Abstract
Purpose.
To determine the etiology and prognosis of visual acuity loss in idiopathic intracranial hypertension (IIH) at presentation and to provide objective measures to predict visual outcome.
Methods.
A retrospective review of 660 patients with IIH (2009–2013) identified 31 patients (4.7%) with 48 eyes having best-corrected visual acuity (BCVA) of 20/25 or worse on initial presentation. Fundus photography, optical coherence tomography (OCT) of the optic disc and macula, and perimetry were used to determine the causes and prognosis of vision loss. Segmentation of the macula OCT was performed using the Iowa Reference Algorithm to determine the retinal ganglion cell-inner plexiform layer complex (GCL-IPL) thickness.
Results.
Outer retinal changes alone caused decreased BCVA at initial presentation in 22 eyes (46%): subretinal fluid in 16, chorioretinal folds in 5, and peripapillary choroidal neovascularization in 1. The vision loss was reversible except for some eyes with chorioretinal folds. Optic neuropathy alone caused decreased BCVA in 10 eyes (21%) and coexisting outer retinal changes and optic neuropathy caused decreased BCVA in 16 eyes (33%). A GCL-IPL thickness less than or equal to 70 μm at initial presentation or progressive thinning of greater than or equal to 10 μm within 2 to 3 weeks compared with baseline correlated with poor visual outcome.
Conclusions.
Visual acuity loss in IIH can be caused by both outer retinal changes and optic neuropathy. Vision loss from outer retinal changes is mostly reversible. The outcome of patients with coexisting outer retinal changes and optic neuropathy or optic neuropathy alone depends on the degree of optic neuropathy, which can be predicted by the GCL-IPL thickness.
Keywords: idiopathic intracranial hypertension, subretinal fluid, optic neuropathy, optical coherence tomography, ganglion cell layer
Decreased visual acuity from idiopathic intracranial hypertension can be caused by reversible retinal changes or fulminant disease with optic neuropathy. A poor visual outcome can be predicted with a ganglion cell-inner plexiform layer complex thickness of less than or equal to 70 μm at presentation or a greater than or equal to 10 μm drop at 2 to 3 weeks.
Idiopathic intracranial hypertension (IIH) is a condition of increased intracranial pressure of unknown cause producing papilledema and visual loss.1–3 The major morbidity of IIH is visual impairment, which can be progressive and insidious.4,5 The visual loss is often reversible if treatment is initiated in a timely fashion, but can be permanent in up to 40% of patients.5,6 The most common visual field defects are enlargement of the physiologic blind spot, nasal steps, and arcuate defects.7,8 If the disease remains untreated, the patient can develop severe irreversible visual field constriction.5
Visual field loss with decrease in visual acuity occurs with advancing optic nerve damage and can progress to blindness. However, some patients present to medical attention with decreased visual acuity. These patients pose a difficult dilemma to the physician, because some may have fulminant IIH, where the vision loss is due to severe papilledema and optic neuropathy,9–11 while others have vision loss from outer retinal changes in the macula.12,13
Past studies have reported that outer retinal changes in the macula associated with IIH include chorioretinal folds,14,15 hyperopic shift,16–18 hemorrhages,19,20 macular edema,19,21–23 subretinal fluid,24 or, rarely, subretinal neovascularization.19,22,25,26 Outer retinal changes in the macula in IIH have been reported to occur with an incidence ranging from 3.2% to 44%, largely dependent on the severity of IIH among the patients included and the methods used to assess for macular pathology.5,12,13,27 Subretinal fluid and macular exudates are common causes of visual acuity loss in IIH, and are often reversible with treatment.12,13,22–24,28
Therefore, distinguishing outer retinal changes from optic neuropathy as the cause of decreased visual acuity is important, because outer retinal changes in the macula affecting visual acuity may be largely reversible,24,28–31 whereas fulminant IIH with optic neuropathy and neuronal loss may require more aggressive management, such as immediate surgical intervention, to prevent further vision loss and irreversible blindness. It is difficult to detect optic neuropathy in IIH, because papilledema obscures the nerve making pallor difficult to evaluate and also prevents accurate assessment of axonal loss by retinal nerve fiber layer (RNFL) thickness measurements determined by optical coherence tomography (OCT) due to the significant swollen axons of the optic nerve.32 In addition, thinning of the RNFL during the disease course may be due to improvement of papilledema or due to axon loss from worsening of the disease.33–35 Evaluating the integrity of the optic nerve in the setting of disc edema could be theoretically accomplished by analyzing the inner layers of the macula, specifically the ganglion cell layer-inner plexiform layer (GCL-IPL).36 Thinning of the macular GCL-IPL layer has been found to have a strong relationship with visual loss in optic nerve diseases such as glaucoma, optic neuritis, ischemic optic neuropathy, hereditary optic neuropathy, toxic optic neuropathy, and optic nerve glioma,37–44 and therefore can theoretically provide accurate measurement of neuronal loss in the presence of papilledema and allow early identification of patients with significant optic neuropathy caused by IIH.
The purpose of this study was to evaluate the prevalence, causes, and prognosis of visual acuity loss in IIH at presentation. In addition, we examined correlations between visual outcomes and GCL-IPL in order to predict reversibility in hopes of being able to identify patients that require urgent aggressive management.
Materials and Methods
Subjects
The study was approved by the University of Iowa (Iowa City, IA, USA) institutional review board and adhered to the tenets of the Declaration of Helsinki. A retrospective review of 660 patients with IIH (2009–2013) identified 31 patients (4.7%) with 48 eyes that had best-corrected or pinhole visual acuity of 20/25 or worse on presentation to our clinic. The average age of patients was 25 years (SD ± 5.5; range, 13–39), with 4 males and 27 females. All patients met the modified Dandy criteria for idiopathic intracranial hypertension.45 Patients were excluded if there was coexisting ophthalmic disease unrelated to IIH or another cause for decreased vision besides IIH. Patients were also excluded if there was a nonorganic component to their vision loss or if their visual field testing was unreliable.
All of the patients were treated medically with acetazolamide and/or topiramate ± surgical intervention with optic nerve sheath fenestration and/or ventriculoperitoneal shunting.
All patients underwent a full neuro-ophthalmologic examination at each visit, which included visual acuity, pinhole visual acuity, and automated Humphrey perimetry (24-2 SITA-standard) and/or kinetic Goldmann perimetry. Goldmann perimetry was typically obtained in patients with visual acuity equal to or worse than 20/40 or in patients who had unreliable automated Humphrey perimetry. For kinetic Goldmann visual field testing, responses to the V4e, I4e, I2e, and I1e stimuli were routinely assessed. Fundus photographs and OCT imaging were performed at the majority of visits. Average follow-up was 4.97 months (SD ± 2.14; range, 2–12 months).
Optical Coherence Tomography
Optical coherence tomography imaging was performed after pupillary dilation using SD-OCT (Cirrus 4000, Cirrus software version 6.0; Carl Zeiss Meditec, Dublin, CA, USA and/or Spectralis HRA+OCT, Spectralis software version 5.3; Heidelberg Engineering, Heidelberg, Germany). Images with signal strength less than 7 were excluded.
