Abstract
Acute myeloid leukemia is a clonal neoplasm derived from myeloid progenitor cells with a varying outcome. The initial goal of treatment is the achievement of complete remission, defined for over 40 years by morphology. However, without additional post-remission treatment the majority of patients relapse. In many cases of acute myeloid leukemia, allogeneic stem cell transplantation offers the best prospects of cure. In 2013, 5608 stem cell transplantations in acute myeloid leukemia were performed in Europe (5228 allogeneic and 380 autologous stem cell transplantations). Most stem cell transplantations are performed in first complete remission. However, despite a considerable reduction in the chance of relapse, in most studies, overall survival benefit of allogeneic stem cell transplantation is modest due to substantial non-relapse mortality. Here we discuss the many factors related to the risk of relapse after allogeneic stem cell transplantation.
Introduction
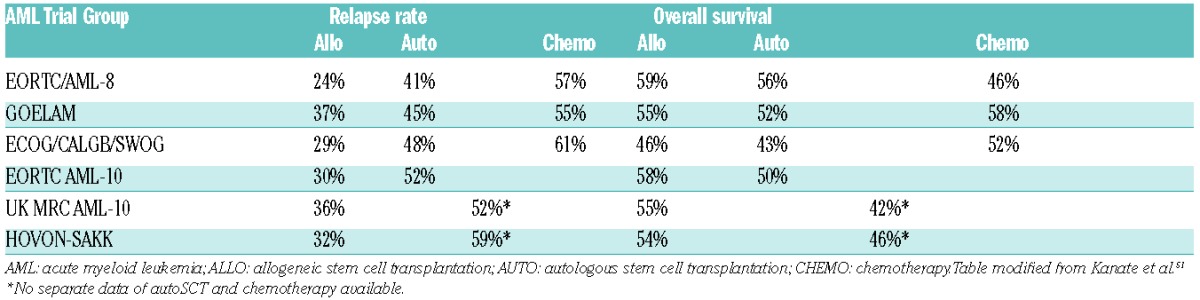
Acute myeloid leukemia (AML) is a clonal neoplasm derived from myeloid progenitor cells with a varying outcome. The initial goal of treatment is the achievement of complete remission (CR), defined for over 40 years by morphology.1 Without additional post-remission treatment, however, the majority of patients relapse. In many cases of AML, allogeneic stem cell transplantation (alloSCT) offers the best prospects of cure. Apart from the conditioning regimen, the anti-leukemic potential is mainly based on the immunological graft-versus-leukemia effect. Indeed, AML is the most frequent indication for alloSCT, as indicated by data from both the European Group for Blood and Marrow Transplantation (EBMT) and the International Bone Marrow Transplant Registry (IBMTR), and the number of patients transplanted for this indication is growing year by year.2 In 2013, 5608 stem cell transplants in AML in Europe were performed [alloSCT: 5228 and autologous (auto) SCT: 380]. The rise in frequency in recent years is due to the application of reduced intensity conditioning (RIC) regimens and the expansion of alternative donor stem cell sources derived from mismatched relatives and unrelated volunteers. The total number of family donors was 2354 (1913 HLA identical and 441 non-identical) while 2863 unrelated transplants were performed, among which there were 211 cord blood transplantations. The majority of patients were transplanted in first complete remission (CR1). A meta-analysis of prospective trials and trials reporting relapse-free survival (RFS) and/or overall survival (OS) outcomes after assigning adult patients with AML in CR1 to undergo alloSCT versus non-alloSCT treatment, based on donor availability (donor vs. no-donor comparisons), showed that, except for good risk AML in CR1, alloSCT gives a significant survival benefit for intermediate and poor risk AML.3 However, despite a considerable reduction in the possibility of relapse, in most studies, OS benefit of alloSCT is modest due to substantial non-relapse mortality (Table 1).4–9 Now we are approaching a situation in which we can identify a suitable donor, either matched unrelated, haplo-identical or cord, for nearly every patient, This means that both disease-related and transplant-related factors should be carefully balanced before proceeding to an allogeneic or non-allogeneic approach after achieving CR.10 Unfortunately, even after alloSCT, a substantial number of AML patients will ultimately relapse, and in these cases survival is very poor.11 Hematologists are now facing the question “which patient should get a transplant in first remission?” Predicting relapse in an individual patient still remains a challenge. Here, we will mainly focus on factors predicting for relapse after allogeneic transplant in AML.
Table 1.
Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission in comparison to autologous stem cell transplantation and chemotherapy.
