3 years of age (Figure 2I). The increase in CD27− IgD− B cells with more malaria exposure was due to an increase in atypical memory B cells (CD19+, CD10−, CD27−, IgD−, CD21−) that were significantly higher in Kisumu, compared to Nandi (Figure 2G). Further characterization of the atypical memory B cells revealed that the Kisumu cohort exhibited higher expression of FcRL4+ than Nandi (Figure 2H). The higher levels of atypical memory B cells in children from Kisumu coincide with lower levels of MZ-like memory B cells (CD19+, CD10−, CD27+, IgD+, IgM+) (Figure 2E).
AID mRNA expression is higher in children from Kisumu and AID expression correlates with a decrease in MZ-like memory
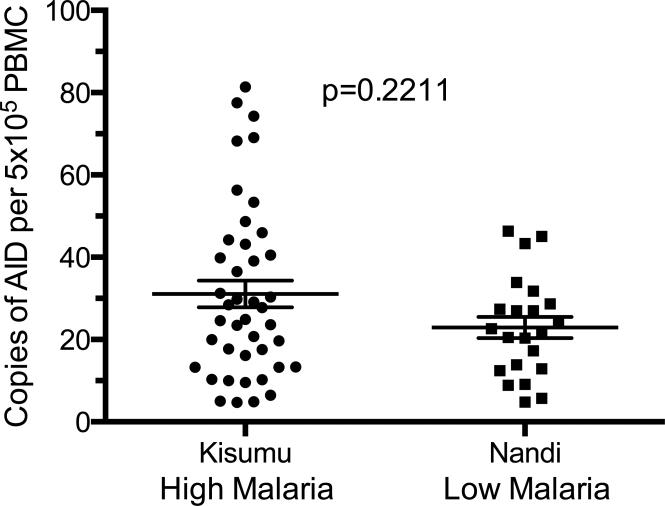
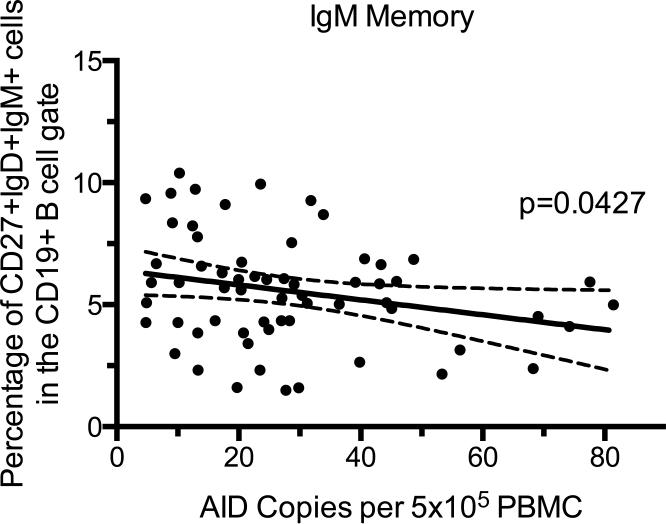
To determine the effects of malaria transmission on the expression of AID in PBMC, RNA was isolated from PBMC, and quantified by RT-qPCR on a per cell basis. All children in our study had detectable AID expression in their PBMC, albeit at low copy number per cell. Children living in Kisumu did not have a significantly (p=0.2211) higher number of AID copies per cell than children in Nandi (Figure 3). Because we had already observed differences in the percentages of B cell subsets between Kisumu and Nandi cohorts, we next wanted to know if any B cell subset correlated with levels of AID expression independent of frequency of malaria exposure by comparing the phenotype data to AID expression from both high and low malaria transmission sites combined. Higher levels of AID expression significantly correlated with a decrease in the MZ-like memory B cell population (Figure 4). There was no significant correlation between AID levels and percentages of other B cell subsets (data not shown).
Figure 3. AID mRNA expression is trending but not significantly increased in PBMC of children in a high malaria transmission region.
AID mRNA copies per 5×105 PBMC from children in high and low malaria transmission regions. Kisumu n=42, Nandi n=22. P-value determined by a Mann-Whitney U-test.
Figure 4. Increased AID expression correlates with a decrease in the MZ-like Memory B cell subset.
