Summary
Animals can detect and consume nutritive sugars without the influence of taste. However, the identity of the taste-independent nutrient sensor and the mechanism by which animals respond to the nutritional value of sugar are unclear. Here, we report that six neurosecretory cells in the Drosophila brain that produce Diuretic hormone 44 (Dh44), a homologue of the mammalian corticotropin-releasing hormone (CRH), were specifically activated by nutritive sugars. Flies in which the activity of these neurons or the expression of Dh44 was disrupted failed to select nutritive sugars. Manipulation of the function of Dh44 receptors had a similar effect. Notably, artificial activation of Dh44 receptor-1 neurons resulted in proboscis extensions, and frequent episodes of excretion. Conversely, reduced Dh44 activity led to decreased excretion. Together, these actions facilitate ingestion and digestion of nutritive foods. We propose that the Dh44 system directs the detection and consumption of nutritive sugars through a positive feedback loop.
Introduction
Sugars in the natural environment can be detected through taste-dependent and taste-independent modalities. Taste-dependent modalities consist mainly of peripheral taste receptor cells such as sweet-sensing cells, which primarily detect the palatability of sugar (see review, Yarmolinsky et al., 2009) Evidence of a taste-independent modality was shown more than 20 years ago when investigators showed that rodents could learn to select a flavored solution when it was paired with an intragastric infusion of nutritive sugars, but not with water or nonnutritive saccharin (see review, Sclafani and Ackroff, 2012). This finding was further demonstrated by experiments using taste-insensitive Trpm5 (−/−) mice, which learn to associate nutritive sugars paired with a conditioned stimulus independent of taste input (de Araujo et al., 2008). Similarly, fruit flies - Drosophila melanogaster - are capable of associating the caloric value of sugars with an odorant to establish a long-term memory (Burke and Waddell, 2011; Fujita and Tanimura, 2011; Musso et al., 2015).
While animals and humans can learn to recognize the nutritional value of sugar during sugar-preference conditioning (Birch et al., 1990; Brunstrom and Mitchell, 2007; Yeomans et al., 2008), Drosophila do not need to be trained to distinguish between nutritive sugars and nonnutritive sugars. Studies have shown that naive flies that had not previously been exposed to nutritive sugars or nonnutritive sugars were still able to select nutritive sugars over nonnutritive ones after periods of food deprivation in a two-choice preference assay (Dus et al., 2011; Miyamoto et al., 2012; Stafford et al., 2012). The post-ingestive preference for a nutritive sugar appears to be mediated by a hardwired neuronal pathway that is activated by the detection of nutritive sugars. However, the molecular and cellular identity of the nutrient sensor and the neural circuitry that allows flies (as well as mammals) to respond to the nutritional value of exogenous sugar is largely unknown.
The postprandial increase in the intestinal and circulating glucose levels plays an important role in the ability of animals to choose conditioned stimuli paired with nutritive sugars. Several studies in rodents showed that intravenous glucose administration is sufficient for preference conditioning, while direct stimulation of the intestinal mucosa was also shown to be important (Mather et al., 1978; Oliveira-Maia et al., 2011; Tordoff and Friedman, 1986; Zukerman et al., 2013). This relationship was further supported by the observation in flies that administrating phlorizin, which lowers hemolymph glycemia by inhibiting sugar transport, blocked the flies’ ability to select nutritive sugars (Dus et al., 2013). Notably, taste-independent sugar conditioning was shown to correlate with the rate of glucose utilization instead of circulating glucose levels in mice (Ren et al., 2010). In humans, the physiological parameter that appears to correlate with preference conditioning is also metabolic responses to glucose (de Araujo et al., 2013). While these studies illustrate that utilizing intracellular glucose is crucial for activating behavioral responses, circulating plasma glucose level is key in determining intracellular glucose concentration.
Indeed, Jean Mayer proposed over five decades ago that feeding is regulated by neurons in the brain that sense circulating blood glucose levels (Mayer, 1953). This “glucostatic hypothesis” was substantiated by the discovery of glucose-sensing neurons in the hypothalamus (Anand et al., 1964; Oomura et al., 1964). These specialized neurons use the products of glucose metabolism to regulate neuronal excitability and neurotransmitter release. Metabolic enzymes such as glucokinase, the AMP-activated protein kinase (AMPK), and the ATP-sensitive K+ (KATP) channel were implicated in mediating this process (Kang et al., 2004; Minokoshi et al., 2004). However, the disruption of KATP channel or AMPK function in glucose-excited pro-opiomelanocortin (POMC) neurons, which impaired their ability to sense glucose, did not result in a discernable feeding phenotype in mice (Claret et al., 2007; Parton et al., 2007). While several populations of glucose-sensing neurons have been identified in the hindbrain and hypothalamus, their biological role in feeding-related behavior is still elusive (Levin, 2007).
In this work, we identified a population of neurons in the fly brain producing the Diuretic hormone 44 neuropeptide (Dh44 - the insect homologue of the mammalian CRH) (Lovejoy and Jahan, 2006) that is essential for mediating taste-independent behavioral responses to the nutritional value of sugar. Calcium imaging revealed that Dh44 neurons are activated by solutions containing nutritive sugars and require a functional glucokinase enzyme to detect these sugars. The Dh44 neuropeptide conveys the information from Dh44 neurons to Dh44 receptor R1 neurons in the brain and R2 cells in the gut, both of which are also required for nutrient selection. Furthermore, artificial activation of Dh44 R1 neurons stimulated rapid proboscis extension reflex (PER) responses, promoting food intake. Flies with activated Dh44 R1 neurons also excreted more frequently, a behavior likely increased by gut motility. Conversely, reduced Dh44 signaling resulted in a lower frequency of excretion. We propose that this putative post-ingestive nutrient sensor activates two pathways: one to promote PER to enhance the ingestion of nutritive foods and another to enhance the gut motility, which would facilitate digestion of greater volumes of the nutritive foods.
Results
The activity of Dh44 neurons is essential for post-ingestive nutrient selection
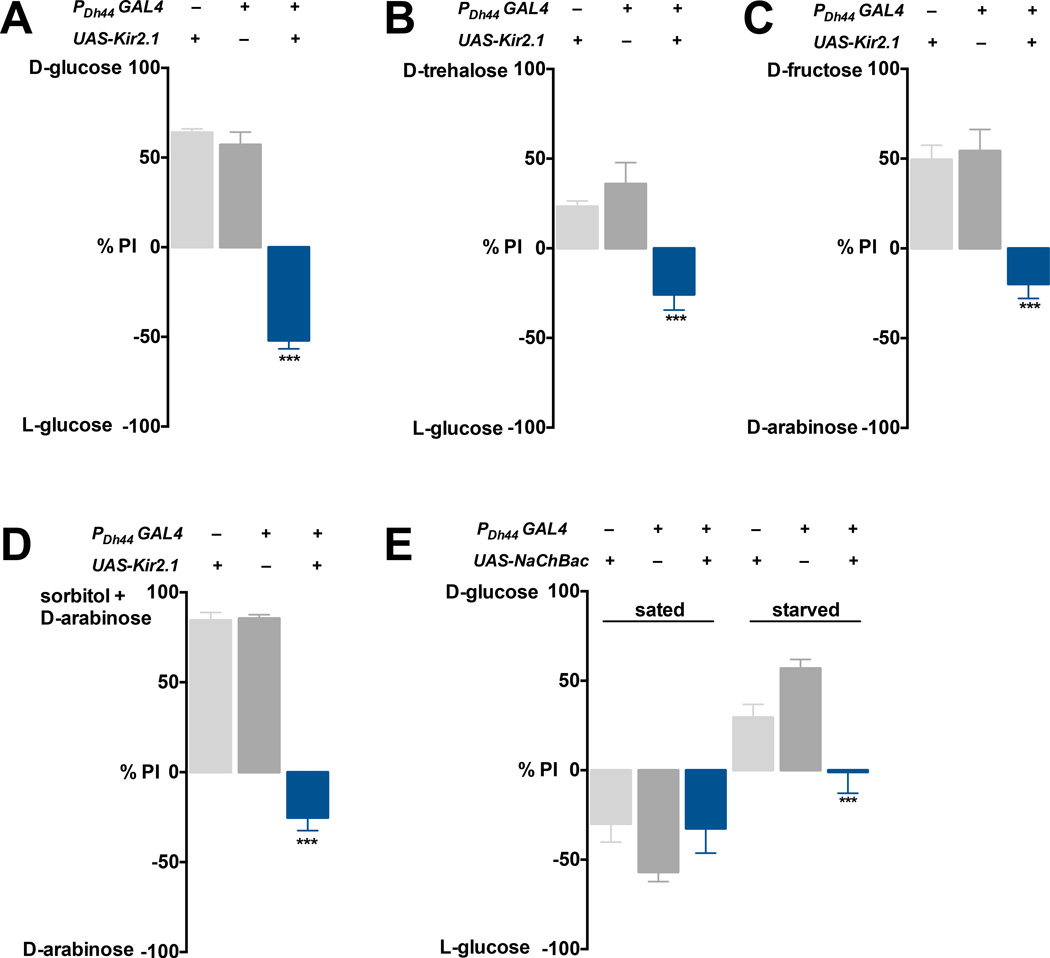
To identify the neural circuitry that underlies the post-ingestive effects of nutritive sugars, we searched for neurons that are required for selection of a nutritive sugar over a nonnutritive sugar after periods of food deprivation. We screened a collection of neuropeptide GAL4 lines crossed to UAS-Tetanus toxin (TNT), which eliminates synaptic transmission, in the two-choice assay. Nearly all of these tested fly lines chose a more concentrated, yet nonnutritive L-glucose (200mM) when they were sated, but developed a preference for nutritive D-glucose (50mM) when they were starved for 18 hours. We found that inactivation of Dh44 neurons by expressing Kir2.1, an inwardly rectifying K+ channel (Nitabach et al., 2002), abolished the preference for D-glucose in starved flies (Figure 1A). We investigated the possibility that Dh44 neurons are also required for flies to select two other sugars that are normally present in the hemolymph: D-trehalose, which like D-glucose is found in abundance and D-fructose, which is found in minute amounts (Miyamoto et al., 2012). We gave flies carrying PDh44-GAL4 and UAS-Kir2.1 a choice between these nutritive sugars and nonnutritive sweeteners. These flies failed to select D-trehalose or D-fructose over higher concentrations of the nonnutritive sweeteners (Figures 1B and 1C). They also failed to respond to the tasteless, yet nutritive sorbitol (Figure 1D). These observations indicate that Dh44 neurons play an important role in mediating the selection of nutritive sugars independent of taste input.