Transplant-related factors in relation to relapse risk after transplantation
Dose intensity is a main determinant for relapse. With increasing dosage, even at the myeloablative (MA) level, the chance of relapse decreases.12 RIC have become popular and these are being increasingly adopted. Although no prospective randomized trials have been completed, these lower intensity conditioning regimens seem to be associated with a higher rate of relapse. A retrospective EBMT comparison of conditioning regimens showed an increase in relapse rate of 23%–39% after MA versus RIC.13 Nevertheless OS benefits for MA are, at best, modest due to the increased non-relapse mortality (NRM) in comparison with RIC. Although the only prospective randomized study comparing RIC versus MA was stopped early because of slow accrual of patients, there was no significant difference in RFS, NRM and OS.14 In general, it is clear that increasing the intensity of the conditioning regimen results in more acute graft-versus-host disease (GvHD) and increased NRM. In several, but not all, studies, more intensive GvHD prophylaxis, for example by pre-transplant administration of anti-thymocyte globulin or T-cell depletion of the graft, resulted in a higher relapse rate, probably due to less GvL. Whether early donor lymphocyte infusion after T-cell depleting strategies or high-dose cyclophosphamide post transplant counterbalance NRM versus relapse is the subject of much debate and investigation.15,16 Chronic GvHD reduced the chance of relapse in many studies; however, in a recent registry study of the Center for International Blood and Marrow Transplant Research (CIBMTR), its impact seemed only clinically relevant for CML after myeloablative alloSCT and not for AML.17 In this study, cGvHD was primarily associated with a higher transplant-related mortality (TRM).
Disease-related characteristics in relation to relapse risk after transplantation
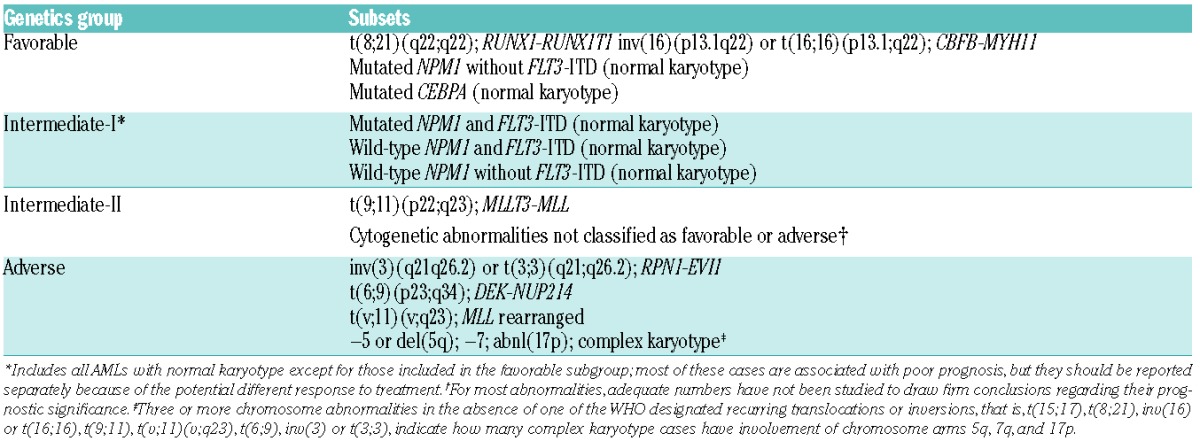
Patient-specific biological factors associated with risk of relapse after transplantation are basically the same as those in patients treated with chemotherapy only. Based on cytogenetic analysis, three risk groups for relapse can be identified, as described by Grimwade et al.18 New categories such as the monosomal karyotype associated with a very poor outcome are also used to estimate the possibility of relapse by various AML trial groups.19 Novel genomic technology has paved the way for the detection of numerous new molecular aberrations, further underlining the heterogeneity of AML, and these are also helpful to establish a prognosis. Some of these molecular aberrancies (mutations in the NPM1, CEBPA and FLT3 genes) have already been incorporated into the widely applied recommendations for standardized reporting of genetic abnormalities by the European LeukemiaNet panel of experts published in 2010 (Table 2).20 Since then, several new molecular aberrancies have been identified, some of which are clearly associated with outcome, while others are less so. The assumption that most of these aberrancies are equally important for risk of relapse after chemotherapy, as well as after transplantation, still has to be proved. Some of these novel genetic data will be discussed below.
Table 2.
