Abstract
Background
Rates of mastectomy with immediate reconstruction are rising. Skin flap necrosis after this procedure is a recognized complication that can impact cosmetic outcomes and patient satisfaction, and, in worst cases, potentially delay adjuvant therapies. Many retrospective studies of this complication have identified variable event rates and inconsistent associated factors.
Methods
We designed a prospective study to capture the rate of skin flap necrosis and pre-, intra-, and post-operative variables with follow-up to 8 weeks post-operatively. Univariate and multivariate analyses were performed for factors associated with skin flap necrosis.
Results
Out of 606 consecutive procedures, 85 (14%) had some level of skin flap necrosis: 46 (8%) mild, 6 (1%) moderate, 31 (5%) severe, and 2 (0.3%) uncategorized. On univariate analysis for any necrosis, smoking, history of breast augmentation, nipple-sparing mastectomy, and time from incision to specimen removal were significant. In multivariate models, nipple-sparing, time from incision to specimen removal, sharp dissection, and previous breast reduction were significant for any necrosis. When looking only at moderate or severe necrosis, BMI, diabetes, nipple-sparing mastectomy, specimen size, and expander size were significant on univariate analysis. Nipple-sparing mastectomy and specimen size were significant on multivariate analysis. Nipple-sparing mastectomy was associated with higher rates of necrosis at every level of severity.
Conclusions
Rates of skin flap necrosis are likely higher than reported in retrospective series. Modifiable technical variables have limited impact on rates of necrosis. Patients with multiple risk factors should be counseled about the risks, especially if they are contemplating nipple-sparing mastectomy.
Keywords: skin flap necrosis, mastectomy, breast reconstruction
INTRODUCTION
Mastectomy is a common procedure which is increasingly chosen by patients for breast cancer management. At present, nearly 40% of women in the United States with breast cancer undergo mastectomy each year, and increasing numbers are opting for immediate reconstruction1,2, making the potential complications of the procedure of great clinical interest.
The most recent national data indicate that the choice of procedure is becoming dichotomized, with women choosing either bilateral mastectomies or breast conservation.1–5 In contrast to lumpectomy, which is an outpatient surgical procedure with very low complication rates, mastectomy with reconstruction generally requires an overnight stay and has higher complication rates. Wound complications, including flap necrosis, are the most common complication and may significantly impact both cosmetic outcomes and costs. Severe flap necrosis may delay adjuvant chemotherapy or radiotherapy. Additionally, an increasing number of women undergoing mastectomy with reconstruction are opting for nipple-sparing procedures. Ischemia of the nipple and areola are common with this procedure, and flap necrosis of this area and the surrounding skin are recognized complications.6,7 As surgical practice and patient choice continue to evolve, it is important to define risks of potential complications to improve patient selection and counseling during the decision-making process.
Reported rates of skin flap necrosis range from 2%–22% in retrospective studies.8–15 The literature is inconsistent due to differing definitions of skin flap necrosis and variable patient selection criteria. Studies have shown many factors to be associated with skin flap necrosis including smoking9,13,14,16, obesity8,9,12,14,15,17–19, incision type11,20–22, age14,16–18, hypertension14,18, tumescence16–18, volume of tissue expander fill17,18, and larger breasts.8,23 To address the limitations in the literature, we designed a prospective study to determine the rate of skin flap necrosis after mastectomy with reconstruction and to identify potentially modifiable factors that could improve patient selection and outcomes.
METHODS
With approval from the Institutional Review Board, the Breast Surgery Service and the Plastic and Reconstructive Surgery Service at Memorial Sloan Kettering Cancer Center developed a list of potentially important patient- and surgeon-level study variables. Pre-, intra-, and post-operative data with follow-up to 8 weeks post-operatively were collected prospectively on all patients undergoing unilateral or bilateral mastectomy and reconstruction from September 10, 2013 to February 28, 2014. There were no exclusion criteria, and we included patients with prior cancer treatment, neoadjuvant chemotherapy, skin-sparing or nipple-sparing mastectomy, and tissue expander, implant, or autologous tissue reconstruction. All surgeons from both services participated. Intraoperative, pre-incision measurements of flap dimensions were performed as diagrammed in Fig 1. At completion of the mastectomy, the Plastic Surgery team determined if over 5cm of dermis was exposed on the mastectomy flaps. The plastics team also trimmed the skin flaps intraoperatively to facilitate wound closure or when vascular compromise was noted based on visual inspection. The indication for trimming was not captured; indocyanine green imaging was not used in this study.
