Abstract
Objective
The aim of this study is to determine whether the cystic periventricular leukomalacia (cPVL) detection rate differs between imaging studies performed at different time points.
Design
We retrospectively reviewed the prospectively collected data of 31,708 infants from the NICHD Neonatal Research Network. Inclusion criteria were infants < 1,000 g birth weight or < 29 weeks’ gestational age who had cranial imaging performed using both early criterion (cranial ultrasound [CUS] < 28 days chronological age) and late criterion (CUS, magnetic resonance imaging, or computed tomography closest to 36 weeks postmenstrual age [PMA]). We compared the frequency of cPVL diagnosed by early and late criteria.
Results
About 664 (5.2%) of the 12,739 infants who met inclusion criteria had cPVL using either early or late criteria; 569 using the late criterion, 250 using the early criterion, and 155 patients at both times. About 95 (14.3%) of 664 cPVL cases seen on early imaging were no longer visible on repeat screening closest to 36 weeks PMA. Such disappearance of cPVL was more common in infants < 26 weeks’ gestation versus infants of 26 to 28 weeks’ gestation (18.5 vs. 11.5%; p = 0.013).
Conclusions
Cranial imaging at both < 28 days chronological age and closest to 36 weeks PMA improves cPVL detection, especially for more premature infants.
Keywords: cystic periventricular leukomalacia, screening cranial imaging, extremely low birth weight preterm infants
Screening neuroimaging helps in detecting clinically silent brain injury in extremely low birth weight (ELBW) preterm infants.1 Cranial ultrasound (CUS) is commonly used in ELBW newborns for routine screening; early CUS examination mostly detects hemorrhagic lesions, whereas a later CUS usually allows diagnosis of white matter abnormalities, including cystic periventricular leukomalacia (cPVL).2 Among all the white matter abnormalities, detection of cPVL on the late CUS examination has the highest correlation with neuropathologic findings3,4 and subsequent neurodevelopmental outcome.5–9
The late timing of the screening neuroimaging for detection of cPVL is intended to maximize cost-effectiveness, by focusing on time points when almost all cases of cPVL are expected to become evident on neuroimaging.10 The timing of “late” ultrasound examination varies with different guidelines for screening; the Canadian Pediatric Society recommends later CUS examination in the6thweekof life,2 and the American Academy of Neurology and the Child Neurology Society recommend it to be done between 36 and 40weeks’ postmenstrual age (PMA).1,11 However, studies in ELBW infants using serial CUS have shown that in some instances cPVL may be apparent for only 1 to 2 weeks before the cysts coalesce, collapse, and then disappear.12,13 If the later cranial screening imaging is deferred until 36 to 40 weeks’ PMA some cases of cPVL, especially in more premature ELBW infants, may be missed because the screening cranial imaging might be performed after the cysts collapsed and disappeared. For example, using the criterion of 36 to 40 weeks, PMA for an infant born at 23 weeks’ gestational age (GA), the later screening imaging would be performed at 13 to 17 weeks’ chronological age (CA). This same infant would be screened earlier, at 29 weeks’ PMA, using the Canadian Pediatric Society CA criterion of 6 weeks. This 7- to 11-week difference in obtaining routine cranial screening imaging may be crucial, if it results in underestimation of cPVL.
We hypothesize that “late” screening cranial imaging only at 36 to 40 weeks PMA as recommended by the American Academy of Neurology and the Child Neurology Society will underestimate the incidence of cPVL in ELBW infants. We also hypothesize that cPVL underdetection at 36 to 40 weeks PMA will increase as GA at birth decreases because of longer delay in obtaining cranial screening imaging in infants who are more preterm. To explore this hypothesis, we assessed the detection of cPVL on screening cranial imaging performed closest to 36 weeks’ PMA (PMA criterion) compared with an earlier time point (CUS performed within 28 days CA) to determine if some cPVL had resolved by the time of later screening cranial imaging. We also compared the cPVL detection rate between ELBW infants born at < 26 weeks’ GA and those born at 26 to 28 weeks’ GA using PMA and CA criteria to determine if the incidence of potentially missed cases of cPVL differs between the two groups. This information might assist clinicians to provide better prognostic information to guide long-term care strategies in high-risk preterm neonates.