The copies of AID per 5×105 PBMC (x-axis) compared to the percentage of IgM Memory B cells (y-axis) for each child from both the high and low malaria transmission regions. P values were determined by correlation analysis. The solid line is a linear regression line and dotted lines represent the 95% confidence interval. n=64.
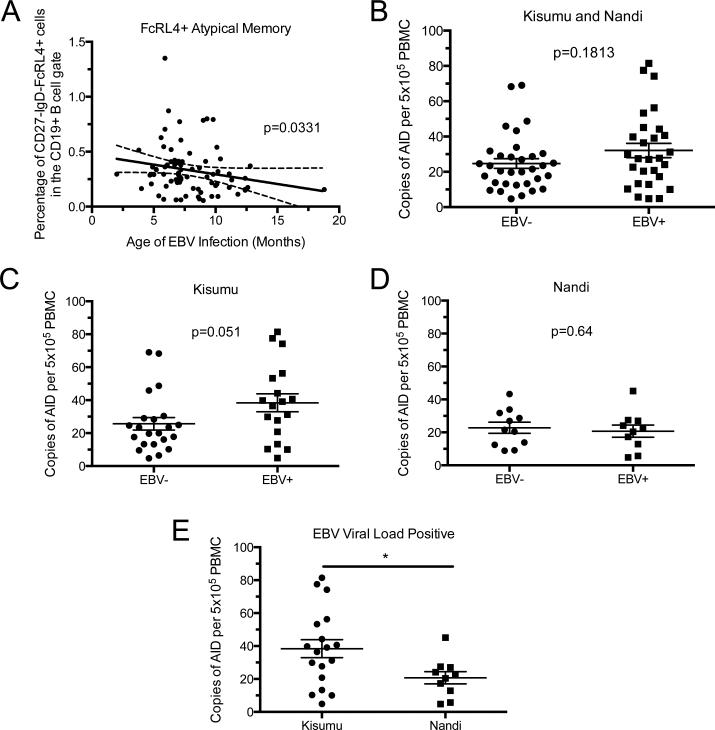
Children that had a detectible EBV viral load had higher AID expression in Kisumu, but not Nandi
As EBV latent proteins have been shown to both activate and repress AID expression in B cell culture systems29,30, we next determined if EBV was associated with AID expression. DNA extracted from whole blood was analyzed by qPCR for EBV viral load. Although all children in this cohort are EBV seropositive26, no difference in the percentage of children with detectable EBV load was observed (52.4% in Kisumu versus 50% in Nandi). The mean viral load for children from Kisumu was 7.66 copies/μg and from Nandi, 9.23 copies/μg and not significantly different between the two sites.
We grouped the study participants based on whether they had measurable EBV viral load at the time of blood draw (3 years old) or no detectible EBV. To determine if the presence of detectable levels of EBV correlated with a change in the levels of AID expression, we compared EBV viral load-positive and-negative children within Kisumu or Nandi. There was an increase in AID expression in the EBV+ children from Kisumu compared to the EBV− children in the same site (Figure 5 A). Children from Nandi had similar levels of AID expression, regardless of whether they were EBV viral load positive or negative (Figure 5 B). When comparing only the EBV+ children between Kisumu and Nandi there was a significantly higher level of AID expression in the blood of the children exposed to higher levels of malaria transmission in Kisumu (Figure 5 C). These data suggest that there is relationship between malaria transmission and EBV infection that leads to increased AID expression.
Figure 5. AID expression is increased in children with PCR-detectable EBV viral load in the high malaria transmission, but not the low malaria transmission regions.
A) AID mRNA expression levels from EBV+ and EBV− children from Kisumu (EBV− n=22, EBV+ n=18) or B) Nandi (EBV− n=11, EBV+ n=10). C) Comparison of EBV+ children from Kisumu versus EBV+ children from Nandi. P-value determined by Mann-Whitney U-test.