Figure 1. Manipulating the activity of Dh44 neurons perturbs post-ingestive nutrient selection.
The food preference of flies that were given a choice between a sweeter, yet nonnutritive sugar (L-glucose or D-arabinose) and a nutritive sugar (D-glucose, D-trehalose, D-fructose, or sorbitol) after 5h (sated) or 18h (starved) food deprivation. A–D) Inactivation of Dh44 neurons by expression of UAS-Kir2.1 transgene using the PDh44-GAL4 driver (blue bars) abolishes preference for nutritive sugars in starved flies. Flies carrying each transgene alone were used as controls (gray bars). Flies were given a choice between A) 200mM L-glucose v. 50mM D-glucose; B) 200mM L-glucose v. 100mM D-trehalose; C) 80mM Darabinose v. 25mM D-fructose; D) 20mM D-arabinose v. 20mM D-arabinose+ 80mM sorbitol. E) Artificial activation of Dh44 neurons by expression of UAS-NaChBac using PDh44-GAL4 abolishes the preference for D-glucose over L-glucose in starved flies, but does not affect sated flies. Flies bearing each transgene alone were used as controls. n=4–10 with each trial comprising approximately 40 flies for this and all subsequent behavior figures. ***P<0.001 (one-way ANOVA with Tukey post-hoc test). Error bars, s.e.m.
We then examined whether artificial activation of Dh44 neurons is sufficient to communicate the reward of nutrient, even when nonnutritive sugars are fed. For this experiment, we generated flies expressing NachBac (Nitabach et al., 2005), a bacterial sodium channel that increases the electrical excitability of neurons under the control of PDh44-GAL4, and tested them in the two-choice assay (D- vs. L-glucose). These flies did not demonstrate a preference for the nutritive sugar even though they had not been fed for 18 hours (Figure 1E). Instead, they consumed both D- and L-glucose indiscriminately, likely because the both glucose enantiomers are perceived as nutritious to these flies when Dh44 neurons are artificially activated. Thus, Dh44 neurons are necessary and sufficient for post-ingestive nutrient sensing behavior. Inactivation or activation of Dh44 neurons, however, did not affect the amount of food intake (Figure S1), suggesting that these neurons selectively control food choice behavior instead of food consumption.
Six Dh44 neurons localized to the pars intercerebralis mediate the behavior
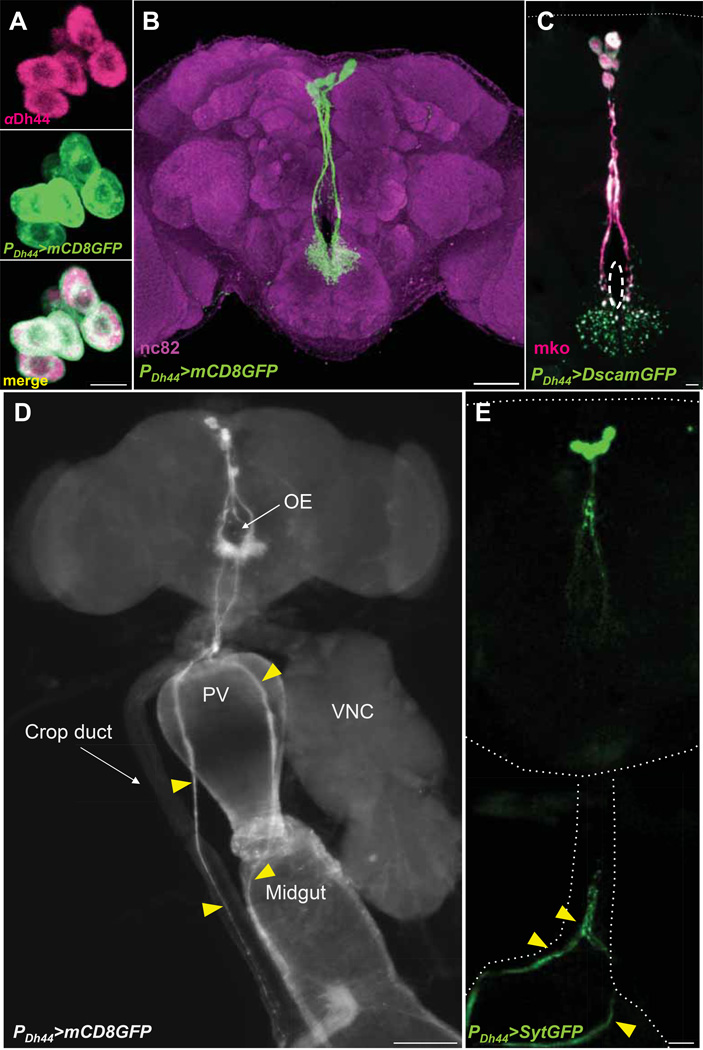
To determine the expression pattern of the PDh44-GAL4 line, we crossed it to UAS-mCD8GFP and found labeled six cells located in the pars intercerebralis (PI) (Figure 2), a region of the fly brain populated with neurosecretory cells. It has been suggested that the pars intercerebralis serves as the functional correlate of the mammalian hypothalamus (de Velasco et al., 2007). The six labeled cells were indeed immunopositive for anti-Dh44 antibody (Zitnan et al., 1993) (Figure 2A). Given that the targeted expression of Dh44 in these cells rescued the behavioral defects caused by the Dh44 mutation (Figure 4B), these neurons are important for mediating selection of nutritive sugars. Dh44 neurons project their neurites to the dorsal region of the subesophageal zone (SEZ) and also extend their lengthy processes along the esophagus to innervate the crop and midgut in the abdomen (Figure 2B and 2D). Using a fluorescent postsynaptic marker, Down Syndrome Cell Adhesion Molecule (Dscam)-GFP (Wang et al., 2004), we traced the dendrites of Dh44 neurons as they arborize in the SEZ (Figure 2C). Conversely, a presynaptic marker, synaptotagmin (Syt)-GFP (Zhang et al., 2002), expressed by the PDh44-GAL4 driver illustrated that these neurons extend their axonal projections along the esophagus to innervate the gut (Figure 2E). In addition to these Dh44 cells located in the brain, three-to-four Dh44 neurons are found in the posterior ventral nerve cord (VNC) (Data not shown).
Figure 2. The expression pattern of the PDh44-GAL4 line.
A) The reporter PDh44-GAL4>mCD8GFP (green) labels Dh44 cells in the PI, visualized by anti-Dh44 antibody (pink) in a z-stack confocal image with 1µm optical sections. Merge is in yellow. Scale bar, 10µm. B) A z-stack image of the brain of a fly carrying PDh44-GAL4 and UAS-mCD8GFP (green) counterstained with the neuropil marker nc82 (magenta). Scale bar, 50µm. C) The dendritic arborization of Dh44 neurons, visualized by PDh44-GAL4>DscamGFP (green), in the dorsal region of the SEZ. Dh44 cell bodies and processes are labeled by a fluorescent marker, Monomeric Kusabira Orange (mko) (pink). Dotted circle depicts the esophagus. Scale bar, 10µm. D) The neurites of PDh44-GAL4>mCD8GFP cells innervate the gut and crop (yellow arrowheads). OE, esophagus; VNC, ventral nerve cord; PV, proventriculus. Scale bar, 100µm. E) The axons of Dh44 neurons, visualized by PDh44-GAL4>SytGFP (green), descend along the esophagus to innervate the gut (yellow arrowheads) revealed in a zstack image. Scale bar, 20µm.
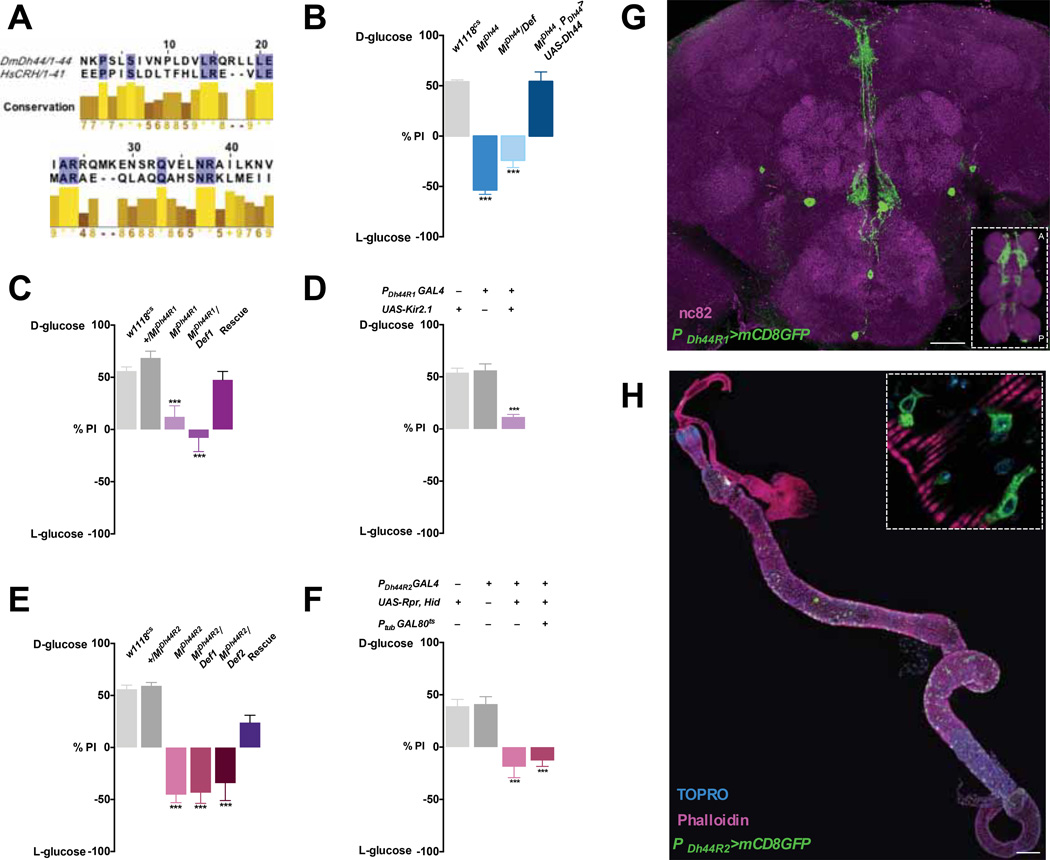
Figure 4. Dh44, Dh44 receptors and their neurons are required for post-ingestive nutrient selection.