Methods/Procedures
Study Design
We retrospectively reviewed the data of all the ELBW preterm infants born and/or cared for at centers of the Eunice Kennedy Shriver NICHD Neonatal Research Network (NRN) over a 10.5-year period and included in the NRN’s Generic Database (GDB) registry. The registry includes maternal and delivery information collected soon after birth and infant data collected prospectively from birth until death, or hospital discharge. If any CUS was performed within 28 days of birth, the registry includes data regarding cystic area(s) in the parenchyma including the presence of cPVL and porencephalic cysts that were documented separately. The exact CA at which cPVL was first detected on < 28 days’ screening CUS was not collected. If cranial imaging (sonogram, computed tomographic [CT] scan, or MRI) was performed after 28 days of life, data are also collected from the imaging study done closest to 36 weeks’ PMA regarding the presence of cPVL, porencephalic cyst, and ventricular size separately. If the infant had more than one imaging modality, results were recorded based on the following hierarchy (highest to lowest) of MRI, sonogram, CT scan. The type of screening cranial imaging (sonogram, CT scan, or MRI) was center dependent. The scans were read by radiologists at each participating center (no central reader), and the neuroimaging findings were abstracted from radiology reports. For the registry, the diagnosis of PVL by CUS was based on characteristic lucencies in the periventricular region (most commonly dorsal and lateral to the external angle of the lateral ventricle and might have been diffuse or focal in distribution along the front to back axis of the head). Increased echogenicity in the periventricular region or ventriculomegaly was not diagnosed as PVL. The Institutional Review Board at each NRN center approved participation in the registry and follow-up studies.
Definition of Study Population
All infants with birth weight between 401 and 999 g inclusive (i.e., ELBW) and/or between 220/7 and 286/7 weeks inclusive GA, who were enrolled in the registry during the study period, survived at least until 36 to 40 weeks’ PMA, and had cranial imaging performed at two time points, that is, within 28 days of birth and again after 28 days and closest to 36 weeks’ PMA, were the focus of this analysis. This study included infants with cranial imaging studies performed at both the time points at an NICHD NRN study center. In this study population, we determined the frequency of cPVL detected using the CA criterion of routine screening cranial sonogram performed within 28 days of birth and the PMA criterion of screening cranial imaging performed after 28 days of life and closest to 36 weeks’ PMA.
Statistical Analyses