Standardized reporting for correlation of cytogenetic and molecular genetic data in acute myeloid leukemia with clinical data.2
Mutations of CCAAT/enhancer binding protein alpha
Mutations of CCAAT/enhancer binding protein alpha (CEBPA) encodes a transcription factor essential for differentiation along the neutrophil lineage. Although already incorporated into the ELN recommendations, it has become clear that only double and not single mutated CEBPA is associated with a favorable outcome.21
Mutations of the nucleophosmin gene
The nucleophosmin gene (NPM1) gene encodes for a protein that shuttles between nucleus and cytoplasm, acting, amongst other things, as a molecular chaperone of histone proteins. In addition, it is involved in other critical cellular functions, such as ribosome biogenesis and transport, and centrosome duplication during the cell cycle. Mutations consist of a 4-base pair insertion that alters the C-terminal end of the protein, leading to a nuclear export signal of the encoded protein, and thus to loss of shuttling activity. Several studies have shown a favorable impact of the NPM1 mutation on outcome in the absence of FLT3 gene mutations. In a donor versus no donor analysis, Rollig et al. recently showed that alloSCT resulted in a significantly prolonged RFS in patients with NPM1+ mutated AML. However, OS was not improved, most probably due to the fact that relapsed NPM1+ AML patients responded well to salvage treatment.22 NPM1+FLT3 ITD-AMLs are now grouped together with the core binding factor (CBF) leukemias and the CEBPA double mutants and have a good prognosis.
Internal tandem duplications of the FMS-like tyrosine kinase 3 gene
Internal tandem duplications (ITDs) of the FMS-like tyrosine kinase 3 gene occur in 20%–30% of AML cases, predominantly in those with normal cytogenetics. They are associated with a poor outcome, especially when the ratio between mutated and non-mutated FLT3 gene is more than 0.51. This is not only due to an increased risk of relapse, but also due to refractoriness to induction treatment.23,24
Expression of the ecotropic viral integration-1 oncogene
Ten percent of AML cases show high ecotropic viral integration-1 (EVI-1) oncogene expression, which predicts for a particularly poor outcome. Besides inv(3)/t(3;3), EVI1(+) is significantly associated with the chromosome abnormalities monosomy 7 and t(11q23). EVI1+ is virtually absent in favorable-risk AML and AML with NPM1 mutations.25
Mutations in DNA-methyltransferase-3A
The DNA-methyltransferase-3A (DNMT3A) enzyme plays a role in DNA methylation by transferring methyl-groups to DNA CpG islands. Mutations in this gene are detected in approximately 20% of AML cases and enriched in normal karyotype AML (CN-AML) with an incidence of between 30%–37%. Various studies have proved to be inconclusive as to the prognostic value for outcome. A recent meta-analysis showed a slightly poor prognostic impact on OS.26,27
Mutations in the additional sex combs-like 1 gene
The additional sex combs-like (ASXL)1 gene is an epigenetic scaffolding protein that assembles epigenetic regulators and transcription factors to specific genomic loci with histone modifications. The incidence of truncating mutations in ASXL1 is between 5%–12% and is always associated with a poor outcome.28
Mutations in the ten-eleven translocation-2 gene
The ten-eleven translocation-2 (TET2) gene is a key enzyme for DNA demethylation and a critical regulator for hematopoietic stem cell homeostasis, whose functional impairment leads to hematologic malignancies. Mutations are frequently found in AML. Although in most studies it is associated with a poor outcome, the prognostic relevance has not been definitively established.29,30
TP53 mutations and 17p abnormalities
The Study Alliance Leukemia (SAL) and other groups reported poor survival data in patients with abn(17p) or TP53-mutated AML.31 In a large retrospective repository-based analysis published by Grossmann et al., patients with TP53-mutated AML had the worst prognosis of all molecularly defined risk groups, with a median OS of 4.6 months and event-free survival (EFS) of 0% at three years.32
Isocitrate dehydrogenase1 and -2 mutations