A) Alignment of the mature peptide sequence of the human CRH and fly Dh44 neuropeptides using Jalview (Waterhouse et al., 2009). Top, Blue color indicates identical residues. Bottom, the degree of amino acid conservation demonstrated by the shade of color (bright yellow to dark brown indicates decreasing similarity), the height of histogram bar, and a numerical score (n>5 high identity, n<5 low identity, n=+ Isoleucine/leucine). B–F) The food preferences in the two-choice assay (50mM D-glucose v. 200mM L-glucose) after 18h starvation were measured in: B) Dh44 mutants (MiDh44 or MiDh44/Def). w1118CS and MiDh44 mutant carrying UAS-Dh44 under the control of PDh44-GAL4 were used as controls. n=3–8; C) Dh44R1 mutants (MiDh44R1 and MiDh44R1/Def). w1118CS, MiDh44R1/+ and MiDh44R1; PDh44R1>UAS-Dh44R1 flies were used as controls. n=3–9; D) Flies harboring PDh44R1-GAL4 and UAS-Kir2.1. Flies carrying each transgene alone were used as controls. n=4–7; E) Dh44R2 mutants (MiDh44R2, MiDh44R2/Def1, and MiDh44R2/Def2). w1118CS, MiDh44R2/+ and MiDh44R2; PDh44R2>UAS-Dh44R2 flies were used as controls. n=3–9; F) flies carrying PDh44R2-GAL4 and UAS-Reaper, Hid, and flies carrying PDh44R2-GAL4, UAS-Hid, and Ptubulin-GAL80ts tested after GAL80ts was inactivated. Flies carrying either PDh44R2-GAL4 or UAS-Reaper, UAS-Hid transgene alone were used as controls. n=3–10. *** P<0.001 (one-way ANOVA with Tukey post-hoc tests). Error bars, s.e.m. G) A z-stack image of the brain of a fly carrying PDh44R1-GAL4 and UAS-mCD8GFP (green) counterstained with the neuropil marker, nc82 (magenta). Scale bar, 50µm. Inset, the expression of PDh44R1-GAL4>mCD8GFP in the VNC. A, Anterior; P, Posterior. H) A z-stack image of the midgut of a fly carrying PDh44R2-GAL4 and UAS-mCD8GFP, counterstained with Phalloidin (pink) and TOPRO (DNA, cyan). Scale bar, 200µm. Inset, Magnified image of PDh44R2-GAL4>mCD8GFP in a subset of enteroendocrine cells (green) in the midgut.
Dh44 neurons are activated by nutritive sugars, and not by nonnutritive sugars
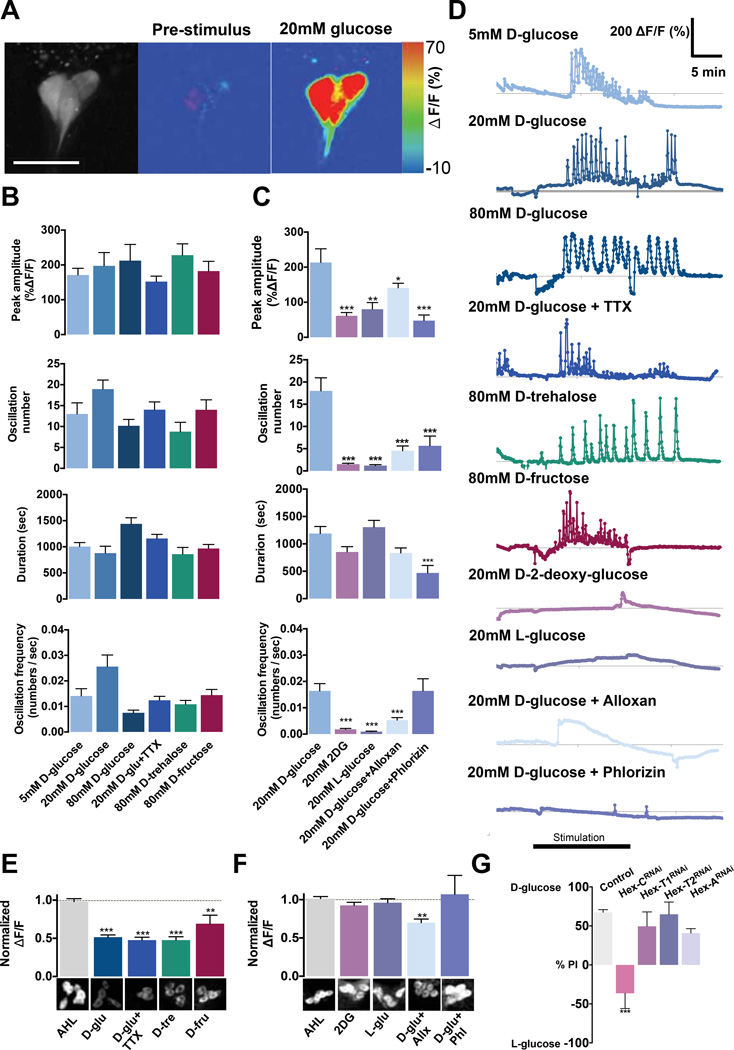
We next asked whether Dh44 neurons respond to nutritive sugars. By performing calcium imaging on ex vivo brain preparations of flies carrying the fluorescent calcium indicator, UAS-GCaMP3.0, and PDh44-GAL4, we found that Dh44 neurons were activated by nutritive D-glucose, D-trehalose, and D-fructose with substantial calcium oscillations (Figures 3A–D and Movie S1). In vivo calcium imaging also showed that Dh44 neurons respond to solutions containing nutritive sugar (Figure S2) (Ai et al., 2010). Sucrose, which was used in the control saline, did not stimulate these neurons (Figure S3), suggesting that only hemolymph sugars are effective. Calcium oscillations are a characteristic of neurosecretory cells and occur when these cells are secreting neuropeptides (Thorner et al., 1988). As the concentration of perfused nutritive sugars was increased, the frequency of these oscillations decreased, yet they were longer in duration. The oscillations were still clearly observed in response to D-glucose concentrations as low as 5mM, and approximately 4 minutes after the brain tissue was exposed to this sugar solution. By contrast, exposures to nonnutritive sugars, L-glucose and 2-deoxy-glucose, resulted in a very limited increase in calcium transit and completely lacked calcium oscillations (Figures 3C and 3D).
Figure 3. Activation of Dh44 neurons by nutritive sugars promotes the secretion of Dh44 neuropeptide.
A–D) The ex vivo brain preparations of flies carrying PDh44-GAL4 and UAS-GCaMP3.0 were exposed to AHL (Adult Hemolymph Like) saline containing different sugars. A) The response (ΔF) of Dh44 neurons to AHL containing 20mM D-glucose (right). Pre-stimulation images show the position of six Dh44 cells (left) and the response to the control AHL containing 20mM sucrose (middle). Scale bar, 20µm. B) Quantification of Dh44 neuronal responses to nutritive sugars: D-glucose (blue bars), D-trehalose (green), and D-fructose (magenta), and D-glucose mixed with tetrodotoxin (TTX, 0.5µM) (bright blue). Peak amplitude (ΔF/F) was obtained by subtracting the pre-stimulation baseline (average of 10–15 frames) from the sugar-evoked peak value; Oscillation number refers to the total number of calcium transients during stimulation; Duration is the length of Dh44 neuronal response to stimulation; Oscillation frequency is calculated as a ratio of oscillation number/duration. n=9–27 cells. C) Quantification of Dh44 neuronal responses to nonnutritive sugars: 2-deoxy-D-glucose (2DG, lavender bars) and L-glucose (light purple), and D-glucose mixed with Alloxan (4 µM), a hexokinase inhibitor (light blue), and D-glucose mixed with phlorizin (1 µM), a glucose transporter inhibitor (purple bar). n=5–19 cells. *P<0.05, **P<0.01, ***P<0.001. D) Representative traces of Dh44 neuronal responses to different sugars. E–F) The immunofluorescence measurement of intracellular Dh44 neuropeptide, probed with anti-Dh44 antibody upon E) stimulation of Dh44 cells with 80mM D-glucose, D-trehalose, D-fructose, D-glucose mixed with TTX (0.5µM), and the control AHL containing 80mM sucrose. n=24–42 cells; F) stimulation of Dh44 cells with 80mM 2-deoxy-glucose, L-glucose and D-glucose mixed with alloxan (Allx) or with phlorizin (Phl). Representative images of Dh44 neurons stimulated by different sugars are shown below. n=22–43 cells. ** p<0.01. *** P<0.001. G) The food preference of flies carrying PDh44-GAL4 and UAS-RNAi for each hexokinase in the two-choice assay (50mM D-glucose v. 200mM L-glucose) after 18h starvation. Flies bearing PDh44-GAL4 alone were used as a control. n=4–6. ***P<0.001 (one-way ANOVA with Tukey post-hoc test). Error bars, s.e.m.