A chi-square test was used to compare the cPVL detection rate using the CA criterion of cranial sonogram within 28 days of birth and the PMA criterion of screening cranial imaging performed closest to 36 weeks’ PMA in the study infants. The cPVL detection rate using PMA and CA criteria were also compared between infants of < 26weeks and infants of 26 to 28weeks using a chi-square test to determine if missing cases of cPVL differ between the two groups.
Demographics and perinatal risk factors were compared between infants with cPVL detected with either the CA or PMA criterion and infants without cPVL using chi-square tests for categorical variables and student t-test for continuous variables. Analysis was performed with SAS version 9.3 (SAS Institute, Cary, NC). A p value < 0.05 was considered significant and no correction was made for multiple comparisons.
Results
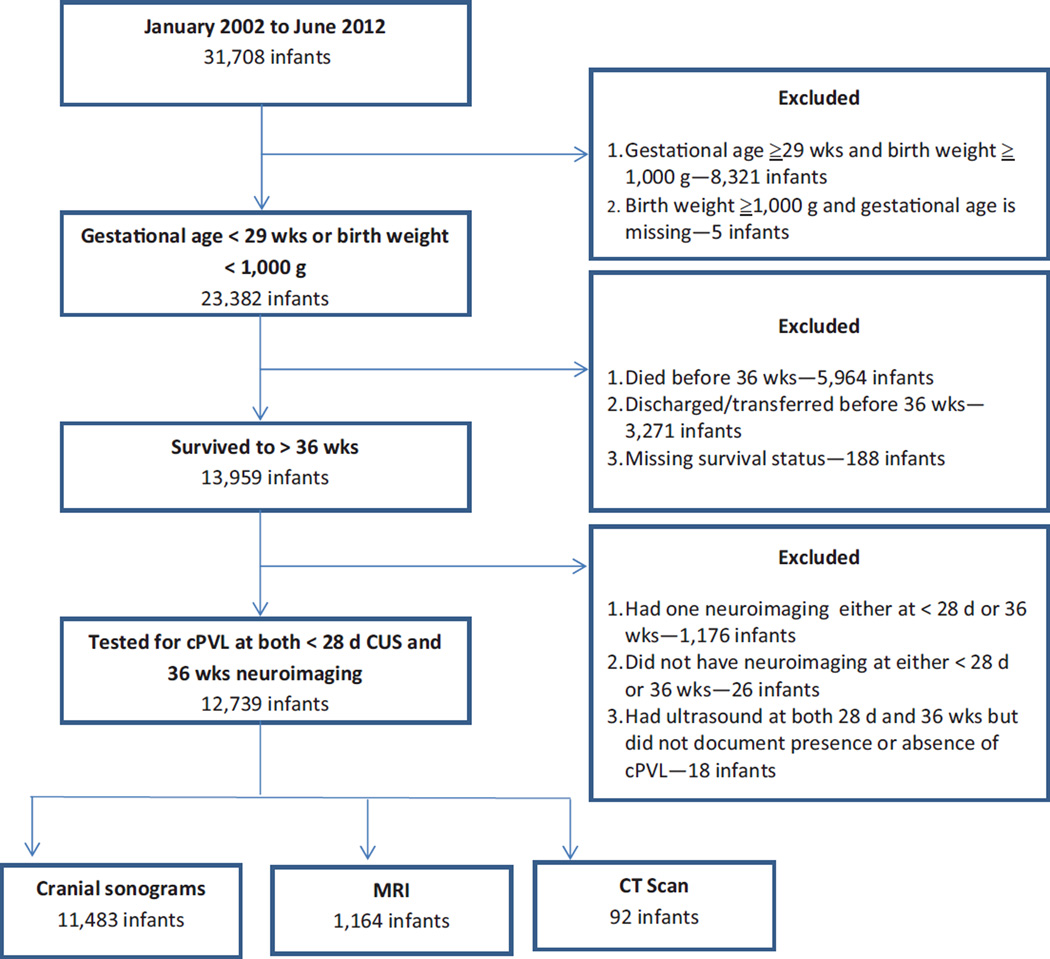
Between January 2002 and June 2012, 31,708 infants were included in the GDB registry. As presented in Fig. 1, 12,739 of the 31,708 infants were < 1,000 g birth weight or < 29 weeks’ GA, and had at least two screening cranial imaging studies—that is, according to CA criterion (CUS < 28 days CA) and as per PMA criterion (CUS, MRI, or CT after 28 days and closest to 36 weeks’ PMA). Overall, 646 (5.2%) of the 12,739 infants had a diagnosis of cPVL by either CUS according to the CA criterion or cranial imaging as per PMA criterion. The median PMA (25th and 75th percentile interquartile range) at screening cranial imaging as per PMA criterion was 346/7 weeks (32 weeks, 362/7 weeks).
Fig. 1.
Details of the study population from the GDB Registry. cPVL, cystic periventricular leukomalacia; CUS, cranial ultrasound; CT, computed tomographic; GDB, Generic Database; MRI, magnetic resonance imaging.
The number of the infants diagnosed to have cPVL on screening imaging either within 28 days of life or closest to 36 weeks PMA, and at both the time points are noted in Table 1. Overall, 250 (2%) infants had cPVL detected using the CA criterion (on CUS within 28 days of birth), and 569 (4.5%) had cPVL detected using the PMA criterion of closest to 36weeks. In 155 (1.2%) infants, cPVL was evident on screening imaging performed at both the time points. The detection rate for cPVL with late cranial imaging closest to 36 weeks’ PMA was higher than screening CUS according to CA criterion (569 of 12,739 or 4.5% vs. 250 of 12,739, or 2.0%; p < 0.0001; odds ratio [OR], 2.3; 95% confidence interval [CI], 2.0–2.7). However, as evident in Table 1, 95 (14.3%) of the 664 cases of cPVL would have been potentially missed if only the late cranial screening imaging closest to 36 weeks’ PMA was obtained. Conversely, 414 (62.3%) of the 664 cases of cPVL would have been potentially missed if the screening CUS was obtained only according to the CA criterion of the CUS within 28 days of birth.
Table 1.
