Abstract
In chronic lymphocytic leukemia the balance between the pro-apoptotic and anti-apoptotic members of the bcl-2 family is involved in the pathogenesis, chemorefractoriness and clinical outcome. Moreover, the recently proposed anti-bcl-2 molecules, such as ABT-199, have emphasized the potential role of of bcl-2 family proteins in the context of target therapies. We investigated bax/bcl-2 ratio by flow cytometry in 502 patients and identified a cut off of 1.50 to correlate bax/bcl-2 ratio with well-established clinical and biological prognosticators. Bax/bcl-2 was 1.50 or over in 263 patients (52%) with chronic lymphocytic leukemia. Higher bax/bcl-2 was associated with low Rai stage, lymphocyte doubling time over 12 months, beta-2 microglobulin less than 2.2 mg/dL, soluble CD23 less than 70 U/mL and a low risk cytogenetic profile (P<0.0001). On the other hand, lower bax/bcl-2 was correlated with unmutated IGHV (P<0.0001), mutated NOTCH1 (P<0.0001) and mutated TP53 (P=0.00007). Significant shorter progression-free survival and overall survival were observed in patients with lower bax/bcl-2 (P<0.0001). Moreover, within IGHV unmutated (168 patients) and TP53 mutated (37 patients) subgroups, higher bax/bcl-2 identified cases with significant longer PFS (P=0.00002 and P=0.039). In multivariate analysis of progression-free survival and overall survival, bax/bcl-2 was an independent prognostic factor (P=0.0002 and P=0.002). In conclusion, we defined the prognostic power of bax/bcl-2 ratio, as determined by a flow cytometric approach, and highlighted a correlation with chemoresistance and outcome in chronic lymphocytic leukemia. Finally, the recently proposed new therapies employing bcl-2 inhibitors prompted the potential use of bax/bcl-2 ratio to identify patients putatively resistant to these molecules.
Introduction
Chronic lymphocytic leukemia (CLL) is a clinically heterogeneous disease characterized by the accumulation of CD5+CD23+ B-cell lymphocytes.1 The genetic and molecular mechanisms involved in the development of the disease are not well known, but recent observations suggest that the modulation of survival signals interfering with apoptosis may be a pivotal tool in the pathogenesis of CLL.2 Bcl-2 (B-cell lymphoma-2) family of antiapoptotic (bcl-2, bcl-xl, bcl-w and mcl-1) and proapoptotic (bax, bak and bok) proteins are critical regulators of apoptosis in CLL.3 In particular, bcl-2 has emerged as the most important protein in predicting survival in CLL between 11 proteins that are implicated in the control of apoptosis, proliferation and differentiation.4 High levels of bcl-2 protein are found in CLL cells even in the absence of classical t(14;18) translocation that is detected frequently in follicular lymphomas.5 In this context, Cimmino et al.6 demonstrated that the loss of microRNAs (miR), miR-15a and miR-16-1, is involved in regulation of the bcl-2 family expression levels. Moreover, bcl-2 family members have also been linked to resistance of chemotherapy in CLL. In particular, Pepper et al.7 demonstrated that high levels of bcl-2 and low levels of bax protein expression are linked to chemoresistance in CLL cells treated with chlorambucil and a remarkable elevation of bax protein was found in CLL cells undergoing apoptosis. Several reports further confirmed that in CLL the balance between the pro- and anti-apoptotic members of the Bcl-2 family determines the chemotherapy sensitivity and survival.8,9 Finally, the recent introduction in the clinical use of novel potent oral pro-apoptotic BH3 peptidomimetics such as ABT-19910,11 and ABT-73712 has further emphasized the importance of bcl-2 family proteins in the context of CLL therapy. In the present study, we addressed the clinical impact of bax/bcl-2 ratio, evaluated by a flow cytometric approach, as independent prognosticator of progression-free survival (PFS) and overall survival (OS) in CLL. In this context, we also correlated the bax/bcl-2 ratio with the other well-established clinical-biological prognosticators in CLL.
Methods
Study design and patients
This is a retrospective study that includes peripheral blood (PB) from 502 CLL patients recruited in a single center during the period 1989–2014. Approval of this study was obtained from the Institutional Review Board of the Centro di Riferimento Oncologico (IRCSS) of Aviano (PN), Italy. Informed consent was provided in accordance with the Declaration of Helsinki. Patients were 279 males and 223 women with a median age of 66 years (range 33–89) at the time of diagnosis. Using the modified Rai staging system, 170 patients had a low stage, 318 an intermediate stage, and 14 a high stage. Median follow up was 7.9 years with 103 deaths and 399 censored patients. Two hundred and ninety-one patients out of 502 (58%) received chemotherapy for their disease. Eighty-seven patients were treated with a combination of chlorambucil plus prednisone and rituximab was added in 11 of them. One hundred and sixty patients received six courses of fludarabine monophosphate (Fludara; Genzyme, Modena, Italy) at 25 mg/m2 intravenously or 30–40 mg/m2 orally for five days every 28 days followed by four courses of rituximab (Mabthera; Roche, Basilea, Switzerland) at 375 mg/m2 for one day every week.13 Twenty-seven patients were treated with six courses of fludarabine, cyclophosphamide and rituximab.14 Finally, 17 patients aged over 60 years received bendamustine at 70 mg/m2 for two days and rituximab for one day at conventional dosages.15
Cellular immunophenotypic analysis
All flow cytometric analyses were performed on a FACSCalibur flow cytometer (BD, Biosciences, CA, USA). The instrument was aligned and calibrated daily with the use of a 4-color mixture of CaliBRITE beads (BD, Biosciences) with FACSComp Sofware (BD, Biosciences), according to the manufacturer’s instructions. Triple-color immunofluorescence analysis of surface antigens was performed by using combinations of phycoerythrin (PE), fluorescein isothiocyanate (FITC), and allophycocyanin (APC) monoclonal antibodies (MoAbs). The following conjugated MoAbs were used: anti-CD23 PE, anti-CD5 FITC, anti-CD5 APC, anti-CD38 PE, anti-CD19 PE, anti-CD19 APC, anti-CD49d PE and anti-CD45 FITC (BD, Biosciences). The peripheral blood (PB) mononuclear cells were analyzed for surface expression of CD19/CD5/CD38, CD19/CD5/CD23 and CD19/CD5/CD49d in order to confirm CLL diagnosis.