To determine whether sugar-induced activation of Dh44 neurons results in the release of Dh44 neuropeptide from these neuronal cells, we incubated Drosophila brains in saline solutions containing different sugars and then probed each brain by using anti-Dh44 antibody to measure the amount of Dh44 left inside the cells. We found significantly less Dh44 immunoreactivity in Dh44 cells of the brains exposed to nutritive sugars compared to those exposed to the control saline (Figure 3E). Among the nutritive sugars, D-glucose and D-trehalose had stronger effects than D-fructose. In contrast, exposures to the nonnutritive sugars, L-glucose and 2-deoxy-glucose, had no effect on Dh44 immunoreactivity (Figure 3F). These findings suggest that sugar-induced activation promotes the release of Dh44 neuropeptide from these neurons.
Consistent with these findings, inactivating Dh44 neurons by UAS-Kir2.1 expression, which resulted in impaired post-ingestive nutrient selection, suppressed the secretion of Dh44 peptide even when the Dh44 cells were stimulated by D-glucose (Figure S4). By contrast, artificial activation of Dh44 neurons by expressing of UAS-NachBac, which was sufficient to communicate the reward of nutrient, released the Dh44 peptide without sugar stimulation (Figure S4). Therefore, manipulating Dh44 neuronal function has distinct effects on the neuronal activity and peptide release, supporting the view that Dh44 neuropeptide is the signal that communicates the information about the rising levels of nutritive sugar in the internal milieu.
Dh44 neuronal responses require sugar entry and a hexokinase
The observation that Dh44 neurons respond specifically to nutritive sugars led us to consider the possibility that intracellular metabolism of nutritive sugars in these cells stimulated the release of Dh44 peptide. The first steps critical for glucose metabolism are the entry of glucose into the Dh44 cells and the conversion of glucose to glucose-6-phosphate by hexokinase. The sugar entry is required for the activation of Dh44 neurons, as an addition of phlorizin, an inhibitor of sugar transporters, in saline significantly reduced glucose-induced calcium oscillations and secretion of the Dh44 peptide (Figure 3C–D and 3F). To determine whether a hexokinase is important for the activation of Dh44 neurons, we used alloxan, a well-known inhibitor of hexokinase (Lenzen et al., 1988), to block glucose metabolism. When Dh44 neurons were stimulated with a mixture of D-glucose and alloxan, calcium oscillations essentially disappeared (Figures 3C and 3D). Consistent with this, glucose-induced secretion of Dh44 from these cells was reduced in fly brains incubated with the mixture of glucose and alloxan compared to glucose alone (Figure 3E and 3F). Furthermore, hexokinase C (Hex-C), one of five hexokinases present in the Drosophila genome, is required for Dh44 neuronal activation, since RNAi-induced knockdown of Hex-C expression in these neurons led to a failure in responding to nutritive sugars in the two-choice assay (Figure 3G); Hex-C RNAi knockdown did not appear to cause anomaly, as the morphology of these neurons was indistinguishable from that of wild-type Dh44 neurons (Figure S5). Intriguingly, unlike other hexokinases, Hex-C is selectively expressed in the brain, fat body and gut of the fly (Gelbart and Emmert, 2013). Pyruvate, the end product of the glycolysis pathway, also activated Dh44 neurons, further supporting a role of this metabolic pathway in the neuronal activation (Figure S6).
The fact that this pathway is autonomously required in Dh44 neurons supports the hypothesis that the function of activated Dh44 neurons is to facilitate the detection of nutritive sugars through direct activation. This hypothesis is further supported by our observation that an addition of tetrodotoxin (TTX, which blocks voltage-gated Na+ channels, and eliminates synaptic transmissions and indirect presynaptic responses) had virtually no effect on the release of Dh44 from Dh44 neurons (Figure 3E) and only an insignificant effect on their neuronal activity in response to D-glucose (Figure 3B).
Dh44, Dh44 receptors, and Dh44 receptor cells mediate post-ingestive nutrient selection
Having shown that Dh44 neurons mediate the secretion of Dh44 upon stimulation by nutritive sugars, we next determined whether the Dh44 gene, the fly homologue of the human CRH (Figure 4A), is required for starved flies to select nutritive sugars during the two-choice assay. We found that Dh44 mutants showed defects in their ability to select D-glucose over L-glucose upon starvation, which do not appear to be caused by aberrant hemolymph glycemia or glycogen levels (Figure 4B and Figure S7). These defects were rescued by the expression of a UAS-Dh44 transgene by the PDh44-GAL4 driver. Additional support for this was provided by the observation that flies with targeted knockdown of the Dh44 transcript in these neurons by Dh44 RNAi were impaired in their ability to develop a preference for D-glucose upon starvation (Data not shown).
Flies, like mammals, have two receptors for this Dh44/CRH neuropeptide: Dh44 R1 and Dh44 R2. In Drosophila, these receptors are both activated by Dh44 peptide (Hector et al., 2009; Johnson et al., 2003) To determine whether these receptors are necessary for flies to be able to select nutritive sugars, we used Dh44 R1 and R2 mutants. These mutants failed to develop a preference for D-glucose over L-glucose in the two-choice assay when starved (Figure 4C and 4E). Moreover, we generated GAL4 lines using the putative promoters for Dh44 R1 and Dh44 R2. Inactivation of Dh44 R1 neurons by expression of UAS-Kir2.1 or ablation of Dh44 R2 cells by expression of UAS-reaper, hid under the control of these GAL4 lines blocked starvation-induced selection of nutritive D-glucose (Figure 4D and 4F). These results indicate that Dh44 R1 and Dh44 R2 receptors and their cells are required for the selection of nutritive sugar.
To further our understanding of how Dh44 R1 and Dh44 R2 contribute to food choice behavior, we examined the expression patterns of the PDh44R1-GAL4 and PDh44R2-GAL4 lines. We found that the PDh44R1-GAL4 line drives the expression of UAS-CD8GFP in approximately ten cells in the fly brain and three-to-four pairs of cells in the VNC (Figure 4G and inset) These cells arborize extensively within the pars intercerebralis and extend processes along the midline, which overlap with the neurites of Dh44 neurons (see Figure 2B), to innervate the dorsal region of the SEZ. The three-to-four pairs of Dh44 R1 cells arborize their neurites throughout the VNC (see Figure 4G inset). In contrast, GFP expression was not observed in the brains of flies carrying PDh44R2-GAL4 and UAS-CD8GFP. Instead, it was seen in a large number of cells in the gut that have the characteristic shape of enteroendocrine cells (Figure 4H and inset).
Artificial activation of Dh44 R1 neurons stimulates rapid PER
We examined flies in which Dh44 R1 neurons are artificially stimulated by expression of NaChBac. In the two-choice assay, these flies chose sweeter L-glucose even when they were starved for extended periods (Figure 5A). This result is similar to the observation obtained in flies carrying PDh44-GAL4 and UAS-NaChBac (Figure 1E) that equally preferred both sugars. Yet, activation of these receptor neurons appeared to be more effective in relieving the preference for nutritive sugar and caused the flies to select more palatable L-glucose, which is detected by intact, external sugar receptors. These illustrate that Dh44 R1 neurons, like Dh44 neurons, are sufficient for mediating post-ingestive nutrient selection.
Figure 5. Artificial activation of Dh44 R1 neurons results in rapid proboscis extension even in absence of food.
A) The food preferences of flies carrying PDh44R1-GAL4 and UAS-NaChBac in the two-choice assay (50mM D-glucose v. 200mM L-glucose) after 5h (sated) and 18h (starved) food deprivation. Flies harboring each transgene alone were used as controls. n=4–10. B) Acute temperature-induced activation of Dh44R1 neurons in flies bearing PDh44R1-GAL4 and UAS-TrpA1 at 30°C promotes robust PER responses in the absence of food. Flies carrying each transgene alone were used as controls, n=12–15. *** P<0.001 (one-way ANOVA with Tukey post-hoc test). Error bars, s.e.m.
Furthermore, we serendipitously observed dramatic increased PER responses, which promote food intake, when Dh44 R1 neurons were artificially activated. We expressed the inducible heat-activated Transient receptor potential A1 cation channel, UAS-TrpA1 (Parisky et al., 2008), in Dh44 R1 neurons and tested the flies at a temperature, 30°C, which triggers inward currents through the channel. The natural feeding pattern is characterized by the repeated extension and retraction of the proboscis, and opening and closing of labella at the tip of the proboscis, which is evoked by contact with food. By contrast, flies with activated Dh44 R1 neurons lifted the rostrum out of the head and opened the labella at a high frequency even in the absence of food. (Figure 5B and Movie S2). These Dh44 R1 neurons do not appear to be motor neurons since axonal projections to labella muscles were not observed. Instead, their processes innervate the dorsal region of the SEZ, where motor neurons reside, send axonal projections to the muscles, and mediate PER responses (Gordon et al., 2008; Manzo et al., 2012).