Cystic PVL in infants of < 1,000 g birth weight or < 29 wks’ gestational age who had neuroimaging at both within 28 d and closest to 36 wks
| cPVL—36 wks | |||
|---|---|---|---|
| cPVL—28 d | No | Yes | Total |
| No | 12,075 | 414 | 12,489 |
| Yes | 95 | 155 | 250 |
| Total | 12,170 | 569 | 12,739 |
Abbreviations: cPVL, cystic periventricular leukomalacia; PVL, periventricular leukomalacia.
Infants with cPVL diagnosed either by CA criterion or PMA criterion were more premature, less likely to be exposed to complete courses of antenatal steroids, and more likely to have a 5-minute Apgar score ≤ 3, had a higher incidence of bronchopulmonary dysplasia (BPD) and late onset sepsis, and had more ventilator days compared with infants without cPVL (all p < 0.05, Table 2). Comparison of demographic and perinatal characteristics between infants with cPVL diagnosed with CA criterion but not with PMA criterion and infants without cPVL is shown in Table 3.
Table 2.
Comparison of demographic and perinatal characteristics between infants with and without cPVL on screening imaging
| Characteristics | All infants < 1,000 g or < 29 wks without cPVL, N = 12,075 |
Cystic PVL with either CA or PMA criterion, N = 664 |
p Value |
|---|---|---|---|
| Birth weight (g), mean ± SD | 864 ± 210 | 855 ± 220 | 0.29 |
| Gestation (wks), mean ± SD | 26.4 ± 1.8 | 25.9 ± 1.8 | < 0.0001a |
| Male | 6,260 (51.9) | 327 (49.3) | 0.19 |
| Pregnancy-induced hypertension | 428 (31.3) | 13 (20.6) | 0.07 |
| Antepartum hemorrhage | 219 (16.0) | 15 (23.8) | 0.10 |
| Any prenatal steroid | 1,236 (10.2) | 59 (8.9) | 0.26 |
| Complete course of prenatal steroid | 977 (79.3) | 33 (56.9) | < 0.0001a |
| Maternal antibiotics < 72 h of delivery | 923 (92.7) | 42 (93.3) | 0.99b |
| Apgar score ≤ 3 at 1 min | 11,271 (93.3) | 635 (95.6) | 0.02a |
| Apgar score ≤ 3 at 5 min | 10,837 (89.8) | 613 (92.3) | 0.03a |
| Delivery room bag and mask | 1,052 (77.0) | 53 (84.1) | 0.18 |
| Delivery room chest compression | 76 (5.6) | 6 (9.5) | 0.17 |
| BPD (O2 at 36 wks) | 2,424 (47.3) | 150 (64.1) | < 0.0001a |
| Steroids for BPD | 1,686 (14.1) | 118 (17.9) | 0.01a |
| Late-onset culture-proven sepsis | 4,009 (33.2) | 268 (40.4) | 0.0001a |
| Ventilator days, mean ± SD | 23.3 ± 26.7 | 34.4 ± 30.4 | < 0.0001a |
Abbreviations: BPD, bronchopulmonary dysplasia; CA, chronological age; cPVL, cystic periventricular leukomalacia; PMA, postmenstrual age; PVL, periventricular leukomalacia; SD, standard deviation.
Note: The percentages are based on nonmissing responses in the registry. The percentages for the complete course of prenatal steroid are among those exposed to any prenatal steroid.
Significantly different at an α level of 0.05. Percentages were tested with a chi-square test, and means were tested with a t-test.
Tested with Fisher exact test. Values are percent and means; CA criterion: Cranial ultrasound within 28 d, PMA criterion: Screening neuroimaging closest to 36 weeks.
Table 3.