Bcl-2 and bax expression
Bcl-2 and bax oncoproteins were evaluated by flow cytometry using the following two MoAbs: 1) Anti-human bcl-2 clone 124 FITC-conjugated MoAb (Dako, Glostrup, Denmark) is an immunoglobulin G1 (IgG1) that reacts specifically with bcl-2 oncoprotein associated with mitochondria, smooth endoplasmic reticulum, and perinuclear membrane;16,17 2) anti-bax clone Ab-2 (Oncogene, Cambridge, MA, USA) is an IgG1 that recognizes bax, normally localized to the cytoplasm, but translocating rapidly to the mitochondria after the induction of an apoptotic signal.18
All samples were tested at diagnosis or before any therapeutic approach. For multiparameter analysis of bcl-2 and bax combined with lymphoid antigens, mononuclear cells were initially incubated with CD19 PE and CD5 APC MoAbs for 30 min at 4°C. Subsequently, the cells, after washing twice in PBS, were fixed and permeabilized with Fix & Perm Cell Permeabilization kit® (Fix&Perm, Caltag Laboratories, Carlsbad, CA, USA). All samples were then incubated at 4°C for 30 min with either 10 μL anti-bcl-2 FITC or 20 μL unconjugated anti-bax. For bax, cells were further incubated at 4°C for 30 min with 100 μL FITC-conjugated F(ab)2 fragment of goat antimouse immunoglobulin (dilution 1:50; Dako, Glostrup; Denmark). Negative controls were performed by incubating cells with non-specific isotype IgG1 antibodies (Figure 1B and F). The results were performed by gating lymphoid cells on a SSC versus FSC plot (Figure 1A and E) and examining bcl-2 and bax as mean fluorescence intensity (MFI) on CD19 positive/CD5 positive cells (Figure 1C, D, G and H).19 Bcl-2 and bax were then evaluated as relative mean fluorescence intensities (rMFIs), calculated as the ratio between bcl-2 or bax MoAbs MFI and non-specific MoAb MFI on B cells. Finally, the results were expressed as an index (bax/bcl-2) obtained by dividing rMFI bax and rMFI bcl-2. Estimation of bax/bcl-2 ratio yelding the best separation of 2 subgroups with different OS and/or PFS probabilities was made by applying various methods including Youden’s index, median value and receiver-operating characteristic (ROC) analysis. Finally, the threshold was set at the bax/bcl-2 median value equal or higher than 1.50 (range 0.27–6.10) confirmed also by ROC analysis (Online Supplementary Figure S1). Two small subsets of patients were studied using 20 μl of two directly conjugated anti–bax MoAbs (clone 2D2), one conjugated with FITC (Santa Cruz Biotechnology, Dallas, TX, USA) and the other with APC (Abcore, Ramona, CA, USA).
Figure 1.
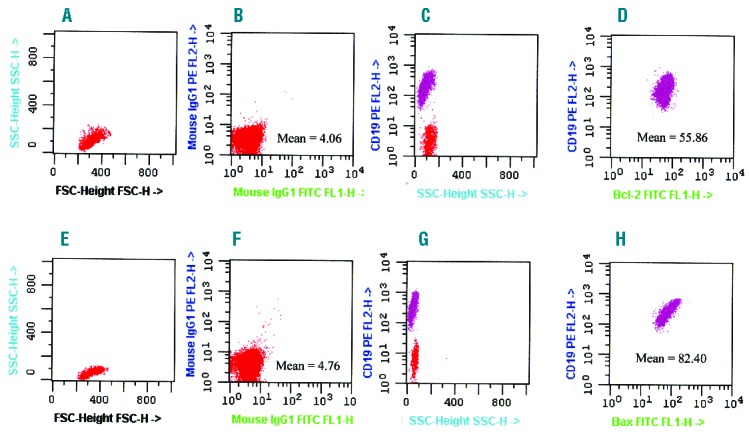
Characteristics of bcl-2 and bax expressions. (A–E) Forward and side scatter of representative gated lymphocytes are shown. (B–F) Mouse IgG1 isotypic antibodies are used as controls for bcl-2 and bax positivities with their mean fluorescence intensities (MFI). (C-G) B-CLL (CD19+) cells (violet) were selected on the gated lymphocytes according to their phenotype. (D-H) Flow cytometric dot plots of bcl-2 and bax (violet) on CD19+ cells on peripheral blood sample of one representative CLL case with reported MFI values.
Soluble CD23 and B2-microglobulin detection
Soluble CD23 (sCD23) immunoenzymometric assay and β2-microglobulin (β2M) determinations were performed as described elsewhere.20 The threshold of positivity was set at 70 U/mL for sCD23 and 2.2 g/L for β2M on the basis of normal laboratory references values.
Interphase FISH
Separate hybridations were carried out for loci on chromosome 11, 12, 13 and 17. For chromosome 11, 13, 12 and 17 specific probes and procedures were used, as reported previously.21
IGHV mutation analysis
Total RNAs were extracted and reverse-transcribed. The resulting cDNAs, checked for first strand synthesis,22 were amplified using a mixture of sense primers annealing to either VH1 through VH6 leader sequences or 5′ends of VH1-VH6 FR1. Purified amplifications were cloned and a minimum of 10–20 colonies for each case were sequenced; CLL specific IGHV transcripts, identified by sharing the same CDR3 in multiple clones were analyzed for percent mutation, as previously described. VH gene sequences deviating more than 2% from the corresponding germline gene were defined as mutated.23
NOTCH1 mutational status and TP53 mutational status
The presence of NOTCH1 mutations was investigated with amplification refractory mutation system (ARMS) PCR for c.7544-7545delCT and by Sanger sequencing of NOTCH1 exon 34.24 Mutation analysis of TP53 exons 2 to 11 was carried out by DNA direct sequencing on an ABI Prism 3130 automated DNA sequence analyzer (Applied Biosystems, Foster City, CA, USA) according to the International Agency for Research on Cancer (IARC) guidelines (www.p53-iarc.fr) and analyzed with the Sequencing Analysis v.5.2 software (Applied Biosystems), as previously reported.25 Mutations were confirmed on both strands on independent amplimers and validated by the IARC TP53 Mutation Database R15, as previously reported.
Statistical analysis
Correlations between bax/bcl-2 ratio and the other biological and clinical variables were based on the two-tailed Fisher exact test. The clinical assessment of CLL patients was based both on the National Cancer Institute Working Group criteria26 and on the International Workshop on Chronic Lymphocytic Leukemia criteria after 2008.27 PFS and OS, measured from diagnosis, were estimated according to the Kaplan-Meier method and compared between groups by means of the log rank test. Cox proportional hazards regression models were used to assess the independent effect of co-variables, treated as dichotomous, both on the PFS and OS.