The Dh44 system is necessary and sufficient for gut motility and excretion
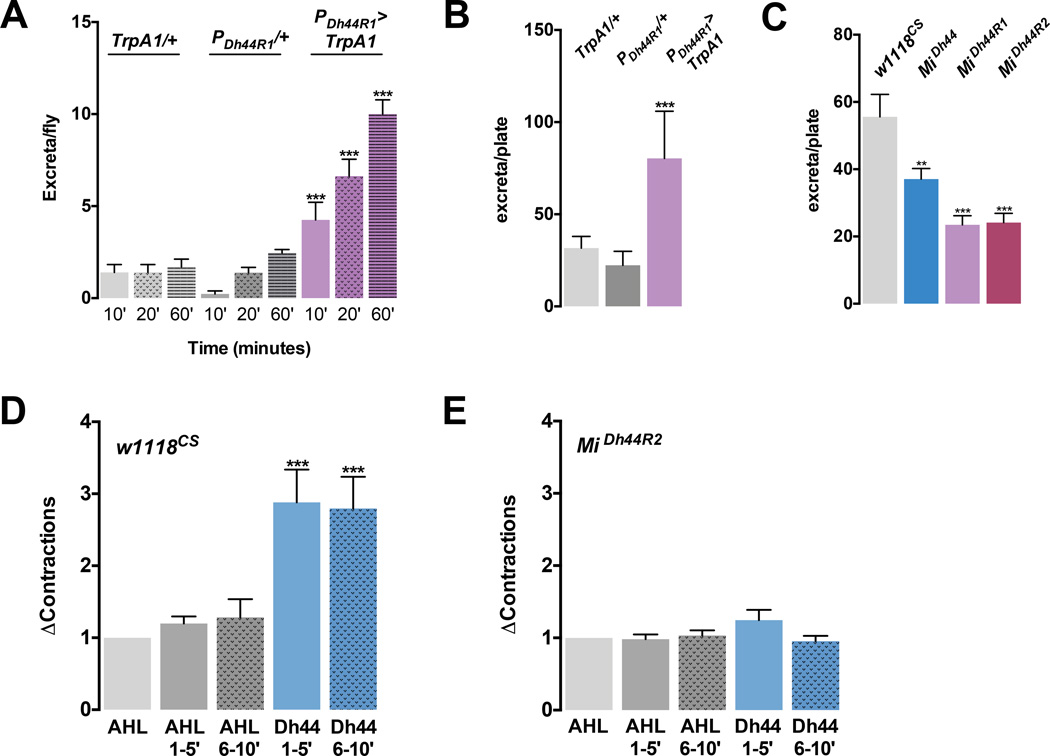
We also observed that artificial activation of Dh44 R1 neurons resulted in a remarkably increased rate of excretion. Individual flies carrying PDh44R1-GAL4 and UAS-TrpA1 excreted significantly higher numbers of waste deposit within 10 minutes and approximately five-fold higher numbers of waste deposit within 60 minutes than the control flies after they were incubated at 30°C (Figure 6A). The number of excreta from a population of 30 flies was three-fold higher than the control flies (Figure 6B). This finding is in accordance with previous reports in which the rapid release of Dh44 into the circulation after meals resulted in increased rates of excretion in other insects (Audsley et al., 1997; Iaboni et al., 1998). Conversely, mutants for Dh44 and Dh44 receptors yielded significantly fewer excreta than the control flies (Figure 6C). This result further supports that the rate of excretion is stimulated by the Dh44 neuropeptide, which is released by the consumption of food containing nutritive sugars.
Figure 6. The Dh44 system is necessary and sufficient for gut motility and excretion.
A) Number of excreta in individual PDh44R1-GAL4>TrpA1 flies starved for 18 hours were measured at different time points at 30°C. Flies carrying each transgene alone were used as controls. n=13–15. ***P<0.001. B–C) Number of excreta in a population of 30 flies: B) flies carrying PDh44R1-GAL4 and UAS-TrpA1, and flies carrying each transgene alone tested at 30°C. n=3. ***P<0.001. Filter papers with the resultant excreta are shown in SI9; C) Dh44, Dh44R1 and Dh44R2 mutants, and w1118CS control flies. n=5–17. **P<0.01, ***P<0.001. D–E) Gut propulsivity of w1118CS (D) or MiDh44R2 mutant (E) in response to the control AHL saline (D–E, gray bars) and AHL containing Dh44 peptide (10−6 µM) (D–E, blue bars). AHL 1–5’ and Dh44 1–5’ refer to minutes 1–5’ incubated in AHL and AHL containing Dh44 peptide; AHL 6–10’ and Dh44 6–10’ refers to minutes 6–10’ incubated in AHL and AHL containing Dh44 peptide, respectively. Δcontractions (y-axis) were calculated by normalizing the number of contractions in AHL containing Dh44 peptide over those in the control AHL. n=9–17 guts. *** P<0.001. (one-way ANOVA with Tukey post-hoc test). Error bars, s.e.m.
CRH in mammals was shown to promote gut motility through CRH receptors in the gut (Tache and Perdue, 2004). We therefore asked whether Dh44 peptide increases the gut motility. The spontaneous contractions of the dissected fly gut were measured after Dh44 peptide was perfused onto the gut preparation. We found that Dh44 at a concentration previously shown to activate Dh44 receptors (Hector et al., 2009; Johnson et al., 2003) stimulated the gut motility approximately three fold compared to the control saline (Figure 6D, Movie S3a and S3b). Conversely, Dh44-induced enhancement of the gut motility was eliminated in mutants for Dh44 R2 (Figure 6E, Movie S4a and S4b) and was unaffected in mutants for Dh44 R1 (Data not shown). These results indicate that coordinated activity of the Dh44 microcircuit regulates appropriate gut motility and excretion.
Flies rapidly detect the nutritional value of sugar
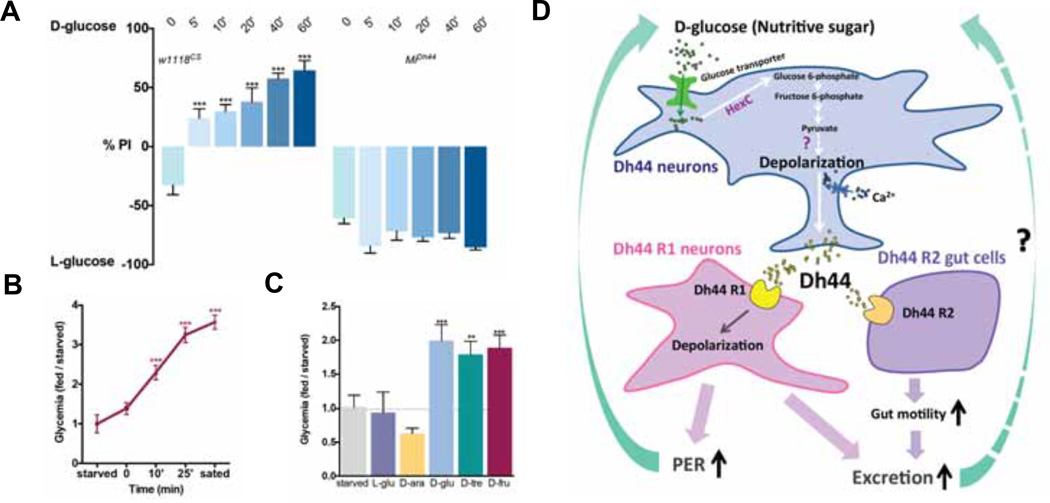
Next, we sought to understand how roaming flies in the two-choice arena readily distinguish a nutritive sugar from a nonnutritive sugar. We investigated the possibility that flies might be capable of detecting the nutritional value of sugars within few minutes of ingesting food. To this end, we carried out a time course study; the preference of flies was scored every few minutes after they began to ingest either D-glucose or L-glucose in the two-choice assay. Intriguingly, we observed that starved wild type flies initially selected sweeter L-glucose, but within 5 minutes, they started to choose nutritive D-glucose (Figure 7A). By contrast, starved Dh44 mutants chose sweeter L-glucose and failed to develop a preference for D-glucose. The finding suggests that the nutritional value of D-glucose is detected in a relatively fast time scale. Consistent with this, a significant increase in hemolymph glycemia was observed within few minutes of ingesting food (Figure 7B and 7C). The rapid detection enables immediate stimulation of innate behavioral programs that lead to the continuation of D-glucose ingestion through increased PER responses and the activation of food processing in the gut (Figure 7D).
Figure 7. Flies promptly detect the nutritional value of sugar.
A) The food preference in the two-choice assay (50mM D-glucose v. 200mM L-glucose), measured at different time points (x-axis) in w1118CS and MiDh44 mutant flies. Time point 0’ is the time at which the majority of flies started to feed. n=4–10, ***P<0.001 (one-way ANOVA with Dunnet post-hoc test). Error bars, s.e.m. B) Measurement of circulating glucose and trehalose levels in 18h-starved male w1118CS flies that were fed with 100mM D-glucose. Their hemolymph was collected at different time points (x-axis) for each measurement. The data is normalized to hemolymph glycemia in 18h-starved flies. 0’ refers to the time at which the majority of flies began to eat. n=6–11. ***P<0.001. C) Measurement of circulating glucose and trehalose levels in 18h-starved male flies that were fed with different sugars at 100mM concentration for 15 minutes. The data is normalized to hemolymph glycemia in 18h-starved flies. n=7–14. **P<0.01, ***P<0.001 with respect to the starved flies. D) The dynamics of Dh44-mediated sugar sensing. Ingestion of nutritive sugar leads to the activation of Dh44 neurons, which results in the release of Dh44 neuropeptide. Dh44 (CRF) subsequently acts on different sites to activate PER responses and to stimulate the gut motility and excretion through a positive feedback loop.
Discussion
The Dh44 system directs the detection and consumption of nutritive sugars
We have identified the molecular and cellular nature of a sensor in the brain that detects the nutritional value of sugar through direct activation by nutritive sugars. Dh44 neurons are activated specifically by nutritive D-glucose, D-trehalose and D-fructose, which are normally found in the hemolymph, and are not activated by nonnutritive sugars or sugars that are not found in the hemolymph. Sugar-induced activation of these six central neurons resulted in secretion of the Dh44 neuropeptide, which transmits a signal to Dh44 R1 and R2 cells. Flies in which the expression of Dh44 or Dh44 receptors is disrupted or the function of Dh44 receptor cells is inactivated failed to develop a preference for nutritive sugar.
Insight into the contribution of the Dh44 downstream effectors to the selection of nutritive sugars was gained by the TrpA1-mediated activation experiment. We made the surprising observation that artificial activation of Dh44 R1 neurons rapidly induced PER responses even in the absence of food. Stimulation of Dh44 R1 neurons also caused the flies to excrete large amounts of waste deposits; conversely, inactivation of the Dh44 circuit resulted in deceleration of gut motility and excretion. Together, we propose that the Dh44 system not only mediates detection of the nutritional content of sugar, but also coordinates the ingestion and digestion of sugar by promoting proboscis extension, and the gut motility and excretion through a positive feedback loop (see Figure 7D).
Dh44 neurons- the post-ingestive nutrient sensor
Two possible mechanisms could explain how flies can make appropriate food choices in the two-choice assay. One mechanism is regulated by a post-ingestive nutrient sensor that detects the nutritional value of D-glucose through direct activation during the postprandial rise in hemolymph glycemia. Another mechanism is mediated by a prescriptive “hunger” sensor that monitors the status of the internal energy reservoir and promotes consumption of nutritive D-glucose after periods of starvation.