For Cirrus OCT imaging, the peripapillary RNFL thickness measurements were obtained using the optic disc cube 200 × 200 Cirrus protocol that covered the 6 × 6 mm2 area centered on the optic disc. The macula measurements were obtained using the macular cube 200 × 200 Cirrus protocol centered on the fovea.
For Spectralis OCT imaging, the scan protocol used captured the central 20° × 15° with a minimum of 19 sections and an automatic real-time averaging of nine frames centered on the optic disc for optic nerve analysis and centered on the fovea for macula analysis.
Macular Segmentation and Measurement
Commercially available segmentation algorithms to assess the GCL-IPL complex are prone to failure when there is significant optic disc edema (Fig. 1). This problem can be overcome by using the Iowa Reference Algorithm (in the public domain, http://www.biomed-imaging.uiowa.edu/downloads), a fully three-dimensional (3D), automated algorithm,46–49 which can accurately measure the macular GCL-IPL complex in the presence of optic disc edema. The incorporation of 3D information allows the Iowa reference algorithm to decrease segmentation error (Fig. 1).46–48 The boundaries of the macular GCL-IPL were defined by the junction between the retinal nerve fiber and ganglion cell layers and the junction between the inner plexiform and inner nuclear layers. The automated segmented layers were inspected for errors and manually corrected if present except for three of the eyes with chorioretinal folds, which were deemed too difficult to correct accurately.
Figure 1.
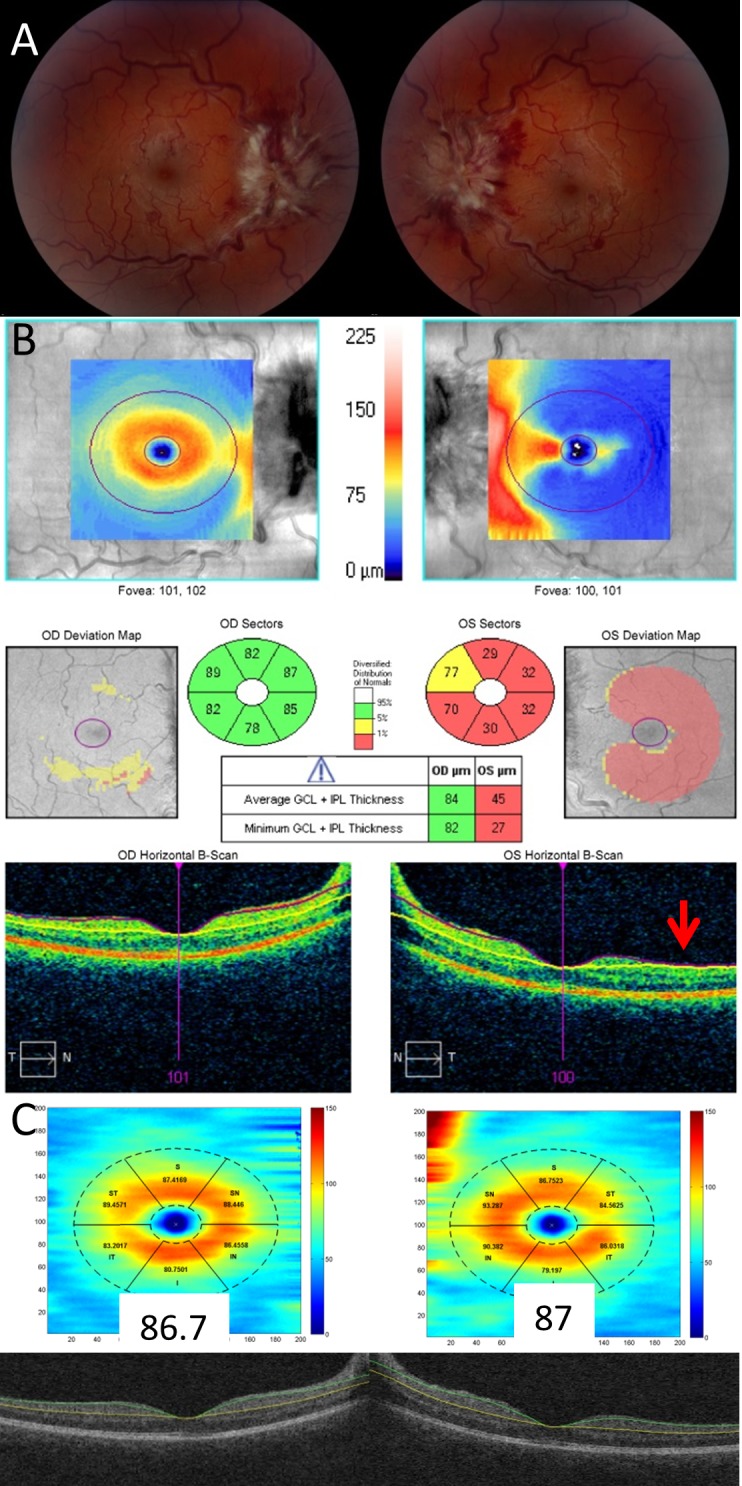
Comparison of the Cirrus and Iowa segmentation algorithms of the GCL-IPL complex in a patient with significant optic disc edema. Fundus photography demonstrates grade IV optic disc edema in both eyes (A). Cirrus segmentation of the GCL-IPL complex correctly segments the right eye, but fails to correctly segment the left eye where the segmentation lines collapse upon one another (red arrow) giving rise to artifactual GCL-IPL thinning (B). The Iowa Reference Algorithm correctly segments the GCL-IPL complex in both eyes (C).
Defining Etiology of Vision Loss
The etiology of vision loss was divided into three main categories: outer retinal changes in the macula alone, combination of outer retinal changes and optic neuropathy, and optic neuropathy alone. The category of outer retinal changes was further divided into three subcategories: subretinal fluid, chorioretinal folds, and choroidal neovascularization. Subretinal fluid was identified on OCT as accumulation of subfoveal fluid. In addition, patients with disruption of the photoreceptor layer on OCT were included in the subcategory of subretinal fluid. Photoreceptor disruption was used as an indicator of recent subretinal fluid because reconstitution of the photoreceptor layer typically takes 1 to 4 weeks after resolution of subretinal fluid in IIH (manuscript in preparation), as has been observed after resolution of subretinal fluid in central serous retinopathy.50 Fundus photographs were also used to identify exudates within the macula that indicated current or prior subretinal fluid. Chorioretinal folds were identified on OCT and fundus photography. A single patient had choroidal neovascularization affecting visual acuity, which was evident on OCT and fundus photography.
Optic neuropathy was identified retrospectively by either the development of optic disc pallor on fundus photography or an average GCL-IPL thickness of less than 70 μm. For comparison, the 95% confidence interval for average GCL-IPL thickness in a healthy young adult is 75 to 95 μm (Zeiss).