Results
Expression of bax/bcl-2 and association with other prognostic factors
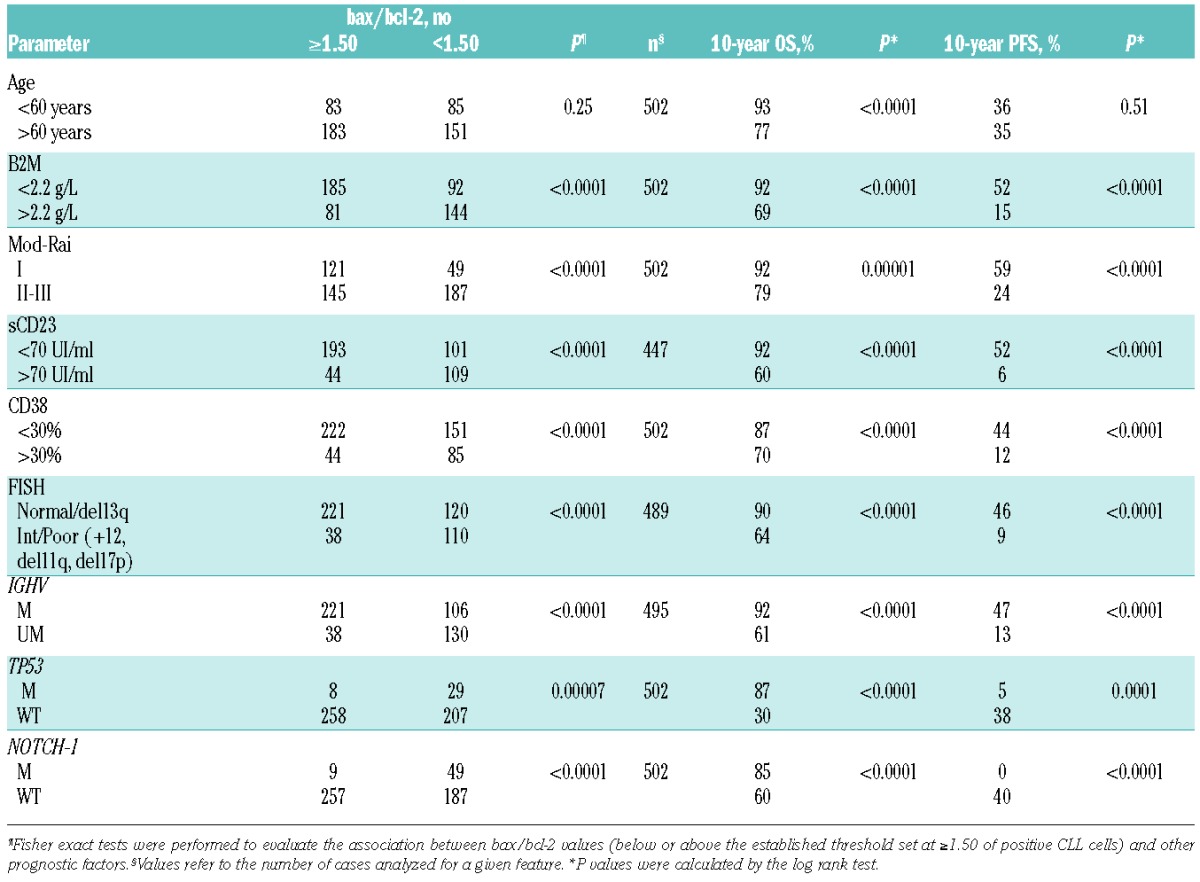
Bax and bcl-2 presented variable pattern of mean fluorescence intensity. Median rMFIs were 12.39 (range 3.85–103.0) for bcl-2 and 17.44 (range 2.20–100.0) for bax, respectively. Median bax/bcl-2 ratio (bax/bcl-2) was 1.50 (range 0.26–6.10). The median bax/bcl-2 ratio was chosen as cut off to discriminate subgroups of cases, being very similar to the best cut off defined by ROC analysis (Online Supplementary Figure S1). Using this cut off, 263 patients (263 of 502, 52%) were bax/bcl-2 positive (≥1.50). An inverse correlation was observed between bax/bcl-2 levels and PB B-cell count (Spearman correlation, r=0.26, P<0.0001). Higher bax/bcl-2 was significantly associated with low Rai stage, lymphocyte doubling time (LDT) over 12 months, β2M less than 2.2 mg/dL and soluble CD23 less than 70 U/mL (P<0.0001) (Table 1). Moreover, lower bax/bcl-2 was significantly associated both with CD38 more than 30% (P<0.0001) (Table 1). and CD49d more than 30% (P=0.010) (Table 1). Four hundred and eighty-nine patients were analyzed by interphase FISH to evaluate deletions in chromosome bands 17p13, 11q23, 13q14, and trisomy of band 12q13. There was a significant correlation between higher bax/bcl-2 and low risk (normal karyotype or del13q) cytogenetics (P<0.0001) (Table 1). Conversely, lower bax/bcl-2 was significantly represented within the intermediate/high risk (trisomy 12, del11q and del17p) classes (P<0.0001) (Table 1). Significant associations were also found between lower bax/bcl-2 and IGHV unmutated status (P<0.0001) or NOTCH1 mutations (P<0.0001) or TP53 mutations (P=0.00007) (Table 1). Moreover, we analyzed a validation cohort of 233 CLL patients recruited in another Institution (Policlinico Tor Vergata) and analyzed in another laboratory located at University Tor Vergata obtaining similar results with regard to bax/bcl-2 ratio and all the above mentioned variables (Online Supplementary Table S1 and Figure S2). Interestingly, 54 of these patients were the same as our series with similar results regarding bax/bcl-2 ratio. We repeated these assays in 20 thawed samples using an anti-bax MoAb APC (Abcore, Ramona, CA, USA) and an anti-bcl-2 MoAb APC (Abcore, Ramona, CA, USA). Then we compared these bax/bcl-2 ratios with the bax/bcl-2 ratios previously obtained on the same patients using fresh cells and an anti-bax unconjugated MoAb. We obtained similar results in 20 of 20 cases (Online Supplementary Table S2) using the same cut off (≥1.50). Interestingly, significant correlations between bax/bcl-2 ratio and some other prognostic factors (Online Supplementary Table S3) were confirmed in 52 patients who were recently studied using an anti-bax FITC MoAb (clone 2D2, Santa Cruz Biotechnology, Dallas, TX, USA) and anti-bcl-2 FITC MoAb (clone 124). In this subset, median rMFI for bax FITC was 18.32 (range 4.0–38.9) and the same value of bax/bcl-2 ratio (≥1.50) was used as cut off. Interestingly, we confirmed the stability over time of bax/bcl-2 ratio in 46 of our untreated patients in which this index was repeated three times along the course of the disease (Online Supplementary Table S4). It is worthy of note that, in some patients treated with fludarabine, we observed that bax/bcl-2 ratio increased progressively from one day post treatment to one month post treatment and this increase was higher in responsive patients (data not shown).
Table 1.
Distribution of prognostic factors in CLL according to bax/bcl-2 values.