Several lines of evidence suggest that Dh44 neurons function as a postingestive nutrient sensor. First, Dh44 neurons are activated specifically by nutritive sugars and not by nonnutritive sugars. Second, Dh44 neurons are capable of directly sensing the nutritional value of sugar, as sugar-induced calcium responses were not eliminated in fly brains treated with TTX, a sodium channel blocker that abolishes synaptic transmission. Third, flies with Hex-C knocked down in these Dh44 neurons had impaired responses to nutritive sugar. Fourth, artificial activation of Dh44 neurons or Dh44 R1 neurons significantly reduced the preference for nutritive sugars even when the flies were starved, because activation of the putative nutrient-sensing pathway was sufficient to communicate the reward of nutrient. Therefore, starved flies carrying PDh44-GAL4 and UAS-NachBac equally preferred D- and L-glucose. This is in contrast to another population of central neurons identified from a screen that functions as a prescriptive hunger sensor. When these neurons were artificially activated, the flies chose a nutritive sugar over a nonnutritive sugar even when they were sated (unpublished data). Finally, either activation or inactivation of Dh44 neurons did not alter the amount of food consumption. This is distinct from manipulating the prescriptive hunger sensor that had substantial effects on the amount of food intake (unpublished data). These support the assertion that the glucose-sensing Dh44 neurons guide flies to recognize the nutritional value of sugar by directly monitoring circulating sugar levels and utilizing sugar molecules.
Flies detect the nutritive value of sugar in a fast time scale
The means by which flies distinguish D-glucose from L-glucose are not understood. It was proposed that flies roaming in the two-choice arena find D-glucose by associating a spatial cue for the location of the D-glucose-containing agar with the nutritional content of D-glucose. The observation that these flies are capable of selecting D-glucose even in the dark (Dus et al., 2013), however, suggests that spatial conditioning is unlikely to be involved in post-ingestive food choice behavior. Rather, the detection of nutritive D-glucose appears to be mediated by a defined population of interoceptive chemosensory neurons that elicits innate behavioral responses, similar to the sweet-evoked chemosensory responses mediated by external sweet receptors. Upon activation by a nutritive sugar, the interoceptive chemosensory neurons stimulate a constellation of behavioral sub-programs that result in a positive feedback for the selection and consumption of nutritive sugar.
Consistent with this hypothesis, the post-ingestive nutrient sensor functions on a fast time scale. Calcium imaging of dissected ex vivo brain preparations, which may not reflect the in vivo context in which ingested foods pass through the digestive tract, showed that the activity of Dh44 neurons is rapidly stimulated when exposed to nutritive sugar. Furthermore, hemolymph glycemia significantly increases as soon as flies start to ingest sugars (Figure 7B). The rise of hemolymph glycemia would readily stimulate the activity of Dh44 neurons, which are located adjacent to Insulin-Producing Cells (IPCs) in the pars intercerebralis that also respond to sugar. Finally, the time-course experiment demonstrated that flies that begin to feed in the two-choice assay are capable of responding correctly to nutritive D-glucose within 5 minutes. These results support the view that flies recognize the nutritional content of D-glucose rapidly after ingestion.
Glucose-sensitive neurons in the brain
It has been five decades since the glucostatic hypothesis was proposed, yet it is still uncertain whether glucose-sensing neurons in the brain have a role in food intake or nutrient selection. Mice that lack a critical signal transducer, AMPK or KATP channel, in their glucose-sensing neurons and thus, lack the ability to sense extracellular glucose, display essentially normal feeding behavior (Claret et al., 2007; Parton et al., 2007). A study in rats also showed a lack of any causal relationship between blood and hypothalamic glucose levels, and daily meal initiations (Dunn-Meynell et al., 2009). Recently, hypothalamic glucose-sensing MCH neurons were shown to respond to and communicate the nutritional value and reward of sugar, but it was not clear whether the glucose-excitability of these MCH neurons mediated the behavioral response (Domingos et al., 2013; Kong et al., 2010). However, central administration of 2-deoxyglucose or insulin-induced hypoglycemia does elicit food intake (Dunn-Meynell et al., 2009; Miselis and Epstein, 1975). It was speculated that extremely low brain glucose levels trigger food intake through the action of unidentified hypothalamic glucose-sensing neurons, which may protect against the dangers of hypoglycemia in mammals (Routh, 2010).
Our study in Drosophila showed that the glucose-excitability of Dh44 neurons mediates starvation-induced selection of nutritive sugars, which depends on the sugar entry and the function of Hex-C to convert the glucose into its metabolic product, glucose-6-phosphate. This step in the glucose metabolic pathway appears to be critical for stimulating the neuronal activity in Dh44 neurons and responding to the nutritional value of sugar. It is noteworthy that Hex-C mRNA is expressed in few regions including the brain, whereas another fly hexokinase, hexokinase A (Hex-A), is expressed in nearly all tissues in the fly. The intracellular glucose metabolism initiated by Hex-C, possibly through the generation of sugar metabolites, is important for detecting the nutritional value of D-glucose that elicits innate preference behavior.
Dh44- a Drosophila homologue of corticotropin-releasing hormone
Since it was discovered 25 years ago, CRH has been characterized as a hypothalamic hormone that communicates stress responses. CRH also plays a significant role in the regulation of energy balance, but the exact nature of its role is controversial. CRH appears to have an anorectic effect in rodents (Richard et al., 2002), but has an opposite effect in humans when calorie intake is stimulated by an infusion of CRH (George et al., 2010). The homology between Drosophila Dh44 and mammalian CRH is approximately 30% and between Drosophila and mammalian receptors is approximately 40%; this suggests that the function of these two systems is conserved. Indeed, mammalian CRH, which is similar in function to Drosophila Dh44, is required for the regulation of gastric and colonic movements; notably, CRH administration was shown to stimulate defecation in rodents (Tache and Perdue, 2004). Furthermore, CRH mediates glucose homeostasis by regulating hypoglycemia-induced counterregulation (CRR) (McCrimmon et al., 2006). CRR triggers a number of responses that limit glucose utilization, promote endogenous glucose production, and lead the animal to seek food. It has been suggested that the function of glucose-sensing neurons is to generate neuroendocrine stress responses to the hypoglycemic challenge, but the identity of these neurons is unknown. It would be interesting to investigate the possibility that CRH neurons, which are expressed in the hypothalamus, are glucose-sensing neurons, and capable of mediating starvation-induced behavioral responses to the nutritional value of sugar in mammals. A stress-responsive CRH system might be co-opted to allow animals to respond to the stress of starvation.
Experimental Procedures
Fly strains
Flies were grown in standard cornmeal-molasses medium at low density at 25°C. w1118 flies backcrossed to Canton-S (CS) 10 generations (referred as w1118CS) kindly provided to us by Dr. Anne Simon were used as control. Dh44 (CG8348, #24345, w1118; Mi{ET1}Dh44[MB07006]), Dh44R1 (CG8422, #23517, w1118; Mi{ET1}Dh44-R1[MB03192]), Dh44R2 (CG12370, w1118; Mi{ET1}Dh44-R2[MB10503], #29129) mutants, deficiencies (#26552, #7731, # 27929, #26388), Dh44 and hexokinases RNAi lines (#35338-Hex-C, #47331-Hex-T2, #46574-Hex-T1, #35155-Hex-A) were obtained from Bloomington Stock Center. UAS-Kir2.1 and tubulin-GAL80ts, and UAS-TNT were from Dr. David Anderson (Caltech, CA); UAS-NaChBac from Dr. Justin Blau (NYU, NY); UAS-Trp1 from Dr. Paul Garrity (Brandeis university, MA); UAS-GCaMP3.0 and UAS-GCaMP6.0 from Dr. Loren Looger and Dr. Jayaraman (Janelia, VA); UAS-mCD8GFP, UAS-Dscam-GFP, UAS-mko and UAS-Synaptotagmin-GFP from Dr. Ann-Shyn Chiang (NTHU, Taiwan); UAS-Reaper, UAS-Hid from Dr. Don Ryoo (NYU, NY).
Transgenic lines
The PDh44-GAL4 line was generated by cloning an 800bp region upstream of the Dh44 promoter into pCasper4-AUG-GAL4X. The PDh44R1-GAL4 and PDh44R2-GAL4 lines were generated in the same way by cloning the ~1kb fragment upstream of the Dh44R1 and Dh44R2 ORF into pCasper4-AUG-GAL4X. The UAS-Dh44, UAS-Dh44R1 and UAS-Dh44R2 rescue constructs were cloned by RT-PCR using total fly RNA and were subsequently subcloned into a pUAST:attb vector. Transgenic flies were generated by Bestgene, Inc.
Two-choice assay
The two-choice preference assay was as previously described (Dus et al., 2011). Briefly, approximately 40 4–8 days old male flies were food deprived in an empty vial with a Kim wipe wetted with 2ml of MilliQ water for 5h or 18h, and then given a choice between two sugars, each color-coded with a tasteless food dye, for two hours. Food preference was scored as percent preference index (% PI) shown in below:
All sugars, except for L-glucose (Carbosynth), were from Sigma.
Immunofluorescence
Immunohistochemistry of the brains was conducted according to the protocol of (Chiang et al., 2011). Gut immunostaining was performed as in (Dus et al., 2013) with an extra step in which flies were fed agar-based food for two days to decrease background. Antibodies used were as follows: mouse anti-nc82 (1:50; Developmental Studies Hybridoma Bank), rabbit anti-GFP IgG (1:500; Invitrogen), goat anti-mouse-biotin (1:200), rabbit anti-Dh44 (1:500) (Zitnan et al., 1993). Secondary antibodies were Alexa Fluor 647-Strepavidin (1:500, Invitrogen), Alexa Fluor 555 goat anti-rabbit IgG (1:500, Invitrogen); TO-PRO3 (1:500; Invitrogen) was used for DNA labeling; Alexa 555-Phalloidin (1:200, Invitrogen) was used for gut immunostaining. Images were acquired by a Zeiss LSM 510 or Zeiss LSM 700 with 1–2µm optical sections at a 1024×1024 or 512×512 resolution.