Although many patients likely had hyperopic shift from posterior globe flattening, these patients were not captured because only patients with best corrected or pinhole visual acuity of 20/25 or worse were included. Patients with hyperopic shift affecting visual acuity achieve a visual acuity of better than 20/25 with refraction or pinhole.
Defining Visual Outcomes
The visual outcomes were analyzed separately for final best corrected or pinhole visual acuity and visual field loss on the final visual field. The visual acuity was expressed as the logMar for analysis. Goldmann perimetry was quantified in an objective manner by measuring and summing the area of each isopter using ImageJ software (http://imagej.nih.gov/ij/; provided in the public domain by the National Institutes of Health, Bethesda, MD, USA) to obtain the visual field volume.51 Only responses to the I4e, I2e, and I1e stimuli were routinely measured in all patients and, consequently, only these were included to quantify the Goldmann visual field volume. The isopters were manually traced using ImageJ. Each isopter was given a relative weight to account for the relative luminance of the energy stimulus (I1e = 1000, I2e = 100, I4e = 10). The volume of the visual field was calculated by multiplying the relative weights with the area of each isopter and summing the values. The Goldmann perimetry results were rank ordered based on the measured visual field volumes. The automated Humphrey perimetry results were ranked based upon mean deviation. Rank ordering of automated and kinetic visual fields as one dataset was accomplished by learning how to relate the two types of perimetry from cases where both were performed on similar dates.
To provide an estimate of vision loss within the ranked fields and confirm the validity of this method of ranking of the fields, the final visual fields were also assigned a severity of visual field loss as described by Wall and George.7 According to the Wall and George classification, grade 5 is blinding visual field loss, grade 4 is marked visual field loss, grade 3 is moderate visual field loss, grade 2 is mild visual field loss, grade 1 is minimal visual field loss, and grade 0 is a normal visual field. The criteria for each of these categories for both manual and automated visual fields can be seen in the manuscript, Wall and George, 1991.7
The final logMar visual acuity and the rank order of final visual field outcomes were compared against the initial visual acuity, initial visual field, initial GCL-IPL thickness, GCL-IPL thickness at 2 to 3 weeks, final GCL-IPL thickness, change of GCL-IPL thickness at 2 to 3 weeks, and the total change of GCL-IPL thickness. Visual acuity outcomes were analyzed with linear regression. The Spearman correlation coefficient was calculated for comparisons against the rank order of the visual fields.
Results
Causes and Prognosis of Vision Loss From IIH at Presentation
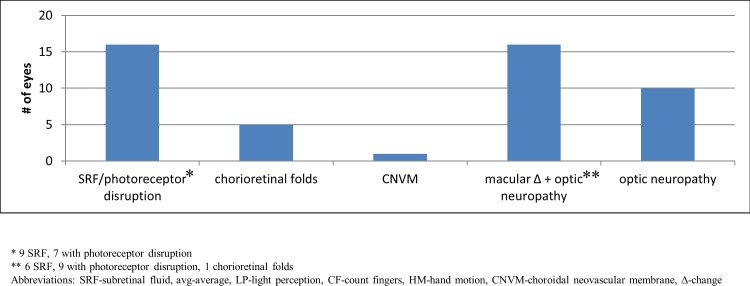
Outer retinal changes alone caused decreased visual acuity at presentation in 22 eyes (46%): subretinal fluid in 16, chorioretinal folds in 5, and peripapillary choroidal neovascularization in 1. Optic neuropathy alone caused decreased visual acuity at presentation in 10 eyes (21%), and coexisting outer retinal changes and optic neuropathy caused decreased visual acuity in 16 eyes (33%; Fig. 2). The majority of eyes presented with modified Frisén grade IV optic disc edema or worse, except for eyes presenting with chorioretinal folds, significant coexisting optic disc pallor, and choroidal neovascularization.52
Figure 2.
Causes of central vision loss from IIH at presentation. Decreased visual acuity at presentation was caused by subretinal fluid/photoreceptor disruption, chorioretinal folds, choroidal neovascularization, combined macular changes and optic neuropathy, and optic neuropathy alone.
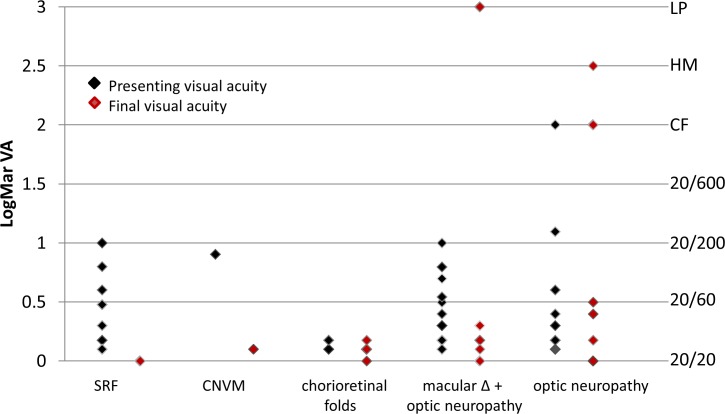
The outer retinal changes in the macula that were responsible for visual acuity loss were largely reversible except for chorioretinal folds. Among the nine patients (16 eyes) with subretinal fluid or evidence of prior subretinal fluid (photoreceptor disruption), all achieved best corrected or pinhole visual acuity of 20/20 with medical treatment alone. One patient with subretinal fluid underwent optic nerve sheath fenestration in one eye early in their course due to intolerance to acetazolamide treatment and recovered to 20/20 vision (Figs. 3, 4). A single patient developed peripapillary choroidal neovascularization with subfoveal fluid and presented with a visual acuity of 20/125, which improved to 20/25 after receiving intravitreal bevacizumab. Among the three patients (five eyes) with chorioretinal folds, the visual acuities did not significantly improve despite treatment of IIH in four of five eyes (Fig. 3).
Figure 3.
Presenting and final visual acuities for each category of vision loss. The presenting visual acuities are shown as black diamonds and the final visual acuities are shown as red diamonds.
Figure 4.