Comparison of demographic and perinatal characteristics between infants with and without cPVL on screening imaging
| Characteristics | All infants < 1,000 g or < 29 wks without cPVL, N = 12,075 |
Cystic PVL with CA criterion but not with PMA criterion, N = 95 |
p Value |
|---|---|---|---|
| Birth weight (g), mean ± SD | 864 ± 210 | 835 ± 208 | 0.18 |
| Gestation (wks), mean ± SD | 26.4 ± 1.8 | 25.4 ± 1.6 | < 0.0001a |
| Male | 6,260 (51.9) | 46 (48.4) | 0.50 |
| PIH | 428 (31.3) | 2 (15.4) | 0.37b |
| APH | 219 (16.0) | 6 (46.2) | 0.004a |
| Any prenatal steroid | 1,236 (10.2) | 12 (12.6) | 0.44 |
| Complete course of steroid | 977 (79.3) | 6 (50.0) | 0.01a |
| Maternal antibiotics < 72 h of delivery | 923 (92.7) | 8 (88.9) | 0.67 |
| Apgar score ≤ 3 at 1 min | 11,271 (93.3) | 91 (95.8) | 0.53b |
| Apgar score ≤ 3 at 5 min | 10,837 (89.8) | 84 (88.4) | 0.67 |
| Delivery room bag and mask | 1,052 (77.0) | 13 (100.0) | < 0.05a,b |
| DR chest compression | 76 (5.6) | 1 (7.7) | 0.99b |
| BPD (O2 at 36 wks) | 2,424 (47.3) | 13 (59.1) | 0.27 |
| Steroids for BPD | 1,686 (14.1) | 9 (9.7) | 0.23 |
| Late-onset culture-proven sepsis | 4,009 (33.2) | 39 (41.1) | 0.11 |
| Ventilator days, mean ± SD | 23.3 ± 26.7 | 34.3 ± 28.8 | 0.0004a |
Abbreviations: APH, antepartum hemorrhage; BPD, bronchopulmonary dysplasia; CA, chronological age; cPVL, cystic periventricular leukomalacia; DR, delivery room; PIH, pregnancy-induced hypertension; PMA, postmenstrual age; PVL, periventricular leukomalacia; SD, standard deviation.
Note: The percentages are based on nonmissing responses in the registry. The percentages for the complete course of prenatal steroid are among those exposed to any prenatal steroid.
Significantly different at an α level of 0.05. Percentages were tested with a chi-square test, and means were tested with a t-test.
Tested with Fisher exact test. Values are percent and means; CA criterion: Cranial ultrasound within 28 d, PMA criterion: Screening neuroimaging closest to 36 weeks.
The frequency of cPVL among infants of < 26 weeks and those 26 to 28 weeks’ GA, noted on screening imaging either at < 28 days or closest to 36 weeks, and at both the time points are shown in Tables 4 and 5. As seen in the tables, 57 (18.6%) of 307 cases of cPVL in infants of < 26 weeks, and 37 (11.5%) of 321 cases of cPVL in infants of 26 to 28 weeks of gestation would have been potentially missed if only late cranial screening imaging was obtained closest to 36 weeks’ PMA. This percent of cPVL detected on the early CUS was higher in infants of < 26 weeks’ gestation compared with infants of 26 to 28 weeks’ gestation (p = 0.013; OR, 1.8; 95% CI, 1.1–2.7) as shown in Table 6.
Table 4.
Comparison of cystic PVL in infants of < 26 wks of gestation (irrespective of birth weight) who had neuroimaging at both within 28 d and closest to 36 wks
| Infants < 26 wks’ gestation | |||
|---|---|---|---|
| cPVL—36 wks | |||
| cPVL—28 d | No | Yes | Total |
| No | 3,894 | 193 | 4,087 |
| Yes | 57 | 57 | 114 |
| Total | 3,951 | 250 | 4,201 |
Abbreviations: cPVL, cystic periventricular leukomalacia; PVL, periventricular leukomalacia.
Table 5.
Comparison of cystic PVL in infants of 26 to 28 wks of gestation (irrespective of birth weight) who had neuroimaging at both within 28 d and closest to 36 wks
| Infants 26–28 wks’ gestation | |||
|---|---|---|---|
| cPVL—36 wks | |||
| cPVL—28 d | No | Yes | Total |
| No | 7,140 | 196 | 7,336 |
| Yes | 37 | 88 | 125 |
| Total | 7,177 | 284 | 7,461 |
Abbreviations: cPVL, cystic periventricular leukomalacia; PVL, periventricular leukomalacia.
Table 6.
Missed cases of cystic PVL if only PMA criterion is used for < 26 wks versus 26 to 28 wks gestation infants
| Infants < 26 wks’ gestation | Infants 26–28 wks’ gestation | OR (95% CI), p Value | |
|---|---|---|---|
| Missed cases of cystic PVL using PMA alone | 57/307 (18.5%) | 37/321 (11.5%) | 1.8 (1.1–2.7), 0.013 |
| Total cases of cystic PVL using both CA and PMA criteria | 307 | 321 |
Abbreviations: CA, chronological age criteriona; CI, confidence interval; PMA, postmenstrual age criterionb; OR, odds ratio; PVL, periventricular leukomalacia.