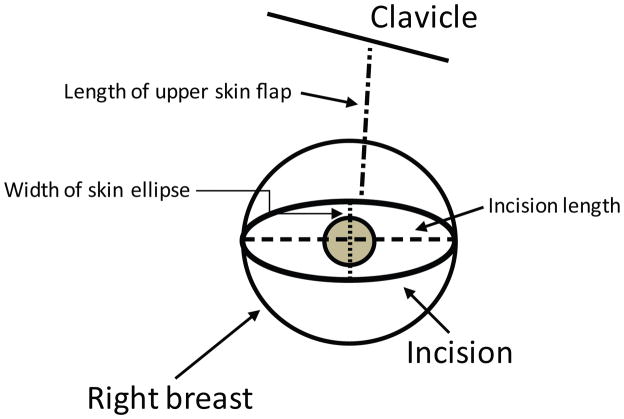
Fig 1.
Diagram of intra-operative pre-incision measurements. Incision length was measured as the horizontal distance between the lateral and medial ends of the incision. Width of skin ellipse was only used for skin-sparing procedures and was measured as the distance between the superior and inferior aspects of the incision. Length of the upper skin flap was measured from the midpoint of the superior incision to the midpoint of the clavicle.
Skin flap necrosis was defined as mild (no intervention needed, fully healed at 8 weeks), moderate (office debridement, fully healed at 8 weeks), or severe (OR debridement, implant loss, or not fully healed at 8 weeks).
All analyses were done per breast, not per patient. Patient characteristics were summarized using frequency and percentage for categorical variables, and median and range for continuous variables. Factors associated with any necrosis and moderate/severe necrosis were identified using univariate logistic regression models with oncologic surgeon and reconstructive surgeon random effects to account for possible correlation between outcomes from the same surgeon. Factors with p<0.1 on univariate analysis were candidates for inclusion in the multivariate models, and backward selection until all variables had p-values of <0.1 was used to create the final models. Because expander size was only defined in the subgroup with implant/tissue expander reconstruction and width of the skin ellipse was only defined in patients with skin-sparing mastectomy, separate models were built on these subgroups to allow these variables. All statistical analysis was performed in SAS 9.2 (SAS Institute, Cary, NC). Two-sided p-values <0.05 were considered significant.
RESULTS
During the study period, 606 mastectomies were performed in 376 patients; there were 146 unilateral mastectomies and 230 bilateral mastectomies. 279 (46%) were for invasive cancer, 69 (11%) were for DCIS, 1 (0.2%) was for malignant phyllodes tumor, and 257 (42%) were risk-reducing mastectomies.24 Median patient age was 48 (22–76) years, and the median body mass index (BMI) was 25.3 (16.5–50); 324 (53.5%) procedures were in overweight patients (BMI>25), and 133 (21.9%) were in obese patients (BMI>30). Patient and operative characteristics are listed in Table 1.
Table 1.
Patient and intraoperative characteristics (all numbers are per breast)
| Characteristic | N (%) or median (range) | ||
|---|---|---|---|
| Patient characteristics | Age | 48 (22–76) | |
| BMI | 25.3 (16.5–50.0) | ||
| Smoking, current or past | 238 (39%) | ||
| Hypertension | 73 (12%) | ||
| Steroid use | 5 (1%) | ||
| Collagen vascular disease | 11 (2%) | ||
| Diabetes | 22 (4%) | ||
| Bra Size (N=586) | A | 64 (11%) | |
| B | 186 (32%) | ||
| C | 175 (30%) | ||
| D or larger | 161 (27%) | ||
| Prior treatments or procedures | Neoadjuvant chemo | 43 (7%) | |
| History of RT | 44 (7%) | ||
| Prior breast biopsy/lumpectomy | 138 (23%) | ||
| Prior breast augmentation | 16 (3%) | ||
| Prior breast reduction | 14 (2%) | ||
| Mastectomy factors | Specimen size (g) (N=520) | 547 (74–2428) | |
| Time to specimen removal (min) (N=532) | 43 (13–233) | ||
| Two breast teams (vs one) (N= 524) | 131 (25%) | ||
| Sharp dissection (vs cautery) | 65 (11%) | ||
| Tumescence | 45 (7%) | ||
| Width skin ellipse (excludes nipple sparing) (cm) (N=478) | 5 (1–23) | ||
| Length upper skin flap (cm) (N=476) | 15 (2–27) | ||
| Length incision (cm) (N=560) | 12 (5–47) | ||
| >5cm flap exposed dermis (N= 489) | 56 (11%) | ||
| Intraoperative trimming of flap (N=483) | 248 (51%) | ||
| Axillary procedures | No axillary procedure | 216 (36%) | |
| SLNB only | 303 (50%) | ||
| SLNB converted to ALND | 45 (8%) | ||
| ALND only | 41 (7%) | ||
| Prior SLNB | 1 (0) | ||
| Reconstructive factors | Expander (vs permanent implant) (N=566) | 551 (97%) | |
| Expander/implant size (excludes autologous flap)(ml)(N=551) | 400 (125–750) | ||
| Intraoperative expander fill (ml) (N=551) | 180 (30–420) | ||
| Cancer characteristics | Invasive carcinoma | 279 (46%) | |
| DCIS | 69 (11%) | ||
| LCIS | 7 (1%) | ||
| Malignant phyllodes | 1 (0) | ||
| No cancer found | 250 (41%) | ||
| Tumor size (cm) (N=361) | 1.5 (0.01–9.8) | ||
BMI, body mass index; RT, radiation therapy; SLNB, sentinel lymph node biopsy; ALND, axillary lymph node dissection; DCIS, ductal carcinoma in situ; LCIS, lobular carcinoma in situ
There were 511 (84%) skin-sparing mastectomies and 95 (16%) nipple-sparing mastectomies included in the study. Tissue expander or implant reconstruction accounted for 567 (94%) of the reconstructive procedures, and the remaining 39 (6%) were autologous tissue reconstruction procedures (10 TRAM, 24 DIEP, 5 latissimus dorsi). Acellular dermal matrix was used in 48 (8.5%) of the expander/implant cases. Of the 230 bilateral procedures (460 breasts), 131 (33% of breasts, data missing for 65 breasts) were performed by two teams of breast surgeons (i.e., a fellow and attending, and two assistants for the case). Twelve breast surgeons and 6 plastic surgeons participated in the study.