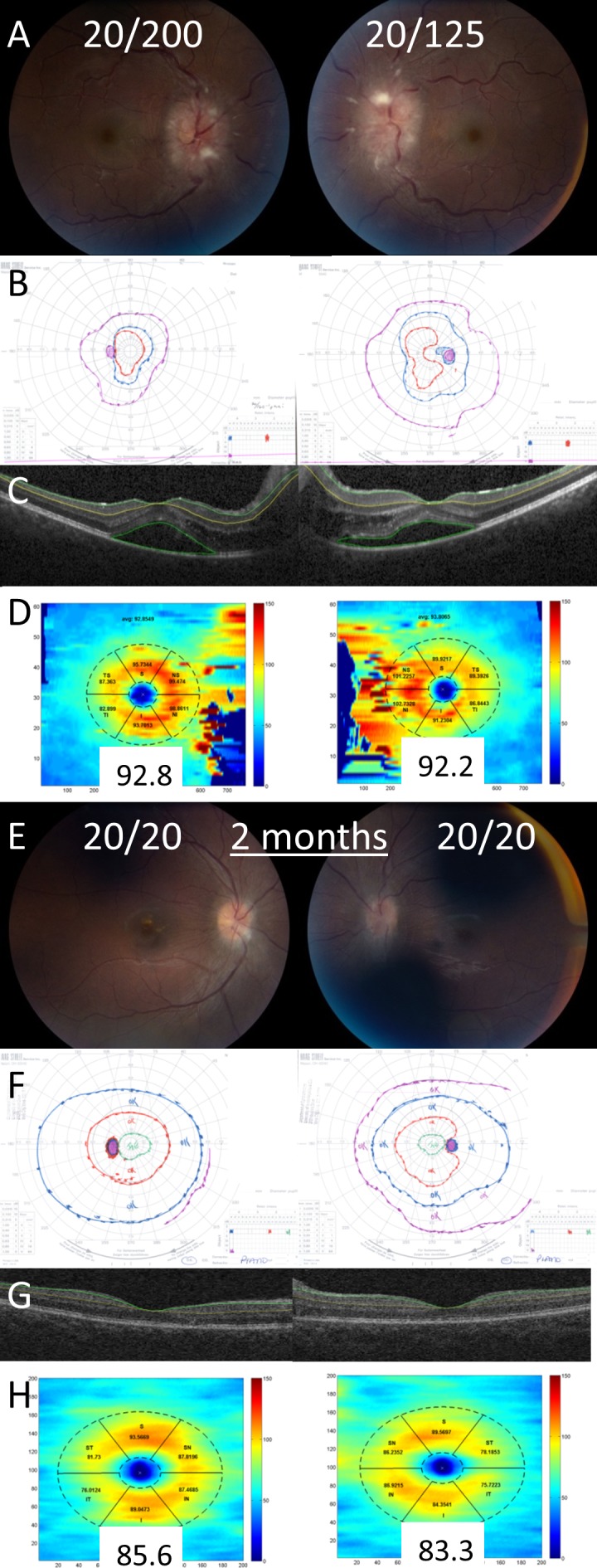
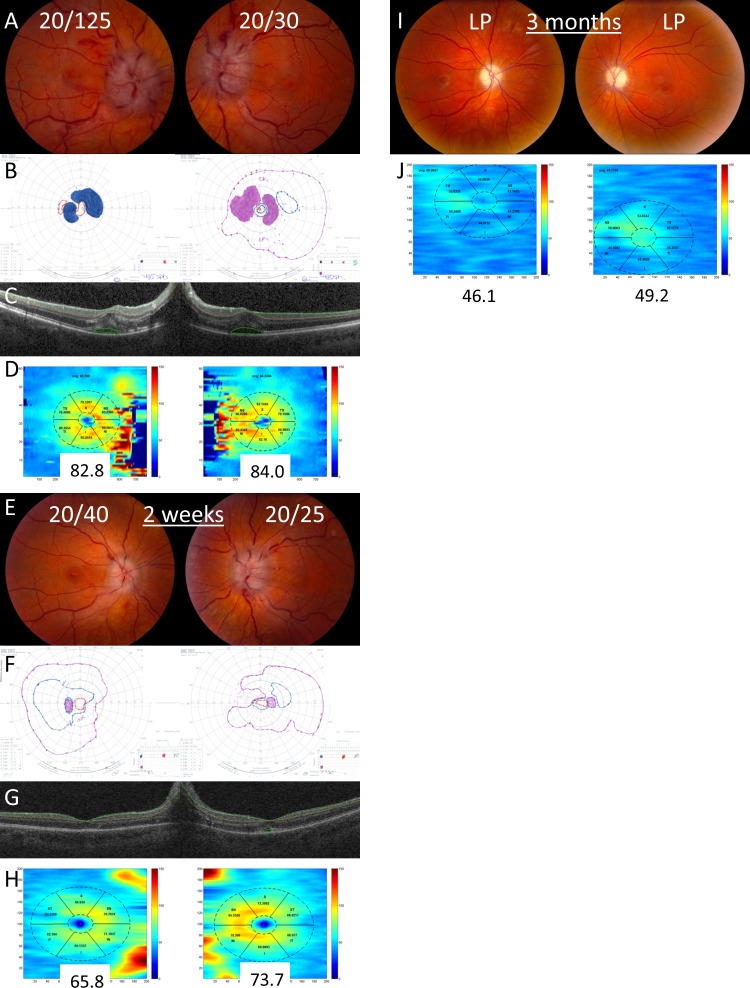
Example of a patient with decreased visual acuity from subretinal fluid. On presentation, the patient had visual acuities of 20/200 in the right eye and 20/125 in the left eye, grade IV optic disc edema in both eyes (A), and constricted Goldmann visual fields (B). Optical coherence tomography of the macula demonstrates subretinal fluid in both eyes (C). The GCL-IPL complex was segmented with the Iowa Reference Algorithm (C) and the average thickness was normal in both eyes (D). After 2 months, visual acuity improved to 20/20 in both eyes, the optic disc edema was significantly improved (E), and the Goldmann visual fields were normal (F). The GCL-IPL was segmented using the Iowa Reference Algorithm (G) and the average thickness remained normal in both eyes (H).
The final visual acuity of patients presenting with optic neuropathy alone or a combination of outer retinal changes in the macula and optic neuropathy was variable, ranging from 20/25 to light perception, with most of the eyes recovering to 20/60 or better (Figs. 3, 5).
Figure 5.
Example of a patient with decreased visual acuity from a combination of subretinal fluid and optic neuropathy with a poor outcome. On initial presentation, the patient had visual acuities of 20/125 in the right eye and 20/30 in the left eye, grade IV optic disc edema in both eyes (A), and constricted, scotomatous Goldmann visual fields (B). Optical coherence tomography of the macula at presentation demonstrates subretinal fluid in both eyes (C). The GCL-IPL complex was segmented with the Iowa Reference Algorithm (C) and the average thickness was normal in both eyes (D). After 2 weeks, visual acuity improved to 20/40 in the right eye and 20/25 in the left eye (E), but the Goldmann visual fields continued to show significant constriction (F). The GCL-IPL was segmented with the Iowa Reference Algorithm (G) and the average thickness was significantly decreased by 16.9 μm in the right and 10.6 μm in the left eye (H). After 3 months, visual acuity had worsened to light perception in both eyes and the optic discs developed severe pallor after resolution of the optic disc edema (I). The GCL-IPL was very thin, showing a 36.7 μm reduction in the right eye and 34.8 μm reduction in the left eye when compared with the initial GCL-IPL thickness (J).
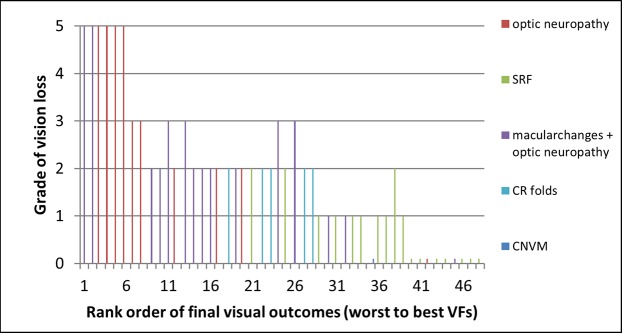
The visual field outcomes of the 48 eyes were put in rank order based on the degree of visual loss on the final visual fields and compared against the Wall and George classification system (Fig. 6).7 This was done because some of the eyes had kinetic Goldmann perimetry, whereas others had static perimetry. The rank order was a means of combining the visual field information, regardless of the perimetry type used. There was a strong correlation between the ranked visual fields and the degree of visual loss (Fig. 6).7
Figure 6.