Relevance of bax/bcl-2 ratio as biological prognostic marker
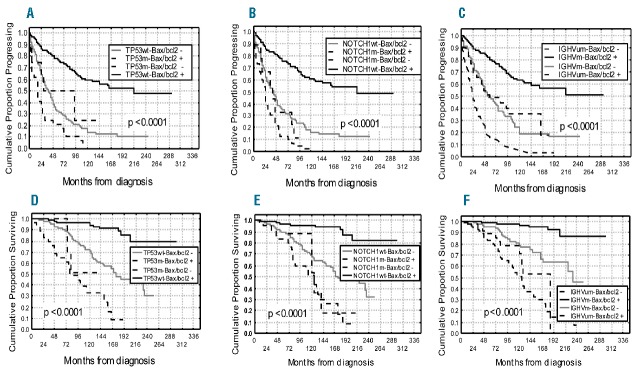
With regard to clinical behavior, a significant correlation was found between bax/bcl-2 and treatment. In particular, 197 of 236 (83%) with lower bax/bcl-2 received chemotherapy, while only 90 (33%) of 263 patients with bax/bcl-2 1.50 or over were treated (P<0.0001). Moreover, 82 (35%) bax/bcl-2 negative patients received two or more treatment regimens versus 25 (9%) bax/bcl-2 positive patients (P<0.0001). Interestingly, patients with lower bax/bcl-2 went more rapidly to treatment (27 months vs. 36 months; P=0.019). It is worthy of note that, in a series of 160 patients treated in induction with fludarabine plus rituximab, all resistant cases (8 patients) were bax/bcl-2 negative, while among patients who achieved a response (152 patients), those bax/bcl-2 positive showed a significant longer response duration compared to bax/bcl-2 negative cases (46% vs. 30% at 4 years; P=0.021) (Figure 2). With regard to clinical outcome, significant shorter PFS and OS were observed in patients with lower bax/bcl-2 (10% vs. 52% at 16 years, P<0.0001 and 46% vs. 79% at 16 years, P<0.0001, respectively) (Figure 3A and B). Similar results were obtained when analyzing the impact on PFS and OS of IGHV gene mutations as well as of TP53 and NOTCH1 mutations (Table 1). A better refinement in the prognostic assessment of PFS and OS was obtained by combining bax/bcl-2 values with those of TP53 and NOTCH1 (Figure 4A and B, and D and E). For all these variables, bax/bcl-2 expression had true additive properties. In particular, positive or negative bax/bcl-2 combined both with wild-type or mutated TP53 or with wild-type or mutated NOTCH1 identified 2 subsets of patients, the former with the best prognosis and the latter with the worst prognosis with regard to both PFS (65% vs. 10% at 8 years, P<0.0001 and 66% vs. 4% at 8 years, P<0.0001) and OS (96% vs. 33% at 10 years, P<0.0001 and 95% vs. 52% at 10 years, P<0.0001). In all combinations, discordant patients showed intermediate outcomes (Figure 4A and B, and D and E). Similar behavior was observed by combining bax/bcl-2 and IGHV mutational status in additional bivariate analyses, the shortest PFS and OS time intervals being found for bax/bcl-2 negative/UM IGHV patients (7% vs. 63% at 10 years and 52% vs. 97% at 10 years; P<0.0001) (Figure 4C and F). To further explore the prognostic impact of bax/bcl-2, we investigated its expression within TP53 mutated (n=37) and IGHV unmutated (n=168) subgroups that notoriously have the worst prognosis. As a matter of fact, higher bax/bcl-2 identified patients with a significant longer PFS (50% vs. 10% and 43% vs. 10% at 7 years; P=0.039 and P=0.00002, respectively) (Figure 5A and B), so confirming its very high prognostic impact.
Figure 2.
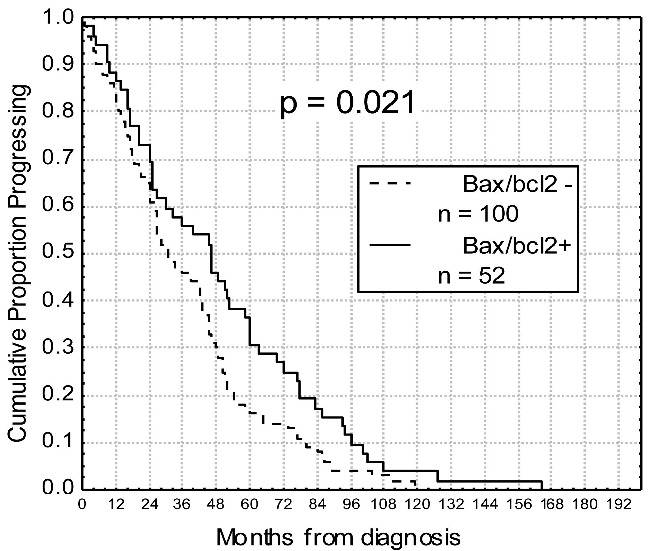
Duration of response after fludarabine and rituximab in relation to bax/bcl-2 values. Progression-free survival after treatment was significantly longer within chronic lymphocytic leukemia subgroup showing bax/bcl-2 ≥1.50 (bax/bcl2+; 46% vs. 30% at 4 years; P=0.021).
Figure 3.
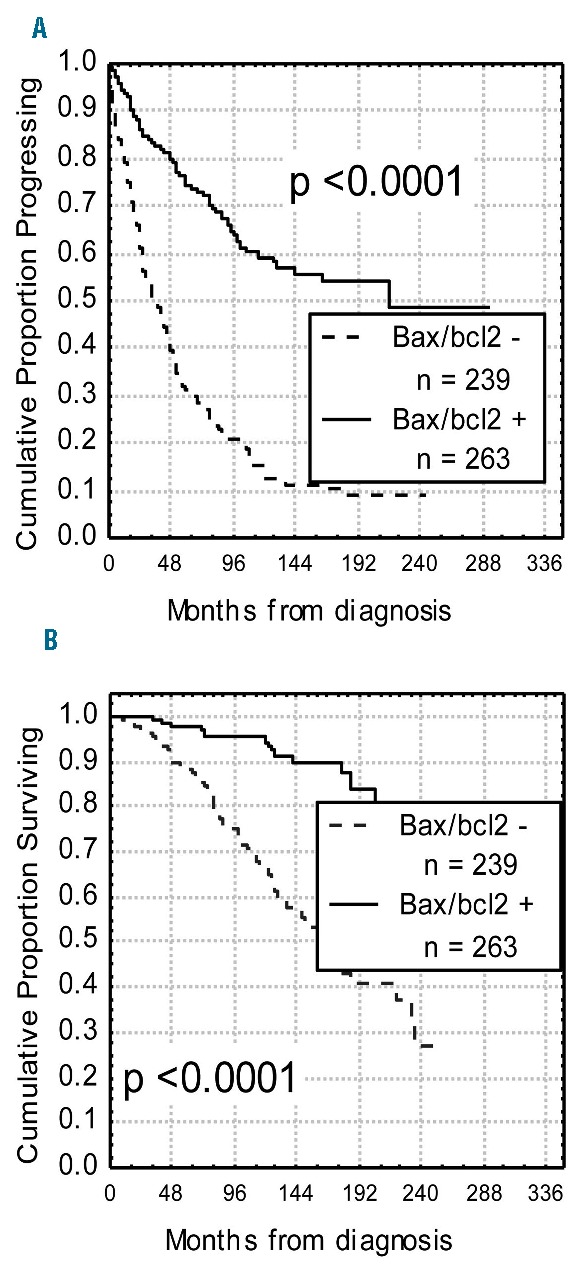
Progression-free survival (PFS) and overall survival (OS) curves based on bax/bcl-2 values. Kaplan-Meier plot comparing PFS (A) and OS (B) based on the detection of bax/bcl-2 ratio ≥1.50 (bax/bcl2+) or <1.50 (bax/bcl2−). Bax/bcl2+ patients experienced both a longer PFS and OS (P<0.0001).
Figure 4.