Any skin flap necrosis
Overall, 85 (14%) breasts in 67 patients had some degree of skin flap necrosis: 46 (8%) mild, 6 (1%) moderate, and 31 (5%) severe. Two (0.3%) patients with skin flap necrosis were not categorized because they received follow-up at other institutions. The median size of the necrotic tissue was reported as the largest single dimension and was 3 (0–24) cm, 9 (1.5–15) cm, and 8 (0.5–26) cm, respectively. 25 of the severe necrosis breasts were categorized as such because they were not healed by 8 weeks postoperatively, 9 breasts underwent debridement in the OR, and 4 implants were lost (Table 2).
Table 2.
Rates of skin flap necrosis by clinical severity
| Degree of Necrosis (N=604) | N (%) |
|---|---|
| Mild (no intervention, healed at 8 weeks) | 46 (8) |
| Moderate (clinical debridement, healed at 8 weeks) | 6 (1) |
| Severe (operative debridement, implant loss, or not healed at 8 weeks) | 31 (5) |
Smoking (current or in the last 6 months) (p=0.05), history of breast augmentation (p<0.01), nipple-sparing mastectomy (p<0.01), and time from incision to specimen removal (p<0.01) were significantly associated with any degree of necrosis by univariate logistic regression analysis. Previous breast reduction, diabetes, sharp dissection, and expander size were not statistically significant, but had p-values of <0.1 and were included in the multivariate models. Two multivariate models were built. One excluded the expander/implant size variable to allow inclusion of all patients, regardless of reconstruction type. A second model included the expander/implant size variable and only included cases undergoing TE or implant reconstruction. In the first model, nipple-sparing (p<0.01), time from incision to specimen removal (p<0.01), sharp dissection (p<0.01), and smoking (p=0.03) were significantly associated with any degree of necrosis (results not shown). In the second model, these factors remained significantly associated with any degree of necrosis, except smoking (p=0.08), and previous breast reduction (p<0.01) was also associated with necrosis (Table 3). No significant differences in rates of necrosis were found between oncologic or plastic surgeons on either analysis.
Table 3.
Factors associated with any skin flap necrosis on univariate (UVA) and multivariate (MVA) analyses. Factors with p≤0.1 on UVA were candidates for MVA; backwards selection was used for determine final factors included in MVA. MVA includes patient with TE/implant reconstruction only. N=502 for MVA.