Isocitrate dehydrogenase1 and -2 (IDH1/2) mutations are found in AML as well in gliomas, both with an incidence of approximately 15%–20%. IDH1 and IDH 2 are critical enzymes in the citric acid cyclus, converting isocitrate to α-ketoglutarate. Due to the mutation, 2 hydroxyglutarate (2-HG) instead of α-ketoglutarate is formed. 2-HG inhibits TET enzymes, which results in hypermethylation. This is thought to suppress expression of tumor suppressor genes. These mutations are mutually exclusive with TET2 mutations. Although the prognostic significance has still not been established, some studies have shown a poor outcome.33,34
RUNX1 mutations
RUNX1 mutations are considered to be associated with a poor prognosis. The incidence (8%–16%) is higher in elderly AML and in secondary AML.35
Mutations in other genes
C-KIT mutations especially have poor prognostic implications in t(8;21) AML and predict for higher relapse rates than unmutated cases; however, MRD status before alloSCT was more predictive than c-kit status.36 The clinical impact of expression levels of numerous other genes such as BAALC, ERG, MN1 etc., still has to be determined. How these new molecular aberrancies could be integrated into a new prognostic algorithm was illustrated by Patel et al. who applied high throughput sequencing of TET2, ASXL1, DNMT3A, CEBPA, PHF6, WT1, TP53, EZH2, RUNX1, PTEN, FLT3, NPM1, HRAS, KRAS, NRAS, KIT, IDH1 and IDH2 in over 500 patients from the ECOG E1900 trial. They showed that the mutational analysis of 9 of these genes could be used to retrospectively classify patients into more precise subgroups with favorable-risk, intermediate-risk, or unfavorable-risk profiles, with marked differences in the overall outcome.37 Another example of how molecular profiling may be helpful has been shown by Grosmann et al.38 In a large cohort of AML patients for whom cytogenetic data was available, they investigated the following molecular alterations: PML-RARA, RUNX1-RUNX1T1, CBFB-MYH11, FLT3-ITD, and MLL-PTD, as well as mutations in NPM1, CEPBA, RUNX1, ASXL1, and TP53. Five distinct prognostic subgroups were identified: 1) very favorable: PML-RARA rearrangement or CEPBA double mutations (OS at 3 years: 82.9%); 2) favorable: RUNX1-RUNX1T1, CBFB-MYH11, or NPM1 mutation without FLT3-ITD (OS at 3 years: 62.6%); 3) intermediate: none of the mutations leading to assignment into groups 1, 2, 4, or 5 (OS at 3 years: 44.2%); 4) unfavorable: MLL-PTD and/or RUNX1 mutation and/or ASXL1 mutation (OS at 3 years: 21.9%); and 5) very unfavorable: TP53 mutation (OS at 3 years: 0%). This profile, based only on molecular abnormalities, provided a more powerful model for prognostication than cytogenetics.38 Taken together, these data indicate that more detailed genetic analysis may lead to improved risk stratification
Clinical features in relation to risk of relapse after transplantation
Apart from cytogenetic and molecular prognostic markers identified at diagnosis, a number of clinical variables that can be assessed either at diagnosis or during induction and consolidation treatment might offer additional prognostic information. These include high WBC count, time to complete remission, day 15 presence of blasts, extramedullary disease, and quantifiable levels of minimal residual disease (MRD) after induction or consolidation therapy. Here we will focus on the prognostic value of MRD.
Minimal residual disease
Despite a multitude of prognostic factors at diagnosis, the outcome of patients is still highly variable and not individually predictable. It thus seems that prognosticators at diagnosis will not enable clinicians to reach the ultimate goal of truly individualized risk assessment. One important issue is that the prognostic impact of these factors in the present risk groups does not take into account the contribution of several cellular resistance mechanisms at diagnosis, and also post-diagnosis factors, which include dosage, compliance, pharmacological resistance, and probably other unknown features. These factors, which corroborate proper risk classification, are only partly covered by inclusion of CR status. However, the ability to define residual disease far below the level of 5% blast cells is changing the landscape of risk classification. This so-called minimal residual disease (MRD) approach currently enables detection of leukemia cells down to levels of 1:1,000–1:106 white blood cells, compared to only 1:20 for morphology.39,40