Progression-free survival (PFS) and overall survival (OS) curves in relation to combined bax/bcl-2 ratio and TP53 or bax/bcl-2 and NOTCH1 or bax/bcl-2 and IGHV status. PFS and OS were significantly longer within the bax/bcl2+ (≥1.50) TP53 wild-type (wt) subgroup (A-D), or within bax/bcl2+ NOTCH1 wt patients (B–E) or within bax/bcl2+IGHV mutated (m) cases (C-F). Discordant patients showed an intermediate outcome.
Figure 5.
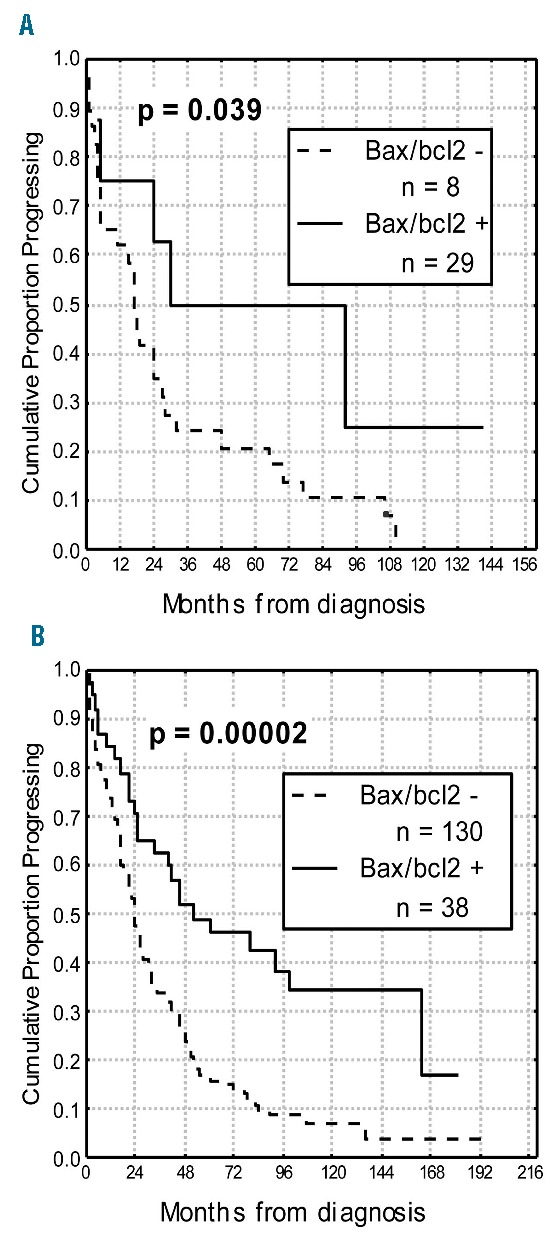
Progression-free survival (PFS) curves based on bax/bcl-2 within IGHV unmutated subgroup and TP53 mutated subgroup. Bax/bcl2+ (≥1.50) patients showed a significant longer PFS both within TP53 mutated subgroup (A) and within IGHV unmutated subset (B).
Multivariate analysis
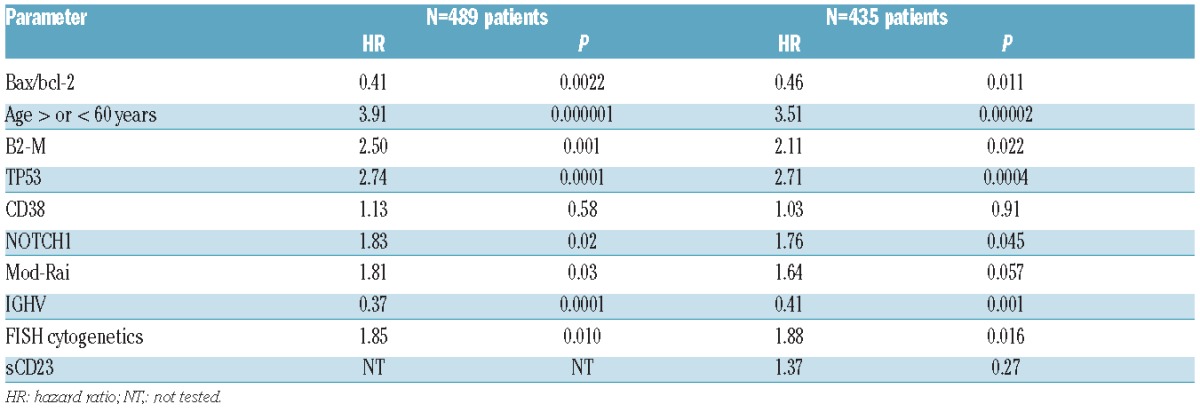
The clinical impact of bax/bcl-2 as independent prognosticator both for PFS and OS was also checked by multivariate Cox proportional hazards analysis applied to models including the prognosticators proven to be significant in univariate analysis (Table 1). In a cohort of 489 patients, in which all the above mentioned prognostic factors were available at the same time, bax/bcl-2 was confirmed to be an independent prognosticator with regard to PFS along with mod-Rai, β2M, FISH, TP53 and IGHV (Table 2). Similarly, multivariate analysis selected age, β2M, bax/bcl-2, FISH, NOTCH1, TP53 and IGHV as significant independent prognosticators for OS (Table 3). Equally, within a subset of 435 patients, in which sCD23 was included, bax/bcl-2 was confirmed again as an independent prognosticator both with regard to PFS and OS (Tables 2 and 3).
Table 2.
Multivariate Cox regression analysis of progression-free survival.
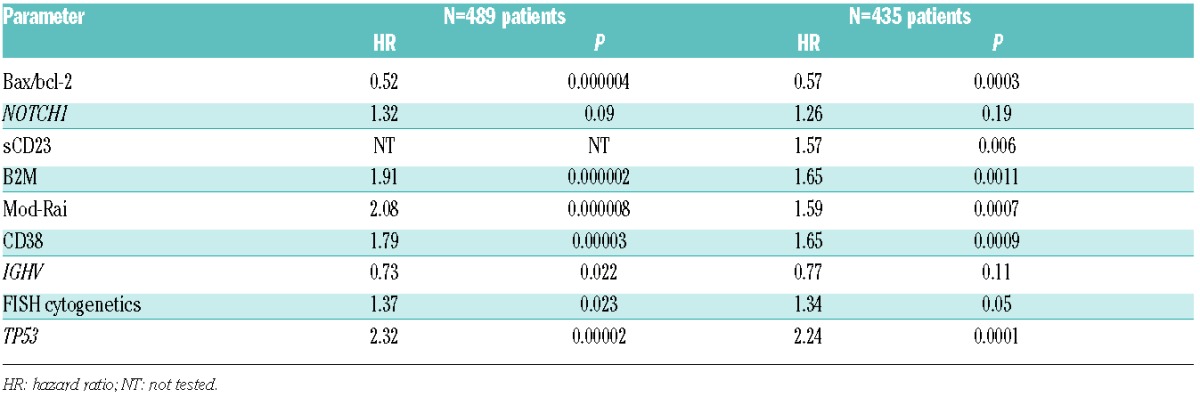
Table 3.