Ranked visual field outcomes compared with the grade of vision loss according to the Wall and George classification. The visual field outcomes of each of the eyes were put in rank order based on the degree of visual loss on the final visual field and compared against the grade of vision loss. The cause of vision loss was color coded so that the separate categories of vision loss could be compared against the ranked visual field outcomes and the grade of vision loss.
Nine of 10 eyes with optic neuropathy were in the bottom half of the ranked visual field outcomes and, therefore, had significant visual field loss with an average of grade 3.2 visual field loss, according to the Wall and George classification (SD ± 1.75, range, 0–5; Fig. 6). Eleven of 15 eyes with combined macular changes and optic neuropathy were in the bottom half of the ranked visual field outcomes, and overall had grade 2.4 vision loss on average (SD ± 1.4, range, 0–5). Patients with subretinal fluid alone achieved nearly normal visual fields and were in the upper half of the ranked visual field outcomes with grade 0.76 vision loss on average (SD ± 0.75, range, 0–2; Fig. 6). Among the 24 eyes in the bottom half of the ranked visual field outcomes, 15 received both medical and surgical intervention (62.5%). Two of the 24 eyes with better visual field outcomes received both medical and surgical intervention (8.3%) and the 22 other eyes with better visual field outcome received medical treatment alone.
GCL-IPL Thickness in Predicting Visual Outcome
There was a weak correlation between the final visual acuity and the macular GCL-IPL thickness at presentation (r2 = 0.392, P < 0.001), at 2 to 3 weeks (r2 = 0.313, P < 0.001), and at the final visit (r2 = 0.345, P < 0.001). The correlation was skewed, because there were only 15 eyes that did not improve to a visual acuity of 20/20 with treatment.
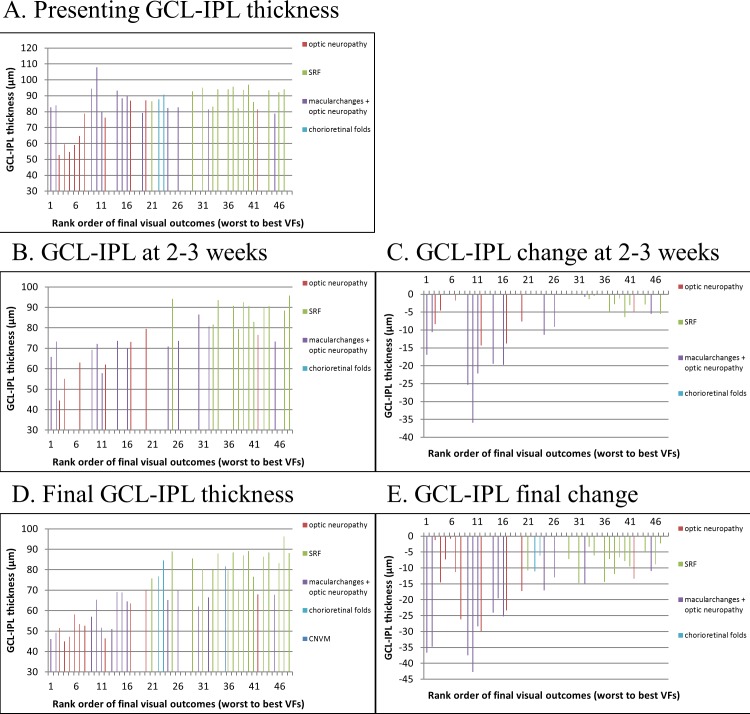
There was a significant correlation between the macular GCL-IPL thickness and the rank order of visual field outcomes (Fig. 7; Table). At presentation, five eyes had a GCL-IPL thickness of less than 70 μm and all of these eyes had poor visual field outcomes, which led to a weak correlation between initial GCL-IPL thickness and final visual field outcome (Spearman Correlation = 0.439, P = 0.005360; Fig. 7A; Table). The rest of the patients had a normal GCL-IPL thickness at presentation, which did not significantly correlate with final visual field outcome. At 2 to 3 weeks, there was a strong correlation between the GCL-IPL thickness and final visual field outcome (Spearman Correlation = 0.76, P < 0.0001; Fig. 7B; Table) and a decrease in the GCL-IPL thickness of more than 10 μm during this period was associated with a poor visual field outcome among eyes presenting with a normal GCL-IPL thickness (Fig. 7C).
Figure 7.
Categories of vision loss and GCL-IPL thickness used as predictors of visual field outcomes. The ranked final visual field outcomes were compared against the initial GCL-IPL thickness (A), GCL-IPL thickness at 2 to 3 weeks (B), change in GCL-IPL thickness at 2 to 3 weeks (C), final GCL-IPL thickness (D), and change in GCL-IPL thickness at final visit (E).
Table.
GCL-IPL Thickness Compared Against Visual Field Outcomes
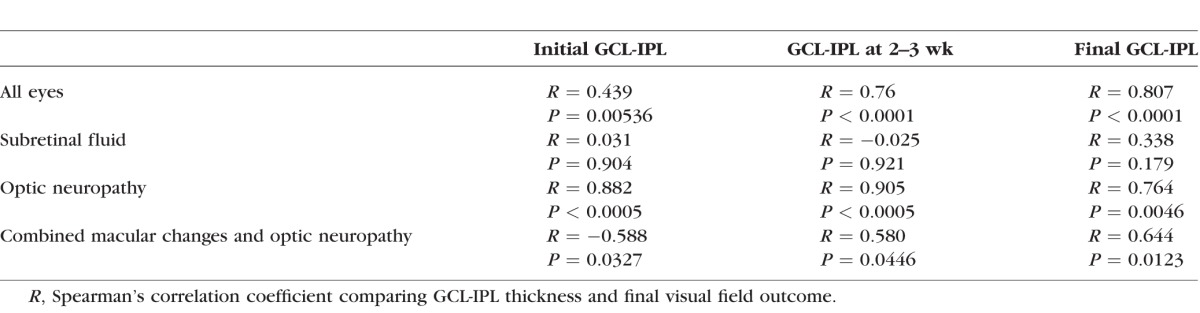
When the categories of vision loss were examined individually, patients with optic neuropathy alone had a significant correlation between final visual outcome and GCL-IPL thicknesses at all time points (Table). Patients with a combination of optic neuropathy and macular changes had a significant correlation between final visual outcome and both GCL-IPL thickness at 2 to 3 weeks and final GCL-IPL thickness, while the initial GCL-IPL thickness had a negative correlation (Table). Patients with macular changes alone had no correlation between GCL-IPL thickness and final visual outcome at any time point (Table).