Methods for detection of minimal residual disease
The most sensitive method at present is real-time quantitative polymerase chain reaction (RQ-PCR). Chimeric fusion genes like PML-RARA, RUNX1-RUNXT1, and CBFB-MYH11 are reliable markers for MRD evaluation; however these genetic abnormalities are only present in approximately 20% of AML cases. NPM1 mutation is an abnormality that occurs more frequently for which mutation-specific RQ-PCR assays have been developed to monitor MRD.41 Another approach for assessment of MRD has gained increasing attention: the use of aberrant (i.e. leukemia-associated) immunophenotypes (LAIP) with flow cytometry. Currently, with this approach, aberrancies may be detected in over 90% of AML cases at diagnosis. These LAIPs consist of normally occurring markers, present in aberrant combinations in AML bone marrow (BM), while only at very low frequencies, or even absent, in normal and regenerating BM.42
Minimal residual disease in clinical studies
During the last 15 years, numerous single institute studies have been carried out, both in adult and pediatric AML, which have established the independent prognostic value of “Immunophenotypic MRD.” The results of the first multicenter (31 sites) multinational prospective MRD study in adult AML (aged 18–60 years) were recently reported by the HOVON/SAKK investigators. In this study, BM MRD was assessed at five different sites in samples obtained after one and 2 cycles of induction therapy and after consolidation therapy.43 MRD measurements were performed in a blinded fashion, i.e. without knowing the patients’ performance, while patients were treated according to protocol also without knowledge of MRD-related data. After all treatment cycles, low MRD values distinguished patients with relatively favorable outcome from those with adverse RFS and OS. In the whole patient group, and in the clinically most interesting subgroup with intermediate risk cytogenetics, MRD was an independent prognostic. After all treatment courses, low MRD values distinguished patients with relatively favorable outcome from those with adverse RFS and OS. Multivariate analysis after cycle 2I, when decisions about consolidation treatment have to be made, confirmed that high MRD values (>0.1% of WBC) was associated with a considerably higher risk of relapse even after adjustment for consolidation treatment time-dependent co-variate risk score and early or later CR. Similar results were obtained by Freeman et al. who used MRD assessments in a group of elderly patients. In addition, MRD determination by means of quantitative PCR of PML-RARA, AML1-ETO, CBFB-MYH11 transcripts and of mutations in NPM1 has also proven to be of value in the clinical arena.44–46
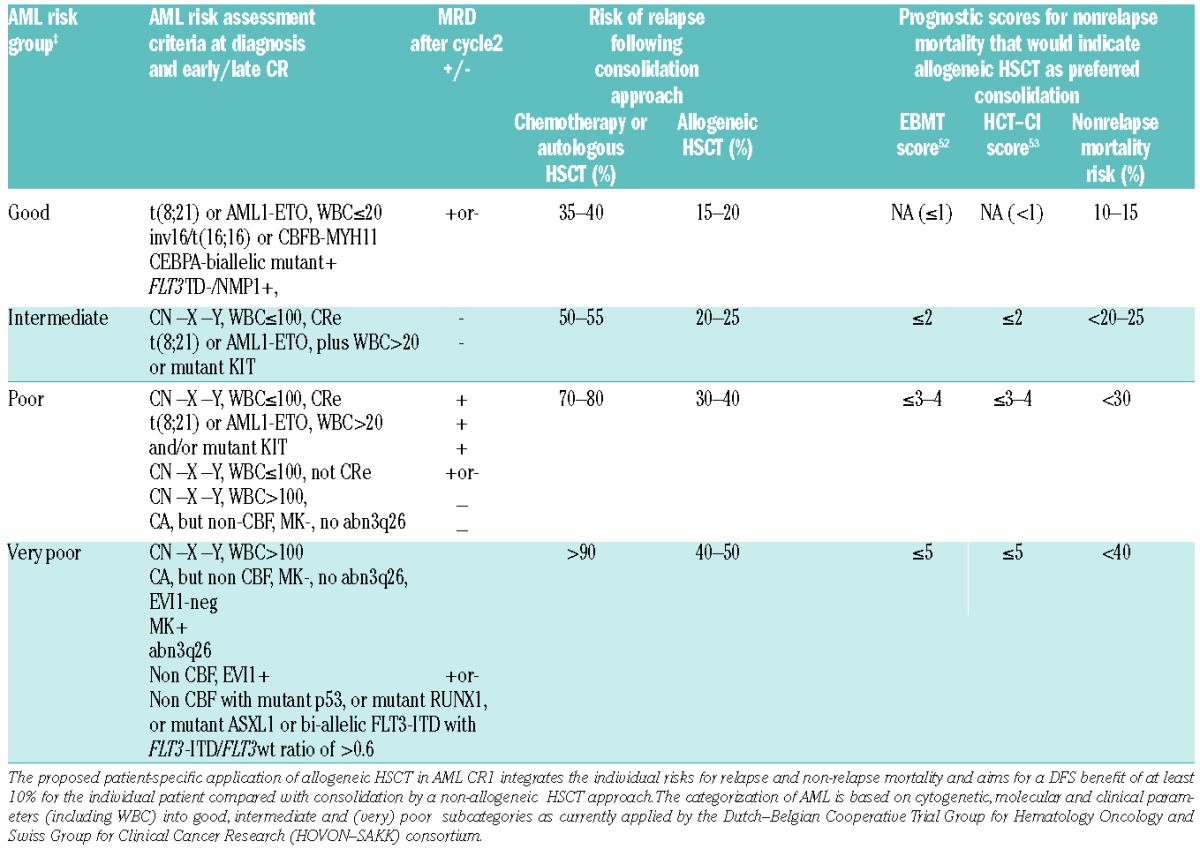
Before transplantation, MRD status in CR1 AML patients was demonstrated to be a highly valuable marker of disease recurrence and shorter OS after transplant. Several studies in patients undergoing myeloablative conditioning all show that the presence of MRD has a negative impact on post-transplant relapse risk.47–49 Recently, Walter et al. showed that the same holds true for non-myeloablative conditioning.50 An integrated risk-adapted approach on allogeneic transplantation for patients with AML is given in Table 3. This is currently being followed in the HOVON-SAKK trials and follows the ELN consensus statement, now further refined by introducing new molecular markers and MRD determination after cycle 2.
Table 3.
Recommendation for allogeneic SCT in AML CR1 based on integrated risk profiles. Adapted by HOVON-SAKK from the ELN recommendation by adding new molecular markers and MRD.10
Conclusion
Risk of relapse after allogeneic stem cell transplantation is a composite of many factors, as described here. An estimation of this risk together with an estimation of expected treatment-related mortality should be used as a guide to determine which patient should be offered an alloSCT as post-remission treatment. It is clear that this must be an individualized, well-balanced clinical decision, which cannot be made from a single recommendation that fits all.