| Factor | UVA OR (95% CI) | p-value | MVA OR (95% CI) | p-value |
|---|---|---|---|---|
| Age (OR per 10-year increase) | 0.90 (0.70–1.15) | 0.398 | ||
| BMI (OR per 1 unit increase) | 1.03(0.99–1.07) | 0.161 | ||
| Cup size C or larger | 1.26(0.78–2.04) | 0.338 | ||
| Smoking, current or past vs none | 1.61 (1.01–2.56) | 0.045 | 1.65 (0.95–2.88) | 0.077 |
| Hypertension | 1.46 (0.77–2.77) | 0.247 | ||
| History of RT | 1.63 (0.75–3.56) | 0.218 | ||
| Collagen disease | 0.69 (0.20–2.37) | 0.560 | ||
| Neoadjuvant chemo | 1.01 (0.41–2.50) | 0.983 | ||
| Prior breast biopsy/lumpectomy | 0.98 (0.56–1.70) | 0.940 | ||
| Prior breast augmentation | 4.16 (1.43–12.04) | 0.009 | * | |
| Prior breast reduction | 3.14 (0.92–10.71) | 0.068 | 4.33 (1.08–17.41) | 0.040 |
| Diabetes | 2.60 (0.97–6.99) | 0.059 | * | |
| Nipple sparing mastectomy (vs skin sparing) | 3.34 (1.88–5.95) | <0.001 | 5.70 (2.71–11.99) | <0.001 |
| Width skin paddle, excl nipple sparing | 1.02 (0.90–1.16) | 0.706 | ||
| Length upper skin flap | 1.08 (0.98–1.19) | 0.114 | ||
| Length incision | 1.00 (0.94–1.05) | 0.883 | ||
| Specimen size (OR per 100 g increase) | 1.05 (0.98–1.12) | 0.168 | ||
| Time incision to spec removal (OR per 10 minute increase) | 1.19 (1.07–1.33) | 0.002 | 1.20 (1.06–1.37) | 0.005 |
| Two team vs one team | 0.92 (0.51–1.66) | 0.777 | ||
| Sharp dissection vs cautery | 2.20 (0.95–5.05) | 0.065 | 5.94 (2.16–16.34) | <0.001 |
| Tumescence | 1.77 (0.78–4.05) | 0.174 | ||
| Expander vs permanent implant | 0.53 (0.14–1.98) | 0.349 | ||
| >5cm flap exposed dermis | 1.58 (0.72–3.45) | 0.252 | ||
| Expander size, excl. autologous flap (OR per 50 ml increase) | 1.12 (0.99–1.26) | 0.061 | 1.15 (1.00–1.32) | 0.052 |
| Intraoperative trimming of flap | 0.93 (0.55–1.57) | 0.786 | ||
| Expander fill volume, excludes autologous flap (OR per 100 ml increase) | 1.12 (0.85–1.48) | 0.412 |
excluded by backwards selection
UVA, univariate; CI, confidence interval; MVA, multivariable; OR, odds ratio; RT, radiation therapy
Moderate or severe necrosis
On univariate analysis of factors associated with moderate or severe necrosis, BMI (p<0.01), diabetes (p<0.01), nipple-sparing mastectomy (p<0.01), specimen size (p=0.03), and expander size (p=0.02) were significant. Width of the skin ellipse was not significant, but had a p<0.1 and was included in the multivariable models.
We first built a multivariate model on the subset of patients who had skin-sparing mastectomy and TE/implant reconstruction that included the expander/implant size and width of skin ellipse variables. Both of these variables were eventually dropped out of the model, which allowed us to include all procedures in the final model. In the final model, only nipple-sparing mastectomy (p<0.01) and specimen size (p<0.01) were significantly associated with moderate or severe necrosis (Table 4). No significant differences were found between oncologic or plastic surgeons in rates of moderate or severe necrosis.
Table 4.
Factors associated with moderate to severe skin flap necrosis on univariate (UVA) and multivariate (MVA) analyses. Factors with p≤0.1 on UVA were candidates for MVA; backwards selection was used for determine final factors included in MVA. N=518 for MVA.
| Factor | UVA OR (95% CI) | p-value | MVA OR (95% CI) | p-value |
|---|---|---|---|---|
| Age (OR per 10-year increase) | 0.81 (0.56–1.17) | 0.271 | ||
| BMI (OR per 1 unit increase) | 1.08 (1.02–1.14) | 0.009 | * | |
| Cup size C or larger | 1.55 (0.76–3.14) | 0.224 | ||
| Smoking, current or past vs none | 1.52 (0.77–2.99) | 0.223 | ||
| Hypertension | 1.05 (0.39–2.83) | 0.924 | ||
| History of RT | 2.08 (0.75–5.77) | 0.160 | ||
| Collagen disease | 1.14 (0.26–5.12 | 0.860 | ||
| Neoadjuvant chemo | 1.28 (0.37–4.45) | 0.698 | ||
| Prior breast biopsy/lumpectomy | 0.80 (0.34–1.89) | 0.612 | ||
| Prior breast augmentation | 1.30 (0.16–10.65) | 0.805 | ||
| Prior breast reduction | 2.83 (0.57–14.06) | 0.203 | ||
| Diabetes | 5.77 (1.86–17.96) | 0.003 | * | |
| Nipple sparing (vs skin sparing) | 3.99 (1.77–8.99) | <0.001 | 12.88 (4.32–38.35) | <0.001 |
| Width skin paddle, (excludes nipple sparing) | 1.15 (0.99–1.33) | 0.073 | * | |
| Length upper skin flap | 1.08 (0.94–1.23) | 0.291 | ||
| Length incision | 1.06 (0.98–1.14) | 0.135 | ||
| Specimen Size (OR per 100 g increase) | 1.10 (1.01–1.20) | 0.030 | 1.24 (1.12–1.37) | <0.001 |