Initial Visual Acuity and Visual Field in Predicting Visual Outcomes
Overall, initial visual acuity did not have a significant correlation with final visual acuity (r2 = 0.185, P = 0.091). However, when each category of vision loss was compared separately, patients with optic neuropathy alone had a significant correlation between initial and final visual acuity (r2 = 0.4691, P = 0.029). There was no correlation between initial and final visual acuities for the other categories of vision loss.
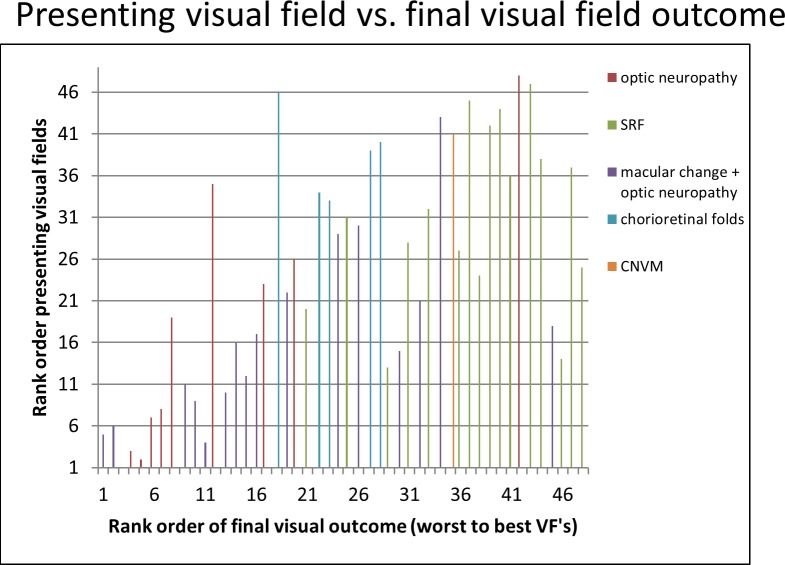
In contrast, the initial visual field had a significant correlation with final visual field outcome (Spearman Correlation = 0.667, P < 0.0001; Fig. 8).
Figure 8.
Initial visual field compared against visual field outcome. The visual fields at presentation were rank ordered and compared against the final visual field. There was a significant correlation between the initial visual field and final visual field outcome, although there were a few outliers (Spearman Correlation = 0.667, P < 0.0001). Each category of vision loss was analyzed separately. There was a significant correlation between the initial visual field and final visual field outcome in patients with a combination of macular changes and optic neuropathy (Spearman Correlation = 0.780, P < 0.0001) and in those with optic neuropathy alone (Spearman Correlation = 0.952, P < 0.0001). Patients with outer retinal changes alone showed no correlation between the initial and final visual fields (Spearman Correlation = 0.294, P = 0.262).
When each category of vision loss was analyzed separately, a significant positive correlation was found between initial and final visual field rank in eyes with a combination of macular changes and optic neuropathy (Spearman Correlation = 0.780, P < 0.0001) and in eyes with optic neuropathy alone (Spearman Correlation = 0.952, P < 0.0001). Eyes with outer retinal changes alone showed no correlation between initial and final visual fields.
Discussion
Our study found that 4.7% of patients with IIH referred to a tertiary center had decreased visual acuity on presentation, defined as best corrected vision of 20/25 or worse. Most (83.3%) of these patients had Frisén grade IV or V optic disc edema. Approximately one-third of these patients had outer retinal changes in the macula, one-third had optic neuropathy, and one-third had a combination of outer retinal changes in the macula and optic neuropathy as the cause of decreased visual acuity.
Our study demonstrates that outer retinal changes in the macula are largely reversible except in the case of chorioretinal folds, which can cause a mild persistent decrease in visual acuity in some instances. Eight of nine patients with subretinal fluid had a full recovery of vision with medical management alone. One patient with subretinal fluid underwent early optic nerve sheath fenestration due to poor tolerance of acetazolamide. These findings are similar to those of a prior study where six of seven patients with subretinal fluid associated with papilledema showed improvement in visual acuity upon resolution of the subretinal fluid with acetazolamide alone.24
In our study, the outcomes of patients with optic neuropathy alone or a combination of optic neuropathy and outer retinal changes were largely dependent on the degree of optic neuropathy, which could be predicted by the GCL-IPL thickness on SD-OCT macular scans. We found that the GCL-IPL thickness at presentation was often normal and, therefore, the initial GCL-IPL thickness was not useful in predicting visual outcome for the majority of patients. However, there was a subset of eyes that had an initial GCL-IPL thickness of less than 70 μm that all had poor visual outcomes. These patients may require immediate aggressive intervention to preserve their remaining vision and ganglion cells. In patients presenting with a normal GCL-IPL thickness, serial GCL-IPL OCT analysis at 2 to 3 weeks was more helpful, where a drop of more than 10 μm compared with the initial GCL-IPL thickness was strongly associated with a poor visual outcome. By combining the subset of eyes with an initial GCL-IPL thickness of less than 70 μm with the eyes having an early decrease in GCL-IPL thickness of more than 10 μm, all eyes with a poor final visual outcome could be predicted within 2 to 3 weeks of presentation.
While the presenting visual acuity had no correlation with final visual acuity in the entire cohort, patients with vision loss from optic neuropathy alone did demonstrate a significant correlation. This supports the notion that vision loss from outer retinal layer changes in the macula is typically reversible, while vision loss from optic neuropathy and inner retinal layer change is less reversible. There was also a correlation between the initial visual field and visual field outcome. This correlation was dominated by patients with optic neuropathy or optic neuropathy in combination with outer retinal changes in the macula.
Patients receiving both medical and surgical management had a worse outcome, likely because of the severity of the disease that led to the decision of surgical intervention rather than surgery causing a worse outcome. However, our findings do suggest that medical therapy alone is often adequate for patients with severe papilledema, because the vast majority of patients in this study had grade IV optic disc edema and the majority of patients with good visual outcomes were treated with medication alone. The idiopathic intracranial hypertension treatment trial (IIHTT) supports the efficacy of acetazolamide in the treatment of IIH.53 The trial examined the effects of acetazolamide against placebo in 165 patients with mild visual field loss (−2 to −7 dB perimetric mean deviation in the worse eye) from IIH and found six treatment failures in the placebo group and only one treatment failure in the acetazolamide group as defined by a worsening of 2 to 3 dB from baseline. All of the patients with treatment failure had high grade papilledema.53 Interestingly, nearly 30% of the study eyes in the IIHTT had BCVA worse than 20/20 on initial presentation.54 Although these patients have not yet been individually evaluated to determine the cause of decreased visual acuity, it is possible that many of them had subtle macular changes that would be amenable to medical treatment.
The greatest limitations to our study are its retrospective nature and variable length of follow-up. The majority of patients were treated in a similar fashion with escalating doses of acetazolamide to a goal of 4000 mg a day within 2 to 4 weeks of diagnosis, then referral for surgical intervention with optic nerve sheath fenestration if their vision continued to worsen. However, some could not tolerate medication and were referred for surgery earlier. Others were referred for surgical intervention earlier because of the perceived severity of the disease. Therefore, there were treatment differences in some of the patients that may or may not have influenced the final visual outcome.