Footnotes
This review article is based on the educational manuscript from the EHA20 meeting (Vienna, June 2015).
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/101/1/20
References
- 1.Bishel HF. Criteria for the evaluation of response for treatment of AML. Blood 1956;11:676–681. [Google Scholar]
- 2.Passweg JR, Baldomero H, Bader P, et al. Hematopoietic SCT in Europe 2013: recent trends in the use of alternative donors showing more haploidentical donors but fewer cord blood transplants. Bone Marrow Transplant. 2015;50(4):476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koreth J, Schlenk R, Kopecky KJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA. 2009;301(22):2349–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cassileth PA, Harrington DP, Appelbaum FR, et al. Chemotherapy compared with autologous or allogeneic bone marrow transplantation in the management of acute myeloid leukemia in first remission. N Engl J Med. 1998;339(23):1649–1656. [DOI] [PubMed] [Google Scholar]
- 5.Zittoun RA, Mandelli F, Willemze R, et al. Autologous or allogeneic bone marrow transplantation compared with intensive chemotherapy in acute myelogenous leukemia. European Organization for Research and Treatment of Cancer (EORTC) and the Gruppo Italiano Malattie Ematologiche Maligne dell’Adulto (GIMEMA) Leukemia Cooperative Groups. N Engl J Med. 1995;332(4):217–223. [DOI] [PubMed] [Google Scholar]
- 6.Harousseau JL, Cahn JY, Pignon B, et al. Comparison of autologous bone marrow transplantation and intensive chemotherapy as postremission therapy in adult acute myeloid leukemia. The Groupe Ouest Est Leucémies Aiguës Myéloblastiques (GOE-LAM) Blood. 1997;90(8):2978–2986. [PubMed] [Google Scholar]
- 7.Burnett AK, Wheatley K, Goldstone AH, et al. The value of allogeneic bone marrow transplant in patients with acute myeloid leukaemia at differing risk of relapse: results of the UK MRC AML 10 trial. Br J Haematol. 2002;118(2):385–400. [DOI] [PubMed] [Google Scholar]
- 8.Suciu S, Mandelli F, de Witte T, et al. Allogeneic compared with autologous stem cell transplantation in the treatment of patients younger than 46 years with acute myeloid leukemia (AML) in first complete remission (CR1): an intention-to-treat analysis of the EORTC/GIMEMAAML-10 trial. Blood. 2003;102(4):1232–1240. [DOI] [PubMed] [Google Scholar]
- 9.Cornelissen JJ, van Putten WL, Verdonck LF, et al. Results of a HOVON/SAKK donor versus no-donor analysis of myeloablative HLA-identical sibling stem cell transplantation in first remission acute myeloid leukemia in young and middle-aged adults: benefits for whom? Blood. 2007;109(9): 3658–3666. [DOI] [PubMed] [Google Scholar]
- 10.Cornelissen JJ, Gratwohl A, Schlenk RF, et al. The European LeukemiaNet AML Working Party consensus statement on allogeneic HSCT for patients with AML in remission: an integrated-risk adapted approach. Nat Rev Clin Oncol. 2012;9(10):579–590. [DOI] [PubMed] [Google Scholar]
- 11.Fathi AT, Chen YB. Treatment of relapse of acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation. Curr Hematol Malig Rep. 2014;9(2):186–192. [DOI] [PubMed] [Google Scholar]
- 12.Appelbaum FR. Dose intensity of preparative regimens for acute myeloid leukemia-one size fits all- or tailor- made? Best Pract Res Clin Hematol. 2010;23(4):509–517. [DOI] [PubMed] [Google Scholar]
- 13.Martino R, de Wreede L, Fiocco M, et al. Comparison of conditioning regimens of various intensities for allogeneic hematopoietic SCT using HLA-identical sibling donors in AML and MDS with <10% BM blasts: a report from EBMT. Bone Marrow Transplant. 2013;48(6):761–770. [DOI] [PubMed] [Google Scholar]
- 14.Bornhäuser M, Kienast J, Trenschel R, et al. Reduced-intensity conditioning versus standard conditioning before allogeneic haemopoietic cell transplantation in patients with acute myeloid leukaemia in first complete remission: a prospective, open-label randomised phase 3 trial. Lancet Oncol. 2012;13(10):1035–1044. [DOI] [PubMed] [Google Scholar]
- 15.Blaise D, Castagna L. Do different conditioning regimens really make a difference? Hematology Am Soc Hematol Educ Program. 2012;2012:237–245. [DOI] [PubMed] [Google Scholar]
- 16.Al-Homsi AS, Roy TS, Cole K, Feng Y, Duffner U. Post-Transplant High-Dose Cyclophosphamide for the Prevention of Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2015;21(4):604–611. [DOI] [PubMed] [Google Scholar]