| Time incision to spec removal (OR per 10 min increase) | 1.08 (0.94–1.24) | 0.282 | ||
| Two team vs one team | 0.91 (0.39–2.11) | 0.819 | ||
| Sharp dissection vs cautery | 0.67 (0.15–3.04) | 0.602 | ||
| Tumescence | 2.29 (0.72–7.26) | 0.160 | ||
| Expander vs permanent implant | 0.76 (0.10–6.10) | 0.799 | ||
| >5cm Flap exposed dermis | 1.43 (0.47–4.37) | 0.531 | ||
| Expander size (excludes autologous flap) (OR per 50 ml increase) | 1.22 (1.03–1.45) | 0.024 | * | |
| Intraoperative trimming of flap | 1.45 (0.68–3.06) | 0.334 | ||
| Expander fill volume, excludes autologous flap (OR per 100 ml increase) | 1.35 (0.90–2.03) | 0.149 |
excluded by backwards selection
UVA, univariate; OR, odds ratio; CI, confidence interval; MVA, multivariable; BMI, body mass index; RT, radiation therapy;
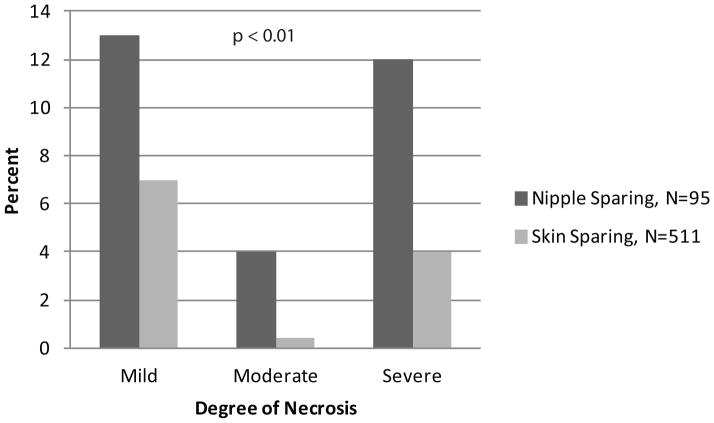
Nipple-sparing mastectomy was associated with significantly more skin flap necrosis at all levels of severity (p<0.01) (Fig 2).
Fig 2.
Comparison of severity of skin flap necrosis by type of mastectomy, skin sparing (N=509) and nipple-sparing (N=95). Nipple-sparing mastectomy was associated with more severe degrees of necrosis (p<0.01).
DISCUSSION
During the study period, the overall rate of skin flap necrosis after mastectomy with reconstruction was 14%. This is higher than many published retrospective reports11–13, but represents a more accurate estimate because prospective data collection allowed us to identify mild necrosis which, by our definition, does not require debridement or a return to the operating room and is unlikely to be well documented in the medical record. We chose to define the degree of necrosis in easily reproducible terms and found that the majority of necrosis was mild, did not delay adjuvant therapy, and likely had little impact on the patient’s experience, though we did not specifically measure this.
Moderate and severe necrosis have a much larger impact on patient outcomes and were much less common. Returns to the operating room and implant loss were rare, with each occuring in <2% of patients. This is lower than previously reported rates of 2.7% and 2.5% from our institution.10 Those studies looked at reconstructive failure out to 6 months after surgery, which likely accounts for the difference. We specifically did not limit our definition of severe necrosis to these events, however, as delayed wound healing without need for return to the operating room can also significantly impact outcomes. If a patient is not healed by 8 weeks postoperatively, this indicates a more severe degree of ischemia and/or wound healing problems, which may be associated with infection, increases the risk of dehiscence, and can potentially delay the receipt of adjuvant therapy.
Though we had very few implants lost, these patients warrant special interest as prior studies have shown that patients who lose their implant have a high rate of foregoing any further reconstruction.25 The low implant loss rate in our study may be due, in part, to our practice of full muscle coverage whenever possible for TE based reconstuction.
Smoking status has been the most consistent patient level factor to be associated with skin flap necrosis after mastectomy with reconstruction, more than doubling the risk of necrosis in prior studies.13,14,16 We did not show this factor to be significantly associated with any skin flap necrosis nor moderate to severe necrosis on multivariate analysis. This may be due to our definition of current or past smoking status, which included those who had quit in the last 6 months.
Prior breast-reduction surgery was also significantly associated with any necrosis in our study. This variable has not been examined in prior studies limiting comparisons. The presence of prior incisions on the breast likely results in more ischemia in the dissected flaps, thereby contributing to necrosis.
Breast size measured by cup size23 and BMI17,19,26 have previously been shown to be associated with skin flap necrosis. We measured these variables as well as specimen size, and clearly all are highly correlated. On multivariable analysis, only specimen size was found to be associated with moderate to severe necrosis, suggesting that it represents a more specific identification of the reason why cup size and BMI have been significant factors in the past.