It is also important to note that commercially available macular segmentation algorithms will commonly fail in patients with significant optic disc edema, giving artifactually thin inner retinal layer measurements. Therefore, measurements of GCL-IPL complex thickness in IIH should be obtained using more robust 3D segmentation algorithms, such as the Iowa Reference Algorithm used in this study.46–48
In summary, outer retinal changes in the macula causing decreased visual acuity in IIH patients at presentation are largely reversible except for chorioretinal folds that can cause mild persistent decreased vision in some instances. The visual prognosis of IIH patients with a combination of outer retinal changes in the macula and optic neuropathy or optic neuropathy alone is largely dependent on the degree of optic neuropathy, which can be predicted by OCT measurement of the GCL-IPL thickness. A GCL-IPL thickness of less than 70 μm early in the disease course or early progressive thinning of more than 10 μm during the first 2 to 3 weeks from presentation are correlated with a poor visual outcome and may indicate the need for more aggressive management.
Acknowledgments
Supported by C9251-C, Rehabilitation Research & Development (RR&D), VA-ORD (RK, MG); R01 EY023279, National Eye Institute (NEI) Subcontract for 3U10 EY017281 (RK, MG, MT, RL); GG003828 with Columbia University for 5 R01 EY02211536, Subcontract NEI (RK); W81XWH-10-1-0736, Department of Defense (RK); 2R01 EY018853, NEI Subcontract for 3U10 (MG); EY017281, NEI (MG); C7098-R Merit Review Grant from the Department of Veterans Affairs (MW); and 1U10EY017281-01A1 (NORDIC) National Institute of Health (MW).
Disclosure: J.J. Chen, None; M.J. Thurtell, None; R.A. Longmuir, None; M.K. Garvin, None; J.-K. Wang, None; M. Wall, None; R.H. Kardon, None
References
- 1. Wall M. Idiopathic intracranial hypertension. Neurol Clin. 2010; 28: 593–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Friedman DI. Idiopathic intracranial hypertension. Curr Pain Headache Rep. 2007; 11: 62–68. [DOI] [PubMed] [Google Scholar]
- 3. Chen J,, Wall M. Epidemiology and risk factors for idiopathic intracranial hypertension. Int Ophthalmol Clin. 2014; 54: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wall M,, George D. Idiopathic intracranial hypertension. A prospective study of 50 patients. Brain. 1991; 114 (pt 1A): 155–180. [PubMed] [Google Scholar]
- 5. Corbett JJ,, Savino PJ,, Thompson HS,, et al. Visual loss in pseudotumor cerebri. Follow-up of 57 patients from five to 41 years and a profile of 14 patients with permanent severe visual loss. Arch Neurol. 1982; 39: 461–474. [DOI] [PubMed] [Google Scholar]
- 6. Wall M. Idiopathic intracranial hypertension. Neurol Clin. 1991; 9: 73–95. [PubMed] [Google Scholar]
- 7. Wall M,, George D. Idiopathic intracranial hypertension. A prospective study of 50 patients. Brain. 1991; 114: 155–180. [PubMed] [Google Scholar]
- 8. Keltner JL,, Johnson CA,, Cello KE,, Wall M;for the NORDIC Idiopathic Intracranial Hypertension Study Group. Baseline visual field findings in the idiopathic intracranial hypertension treatment trial (IIHTT). Invest Ophthalmol Vis Sci. 2014; 55: 3200–3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thambisetty M,, Lavin PJ,, Newman NJ,, Biousse V. Fulminant idiopathic intracranial hypertension. Neurology. 2007; 68: 229–232. [DOI] [PubMed] [Google Scholar]
- 10. Mensah A,, Milea D,, Jensen R,, Fledelius H. Persistent visual loss in malignant idiopathic intracranial hypertension. Acta Ophthalmol. 2009; 87: 934–936. [DOI] [PubMed] [Google Scholar]
- 11. Pollock SC., Acute papilledema and visual loss in a patient with pseudotumor cerebri. Arch Ophthalmol. 1987; 105: 752–753. [DOI] [PubMed] [Google Scholar]
- 12. Talks SJ,, Mossa F,, Elston JS. The contribution of macular changes to visual loss in benign intracranial hypertension. Eye (Lond). 1998; 12 (pt 5): 806–808. [DOI] [PubMed] [Google Scholar]
- 13. Mitchell DJ,, Steahly LP. Pseudotumor cerebri and macular disease. Retina. 1989; 9: 115–117. [DOI] [PubMed] [Google Scholar]
- 14. Bird AC,, Sanders MD. Choroidal folds in association with papilloedema. Br J Ophthalmol. 1973; 57: 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Griebel SR,, Kosmorsky GS. Choroidal folds associated with increased intracranial pressure. Am J Ophthalmol. 2000; 129: 513–516. [DOI] [PubMed] [Google Scholar]
- 16. Jacobson DM. Intracranial hypertension and the syndrome of acquired hyperopia with choroidal folds. J Neuroophthalmol. 1995; 15: 178–185. [PubMed] [Google Scholar]
- 17. Sharma M,, Volpe NJ,, Patel T,, Kimmel A. Intracranial hypertension associated with acquired hyperopia and choroidal folds. Retina. 1999; 19: 260–262. [DOI] [PubMed] [Google Scholar]
- 18. Murdoch D,, Merriman M. Acquired hyperopia with choroidal folds. Clin Experiment Ophthalmol. 2002; 30: 292–294. [DOI] [PubMed] [Google Scholar]
- 19. Morris AT,, Sanders MD. Macular changes resulting from papilloedema. Br J Ophthalmol. 1980; 64: 211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Keane JR. Papilledema with unusual ocular hemorrhages. Arch Ophthalmol. 1981; 99: 262–263. [DOI] [PubMed] [Google Scholar]
- 21. Rush JA. Hard Retinal exudates and visual loss due to papilledema. Ann Ophthalmol. 1982; 14: 168–172. [PubMed] [Google Scholar]
- 22. Carter SR,, Seiff SR. Macular changes in pseudotumor cerebri before and after optic nerve sheath fenestration. Ophthalmology. 1995; 102: 937–941. [DOI] [PubMed] [Google Scholar]
- 23. Gittinger JW,, Jr, Asdourian GK. Macular abnormalities in papilledema from pseudotumor cerebri. Ophthalmology. 1989; 96: 192–194. [DOI] [PubMed] [Google Scholar]
- 24. Hoye VJ,, III, Berrocal AM,, Hedges TR,, III, Amaro-Quireza ML. Optical coherence tomography demonstrates subretinal macular edema from papilledema. Arch Ophthalmol. 2001; 119: 1287–1290. [DOI] [PubMed] [Google Scholar]
- 25. Akova YA,, Kansu T,, Yazar Z,, Atabay C,, Karagoz Y,, Duman S. Macular subretinal neovascular membrane associated with pseudotumor cerebri. J Neuroophthalmol. 1994; 14: 193–195. [PubMed] [Google Scholar]
- 26. Jamison RR. Subretinal neovascularization and papilledema associated with pseudotumor cerebri. Am J Ophthalmol. 1978; 85: 78–81. [DOI] [PubMed] [Google Scholar]