Multivariate Cox regression analyses of overall survival.
Discussion
In CLL malignant clonal B cells accumulate because of dysregulated production and inappropriately prolonged survival due to impairment of apoptosis.2 In fact, the alteration of some apoptotic mechanisms such as the balance between the pro- (bax) and anti-apoptotic members (bcl-2) of the bcl-2 family, is recognized to be an important factor not only in the pathogenesis but also in the development of chemorefractoriness in CLL.7 Thus, measures of bax/bcl-2 could provide information on the intrinsic chemosensitivity of CLL influencing response to treatment and clinical outcome.
In the present study, we addressed the clinical impact of bax/bcl-2 ratio, evaluated with a flow cytometric approach, as independent prognostic factor in a large homogeneous series of 502 patients affected by CLL. We had previously demonstrated that the quantification of bcl-2 and bax, measuring fluorescence intensity by flow cytometry, is technically adequate and useful for a correct evaluation of clinical and prognostic correlations both in acute myeloid leukemia17 and in CLL.19 In the present study, we used a well-standardized and simple flow cytometric method for bcl-2 and bax determinations. In particular the methodological feasibility and reproducibility of this flow cytometric approach was confirmed by the finding that superimposable results of bax/bcl-2 ratios were obtained in a series of CLL cases for which bax/bcl-2 measures from fresh PB samples were repeated in two different laboratories (Online Supplementary Table S1), or when two directly conjugated anti-bax MoAbs were employed (Online Supplementary Tables S2 and S3).
The robustness of this flow cytometry-based method was also highlighted by the fact that in a series of 46 untreated patients in which the bax/bcl-2 measures were performed three times along the course of the disease, but always before any treatment, the bax/bcl-2 ratio was seen to be stable over time (Online Supplementary Table S4). This stability of the ratio underlines a reliable use of bax/bcl-2 ratio as prognostic marker, although further analysis is required to clarify this important issue. However, to the best of our knowledge, this is the largest study in CLL patients on clinical significance of bax/bcl-2 index by flow cytometry. Clinically, by using a cut off of 1.50, representing the median value of bax/bcl-2 ratio of our case cohort, and further confirmed as best cut off by ROC analysis, we found significant correlations of bax/bcl-2 ratio both with tumor burden indicators (PB B lymphocytes, Rai stages, β2M, sCD23 and LDT) and with other biological prognosticators (CD38, CD49d, IGHV mutations, cytogenetic abnormalities, NOTCH1 and TP53). Specifically, we observed that a higher bax/bcl-2 ratio showed a strict correlation with some favorable clinical features such as low PB B cells and low/intermediate Rai stages. Moreover, low levels of sCD23 and β2M, known markers of disease activity in CLL, were correlated with higher apoptosis levels,28 although previously published studies did not find any significant association between some apoptosis regulation proteins such as bcl-2, mcl-1 and Rai stages, while their correlation with CD23 or β2M was not investigated.29–32 Most of these studies involved a small number of patients and methods other than flow cytometry, such as Western blotting, immunoblotting and PCR.33 However, Kitada et al.32 demonstrated that high bcl-2/bax ratio was associated with elevated white blood cell count, similar to that found in our series. Moreover, we noted also that LDT was significantly longer in bax/bcl-2 positive patients, thus suggesting a significant correlation between this ratio and low proliferation.
From a biological point of view, we found a significant relationship between this index and some well-known biological prognosticators. First, we observed a significant lower bax/bcl-2 ratio in CD38 and CD49d positive patients. It is well known that CD38 and CD49d overexpression are adverse prognostic markers in CLL19,34,35 and it has been reported that interactions involving CD38 and CD49d prevent spontaneous and drug-induced apoptosis of normal and neoplastic B cells.36 Interestingly, co-culture of CLL cells with human vascular endothelial cells determined a simultaneous overexpression of CD38, CD49d and bcl-2 with an enhanced tumor cell survival targeting NF-κB activation.37 Recently, it has been observed that CD49d was nearly universally expressed in trisomy 12 CLL patients.36 In our study, we demonstrated a significant correlation between lower bax/bcl-2 ratio and trisomy 12 (Table 1), so suggesting that this ratio may represent a further mechanism of chemoresistance in this subset. A strict association was also observed between bax/bcl-2 negative patients and other high risk (del11q and del17p) classes (Table 1), thus confirming the clinical impact of the apoptotic mechanisms in these poor risk cytogenetic subsets. Conversely, higher bax/bcl-2 was significantly represented in low risk (normal or del13q) cytogenetic groups (Table 1).
In addition, we found that a lower bax/bcl-2 ratio was associated with NOTCH1 mutations (Table 1), unfavorable prognosticator in CLL,38–40 in keeping with previous studies showing that NOTCH1 mutations activate NFkB pathway resulting in upregulations of target genes such as bcl-2 in T-acute lymphoblastic leukemia.41,42
Significant associations were also found between lower bax/bcl-2 ratio and UM-IGHV or TP53 mutations (P<0.0001) (Table 1), thus confirming that biological mechanisms related to defective apoptosis play a pivotal role both in the chemoresistance and in the unfavorable clinical course of these patient subgroups.
Results of this study also confirmed a correlation between bax/bcl-2 levels and chemoresistance in CLL, in keeping with previous studies.7,33,43,44 In particular, significant differences in treatment histories were found between bax/bcl-2 positive and negative patients. In fact, bax/bcl-2 negative patients underwent treatment more frequently and rapidly than bax/bcl-2 positive patients. Moreover, they presented a shorter response duration (Figure 2) and received, significantly, more chemotherapy regimens than patients with higher levels of bax/bcl-2.
Clinically, we demonstrated that the bax/bcl-2 ratio had an impact for PFS and OS in our large series of CLL patients, together with CD38, IGHV mutations, cytogenetic abnormalities, NOTCH1 and TP53 (Table 1). Moreover, bax/bcl-2 ratio was confirmed to be an independent prognostic factor with regard both to PFS and OS in multivariate analysis, considering 489 patients in which all the most important recognized prognostic factors were included (Tables 2 and 3).
Furthermore, bax/bcl-2 ratio demonstrated significant additive prognostic properties by the fact that positive or negative bax/bcl-2 combined both with wild-type or mutated TP53 or with wild-type or mutated NOTCH1 identified 2 subsets of patients, the former with the best prognosis and the latter with the worst prognosis with regard to both PFS and OS (Figure 4). The superior prognostic capacity of bax/bcl-2 with respect both to IGHV mutation status and TP53 mutations25,45,46 was corroborated by the observation that higher bax/bcl-2 ratio identified patients with a significant longer PFS within IGHV unmutated and TP53 mutated subgroups, notoriously with the worst prognosis (Figure 5).