- 17.Boyiadzis M, Arora M, Klein J, et al. Impact of chronic graft-versus-host disease on late relapse and survival on 7489 patients after myeloablative allogeneic hematopoietic cell transplantation for leukemia. Clin Cancer Res. 2015;21(9):2020–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grimwade D, Hills RK, Moorman AV, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116(3):354–365. [DOI] [PubMed] [Google Scholar]
- 19.Breems DA, Van Putten WL, De Greef GE, et al. Monosomal karyotype in acute myeloid leukemia: a better indicator of poor prognosis than a complex karyotype. J Clin Oncol. 2008;26(29):4791–4797. [DOI] [PubMed] [Google Scholar]
- 20.Döhner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–474. [DOI] [PubMed] [Google Scholar]
- 21.Taskesen E, Bullinger L, Corbacioglu A, et al. Prognostic impact, concurrent genetic mutations, and gene expression features of AML with CEBPA mutations in a cohort of 1182 cytogenetically normal AML patients: further evidence for CEBPA double mutant AML as a distinctive disease entity. Blood. 2011;117(8):2469–2475. [DOI] [PubMed] [Google Scholar]
- 22.Röllig C, Bornhäuser M, Kramer M, et al. Allogeneic Stem-Cell Transplantation in Patients With NPM1-Mutated Acute Myeloid Leukemia: Results From a Prospective Donor Versus No-Donor Analysis of Patients After Upfront HLA Typing Within the SAL-AML 2003 Trial. J Clin Oncol. 2015;33(5):403–410. [DOI] [PubMed] [Google Scholar]
- 23.Kiyoi H, Yanada M, Ozekia K. Clinical significance of FLT3 in leukemia. Int J Hematol. 2005;82(2):85–92. [DOI] [PubMed] [Google Scholar]
- 24.Schlenk RF, Kayser S, Bullinger L, et al. Differential impact of allelic ratio and insertion site in FLT3-ITD-positive AML with respect to allogeneic transplantation. Blood. 2014;124(23):3441–3449. [DOI] [PubMed] [Google Scholar]
- 25.Gröschel S, Lugthart S, Schlenk RF, et al. High EVI1 expression predicts outcome in younger adult patients with acute myeloid leukemia and is associated with distinct cytogenetic abnormalities. J Clin Oncol. 2010;28(12):2101–2107. [DOI] [PubMed] [Google Scholar]
- 26.Gaidzik VI, Schlenk RF, Paschka P, et al. Clinical impact of DNMT3A mutations in younger adult patients with acute myeloid leukemia: results of the AML Study Group (AMLSG). Blood. 2013;121(23):4769–4777. [DOI] [PubMed] [Google Scholar]
- 27.Tie R, Zhang T, Fu H, et al. Association between DNMT3A mutations and prognosis of adults with de novo acute myeloid leukemia: a systematic review and meta-analysis. PLoS One. 2014;9(6):e93353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schnittger S, Eder C, Jeromin S, et al. ASXL1 exon 12 mutations are frequent in AML with intermediate risk karyotype and are independently associated with an adverse outcome. Leukemia. 2013;27(1):82–91. [DOI] [PubMed] [Google Scholar]
- 29.Damm F, Markus B, Thol F, et al. TET2 mutations in cytogenetically normal acute myeloid leukemia: clinical implications and evolutionary patterns. Genes Chromosomes Cancer. 2014;53(10):824–832. [DOI] [PubMed] [Google Scholar]
- 30.Gaidzik VI, Paschka P, Späth D, et al. TET2 mutations in acute myeloid leukemia (AML): results from a comprehensive genetic and clinical analysis of the AML study group. J Clin Oncol. 2012;30(12):1350–1357. [DOI] [PubMed] [Google Scholar]
- 31.Seifert H, Mohr B, Thiede C, et al. The prognostic impact of 17p (p53) deletion in 2272 adults with acute myeloid leukemia. Leukemia. 2009;23(4):656–663. [DOI] [PubMed] [Google Scholar]
- 32.Middeke JM, Beelen D, Stadler M, et al. Outcome of high-risk acute myeloid leukemia after allogeneic hematopoietic cell transplantation: negative impact of abnl(17p) and -5/5q- Blood. 2012;120(12): 2521–2528. [DOI] [PubMed] [Google Scholar]
- 33.Kats LM, Reschke M, Taulli R, et al. Protooncogenic role of mutant IDH2 in leukemia initiation and maintenance. Cell Stem Cell. 2014;14(3):329–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nomdedéu J, Hoyos M, Carricondo M, et al. Adverse impact of IDH1 and IDH2 mutations in primary AML: experience of the Spanish CETLAM group. Leuk Res. 2012;36(8):990–997. [DOI] [PubMed] [Google Scholar]