Cranial ultrasound within 28 d.
Screening neuroimaging closest to 36 wks.
Discussion
Cranial ultrasonography remains the preferred neuroimaging technique for routine screening of intracranial pathology in ELBW newborn infants. Routine screening of cPVL with CUS provides critical information that may alter prognosis and treatment programs.1,2 Using a large cohort of ELBW infants with cPVL, we report that the detection rate of cPVL from screening neuroimaging closest to 36 weeks’ PMA is higher than from CUS at < 28 days CA. However, to maximize the detection of cPVL screening, cranial imaging at both the time points may be necessary. As is evident from our study, almost one in seven cases of cPVL will potentially remain underdetected or missed if additional cranial imaging is not performed at < 28 days’ CA and screening imaging for cPVL is obtained only closest to 36 weeks’ PMA. We have also shown that without screening cranial imaging at multiple time points, cPVL is more likely to be missed in infants of < 26 weeks’ gestation compared with infants of 26 to 28 weeks’ gestation.
Our findings suggest that a restrictive policy of late screening cranial imaging for detection of cPVL until at 36 weeks’ PMA, may result in fewer diagnoses of cPVL, compared with more liberal use of serial screening cranial imaging studies. A policy of using time from birth and not only weeks of gestation for late screening cranial imaging could potentially be beneficial in better identification of cPVL. Our findings extend those first reported in the NICHD NRN study using the generic birth registry and the Benchmarking study data in which 21 of the 905 low-birth-weight infants had the diagnosis of cPVL based on the CUS at either the study closest to 28 days or 36 weeks’ PMA.14 However, only 8 of the 21 infants in that study had cPVL on both the 28- and 36-week study, suggesting that many cases of cPVL might be missed if cranial screening imaging is performed only at < 28 days’ CA, or only at 36 to 40 weeks’ PMA. Similar observations were made in another report that evaluated the evolution of postnatally acquired periventricular leukomalacia with frequent serial CUS studies.12 We extend these observations by demonstrating that screening cranial imaging at multiple time points in a large cohort of ELBW infants improves detection of cPVL in ELBW infants. Indeed, this study increases the external validity of the recommendations regarding screening cranial imaging,10,15 and focuses on the group of very immature infants including the population of infants born before 26 weeks of GA as most of the previous studies were done on infants born with a higher birth weight or GA.16
Cystic PVL may be first seen approximately 3 weeks after injury on routine screening CUS.17 Cystic PVL of antenatal onset is typically evident by 2weeks of age.18 The spectrum of cPVL, including the timing of emergence of cysts using CUS has been extensively studied by De Vries et al and Pierrat et al who reported that most cases of cPVL manifest at around 2 to 4 weeks of CA.9,16,19 While we demonstrate the need for screening cranial imaging at both < 28 days’ CA, and 36 weeks’ PMA, we were unable to determine the exact age of initial detection of cPVL by CUS within the first 28 days using this data set. Irrespective of the timing when the cysts first appeared before 28 days, the results of our study indicate that screening cranial imaging at multiple time points will improve the detection of cPVL. Another limitation of this report is that additional CUS that may have been obtained between the < 28 days’ CA and closest to 36 weeks’ PMA were not recorded, hence, we cannot comment on cPVL (which may be related to conditions such as late-onset sepsis or necrotizing enterocolitis) occurring between < 28 days’ CA and the closest to 36weeks’ PMA cranial imaging studies. This limits our ability to draw conclusions about optimal timing of additional screening cranial imaging for “late onset” cPVL detection in the potentially long interval between the < 28 days’ CA, and 36 weeks’ PMA. Furthermore, the diagnostic accuracy of cPVL may differ between CUS, MRI, and CT scan yet we compared < 28 days’ CUS to any type of late imaging, although the overwhelming majority of which were CUS.