Discussion
A key step to the development of eBL is the translocation of c-myc oncogene to the control of the immunoglobulin promoter. This event is thought to require the activity of AID31,32, which is tightly regulated under normal circumstances33. It has been suggested that chronic activation of AID expression by P. falciparum or HIV could be the link to the increased risk for B cell malignancies in the infected populations1. However, there have been no studies to date demonstrating an increase in AID expression correlated with malaria infection. Studies of HIV associated non-Hodgkin's lymphoma show that AID expression is detectable in peripheral blood before diagnosis with lymphoma, but the B cell subset that expresses AID is unknown10. Several groups have demonstrated the effects of Plasmodium infection on peripheral blood B cells22,27,34, with a particular emphasis on memory B cells, due to the lack of long-lived immunity to malaria. Memory B cell subsets are also important in the context of BL development, because phenotyping of malignant cells has shown a significant amount of SHM, suggesting BL is derived from a post-germinal center B cell35. Our study is unique because we enrolled children from geographically proximate regions in western Kenya that were at a high or low risk for eBL due to differences in malaria transmission between the sites. In our high-risk cohort from the malaria endemic region of Kisumu, we observed that the children with a detectible EBV viral load in Kisumu had significantly higher expression of AID than children with detectible EBV in Nandi. Memory B cell populations in our cohorts were significantly altered by high frequency malaria transmission. Children from Kisumu had lower levels of immature B cells and MZ-like memory, but higher percentages of atypical memory B cells. High percentages of atypical memory, and low levels of MZ-like memory B cell subsets, correlated with increased AID expression when analyzing the combined data from both sites. Together these data suggest that AID expression is associated with a high frequency of malaria transmission, and B cell subset changes that are consistent with chronic stimulation.
AID expression in B cells is required for normal humoral immune functions, such as SHM and CSR, but those processes are normally restricted to germinal centers in secondary lymphoid organs9. The detection of AID in B cells of children that do not have non-Hodgkin's lymphoma is important because it suggests that the role of malaria in the etiology of BL could be due to the ability of Plasmodium to induce AID expression and result in c-myc translocation. Another hypothesis for the etiology of BL has been that malaria leads to the loss of T cell control over EBV-infected cells28. Our data does not rule out this hypothesis, but suggest an additional role for malaria within that model. In addition, we measured higher AID expression in children from Kisumu with a positive EBV viral load compared to the children with no or undetectable virus in their peripheral blood, but EBV viral load-positive children in Nandi had similar levels of AID compared to EBV negative children. These date suggest that EBV alone does not contribute to AID expression and that increased malaria exposure results in higher AID expression only in the presence of EBV. Malaria may be playing a role in upregulating AID activity resulting in increased EBV viral load due to the preference of EBV to infect B cells with a mutated immunoglobulin36. Therefore, there is a possible synergy occurring between malaria and EBV that could lead to increased AID expression.
Multi-parameter B cell phenotyping of children from regions of endemic and sporadic malaria transmission has not been performed with the breadth of our 11-color panel. Previous studies performed by our group on the Kisumu and Nandi cohorts were unable to definitively distinguish memory B cell subsets27. In addition, previously published studies from malaria endemic regions have primarily been performed on samples from adults. The cohort of children in our study is unique because we have followed them clinically from 1 month to 36 months old. Trends in B cell subset percentages are seen throughout the sampling period for these children and they remain steady through our analysis at 3 years of age. We previously demonstrated that there is a decrease in non-class switched (CD27+IgD+) and an increase in CD27−IgD− memory B cell subsets in the Kisumu cohort27. Our current findings confirm these trends, but we were able to better define these populations as MZ-like memory B cells (CD27+IgD+IgM+) and atypical memory B cells (CD27−IgD−CD21−).
With the goal of understanding the lack of long-lived immunity, several groups have reported the effects of malaria on B cell subsets22,34. There are several changes in memory B cell populations, including an increase in atypical memory B cells in people living in malaria endemic regions22. Atypical memory B cells have been described as ‘exhausted’ memory B cells because they are functionally inactive21. There are some groups that refer to these as anergic B cells, and it has been suggested that they could function as B regulatory cells rather than memory B cells37. In the present study, we show an increase in atypical memory B cells in children, consistent with what is seen in adults. However, the expansion of atypical memory B cells in the children from Kisumu is not as great as that demonstrated by other groups, and the percentage FcRL4+ atypical memory B cells of total B cells is less than 1%22. There is some inconsistency in gating strategy for memory B cell subsets among other groups and we attempted to define the memory subsets taking into account the various methods used before38. We defined atypical B cells by their cell surface phenotype that was CD19+, CD10−, CD24−, CD27−, IgD−, and CD21−. The addition of gating on IgD-negative B cells could account for the lower levels of atypical memory B cells in our study. In order for a B cell to be ‘memory’ it must have experienced antigen, and therefore should be IgD-negative39. The chronic stimulation of B cells through the BCR can lead to the anergic phenotype of the cells in the atypical B cell pool, and therefore the increase in atypical B cells could be a result of repeated activation and have no functional role in malaria immunity.