Calcium Imaging
Adult fly brains were dissected with sugar-free AHL solution and immobilized with fine tungsten pins on a SYLGARD-based perfusion chamber. During perfusion, appropriate concentrations of sucrose were added to sugar-free AHL before and after the stimulus to balance the difference in osmolarity. Each brain was recorded for 500 frames in total (512 × 512 pixel; 1 frame per 5 second); the first 100 frames were recorded before each stimulus was presented and the next 200 frames were recorded during the exposure to sugar/drug, and the following 200 frames were recorded during washout. Solutions in the perfusion chamber were operated by pinch valves, which were controlled by a ValveBank® controller (AutoMate Scientific). Changes in the fluorescence intensity were recorded with a Prairie two-photon microscope with 40× water immersion lens (Olympus). Pseudo-color images and image analyses were performed using ImageJ. Note that a single outliner was removed from quantifying the glucose + phlorizin dataset (1/9).
Measurement of intracellular Dh44
The brains of 18h-starved flies were rapidly dissected in sugar-free AHL, incubated in either AHL saline (108 mM NaCl, 8.2 mM MgCl2, 4 mM NaHCO3, 1 mM NaH2PO4, 2 mM CaCl2, 5 mM KCl, 5 mM HEPES + 80mM sucrose to balance the osmolarity) or AHL+ 80mM sugars for 30 minutes, then fixed and stained with anti-Dh44 antibody as per immunofluorescence protocol described above. Image acquisition was conducted using a Zeiss LSM 510 confocal microscope with a fixed gain setting between samples. ImageJ software was used to quantify the fluorescence intensity per cell.
Measurement of gut motility
The guts from 18h-starved flies were dissected in AHL without disrupting attached tissues and without removing the head, muscles or fat (Talsma et al., 2012). The exposed gut was pinned onto a Sylgard plate with fine tungsten pins through the proboscis and a small piece of cuticle attached to the end of the gut, and bathed in 13µl of AHL. Each gut was imaged with a Zeiss High-speed camera (2 frames/sec) connected to a stereomicroscope with 0.6× magnification. After 5 minutes in AHL, the solution was removed by capillary action and replaced with 13µl AHL containing 10−6 µM Dh44 peptide or AHL alone. Video acquisition rapidly restarted for 10 minutes. Each video was processed with the Zeiss AxioPlan 4.8 software and converted into a MP4 file with 7 frames/second. Quantification of gut contraction was conducted by visually counting for one minute after an addition of the solution to avoid diffusion artifacts: In Figure 6, AHL 1–5’ and Dh44 1–5’ refer to 4-minute long incubation (1–5 minutes). AHL 6–10’ and Dh44 6–10’ refer to 4-minute long incubation (6–10 minutes). The real-time video was accelerated 4 times (1 minute in the real-time equates 15 seconds in the video). Contractions of each gut were normalized to the number of contractions in the initial AHL solution.
PER
A 18h-starved fly was gently trapped into a chopped p200 tip to expose the head and forelegs to stimuli. Each tip was placed perpendicularly onto a slide covered with clay and positioned at the bottom of a stereomicroscope in a room heated to 30°C. After 5 minutes, each fly was observed throug h the objective of the microscope and their PER responses were counted. To obtain a video, flies were gently trapped into a glass Pasteur pipette with a small cotton plug and transferred to a 30°C heated room for 5 minutes whe re the footage was captured using a Zeiss high-speed camera and stereomicroscope at 2 frames/second.
Excretion assay
Single-fly assay: a starved single male fly was gently introduced into a glass Pasteur pipette sealed with a small cotton plug and ~5µl water to prevent desiccation and immediately transferred to a 30°C h eated room. The number of excretion (visible on the glass wall) was counted after 10’, 20’, and 60’.
Population assays: 30 males flies previously fed food + 0.1% blue dye (erioglaucine) for 3 days were introduced into a 5cm plastic Petri dish containing filter paper. These flies were either kept in room temperature or immediately transferred to a 30°C heated room for 60 minutes. T he numbers of excretion on the filter paper were quantified visually by using a stereomicroscope.
Measurement of hemolymph glycemia
Hemolymph glycemia was measured as previously described (Dus et al., 2011).
Statistics
GraphPad Prism software was used for all graphs and statistical analyses. Student’s t-test or one-way ANOVA were used.
Supplementary Material
Acknowledgments
We thank Drs. Anne Simon, Justin Blau, Ann-Shyn Chiang, Paul Garrity, Dusan Zitnan, the VDRC and the Bloomington stock center for fly stocks and reagents. We thank Drs. Jesus Torres-Vazquez, Steven Burden, Joel Belasco, Niels Ringstad for allowing us use equipment in their labs. We thank the Suh lab and Drs. Jessica Treisman, Claude Desplan and Gord Fishell for helpful comments on our manuscript. This work is supported by the NIH career development grant (NIDDK: 1K99DK097141) to MD and NIH RO1 grants (NIGMS: RO1GM08946 and NIDCD: RO1DC01279), Skirball Collaborative Award and the Irma T. Hirschl/Weill Caulier Trust Award to GSBS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
GSBS, MD, and JSYL designed, analyzed, and interpreted the experiments. GSBS wrote the manuscript with MD and other authors. MD performed the screen, all the behavioral experiments, immunostaining, excretion, glycemia and glycogen measurements. JYSL performed all the calcium imaging experiments and immunostaining. KG made the PDh44R1-GAL4 and PDh44R2-GAL4 drivers, and rescue constructs. SM showed DH44 neuronal projections in the gut. TDT and ACH constructed neuropeptide GAL4 lines. EG and CMJ helped excretion measurement. GSBS conceived and supervised the project.
References
- Ai M, Min S, Grosjean Y, Leblanc C, Bell R, Benton R, Suh GS. Acid sensing by the Drosophila olfactory system. Nature. 2010;468:691–695. doi: 10.1038/nature09537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand BK, Chhina GS, Sharma KN, Dua S, Singh B. Activity of Single Neurons in the Hypothalamic Feeding Centers: Effect of Glucose. The American journal of physiology. 1964;207:1146–1154. doi: 10.1152/ajplegacy.1964.207.5.1146. [DOI] [PubMed] [Google Scholar]
- Audsley N, Goldsworthy GJ, Coast GM. Circulating levels of Locusta diuretic hormone: the effects of feeding. Peptides. 1997;18:59–65. doi: 10.1016/s0196-9781(96)00234-3. [DOI] [PubMed] [Google Scholar]
- Birch LL, McPhee L, Steinberg L, Sullivan S. Conditioned flavor preferences in young children. Physiology & behavior. 1990;47:501–505. doi: 10.1016/0031-9384(90)90116-l. [DOI] [PubMed] [Google Scholar]
- Brunstrom JM, Mitchell GL. Flavor-nutrient learning in restrained and unrestrained eaters. Physiology & behavior. 2007;90:133–141. doi: 10.1016/j.physbeh.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Burke CJ, Waddell S. Remembering nutrient quality of sugar in Drosophila. Current biology : CB. 2011;21:746–750. doi: 10.1016/j.cub.2011.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang AS, Lin CY, Chuang CC, Chang HM, Hsieh CH, Yeh CW, Shih CT, Wu JJ, Wang GT, Chen YC, et al. Three-dimensional reconstruction of brain-wide wiring networks in Drosophila at single-cell resolution. Current biology : CB. 2011;21:1–11. doi: 10.1016/j.cub.2010.11.056. [DOI] [PubMed] [Google Scholar]
- Claret M, Smith MA, Batterham RL, Selman C, Choudhury AI, Fryer LG, Clements M, Al-Qassab H, Heffron H, Xu AW, et al. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. The Journal of clinical investigation. 2007;117:2325–2336. doi: 10.1172/JCI31516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araujo IE, Lin T, Veldhuizen MG, Small DM. Metabolic regulation of brain response to food cues. Current biology : CB. 2013;23:878–883. doi: 10.1016/j.cub.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MA, Simon SA. Food reward in the absence of taste receptor signaling. Neuron. 2008;57:930–941. doi: 10.1016/j.neuron.2008.01.032. [DOI] [PubMed] [Google Scholar]
- de Velasco B, Erclik T, Shy D, Sclafani J, Lipshitz H, McInnes R, Hartenstein V. Specification and development of the pars intercerebralis and pars lateralis, neuroendocrine command centers in the Drosophila brain. Developmental biology. 2007;302:309–323. doi: 10.1016/j.ydbio.2006.09.035. [DOI] [PubMed] [Google Scholar]
- Domingos AI, Sordillo A, Dietrich MO, Liu ZW, Tellez LA, Vaynshteyn J, Ferreira JG, Ekstrand MI, Horvath TL, de Araujo IE, et al. Hypothalamic melanin concentrating hormone neurons communicate the nutrient value of sugar. eLife. 2013;2:e01462. doi: 10.7554/eLife.01462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn-Meynell AA, Sanders NM, Compton D, Becker TC, Eiki J, Zhang BB, Levin BE. Relationship among brain and blood glucose levels and spontaneous and glucoprivic feeding. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:7015–7022. doi: 10.1523/JNEUROSCI.0334-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dus M, Ai M, Suh GS. Taste-independent nutrient selection is mediated by a brain-specific Na+ /solute co-transporter in Drosophila. Nature neuroscience. 2013;16:526–528. doi: 10.1038/nn.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dus M, Min S, Keene AC, Lee GY, Suh GS. Taste-independent detection of the caloric content of sugar in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:11644–11649. doi: 10.1073/pnas.1017096108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Tanimura T. Drosophila evaluates and learns the nutritional value of sugars. Current biology : CB. 2011;21:751–755. doi: 10.1016/j.cub.2011.03.058. [DOI] [PubMed] [Google Scholar]