With advances in diagnostic imaging, it is now known that in most ELBW infants, periventricular white matter injury is more diffuse and more common than previously appreciated.20,21 Although other imaging studies including brain MRI have been shown to detect more white matter abnormalities (diffuse noncystic and cystic) compared with simultaneous cranial ultrasonography examination, the significance of these additional findings is not clear and cranial ultrasonography remains the preferred neuroimaging technique for routine screening of cPVL in newborns, because of its ease of performance.1 On the basis of the MRI findings, some authors suggest that cPVL has become less common over the last two decades.20,21 However, most of these studies incorporated MRI obtained closest to 36 to 40 weeks’ PMA because MRI is more feasible when the infant is more mature.22,23 The results of this study suggest that the latter imaging strategy may result in cystic PVL being underdetected, and that this will be more frequent in the most premature ELBW infants.
The availability of the large NICHD NRN GDB registry with a large number of infants diagnosed with cPVL is a major strength of this study. However, a major limitation is that the NRN database is not designed for the evaluation of temporal progression of intracranial abnormalities on screening imaging. We also acknowledge that our results may not address infants who have died, as the study is focused on infants who survived to have screening cranial imaging at both the time periods. Lack of a central reader for CUS may be considered a limitation; however, it is recognized that “local” reading of CUS can be reliable for major brain injury.24 In conclusion, our study supports the need for routine screening cranial imaging at both < 28 days’ CA and closest to 36 weeks’ PMA to improve detection of cPVL in ELBW infants, especially for more premature infants.
Acknowledgments
Funding Source
The National Institutes of Health and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) provided grant support for the Neonatal Research Network’s GDB Study through cooperative agreements. Although NICHD staff did have input into the study design, conduct, analysis, and manuscript drafting, the comments and views of the authors do not necessarily represent the views of the NICHD.
Footnotes
Conflict of Interest
None.
References
- 1.Ment LR, Bada HS, Barnes P, et al. Practice parameter: neuroimaging of the neonate: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2002;58(12):1726–1738. doi: 10.1212/wnl.58.12.1726. [DOI] [PubMed] [Google Scholar]
- 2.Canadian Paediatric Society. Routine screening cranial ultrasound examinations for the prediction of long term neurodevelopmental outcomes in preterm infants. Paediatr Child Health (Oxford) 2001;6(1):39–52. doi: 10.1093/pch/6.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trounce JQ, Fagan D, Levene MI. Intraventricular haemorrhage and periventricular leucomalacia: ultrasound and autopsy correlation. Arch Dis Child. 1986;61(12):1203–1207. doi: 10.1136/adc.61.12.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bejar R, Coen RW, Merritt TA, et al. Focal necrosis of the white matter (periventricular leukomalacia): sonographic, pathologic, and electroencephalographic features. AJNR Am J Neuroradiol. 1986;7(6):1073–1079. [PMC free article] [PubMed] [Google Scholar]
- 5.Bass WT. Periventricular leukomalacia. Neoreviews. 2011;12:e76–e84. [Google Scholar]
- 6.Bass WT, Jones MA, White LE, Montgomery TR, Aiello F, III, Karlowicz MG. Ultrasonographic differential diagnosis and neurodevelopmental outcome of cerebral white matter lesions in premature infants. J Perinatol. 1999;19(5):330–336. doi: 10.1038/sj.jp.7200190. [DOI] [PubMed] [Google Scholar]
- 7.Piecuch RE, Leonard CH, Cooper BA, Sehring SA. Outcome of extremely low birth weight infants (500 to 999 grams) over a 12-year period. Pediatrics. 1997;100(4):633–639. doi: 10.1542/peds.100.4.633. [DOI] [PubMed] [Google Scholar]
- 8.Pinto-Martin JA, Riolo S, Cnaan A, Holzman C, Susser MW, Paneth N. Cranial ultrasound prediction of disabling and nondisabling cerebral palsy at age two in a low birth weight population. Pediatrics. 1995;95(2):249–254. [PubMed] [Google Scholar]