- 27. Rush JA. Pseudotumor cerebri: clinical profile and visual outcome in 63 patients. Mayo Clin Proc. 1980; 55: 541–546. [PubMed] [Google Scholar]
- 28. Schirmer CM,, Hedges TR,, III. Mechanisms of visual loss in papilledema. Neurosurg Focus. 2007; 23: E5. [DOI] [PubMed] [Google Scholar]
- 29. Frisen L,, Holm M. Visual field defects associated with chorioretinal folds. : Henkes HE. Documenta Ophthalmologica Proceedings: Second International Visual Field Symposium. Vol 14. Tübingen: The Hague; 1977: 327–330. [Google Scholar]
- 30. Corbett JJ,, Jacobson DM,, Mauer RC,, Thompson HS. Enlargement of the blind spot caused by papilledema. Am J Ophthalmol. 1988; 105: 261–265. [DOI] [PubMed] [Google Scholar]
- 31. Tawse KL,, Hedges TR,, III,, Gobuty M,, Mendoza-Santiesteban C. Optical coherence tomography shows retinal abnormalities associated with optic nerve disease. Br J Ophthalmol. 2014; 98 (suppl 2): ii30–ii33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Scott CJ,, Kardon RH,, Lee AG,, Frisen L,, Wall M. Diagnosis and grading of papilledema in patients with raised intracranial pressure using optical coherence tomography vs clinical expert assessment using a clinical staging scale. Arch Ophthalmol. 2010; 128: 705–711. [DOI] [PubMed] [Google Scholar]
- 33. Marzoli SB,, Ciasca P,, Curone M,, et al. Quantitative analysis of optic nerve damage in idiopathic intracranial hypertension (IIH) at diagnosis. Neurol Sci. 2013; 34 (suppl 1): S143–S145. [DOI] [PubMed] [Google Scholar]
- 34. Yri HM,, Wegener M,, Sander B,, Jensen R. Idiopathic intracranial hypertension is not benign: a long-term outcome study. J Neurol. 2012; 259: 886–894. [DOI] [PubMed] [Google Scholar]
- 35. Skau M,, Sander B,, Milea D,, Jensen R. Disease activity in idiopathic intracranial hypertension: a 3-month follow-up study. J Neurol. 2011; 258: 277–283. [DOI] [PubMed] [Google Scholar]
- 36. Kardon RH., Role of the macular optical coherence tomography scan in neuro-ophthalmology. J Neuroophthalmol. 2011; 31: 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Akiyama H,, Kashima T,, Li D,, Shimoda Y,, Mukai R,, Kishi S. Retinal ganglion cell analysis in Leber's hereditary optic neuropathy. Ophthalmology. 2013; 120: 1943–1944, e1945. [DOI] [PubMed] [Google Scholar]
- 38. Medeiros FA,, Lisboa R,, Weinreb RN,, Liebmann JM,, Girkin C,, Zangwill LM. Retinal ganglion cell count estimates associated with early development of visual field defects in glaucoma. Ophthalmology. 2013; 120: 736–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cho JW,, Sung KR,, Lee S,, et al. Relationship between visual field sensitivity and macular ganglion cell complex thickness as measured by spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2010; 51: 6401–6407. [DOI] [PubMed] [Google Scholar]
- 40. Tan O,, Chopra V,, Lu AT,, et al. Detection of macular ganglion cell loss in glaucoma by Fourier-domain optical coherence tomography. Ophthalmology. 2009; 116: 2305–2314, e2301–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gu S,, Glaug N,, Cnaan A,, Packer RJ,, Avery RA. Ganglion cell layer-inner plexiform layer thickness and vision loss in young children with optic pathway gliomas. Invest Ophthalmol Vis Sci. 2014; 55: 1402–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mwanza JC,, Durbin MK,, Budenz DL,, et al. Glaucoma diagnostic accuracy of ganglion cell-inner plexiform layer thickness: comparison with nerve fiber layer and optic nerve head. Ophthalmology. 2012; 119: 1151–1158. [DOI] [PubMed] [Google Scholar]
- 43. Walter SD,, Ishikawa H,, Galetta KM,, et al. Ganglion cell loss in relation to visual disability in multiple sclerosis. Ophthalmology. 2012; 119: 1250–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ronnback C,, Milea D,, Larsen M. Imaging of the macula indicates early completion of structural deficit in autosomal-dominant optic atrophy. Ophthalmology. 2013; 120: 2672–2677. [DOI] [PubMed] [Google Scholar]
- 45. Smith JL. Whence pseudotumor cerebri? J Clin Neuroophthalmol. 1985; 5: 55–56. [PubMed] [Google Scholar]
- 46. Lee K,, Niemeijer M,, Garvin MK,, Kwon YH,, Sonka M,, Abramoff MD. Segmentation of the optic disc in 3-D OCT scans of the optic nerve head. IEEE Trans Med Imaging. 2010; 29: 159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Garvin MK,, Abramoff MD,, Kardon R,, Russell SR,, Wu X,, Sonka M. Intraretinal layer segmentation of macular optical coherence tomography images using optimal 3-D graph search. IEEE Trans Med Imaging. 2008; 27: 1495–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Garvin MK,, Abramoff MD,, Wu X,, Russell SR,, Burns TL,, Sonka M. Automated 3-D intraretinal layer segmentation of macular spectral-domain optical coherence tomography images. IEEE Trans Med Imaging. 2009; 28: 1436–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang JK,, Kardon RH,, Kupersmith MJ,, Garvin MK. Automated quantification of volumetric optic disc swelling in papilledema using spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012; 53: 4069–4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ojima Y,, Tsujikawa A,, Yamashiro K,, Ooto S,, Tamura H,, Yoshimura N. Restoration of outer segments of foveal photoreceptors after resolution of central serous chorioretinopathy. Jpn J Ophthalmol. 2010; 54: 55–60. [DOI] [PubMed] [Google Scholar]
- 51. Schindler EI,, Nylen EL,, Ko AC,, et al. Deducing the pathogenic contribution of recessive ABCA4 alleles in an outbred population. Hum Mol Genet. 2010; 19: 3693–3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Frisen L. Swelling of the optic nerve head: a staging scheme. J Neurol Neurosurg Psychiatry. 1982; 45: 13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wall M,, McDermott MP,, Kieburtz KD,, et al. Effect of acetazolamide on visual function in patients with idiopathic intracranial hypertension and mild visual loss: the idiopathic intracranial hypertension treatment trial. JAMA. 2014; 311: 1641–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wall M,, Kupersmith MJ,, Kieburtz KD,, et al. The idiopathic intracranial hypertension treatment trial: clinical profile at baseline. JAMA Neurol. 2014; 71: 693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]