The capacity of bax/bcl-2 index to identify subsets of patients with a different apoptotic profile that reflects prognosis could be of interest in the light of the recently introduced bcl-2 inhibitors such as ABT-199, a 2nd-generation BH3 mimetic, highly selective for bcl-2 with demonstrated high rates of activity in relapsed/refractory CLL patients.3,10 In this regard, the bax/bcl-2 ratio, determined with the flow cytometric approach, as proposed in the present study, could represent a proper predictive bio-marker to identify CLL patients who could benefit from the use of ABT-199 or who, on the contrary, could be more effectively treated with conventional chemotherapy. Experiments of apoptosis testing ABT-199 on CLL cells in order to use bax/bcl-2 ratio for identifying the sensitivity degree of patients and thus monitoring the anti-bcl-2 therapy are in progress at our Institution (data not shown). In this context, antibodies able to detect bax conformational changes could be useful to characterize apoptosis levels.47
In conclusion, in the present study, we defined the prognostic power of bax/bcl-2 ratio, as determined by a flow cytometric approach, easily transferable to routine laboratory practice. Moreover, we highlighted the correlation of these two mitochondrial apoptotic proteins with chemoresistance and clinical outcome in CLL. Finally, the recently proposed new therapies employing bcl-2 inhibitors, such as ABT-199, prompted the potential use of bax/bcl-2 ratio to identify CLL cases putatively resistant to these molecules.
Acknowledgments
Finally, all the authors thank the members of our Department of Haematology clinical staff for their invaluable support to our CLL clinical research program. Part of of this article was presented orally at the 56th Annual Meeting of the American Society of Hematology, San Francisco, CA, USA, December 6–9, 2014.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/101/1/77
Funding
This study was supported in part by Ministero dell’Università e della Ricerca Scientifica e Tecnologica (MURST), Programmi di Ricerca di Interesse Nazionale, 2005; Ministero della Salute (Ricerca Finalizzata Istituto di Ricovero e Cura a Carattere Scientifico [IRCCS], Rome, Italy; the Associazione Italiana Ricerca Cancro (AIRC), Investigator Grant IG-13227; Progetto Ricerca Finalizzata I.R.C.C.S. n. RF-2009-1469205, n. RF-2010-2307262; Progetto Giovani Ricercatori n.GR-2009-1475467, n.GR-2010-2317594, n.GR-2011-02347441, n.GR2011-02346826; Ministero della Salute, Rome, Italy; Fondazione Cariplo (grant 2012-0689); Associazione Italiana contro leLeucemie, Linfomi e Mielomi (AIL), Venezia Section, Pramaggiore Group, Italy; Fondazione per la Vita di Pordenone, Italy; Ricerca Scientifica Applicata, Regione Friuli Venezia Giulia (“Linfonet” Project), Trieste, Italy; “5×1000 Intramural Program”, Centro di Riferimento Oncologico, Aviano, Italy. F.A. is supported by a Beat-Leukemia fellowship.
References
- 1.Rozman C, Montserrat E. Chronic Lymphocytic leukemia. N Engl J Med. 1995;333(16):1052–1057. [DOI] [PubMed] [Google Scholar]
- 2.Chiorazzi N, Rai KR, Ferrarini M. Chronic Lymphocytic leukemia. N Engl J Med. 2005;352(8):804–815. [DOI] [PubMed] [Google Scholar]
- 3.Scarfò L, Ghia P. Reprogramming cell death: BCL2 family inhibition in hematological malignancies. Immunol Lett. 2013;155(1–2):36–39. [DOI] [PubMed] [Google Scholar]
- 4.Faderl S, Keating MJ, Do KA, et al. Expression profile of 11 proteins and their prognostic significance in patients with chronic lymphocytic leukemia (CLL.). Leukemia. 2002;16(6):1045–1052. [DOI] [PubMed] [Google Scholar]
- 5.Hanada M, Delia D, Aiello A, Stadtmauer E, Reed JC. Bcl-2 gene hypomethylation and high levels espression in B-cell chronic lymphocytic leukemia. Blood. 1993;82(6):1820–1828. [PubMed] [Google Scholar]
- 6.Cimmino A, Calin GA, Fabbri M, et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci USA. 2005;102(39):13944–13949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pepper C, Hoy T, Bentley P. Elevated bcl-2/bax are a consistent feature of apoptosis resistance in B-cell chronic lymphocytic leukemia and are correlated with in vivo chemoresistance. Leuk Lymphoma. 1998;28(3–4):355–361. [DOI] [PubMed] [Google Scholar]
- 8.Fegan C, Pepper C. Apoptosis deregulation in CLL. Adv Exp Med Biol. 2013;792:151–171. [DOI] [PubMed] [Google Scholar]
- 9.Williamson KE, Kelly JD, Hamilton PW, McManus D, Johnston SR. Bcl-2/Bax ratios in chronic lymphocytic leukaemia and their correlation with in vitro apoptosis and clinical resistance. Br J Cancer. 1998;78(4):553–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Souers AJ, Leverson JD, Boghaert ER, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19(2):202–208. [DOI] [PubMed] [Google Scholar]
- 11.Davids MS, Letai A. ABT-199: a new hope for selective BCL-2 inhibition. Cancer Cell. 2013;23(2):139–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Del Gaizo Moore V, Brown JR, Certo M, Love TM, Novina CD, Letai A. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J Clin Invest. 2007;117(1):112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Del Poeta G, Del Principe MI, Consalvo MA, et al. The addition of rituximab to fludarabine improves clinical outcome in untreated patients with ZAP-70 negative chronic lymphocytic leukemia. Cancer. 2005;104(12):2743–2752. [DOI] [PubMed] [Google Scholar]
- 14.Hallek M, Fisher K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukemia: a randomized, open-label, phase 3 trial. Lancet. 2010;376(9747):1164–1174. [DOI] [PubMed] [Google Scholar]
- 15.Cuneo A, Marchetti M, Marosi G, et al. Appropriate use of bendamustine in first-line therapy of chronic lymphocytic leukemia. Recommendations SIE; SIES, GITMO Group. Leuk Res. 2014;38(11):1269–1277. [DOI] [PubMed] [Google Scholar]
- 16.Korsmeyer SJ. Bcl-2 initiates a new category of oncogenes regulators of cell death. Blood. 1992;80(4):879–886. [PubMed] [Google Scholar]
- 17.Del Poeta G, Venditti A, Del Principe MI, et al. Amount of spontaneous apoptosis detected by Bax/Bcl-2 ratio predicts outcome in acute myeloid leukemia (AML). Blood. 2003;101(6):2125–2131. [DOI] [PubMed] [Google Scholar]
- 18.Wolter KG, Hsu Y-T, Smith CL, Nechushtan A, Xi X-G, Youle RJ. Movement of bax from cytosol to mitochondria during apoptosis. J Cell Biol. 1997;139(5):1281–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Del Poeta G, Del Principe MI, Maurillo L, et al. Spontaneuos apoptosis and proliferation detected by BCL-2 and CD71 proteins are important progression indicators within ZAP-70 negative chronic lymphocytic leukemia. Leuk Lymphoma. 2010;51(1):95–106. [DOI] [PubMed] [Google Scholar]