Though technical variables had limited impact in our study, there are a few that merit discussion. Tumescence has been shown in prior studies to be associated with skin flap necrosis16,17, but this was not a significant factor in our study, consistent with a study by Khavanin et al in 2014.26 These varying results could be a signal of surgeon or institution variability. In our study, sharp dissection (knife versus cautery) was associated with any necrosis, but not moderate to severe necrosis, and this has not been previously identified as a risk factor. Time to specimen removal was also a significant risk factor for any necrosis, but not moderate to severe necrosis. This variable, too, has not been identified in prior studies and is likely related to specimen size and surgeon experience. All of the attending surgeons in this study are breast specialists, but time in practice ranged from 1 to more than 20 years, and trainees did participate in the majority of cases. The level of participation varied, and we were not able to capture this variability in our data. It is possible that rates may be even higher in centers with less experienced surgeons.
Nipple-sparing mastectomy was the most significant predictor of skin flap necrosis and has been associated with higher complication rates in multiple studies.11,23,27 Our study confirms that patients choosing this procedure have a significant risk not only of necrosis of the nipple areolar complex but of skin flap necrosis also. While this may not deter patients from this choice, it does warrant a more extensive conversation to ensure they understand the potential outcomes. Studies on other modifiable variables that may decrease complications for this procedure, such as incision type11,23,27, are inconsistent. While the long-term oncologic outcomes for nipple-sparing mastectomy are still not well established, it is clear that the acute complication rates are significantly higher than complication rates in skin-sparing mastectomy. This may be offset with the overall higher satisfaction with outcomes in these patients.28,29 The level of satisfaction, however, is affected by the occurrence of post-operative complications, including skin flap necrosis, again warranting a frank discussion with patients who choose this procedure. These patients may benefit from nipple-areola delay procedures aiming to improve the blood supply of the nipple areola complex, an approach which merits further investigation.
Identification of patients undergoing nipple-sparing mastectomy with the highest risk of ischemic complications could be useful. A study from Stanford used intraoperative skin perfusion assessment using laser-assisted indocycanine green angiography (SPY Elite) prior to mastectomy to identify patterns of perfusion associated with ischemic complications of the nipple-areolar complex.30 This technique has also been applied to skin-sparing mastectomy and was found to correlate well with patient outcomes.31 While this method may help to identify patients at higher risk for necrosis, it is not cost-effective if applied broadly32, and we did not use this method in our study. Even with optimal patient selection, the SPY information may not be clinically useful as most surgeons are hesitant to default to primary removal of the nipple-areolar complex even when the SPY results indicate extremely poor perfusion.
Though we collected data on a large number of procedures, some variables are underrepresented. Prior radiation therapy has been studied as a risk factor for skin and wound complications after mastectomy9,17,19,33,34, and is underrepresented in our study population, with only 7% of patients having prior radiation. We also have no specific data on traction injury, though flap length, incision size, and time to specimen removal may be surrogates of this. In addition, there are likely other modes of injury that contribute to flap necrosis.
While the rates of skin flap necrosis do vary somewhat by institution and individual, this study provides evidence that the rates of any necrosis are likely higher than reported in many restrospective series. While modifiable technical variables had little impact on rates of clinically significant skin flap necrosis, patients with multiple minor risk factors may be targeted for counseling regarding this risk, especially if they are contemplating nipple-sparing mastectomy. Discussing the potential for wound healing complications is especially important for patients electing risk reducing mastectomy. Studies to evaluate interventions to decrease the incidence of flap necrosis in high-risk patients are warranted.
Synopsis.
Skin flap necrosis after mastectomy with reconstruction is a recognized complication. Here we identified multiple risk factors, but found little impact of modifiable technical variables. Nipple-sparing mastectomy was associated with higher rates of necrosis at every level of severity.
Footnotes
Disclosure: This study, funded in part by NIH/NCI Cancer Center Support Grant P30 CA008748, was presented as an oral presentation at the 2015 Society of Surgical Oncology Annual Meeting.