- 35.Gaidzik VI, Bullinger L, Schlenk RF, et al. RUNX1 mutations in acute myeloid leukemia: results from a comprehensive genetic and clinical analysis from the AML study group. J Clin Oncol. 2011;29(10): 1364–1372. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Wu DP, Liu QF, et al. In adults with t(8;21)AML, posttransplant RUNX1/RUNX1T1-based MRD monitoring, rather than c-KIT mutations, allows further risk stratification. Blood. 2014;124(12): 1880–1886. [DOI] [PubMed] [Google Scholar]
- 37.Patel JP, Gönen M, Figueroa ME, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366(12):1079–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grossmann V, Schnittger S, Kohlmann A, et al. A novel hierarchical prognostic model of AML solely based on molecular mutations. Blood. 2012;120(15):2963–2972. [DOI] [PubMed] [Google Scholar]
- 39.Grimwade D, Freeman SD. Defining minimal residual disease in acute myeloid leukemia: which platforms are ready for “prime time”? Blood. 2014;124(23):3345–3355. [DOI] [PubMed] [Google Scholar]
- 40.Ossenkoppele GJ, Schuurhuis GJ. MRD in AML: it is time to change the definition of remission. Best Pract Res Clin Haematol. 2014;27(3–4):265–271. [DOI] [PubMed] [Google Scholar]
- 41.Gorello P, Cazzaniga G, Alberti F, et al. Quantitative assessment of minimal residual disease in acute myeloid leukemia carrying nucleophosmin (NPM1) gene mutations. Leukemia. 2006;20(6):1103–1108. [DOI] [PubMed] [Google Scholar]
- 42.Ossenkoppele GJ, van de Loosdrecht AA, Schuurhuis GJ. Review of the relevance of aberrant antigen expression by flow cytometry in myeloid neoplasms. Br J Haematol. 2011;153(4):421–436. [DOI] [PubMed] [Google Scholar]
- 43.Terwijn M, van Putten WL, Kelder A, et al. High prognostic impact of flow cytometric minimal residual disease detection in acute myeloid leukemia: data from the HOVON/SAKK AML 42A study. J Clin Oncol. 2013;31(31):3889–3897. [DOI] [PubMed] [Google Scholar]
- 44.Gabert J, Beillard E, van der Velden VH, et al. Standardization and quality control studies of ‘real-time’ quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia - a Europe Against Cancer program. Leukemia. 2003;17(12):2318–2357. [DOI] [PubMed] [Google Scholar]
- 45.Jourdan E, Boissel N, Chevret S, et al. French AML Intergroup Prospective evaluation of gene mutations and minimal residual disease in patients with core binding factor acute myeloid leukemia. Blood. 2013; 121(12):2213–2223. [DOI] [PubMed] [Google Scholar]
- 46.Grimwade D, Jovanovic JV, Hills RK, et al. Prospective minimal residual disease monitoring to predict relapse of acute promyelocytic leukemia and to direct pre-emptive arsenic trioxide therapy. J Clin Oncol. 2009;27(22):3650–3658. [DOI] [PubMed] [Google Scholar]
- 47.Walter RB, Buckley SA, Pagel JM, et al. Significance of minimal residual disease before myeloablative allogeneic hematopoietic cell transplantation for AML in first and second complete remission. Blood. 2013;122(10):1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walter RB, Gooley TA, Wood BL, et al. Impact of pretransplantation minimal residual disease, as detected by multiparametric flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute myeloid leukemia. J Clin Oncol. 2011;29(9):1190–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buckley SA, Appelbaum FR, Walter RB. Prognostic and therapeutic implications of minimal residual disease at the time of transplantation in acute leukemia. Bone Marrow Transplant. 2013;48(5):630–641. [DOI] [PubMed] [Google Scholar]
- 50.Walter RB, Gyurkocza B, Storer BE, et al. Comparison of minimal residual disease as outcome predictor for AML patients in first complete remission undergoing myeloablative or nonmyeloablative allogeneic hematopoietic cell transplantation. Leukemia. 2015;29(1):137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kanate AS, Pasquini MC, Hari PN, Hamadani M. Allogeneic hematopoietic cell transplant for acute myeloid leukemia: Current state in 2013 and future directions. World J Stem Cells. 2014;6(2):69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gratwohl A, Stern M, Brand R, et al. Risk score for outcome after allogeneic hematopoietic stem cell transplantation. Cancer. 2009;115(20):4715–4726. [DOI] [PubMed] [Google Scholar]
- 53.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]