IgM memory B cells have been described as marginal zone-like B cells, but recent evidence suggests that they are more similar to class-switched memory B cells than innate-like B cells40. MZ-like memory B cells are a lower percentage of total B cells in children from Kisumu compared to Nandi as shown in previous studies by our group at 12, 18, and 24 months of age27. In the present study, we see that this trend continues through 36 months of age. Additionally, there was a significant correlation of higher AID expression with a decrease in MZ-like memory B cells. The negative correlation of AID and MZ-like memory suggests that MZ-like memory B cells are not contributing to the expression of AID in PBMC. MZ-like memory B cells may be reduced in malaria endemic regions due to chronic BCR stimulation resulting in a down-regulation of IgM on B cells. The decrease in AID expression associated with higher levels of MZ-like memory B cells could be due to the fact that higher AID levels would lead to increased class switching and therefore lower IgM expression. Further studies are necessary to identify the cell type(s) responsible for increased AID expression.
The frequency of BL is estimated to be around 5 cases per 100,000 children living in equatorial Africa20,24,41. Therefore, despite our detection of increased AID expression in children from a region with a high malaria frequency the likelihood of a child in our cohort developing BL is small, because our study started with less than 300 participants. Previous studies by Epeldegui et al have shown that AID is expressed in the peripheral blood of people infected with HIV that went on to develop non-Hodgkin's lymphoma10. The patient population of the Epeldegui et al study has several key differences compared to the current study of cohorts from Kisumu and Nandi. The use of the Multicenter AIDS Cohort Study patient population, which consisted of 6,972 patients followed longitudinally from the mid 1980's to present, had advantages such as the length of patient exposure to HIV (5 years of more), the age of sampling (adulthood), and most importantly, the ability to select a group that had already developed lymphoma10. We hypothesize that if we were able to look at the expression of AID before the development of BL we would detect similarly significant increases in AID expression, as shown in the Epeldegui et al study. Unfortunately, the low frequency of BL and the limited resources available, did not allow for such a study in children in western Kenya.
The results of this study demonstrate that elevated AID expression and shifts towards higher atypical memory B cell subset frequency could be a consequence of a high frequency of malaria exposure in 3 year-old children from a region associated with a high risk for BL. High levels of AID were found to be primarily in children with detectable levels of EBV in the Kisumu District, suggesting that there was a synergistic effect of malaria and EBV. These data further provide evidence of altered memory B cell subsets in children with high malaria exposure leading to increased levels of atypical memory B cells and decreased MZ-like memory. Additionally, there was a significant correlation with AID expression and a decrease in MZ-like memory regardless of the level of malaria exposure. Further research is necessary to determine how EBV and malaria act on these cells to increase AID expression and lead to malignancy.
Novelty and Impact Statement.
The present study is the first to demonstrate a link between AID expression and exposure to malaria in the peripheral blood of children. Additionally, we found that AID is linked to detectable EBV in children living in a region of high malaria transmission. These findings provide a better understanding of how malaria and EBV co-infection can increase the risk of developing Burkitt's lymphoma.
Acknowledgments
This work was funded by a grants awarded to RR by Alex's Lemonade Stand and R01CA102667 and grants awarded to IS by R37AI049660, R01AI084808, P01AI078907. We thank Julie Ritchie and Nancy Fiore for excellent technical assistance.