References
- 1.Habermann EB, Abbott A, Parsons HM, et al. Are mastectomy rates really increasing in the United States? J Clin Oncol. 2010;28(21):3437–41. doi: 10.1200/JCO.2009.27.6774. [DOI] [PubMed] [Google Scholar]
- 2.Habermann EB, Thomsen KM, Hieken TJ, et al. Impact of Availability of Immediate Breast Reconstruction on Bilateral Mastectomy Rates for Breast Cancer across the United States: Data from the Nationwide Inpatient Sample. Ann Surg Oncol. 2014;21(10):3290–6. doi: 10.1245/s10434-014-3924-y. [DOI] [PubMed] [Google Scholar]
- 3.Tuttle TM, Habermann EB, Grund EH, et al. Increasing use of contralateral prophylactic mastectomy for breast cancer patients: a trend toward more aggressive surgical treatment. J Clin Oncol. 2007;25(33):5203–9. doi: 10.1200/JCO.2007.12.3141. [DOI] [PubMed] [Google Scholar]
- 4.McGuire KP, Santillan AA, Kaur P, et al. Are mastectomies on the rise? A 13-year trend analysis of the selection of mastectomy versus breast conservation therapy in 5865 patients. Ann Surg Oncol. 2009;16(10):2682–90. doi: 10.1245/s10434-009-0635-x. [DOI] [PubMed] [Google Scholar]
- 5.Yao K, Stewart AK, Winchester DJ, et al. Trends in contralateral prophylactic mastectomy for unilateral cancer: a report from the National Cancer Data Base, 1998–2007. Ann Surg Oncol. 2010;17(10):2554–62. doi: 10.1245/s10434-010-1091-3. [DOI] [PubMed] [Google Scholar]
- 6.Algaithy ZK, Petit JY, Lohsiriwat V, et al. Nipple sparing mastectomy: can we predict the factors predisposing to necrosis? Eur J Surg Oncol. 2012;38(2):125–9. doi: 10.1016/j.ejso.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Murthy V, Chamberlain RS. Defining a place for nipple sparing mastectomy in modern breast care: an evidence based review. Breast J. 2013;19(6):571–81. doi: 10.1111/j.1524-4741.2011.01220.x. [DOI] [PubMed] [Google Scholar]
- 8.Davies K, Allan L, Roblin P, et al. Factors affecting post-operative complications following skin sparing mastectomy with immediate breast reconstruction. Breast. 2011;20(1):21–5. doi: 10.1016/j.breast.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Pinsolle V, Grinfeder C, Mathoulin-Pelissier S, et al. Complications analysis of 266 immediate breast reconstructions. J Plast Reconstr Aesthet Surg. 2006;59(10):1017–24. doi: 10.1016/j.bjps.2006.03.057. [DOI] [PubMed] [Google Scholar]
- 10.Cordeiro PG, McCarthy CM. A single surgeon’s 12-year experience with tissue expander/implant breast reconstruction: part I. A prospective analysis of early complications. Plast Reconstr Surg. 2006;118(4):825–31. doi: 10.1097/01.prs.0000232362.82402.e8. [DOI] [PubMed] [Google Scholar]
- 11.Garwood ER, Moore D, Ewing C, et al. Total skin-sparing mastectomy: complications and local recurrence rates in 2 cohorts of patients. Ann Surg. 2009;249(1):26–32. doi: 10.1097/SLA.0b013e31818e41a7. [DOI] [PubMed] [Google Scholar]
- 12.Chang DW, Wang B, Robb GL, et al. Effect of obesity on flap and donor-site complications in free transverse rectus abdominis myocutaneous flap breast reconstruction. Plast Reconstr Surg. 2000;105(5):1640–8. doi: 10.1097/00006534-200004050-00007. [DOI] [PubMed] [Google Scholar]
- 13.Chang DW, Reece GP, Wang B, et al. Effect of smoking on complications in patients undergoing free TRAM flap breast reconstruction. Plast Reconstr Surg. 2000;105(7):2374–80. doi: 10.1097/00006534-200006000-00010. [DOI] [PubMed] [Google Scholar]
- 14.McCarthy CM, Mehrara BJ, Riedel E, et al. Predicting complications following expander/implant breast reconstruction: an outcomes analysis based on preoperative clinical risk. Plast Reconstr Surg. 2008;121(6):1886–92. doi: 10.1097/PRS.0b013e31817151c4. [DOI] [PubMed] [Google Scholar]
- 15.Spear SL, Ducic I, Cuoco F, et al. Effect of obesity on flap and donor-site complications in pedicled TRAM flap breast reconstruction. Plast Reconstr Surg. 2007;119(3):788–95. doi: 10.1097/01.prs.0000252003.14537.d2. [DOI] [PubMed] [Google Scholar]
- 16.Mlodinow AS, Fine NA, Khavanin N, et al. Risk factors for mastectomy flap necrosis following immediate tissue expander breast reconstruction. J Plast Surg Hand Surg. 2014 doi: 10.3109/2000656X.2014.884973. [DOI] [PubMed] [Google Scholar]
- 17.Chun YS, Verma K, Rosen H, et al. Use of tumescent mastectomy technique as a risk factor for native breast skin flap necrosis following immediate breast reconstruction. Am J Surg. 2011;201(2):160–5. doi: 10.1016/j.amjsurg.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Khavanin N, Jordan S, Lovecchio F, et al. Synergistic interactions with a high intraoperative expander fill volume increase the risk for mastectomy flap necrosis. J Breast Cancer. 2013;16(4):426–31. doi: 10.4048/jbc.2013.16.4.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hultman CS, Daiza S. Skin-sparing mastectomy flap complications after breast reconstruction: review of incidence, management, and outcome. Ann Plast Surg. 2003;50(3):249–55. doi: 10.1097/01.sap.0000046784.70583.e1. discussion 55. [DOI] [PubMed] [Google Scholar]