- 9.De Vries LS, Van Haastert IL, Rademaker KJ, Koopman C, Groenendaal F. Ultrasound abnormalities preceding cerebral palsy in high-risk preterm infants. J Pediatr. 2004;144(6):815–820. doi: 10.1016/j.jpeds.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 10.Perlman JM, Rollins N. Surveillance protocol for the detection of intracranial abnormalities in premature neonates. Arch Pediatr Adolesc Med. 2000;154(8):822–826. doi: 10.1001/archpedi.154.8.822. [DOI] [PubMed] [Google Scholar]
- 11.McCrea HJ, Ment LR. The diagnosis, management, and postnatal prevention of intraventricular hemorrhage in the preterm neonate. Clin Perinatol. 2008;35(4):777–792. vii. doi: 10.1016/j.clp.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burdjalov V, Srinivasan P, Baumgart S, Spitzer AR. Handheld, portable ultrasound in the neonatal intensive care nursery: a new, inexpensive tool for the rapid diagnosis of common neonatal problems. J Perinatol. 2002;22(6):478–483. doi: 10.1038/sj.jp.7210782. [DOI] [PubMed] [Google Scholar]
- 13.Dubowitz LMS, Bydder GM, Mushin J. Developmental sequence of periventricular leukomalacia. Correlation of ultrasound, clinical, and nuclear magnetic resonance functions. Arch Dis Child. 1985;60(4):349–355. doi: 10.1136/adc.60.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shankaran S, Langer JC, Kazzi SN, Laptook AR, Walsh M National Institute of Child Health and Human Development Neonatal Research Network. Cumulative index of exposure to hypocarbia and hyperoxia as risk factors for periventricular leukomalacia in low birth weight infants. Pediatrics. 2006;118(4):1654–1659. doi: 10.1542/peds.2005-2463. [DOI] [PubMed] [Google Scholar]
- 15.de Vries LS, Benders MJ, Groenendaal F. Imaging the premature brain: ultrasound or MRI? Neuroradiology. 2013;55(Suppl 2):13–22. doi: 10.1007/s00234-013-1233-y. [DOI] [PubMed] [Google Scholar]
- 16.Pierrat V, Duquennoy C, van Haastert IC, Ernst M, Guilley N, de Vries LS. Ultrasound diagnosis and neurodevelopmental outcome of localised and extensive cystic periventricular leucomalacia. Arch Dis Child Fetal Neonatal Ed. 2001;84(3):F151–F156. doi: 10.1136/fn.84.3.F151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayakawa F, Okumura A, Kato T, Kuno K, Watanabe K. Determination of timing of brain injury in preterm infants with periventricular leukomalacia with serial neonatal electroencephalography. Pediatrics. 1999;104(5 Pt 1):1077–1081. doi: 10.1542/peds.104.5.1077. [DOI] [PubMed] [Google Scholar]
- 18.Ito T, Hashimoto K, Kadowaki K, et al. Ultrasonographic findings in the periventricular region in premature newborns with antenatal periventricular leukomalacia. J Perinat Med. 1997;25(2):180–183. doi: 10.1515/jpme.1997.25.2.180. [DOI] [PubMed] [Google Scholar]
- 19.DeVries LS, Eken P, Dubowitz LMS. The spectrum of leucomalacia using cranial ultrasound. Behav Brain Res. 1992;49(1):1–6. doi: 10.1016/s0166-4328(05)80189-5. [DOI] [PubMed] [Google Scholar]
- 20.Volpe JJ. Cerebral white matter injury of the premature infant-more common than you think. Pediatrics. 2003;112(1 Pt 1):176–180. doi: 10.1542/peds.112.1.176. [DOI] [PubMed] [Google Scholar]
- 21.Khwaja O, Volpe JJ. Pathogenesis of cerebral white matter injury of prematurity. Arch Dis Child Fetal Neonatal Ed. 2008;93(2):F153–F161. doi: 10.1136/adc.2006.108837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maalouf EF, Duggan PJ, Counsell SJ, et al. Comparison of findings on cranial ultrasound and magnetic resonance imaging in preterm infants. Pediatrics. 2001;107(4):719–727. doi: 10.1542/peds.107.4.719. [DOI] [PubMed] [Google Scholar]
- 23.Dyet LE, Kennea N, Counsell SJ, et al. Natural history of brain lesions in extremely preterm infants studied with serial magnetic resonance imaging from birth and neurodevelopmental assessment. Pediatrics. 2006;118(2):536–548. doi: 10.1542/peds.2005-1866. [DOI] [PubMed] [Google Scholar]
- 24.Hintz SR, Slovis T, Bulas D, et al. NICHD Neonatal Research Network. Interobserver reliability and accuracy of cranial ultrasound scanning interpretation in premature infants. J Pediatr. 2007;150(6):592–596. 596.e1–596.e5. doi: 10.1016/j.jpeds.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]