References
- 1.Molyneux EM, Rochford R, Griffin B, Newton R, Jackson G, Menon G, Harrison CJ, Israels T, Bailey S. Burkitt's lymphoma. Lancet. 2012;379:1234–44. doi: 10.1016/S0140-6736(11)61177-X. [DOI] [PubMed] [Google Scholar]
- 2.Burkitt DP. Etiology of Burkitt's lymphoma--an alternative hypothesis to a vectored virus. J Natl Cancer Inst. 1969;42:19–28. [PubMed] [Google Scholar]
- 3.Kennedy G, Komano J, Sugden B. Epstein-Barr virus provides a survival factor to Burkitt's lymphomas. Proc Natl Acad Sci U S A. 2003;100:14269–74. doi: 10.1073/pnas.2336099100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zur Hausen H, Schulte-Holthausen H, Klein G, Henle W, Henle G, Clifford P, Santesson L. EBV DNA in biopsies of Burkitt tumours and anaplastic carcinomas of the nasopharynx. Nature. 1970;228:1056–8. doi: 10.1038/2281056a0. [DOI] [PubMed] [Google Scholar]
- 5.Erikson J, ar-Rushdi A, Drwinga HL, Nowell PC, Croce CM. Transcriptional activation of the translocated c-myc oncogene in burkitt lymphoma. Proc Natl Acad Sci U S A. 1983;80:820–4. doi: 10.1073/pnas.80.3.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lombardi L, Newcomb EW, Dalla-Favera R. Pathogenesis of Burkitt lymphoma: expression of an activated c-myc oncogene causes the tumorigenic conversion of EBV-infected human B lymphoblasts. Cell. 1987;49:161–70. doi: 10.1016/0092-8674(87)90556-3. [DOI] [PubMed] [Google Scholar]
- 7.Gutiérrez MI, Bhatia K, Cherney B, Capello D, Gaidano G, Magrath I. Intraclonal molecular heterogeneity suggests a hierarchy of pathogenetic events in Burkitt's lymphoma. Ann Oncol Off J Eur Soc Med Oncol ESMO. 1997;8:987–94. doi: 10.1023/a:1008265304712. [DOI] [PubMed] [Google Scholar]
- 8.Greeve J, Philipsen A, Krause K, Klapper W, Heidorn K, Castle BE, Janda J, Marcu KB, Parwaresch R. Expression of activation-induced cytidine deaminase in human B-cell non-Hodgkin lymphomas. Blood. 2003;101:3574–80. doi: 10.1182/blood-2002-08-2424. [DOI] [PubMed] [Google Scholar]
- 9.Klein U, Dalla-Favera R. Germinal centres: role in B-cell physiology and malignancy. Nat Rev Immunol. 2008;8:22–33. doi: 10.1038/nri2217. [DOI] [PubMed] [Google Scholar]
- 10.Epeldegui M, Breen EC, Hung YP, Boscardin WJ, Detels R, Martínez-Maza O. Elevated expression of activation induced cytidine deaminase in peripheral blood mononuclear cells precedes AIDS-NHL diagnosis. AIDS Lond Engl. 2007;21:2265–70. doi: 10.1097/QAD.0b013e3282ef9f59. [DOI] [PubMed] [Google Scholar]
- 11.Rastelli J, Hömig-Hölzel C, Seagal J, Müller W, Hermann AC, Rajewsky K, Zimber-Strobl U. LMP1 signaling can replace CD40 signaling in B cells in vivo and has unique features of inducing class-switch recombination to IgG1. Blood. 2008;111:1448–55. doi: 10.1182/blood-2007-10-117655. [DOI] [PubMed] [Google Scholar]
- 12.Moormann AM, Chelimo K, Sumba OP, Lutzke ML, Ploutz-Snyder R, Newton D, Kazura J, Rochford R. Exposure to holoendemic malaria results in elevated Epstein- Barr virus loads in children. J Infect Dis. 2005;191:1233–8. doi: 10.1086/428910. [DOI] [PubMed] [Google Scholar]
- 13.Donati D, Zhang LP, Chêne A, Chen Q, Flick K, Nyström M, Wahlgren M, Bejarano MT. Identification of a polyclonal B-cell activator in Plasmodium falciparum. Infect Immun. 2004;72:5412–8. doi: 10.1128/IAI.72.9.5412-5418.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parroche P, Lauw FN, Goutagny N, Latz E, Monks BG, Visintin A, Halmen KA, Lamphier M, Olivier M, Bartholomeu DC, Gazzinelli RT, Golenbock DT. Malaria hemozoin is immunologically inert but radically enhances innate responses by presenting malaria DNA to Toll-like receptor 9. Proc Natl Acad Sci U S A. 2007;104:1919–24. doi: 10.1073/pnas.0608745104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He B, Qiao X, Cerutti A. CpG DNA induces IgG class switch DNA recombination by activating human B cells through an innate pathway that requires TLR9 and cooperates with IL-10. J Immunol Baltim Md 1950. 2004;173:4479–91. doi: 10.4049/jimmunol.173.7.4479. [DOI] [PubMed] [Google Scholar]