- Gelbart WM, Emmert DB. FlyBase High Throughput Expression Pattern Data. ModENCODE. 2013 [Google Scholar]
- George SA, Khan S, Briggs H, Abelson JL. CRH-stimulated cortisol release and food intake in healthy, non-obese adults. Psychoneuroendocrinology. 2010;35:607–612. doi: 10.1016/j.psyneuen.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MD, Manzo A, Scott K. Fly neurobiology: development and function of the brain. Meeting on the Neurobiology of Drosophila. EMBO reports. 2008;9:239–242. doi: 10.1038/embor.2008.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hector CE, Bretz CA, Zhao Y, Johnson EC. Functional differences between two CRF-related diuretic hormone receptors in Drosophila. The Journal of experimental biology. 2009;212:3142–3147. doi: 10.1242/jeb.033175. [DOI] [PubMed] [Google Scholar]
- Iaboni A, Holman GM, Nachman RJ, Orchard I, Coast GM. Immunocytochemical localisation and biological activity of diuretic peptides in the housefly, Musca domestica. Cell and tissue research. 1998;294:549–560. doi: 10.1007/s004410051205. [DOI] [PubMed] [Google Scholar]
- Johnson EC, Bohn LM, Barak LS, Birse RT, Nassel DR, Caron MG, Taghert PH. Identification of Drosophila neuropeptide receptors by G protein-coupled receptors-beta-arrestin2 interactions. The Journal of biological chemistry. 2003;278:52172–52178. doi: 10.1074/jbc.M306756200. [DOI] [PubMed] [Google Scholar]
- Kang L, Routh VH, Kuzhikandathil EV, Gaspers LD, Levin BE. Physiological and molecular characteristics of rat hypothalamic ventromedial nucleus glucosensing neurons. Diabetes. 2004;53:549–559. doi: 10.2337/diabetes.53.3.549. [DOI] [PubMed] [Google Scholar]
- Kong D, Vong L, Parton LE, Ye C, Tong Q, Hu X, Choi B, Bruning JC, Lowell BB. Glucose stimulation of hypothalamic MCH neurons involves K(ATP) channels, is modulated by UCP2, and regulates peripheral glucose homeostasis. Cell metabolism. 2010;12:545–552. doi: 10.1016/j.cmet.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzen S, Freytag S, Panten U. Inhibition of glucokinase by alloxan through interaction with SH groups in the sugar-binding site of the enzyme. Molecular pharmacology. 1988;34:395–400. [PubMed] [Google Scholar]
- Levin BE. Neuronal glucose sensing: still a physiological orphan? Cell metabolism. 2007;6:252–254. doi: 10.1016/j.cmet.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Lovejoy DA, Jahan S. Phylogeny of the corticotropin-releasing factor family of peptides in the metazoa. General and comparative endocrinology. 2006;146:1–8. doi: 10.1016/j.ygcen.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Manzo A, Silies M, Gohl DM, Scott K. Motor neurons controlling fluid ingestion in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:6307–6312. doi: 10.1073/pnas.1120305109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather P, Nicolaidis S, Booth DA. Compensatory and conditioned feeding responses to scheduled glucose infusions in the rat. Nature. 1978;273:461–463. doi: 10.1038/273461a0. [DOI] [PubMed] [Google Scholar]
- Mayer J. Glucostatic mechanism of regulation of food intake. The New England journal of medicine. 1953;249:13–16. doi: 10.1056/NEJM195307022490104. [DOI] [PubMed] [Google Scholar]
- McCrimmon RJ, Song Z, Cheng H, McNay EC, Weikart-Yeckel C, Fan X, Routh VH, Sherwin RS. Corticotrophin-releasing factor receptors within the ventromedial hypothalamus regulate hypoglycemia-induced hormonal counterregulation. The Journal of clinical investigation. 2006;116:1723–1730. doi: 10.1172/JCI27775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- Miselis RR, Epstein AN. Feeding induced by intracerebroventricular 2-deoxy-D-glucose in the rat. The American journal of physiology. 1975;229:1438–1447. doi: 10.1152/ajplegacy.1975.229.5.1438. [DOI] [PubMed] [Google Scholar]
- Miyamoto T, Slone J, Song X, Amrein H. A fructose receptor functions as a nutrient sensor in the Drosophila brain. Cell. 2012;151:1113–1125. doi: 10.1016/j.cell.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso PY, Tchenio P, Preat T. Delayed dopamine signaling of energy level builds appetitive long-term memory in Drosophila. Cell reports. 2015;10:1023–1031. doi: 10.1016/j.celrep.2015.01.036. [DOI] [PubMed] [Google Scholar]
- Nitabach MN, Blau J, Holmes TC. Electrical silencing of Drosophila pacemaker neurons stops the free-running circadian clock. Cell. 2002;109:485–495. doi: 10.1016/s0092-8674(02)00737-7. [DOI] [PubMed] [Google Scholar]
- Nitabach MN, Sheeba V, Vera DA, Blau J, Holmes TC. Membrane electrical excitability is necessary for the free-running larval Drosophila circadian clock. J Neurobiol. 2005;62:1–13. doi: 10.1002/neu.20053. [DOI] [PubMed] [Google Scholar]
- Oliveira-Maia AJ, Roberts CD, Walker QD, Luo B, Kuhn C, Simon SA, Nicolelis MA. Intravascular food reward. PloS one. 2011;6:e24992. doi: 10.1371/journal.pone.0024992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oomura Y, Kimura K, Ooyama H, Maeno T, Iki M, Kuniyoshi M. Reciprocal Activities of the Ventromedial and Lateral Hypothalamic Areas of Cats. Science. 1964;143:484–485. doi: 10.1126/science.143.3605.484. [DOI] [PubMed] [Google Scholar]
- Parisky KM, Agosto J, Pulver SR, Shang Y, Kuklin E, Hodge JJ, Kang K, Liu X, Garrity PA, Rosbash M, et al. PDF cells are a GABA-responsive wake-promoting component of the Drosophila sleep circuit. Neuron. 2008;60:672–682. doi: 10.1016/j.neuron.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang CY, Xu C, Vianna CR, Balthasar N, Lee CE, et al. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007;449:228–232. doi: 10.1038/nature06098. [DOI] [PubMed] [Google Scholar]
- Ren X, Ferreira JG, Zhou L, Shammah-Lagnado SJ, Yeckel CW, de Araujo IE. Nutrient selection in the absence of taste receptor signaling. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:8012–8023. doi: 10.1523/JNEUROSCI.5749-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard D, Lin Q, Timofeeva E. The corticotropin-releasing factor family of peptides and CRF receptors: their roles in the regulation of energy balance. European journal of pharmacology. 2002;440:189–197. doi: 10.1016/s0014-2999(02)01428-0. [DOI] [PubMed] [Google Scholar]
- Routh VH. Glucose sensing neurons in the ventromedial hypothalamus. Sensors. 2010;10:9002–9025. doi: 10.3390/s101009002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A, Ackroff K. Role of gut nutrient sensing in stimulating appetite and conditioning food preferences. American journal of physiology Regulatory, integrative and comparative physiology. 2012;302:R1119–R1133. doi: 10.1152/ajpregu.00038.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford JW, Lynd KM, Jung AY, Gordon MD. Integration of taste and calorie sensing in Drosophila. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:14767–14774. doi: 10.1523/JNEUROSCI.1887-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tache Y, Perdue MH. Role of peripheral CRF signalling pathways in stress-related alterations of gut motility and mucosal function. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2004;16(Suppl 1):137–142. doi: 10.1111/j.1743-3150.2004.00490.x. [DOI] [PubMed] [Google Scholar]
- Talsma AD, Christov CP, Terriente-Felix A, Linneweber GA, Perea D, Wayland M, Shafer OT, Miguel-Aliaga I. Remote control of renal physiology by the intestinal neuropeptide pigment-dispersing factor in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:12177–12182. doi: 10.1073/pnas.1200247109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorner MO, Holl RW, Leong DA. The somatotrope: an endocrine cell with functional calcium transients. The Journal of experimental biology. 1988;139:169–179. doi: 10.1242/jeb.139.1.169. [DOI] [PubMed] [Google Scholar]
- Tordoff MG, Friedman MI. Hepatic portal glucose infusions decrease food intake and increase food preference. The American journal of physiology. 1986;251:R192–R196. doi: 10.1152/ajpregu.1986.251.1.R192. [DOI] [PubMed] [Google Scholar]
- Wang J, Ma X, Yang JS, Zheng X, Zugates CT, Lee CH, Lee T. Transmembrane/juxtamembrane domain-dependent Dscam distribution and function during mushroom body neuronal morphogenesis. Neuron. 2004;43:663–672. doi: 10.1016/j.neuron.2004.06.033. [DOI] [PubMed] [Google Scholar]
- Waterhouse AM, Procter JB, Martin DM, Clamp M, Barton GJ. Jalview Version 2--a multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25:1189–1191. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarmolinsky DA, Zuker CS, Ryba NJ. Common sense about taste: from mammals to insects. Cell. 2009;139:234–244. doi: 10.1016/j.cell.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeomans MR, Leitch M, Gould NJ, Mobini S. Differential hedonic, sensory and behavioral changes associated with flavor-nutrient and flavor-flavor learning. Physiology & behavior. 2008;93:798–806. doi: 10.1016/j.physbeh.2007.11.041. [DOI] [PubMed] [Google Scholar]
- Zhang YQ, Rodesch CK, Broadie K. Living synaptic vesicle marker: synaptotagmin-GFP. Genesis. 2002;34:142–145. doi: 10.1002/gene.10144. [DOI] [PubMed] [Google Scholar]
- Zitnan D, Sehnal F, Bryant PJ. Neurons producing specific neuropeptides in the central nervous system of normal and pupariation-delayed Drosophila. Developmental biology. 1993;156:117–135. doi: 10.1006/dbio.1993.1063. [DOI] [PubMed] [Google Scholar]
- Zukerman S, Ackroff K, Sclafani A. Post-oral appetite stimulation by sugars and nonmetabolizable sugar analogs. American journal of physiology Regulatory, integrative and comparative physiology. 2013;305:R840–R853. doi: 10.1152/ajpregu.00297.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.