- 20.Del Poeta G, Maurillo L, Venditti A, et al. Clinical significance of CD38 expression in chronic lymphocytic leukemia. Blood. 2001;98(9):2633–2639. [DOI] [PubMed] [Google Scholar]
- 21.Del Principe MI, Del Poeta G, Buccisano F, et al. Clinical significance of ZAP-70 protein expression in B-cell chronic lymphocytic leukemia. Blood. 2006;108(3):853–861. [DOI] [PubMed] [Google Scholar]
- 22.Bomben D, Dal Bo M, Zucchetto A, et al. Mutational status of IgV(H) genes in B-cell chronic lymphocytic leukemia and prognosis: percent mutations or antigen-driven selection. Leukemia. 2005;19(8):1490–1492. [DOI] [PubMed] [Google Scholar]
- 23.Degan M, Bomben R, Dal Bo M, et al. Analysis of IgV gene mutations in B cell chronic lymphocytic leukaemia according to antigen-driven selection identifies subgroups with different prognosis and usage of canonical somatic hypermutation machinery. Br J Haematol. 2004;126(1):29–42. [DOI] [PubMed] [Google Scholar]
- 24.Rossi D, Rasi S, Spina V, et al. Integrated mutational and cytogenetic analysis identifies new prognostic subgroups in chronic lymphocytic leukemia. Blood. 2013; 121(8):1403–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rossi D, Cerri M, Deambrogi C, et al. The prognostic value of TP53 mutations in chronic lymphocytic leukemia is independent of del17p13: implications of overall survival and chemorefractoriness. Clin Cancer Res. 2009;15(3):995–1004. [DOI] [PubMed] [Google Scholar]
- 26.Cheson BD, Bennett JM, Grever M, et al. National Cancer Institute Sponsored Working Group Guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood. 1996;87(12):4990–4997. [PubMed] [Google Scholar]
- 27.Hallek M, Cheson BD, Catovsky D, et al. International Workshop on Chronic Lymphocytic Leukemia. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446–5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saka B, Atkan M, Sami U, Oner D, Sanem O, Dincol G. Prognostic importance of soluble CD23 in B-cell chronic lymphocytic leukemia. Clin Lab Haematol. 2006; 28(1):30–35. [DOI] [PubMed] [Google Scholar]
- 29.Binet JL, Plunkett W, Robertson B, et al. What does apoptosis mean in CLL? Leuk Lymphoma. 1996;Suppl 2:47–52. [DOI] [PubMed] [Google Scholar]
- 30.Robertson LE, Plunkett W, McConnel M, Keating MJ, McDonnel TJ. Bcl-2 expression in chronic lymphocytic leukemia is associated with a progressive pattern of disease. Leukemia. 1996;10(3):456–459. [PubMed] [Google Scholar]
- 31.Gottardi D, Alfarano A, De Leo AM, et al. In leukaemic CD5+ B cells the expression of BCL-2 gene family is shifted toward protection from apoptosis. Br J Haematol. 1996;94(4):612–618. [DOI] [PubMed] [Google Scholar]
- 32.Kitada S, Andersen J, Akar S, et al. Expression of apoptosis-regulating proteins in chronic lymphocytic leukemia: correlations with in vitro and in vivo chemoresponses. Blood. 1998;91(9):3379–3389. [PubMed] [Google Scholar]
- 33.Saxena A, Viswanathan S, Moshynska O, Tandon P, Sankaran K, Sheridan DP.Mcl-1 and Bcl-2/Bax ratio are associated with treatment response but not with Rai stage in B-cell chronic lymphocytic leukemia. Am J Hematol. 2004;75(1):22–33. [DOI] [PubMed] [Google Scholar]
- 34.Damle RN, Wasil T, Fais F, et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 1999;94(6):1840–1847. [PubMed] [Google Scholar]
- 35.Gattei V, Bulian P, Del Principe MI, et al. Relevance of CD49d protein expression as overall survival and progressive disease prognosticator in chronic lymphocytic leukemia. Blood. 2008;111(2):865–873. [DOI] [PubMed] [Google Scholar]
- 36.Zucchetto A, Benedetti D, Tripodo C, et al. CD38/CD31, the CCL3 and CCL4 chemokines, and CD49d vascular cell adhesion molecule-1 are interchained by sequential events sustaining chronic lymphocytic leukemia cell survival. Cancer Res. 2009;69(9):4001–4009. [DOI] [PubMed] [Google Scholar]
- 37.Buggins AG, Pepper C, Patten PE, et al. Interaction with vascular endothelium enhances survival in primary chronic lymphocytic leukemia cells via NF-kB activation and de novo gene transcription. Cancer Res. 2010;70(19):7523–7533. [DOI] [PubMed] [Google Scholar]
- 38.Fabbri G, Rasi S, Rossi D, et al. Analysis of the chronic lymphocytic leukemia coding genome: role of NOTCH1 mutational activation. J Exp Med. 2011;208(7):1389–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Puente XS, Pinyol M, Quesada V, et al. Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature. 2011;475(7354):101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Del Giudice I, Rossi D, Chiaretti S, et al. NOTCH1 mutations in +12 chronic lymphocytic leukemia (CLL) confer an unfavorable prognosis, induce a distinctive transcriptional profiling and refine the intermediate prognosis of +12 CLL. Haematologica. 2012;97(3):437–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koch U, Radtke F. Notch and cancer: a double-edged sword. Cell Mol Life Sci. 2007; 64(21):2746–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vilimas T, Mascarenhas J, Palomero T, et al. Targeting the NF-kappaB signaling pathway in Notch1-induced T-cell leukemia. Nat Med. 2007;13(1):70–77. [DOI] [PubMed] [Google Scholar]
- 43.Faria JR, Yamamoto M, Faria RMD, Kerbauy J, Oliveira JSR. Fludarabine induces apoptosis in chronic lymphocytic leukemia- the role of P53,Bcl-2, Bax, Mcl-1, and Bag-1 proteins. Braz J Med Biol Res. 2006;39(3):327–333. [DOI] [PubMed] [Google Scholar]
- 44.Molica S, Dattilo A, Giulino C, Levato D, Levato L. Increased bcl-2/bax ratio in B-cell chronic lymphocytic leukemia is associated with progressive pattern of disease. Haematologica. 1998;83(12):1122–1124. [PubMed] [Google Scholar]
- 45.Zenz T, Kröber A, Scherer K, et al. Monoallelic TP53 inactivation is associated with poor prognosis in chronic lymphocytic leukemia: results from a detailed genetic characterization with long-term follow-up. Blood. 2008;112(8):3322–3329. [DOI] [PubMed] [Google Scholar]
- 46.Dicker F, Herholz H, Schnittger S, et al. The detection of TP53 mutations in chronic lymphocytic leukemia independently predicts rapid disease progression and is highly correlated with a complex aberrant karyotype. Leukemia. 2009;23(1):117–124. [DOI] [PubMed] [Google Scholar]
- 47.Bellosillo B, Villamor N, Lopez-Guillermo A, et al. Spontaneous and drug-induced apoptosis is mediated by conformational changes of bax and bak in B-cell chronic lymphocytic leukemia. Blood. 2002; 100(5):1810–1816. [DOI] [PubMed] [Google Scholar]