- 16.Gowda DC. TLR-mediated cell signaling by malaria GPIs. Trends Parasitol. 2007;23:596–604. doi: 10.1016/j.pt.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Kassa D, Petros B, Mesele T, Hailu E, Wolday D. Characterization of peripheral blood lymphocyte subsets in patients with acute Plasmodium falciparum and P. vivax malaria infections at Wonji Sugar Estate, Ethiopia. Clin Vaccine Immunol CVI. 2006;13:376–9. doi: 10.1128/CVI.13.3.376-379.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asito AS, Moormann AM, Kiprotich C, Ng'ang'a ZW, Ploutz-Snyder R, Rochford R. Alterations on peripheral B cell subsets following an acute uncomplicated clinical malaria infection in children. Malar J. 2008;7:238. doi: 10.1186/1475-2875-7-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roestenberg M, Teirlinck AC, McCall MBB, Teelen K, Makamdop KN, Wiersma J, Arens T, Beckers P, van Gemert G, van de Vegte-Bolmer M, van der Ven AJAM, Luty AJF, et al. Long-term protection against malaria after experimental sporozoite inoculation: an open-label follow-up study. Lancet. 2011;377:1770–6. doi: 10.1016/S0140-6736(11)60360-7. [DOI] [PubMed] [Google Scholar]
- 20.Rainey JJ, Omenah D, Sumba PO, Moormann AM, Rochford R, Wilson ML. Spatial clustering of endemic Burkitt's lymphoma in high-risk regions of Kenya. Int J Cancer J Int Cancer. 2007;120:121–7. doi: 10.1002/ijc.22179. [DOI] [PubMed] [Google Scholar]
- 21.Moir S, Ho J, Malaspina A, Wang W, DiPoto AC, O'Shea MA, Roby G, Kottilil S, Arthos J, Proschan MA, Chun T-W, Fauci AS. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med. 2008;205:1797–805. doi: 10.1084/jem.20072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiss GE, Crompton PD, Li S, Walsh LA, Moir S, Traore B, Kayentao K, Ongoiba A, Doumbo OK, Pierce SK. Atypical memory B cells are greatly expanded in individuals living in a malaria-endemic area. J Immunol Baltim Md 1950. 2009;183:2176–82. doi: 10.4049/jimmunol.0901297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thorley-Lawson DA, Allday MJ. The curious case of the tumour virus: 50 years of Burkitt's lymphoma. Nat Rev Microbiol. 2008;6:913–24. doi: 10.1038/nrmicro2015. [DOI] [PubMed] [Google Scholar]
- 24.Rainey JJ, Mwanda WO, Wairiumu P, Moormann AM, Wilson ML, Rochford R. Spatial distribution of Burkitt's lymphoma in Kenya and association with malaria risk. Trop Med Int Heal TM IH. 2007;12:936–43. doi: 10.1111/j.1365-3156.2007.01875.x. [DOI] [PubMed] [Google Scholar]
- 25.Moormann AM, Heller KN, Chelimo K, Embury P, Ploutz-Snyder R, Otieno JA, Oduor M, Münz C, Rochford R. Children with endemic Burkitt lymphoma are deficient in EBNA1-specific IFN-gamma T cell responses. Int J Cancer J Int Cancer. 2009;124:1721–6. doi: 10.1002/ijc.24014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piriou E, Asito AS, Sumba PO, Fiore N, Middeldorp JM, Moormann AM, Ploutz- Snyder R, Rochford R. Early age at time of primary Epstein-Barr virus infection results in poorly controlled viral infection in infants from Western Kenya: clues to the etiology of endemic Burkitt lymphoma. J Infect Dis. 2012;205:906–13. doi: 10.1093/infdis/jir872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asito AS, Piriou E, Jura WGZO, Ouma C, Odada PS, Ogola S, Fiore N, Rochford R. Suppression of circulating IgD+CD27+ memory B cells in infants living in a malaria-endemic region of Kenya. Malar J. 2011;10:362. doi: 10.1186/1475-2875-10-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moormann AM, Chelimo K, Sumba PO, Tisch DJ, Rochford R, Kazura JW. Exposure to holoendemic malaria results in suppression of Epstein-Barr virus-specific T cell immunosurveillance in Kenyan children. J Infect Dis. 2007;195:799–808. doi: 10.1086/511984. [DOI] [PubMed] [Google Scholar]