- 20.Meretoja TJ, von Smitten KA, Kuokkanen HO, et al. Complications of skin-sparing mastectomy followed by immediate breast reconstruction: a prospective randomized study comparing high-frequency radiosurgery with conventional diathermy. Ann Plast Surg. 2008;60(1):24–8. doi: 10.1097/SAP.0b013e31804a8627. [DOI] [PubMed] [Google Scholar]
- 21.Rinker B, Thornton BP. Skin-sparing mastectomy and immediate tissue expander breast reconstruction in patients with macromastia using the Passot breast reduction pattern. Ann Plast Surg. 2014;72(6):S158–64. doi: 10.1097/01.sap.0000435768.51143.c9. [DOI] [PubMed] [Google Scholar]
- 22.Peled AW, Foster RD, Ligh C, et al. Impact of Total Skin-Sparing Mastectomy Incision Type on Reconstructive Complications following Radiation Therapy. Plast Reconstr Surg. 2014;134(2):169–75. doi: 10.1097/PRS.0000000000000386. [DOI] [PubMed] [Google Scholar]
- 23.Gould DJ, Hunt KK, Liu J, et al. Impact of surgical techniques, biomaterials, and patient variables on rate of nipple necrosis after nipple-sparing mastectomy. Plast Reconstr Surg. 2013;132(3):330e–8e. doi: 10.1097/PRS.0b013e31829ace49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.King TA, Sakr R, Patil S, et al. Clinical management factors contribute to the decision for contralateral prophylactic mastectomy. J Clin Oncol. 2011;29(16):2158–64. doi: 10.1200/JCO.2010.29.4041. [DOI] [PubMed] [Google Scholar]
- 25.Peled AW, Stover AC, Foster RD, et al. Long-term reconstructive outcomes after expander-implant breast reconstruction with serious infectious or wound-healing complications. Ann Plast Surg. 2012;68(4):369–73. doi: 10.1097/SAP.0b013e31823aee67. [DOI] [PubMed] [Google Scholar]
- 26.Khavanin N, Fine NA, Bethke KP, et al. Tumescent technique does not increase the risk of complication following mastectomy with immediate reconstruction. Ann Surg Oncol. 2014;21(2):384–8. doi: 10.1245/s10434-013-3311-0. [DOI] [PubMed] [Google Scholar]
- 27.Wang F, Peled AW, Garwood E, et al. Total Skin-Sparing Mastectomy and Immediate Breast Reconstruction: An Evolution of Technique and Assessment of Outcomes. Ann Surg Oncol. 2014 doi: 10.1245/s10434-014-3915-z. [DOI] [PubMed] [Google Scholar]
- 28.Didier F, Radice D, Gandini S, et al. Does nipple preservation in mastectomy improve satisfaction with cosmetic results, psychological adjustment, body image and sexuality? Breast Cancer Res Treat. 2009;118(3):623–33. doi: 10.1007/s10549-008-0238-4. [DOI] [PubMed] [Google Scholar]
- 29.Yueh JH, Houlihan MJ, Slavin SA, et al. Nipple-sparing mastectomy: evaluation of patient satisfaction, aesthetic results, and sensation. Ann Plast Surg. 2009;62(5):586–90. doi: 10.1097/SAP.0b013e31819fb1ac. [DOI] [PubMed] [Google Scholar]
- 30.Wapnir I, Dua M, Kieryn A, et al. Intraoperative imaging of nipple perfusion patterns and ischemic complications in nipple-sparing mastectomies. Ann Surg Oncol. 2014;21(1):100–6. doi: 10.1245/s10434-013-3214-0. [DOI] [PubMed] [Google Scholar]
- 31.Munabi NC, Olorunnipa OB, Goltsman D, et al. The ability of intra-operative perfusion mapping with laser-assisted indocyanine green angiography to predict mastectomy flap necrosis in breast reconstruction: a prospective trial. J Plast Reconstr Aesthet Surg. 2014;67(4):449–55. doi: 10.1016/j.bjps.2013.12.040. [DOI] [PubMed] [Google Scholar]
- 32.Kanuri A, Liu AS, Guo L. Whom should we SPY? A cost analysis of laser-assisted indocyanine green angiography in prevention of mastectomy skin flap necrosis during prosthesis-based breast reconstruction. Plast Reconstr Surg. 2014;133(4):448e–54e. doi: 10.1097/PRS.0000000000000025. [DOI] [PubMed] [Google Scholar]
- 33.Kronowitz SJ. Current status of implant-based breast reconstruction in patients receiving postmastectomy radiation therapy. Plast Reconstr Surg. 2012;130(4):513e–23e. doi: 10.1097/PRS.0b013e318262f059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sbitany H, Wang F, Saeed L, et al. Immediate implant-based breast reconstruction following total skin-sparing mastectomy in women with a history of augmentation mammaplasty: assessing the safety profile. Plast Reconstr Surg. 2014;134(1):1–9. doi: 10.1097/PRS.0000000000000293. [DOI] [PubMed] [Google Scholar]