- 29.Tobollik S, Meyer L, Buettner M, Klemmer S, Kempkes B, Kremmer E, Niedobitek G, Jungnickel B. Epstein-Barr virus nuclear antigen 2 inhibits AID expression during EBV-driven B-cell growth. Blood. 2006;108:3859–64. doi: 10.1182/blood-2006-05-021303. [DOI] [PubMed] [Google Scholar]
- 30.Gourzi P, Leonova T, Papavasiliou FN. Viral induction of AID is independent of the interferon and the Toll-like receptor signaling pathways but requires NF-kappaB. J Exp Med. 2007;204:259–65. doi: 10.1084/jem.20061801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramiro AR, Jankovic M, Eisenreich T, Difilippantonio S, Chen-Kiang S, Muramatsu M, Honjo T, Nussenzweig A, Nussenzweig MC. AID is required for c myc/IgH chromosome translocations in vivo. Cell. 2004;118:431–8. doi: 10.1016/j.cell.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 32.Robbiani DF, Bothmer A, Callen E, Reina-San-Martin B, Dorsett Y, Difilippantonio S, Bolland DJ, Chen HT, Corcoran AE, Nussenzweig A, Nussenzweig MC. AID is required for the chromosomal breaks in c-myc that lead to c-myc/IgH translocations. Cell. 2008;135:1028–38. doi: 10.1016/j.cell.2008.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zaheen A, Boulianne B, Parsa J-Y, Ramachandran S, Gommerman JL, Martin A. AID constrains germinal center size by rendering B cells susceptible to apoptosis. Blood. 2009;114:547–54. doi: 10.1182/blood-2009-03-211763. [DOI] [PubMed] [Google Scholar]
- 34.Nogaro SI, Hafalla JC, Walther B, Remarque EJ, Tetteh KKA, Conway DJ, Riley EM, Walther M. The breadth, but not the magnitude, of circulating memory B cell responses to P. falciparum increases with age/exposure in an area of low transmission. PloS One. 2011;6:e25582. doi: 10.1371/journal.pone.0025582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bellan C, Lazzi S, Hummel M, Palummo N, de Santi M, Amato T, Nyagol J, Sabattini E, Lazure T, Pileri SA, Raphael M, Stein H, et al. Immunoglobulin gene analysis reveals 2 distinct cells of origin for EBV-positive and EBV-negative Burkitt lymphomas. Blood. 2005;106:1031–6. doi: 10.1182/blood-2005-01-0168. [DOI] [PubMed] [Google Scholar]
- 36.Heath E, Begue-Pastor N, Chaganti S, Croom-Carter D, Shannon-Lowe C, Kube D, Feederle R, Delecluse H-J, Rickinson AB, Bell AI. Epstein-Barr virus infection of naïve B cells in vitro frequently selects clones with mutated immunoglobulin genotypes: implications for virus biology. PLoS Pathog. 2012;8:e1002697. doi: 10.1371/journal.ppat.1002697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andrews SF, Wilson PC. The anergic B cell. Blood. 2010;115:4976–8. doi: 10.1182/blood-2010-03-276352. [DOI] [PubMed] [Google Scholar]
- 38.Wei C, Jung J, Sanz I. OMIP-003: phenotypic analysis of human memory B cells. Cytom Part J Int Soc Anal Cytol. 2011;79:894–6. doi: 10.1002/cyto.a.21112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geisberger R, Lamers M, Achatz G. The riddle of the dual expression of IgM and IgD. Immunology. 2006;118:429–37. doi: 10.1111/j.1365-2567.2006.02386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seifert M, Küppers R. Molecular footprints of a germinal center derivation of human IgM+(IgD+)CD27+ B cells and the dynamics of memory B cell generation. J Exp Med. 2009;206:2659–69. doi: 10.1084/jem.20091087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Magrath I. Epidemiology: clues to the pathogenesis of Burkitt lymphoma. Br J Haematol. 2012;156:744–56. doi: 10.1111/j.1365-2141.2011.09013.x. [DOI] [PubMed] [Google Scholar]