Oral iron replacement is considered standard front-line therapy for iron-deficiency anemia.1 The effectiveness of oral iron is compromised by lack of absorption, gastrointestinal perturbation, and non-adherence to treatment estimated to range from 10% at day 14 of therapy to 32% after two months.2–4 The timing for an optimal switch from oral to intravenous (IV) iron replacement therapy is not well characterized in patient care or treatment algorithms. Currently, there are no markers routinely used in clinical practice to predict response to oral iron. The oral iron challenge test is rarely used since it has not been extensively validated.1 The primary goal of our study was to identify a practical and sensitive predictor of non-response to oral iron replacement therapy to inform decisions around transitioning to intravenous therapy in patients unlikely to benefit from oral iron. We conducted a secondary analysis of data from five randomized clinical trials (RCT) of oral iron versus intravenous (IV) ferric carboxymaltose to characterize response to oral iron therapy.
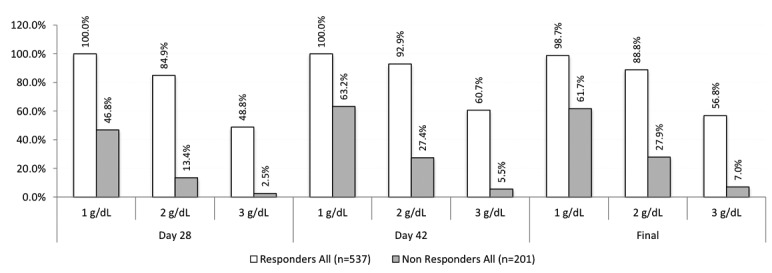
Complete data from the oral arms of five RCTs of iron-deficiency anemia in heavy uterine bleeding (n=1), inflammatory bowel diseases (n=1), post-partum anemia (n=2), and other etiologies (n=1) were combined for assessment of response to oral iron.4–8 Inclusion criteria for the trials were similar, most included a hemoglobin (Hb) of 10–11 g/dL or less and a ferritin less than 100 ng/mL. Responders were defined as subjects with an Hb increase of 1.0 g/dL or more at day 14 and non-responders were subjects with an Hb increase of less than 1.0 g/dL at day 14. The analysis population was the modified intention-to-treat population of subjects randomized to oral iron in each trial who received at least one 1 dose of assigned study medication, and had 1 or more post-baseline Hb tests. Base-line and outcome data at day 14, day 28, and the interval from days 42 to 56 were evaluated using Fisher’s exact test for categorical variables and one-way analysis of variance for continuous variables. Sensitivity, specificity, and predictive values were calculated using online tools provided by GraphPad Software Inc., La Jolla, CA, USA (www.graphpad.com). All statistical tests were post hoc, with no adjustment to Type I error for multiple comparisons.
The cohort of 738 subjects was composed of 537 responders and 201 non-responders who participated in one of five RCTs (Table 1). Responders, compared to non-responders, were younger (32.9 vs. 39.7 years), had lower BMI (27.9 vs. 31.1 kg/m2) and a lower base-line Hb (9.1 vs. 9.8 g/dL). Treatment compliance with oral iron was reported in four trials and ranged from 83.9% to 98.5% (Table 1). At day 28, the number of patients with an Hb increase of 1.0 g/dL or more from baseline was higher for responders than non-responders (100% vs. 46.8%) and at day 42-56 the number of patients with an Hb increase of 2 g/dL or more was higher for responders than non-responders (92.9% vs. 27.4%) (Figure 1). Following peak responses in Hb of 1.0 g/dL at day 28 and 2.0 g/dL at day 42-56, further incremental increases in Hb were negligible. The sensitivity, specificity and positive predictive value of an Hb increase of 1.0 g/dL or more at day 14 were 90.1%, 79.3% and 92.9%, respectively. In the stratified analysis according to IDA etiology, the day 14 treatment response of Hb of 1.0 g/dL or more was more robust for subjects with post-partum anemia (95.5%) than subjects with heavy uterine bleeding (56.3%), gastrointestinal (53.5%) and other etiologies (42.9%).
Table 1.
Summary of included randomized controlled trials.
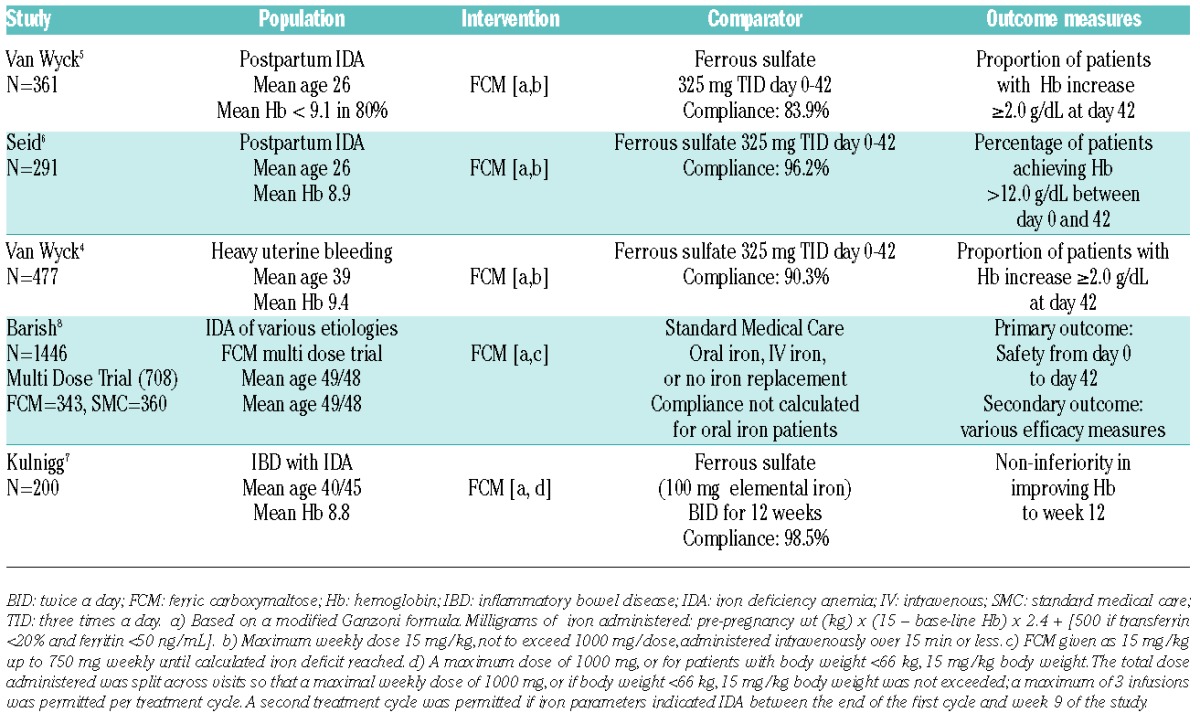
Figure 1.
Subsequent hemoglobin trend according to 14-day hemoglobin response. Responders (those with a ≥1 g/dL rise in hemoglobin by day 14) were more likely to manifest durable and more robust increments than non-responders (those with <1 g/dL rise in hemoglobin by day 14).
The major finding from this pooled analysis of RCT data in iron-deficiency anemia was that day 14 Hb treatment response to oral iron was an accurate predictor of longer-term and sustained treatment response to continued oral iron supplementation. Day 14 Hb may be a useful tool for clinicians in determining whether and when to transition patients from oral to IV iron. While our findings provide a practical approach to the monitoring of oral iron treatment response in iron-deficiency anemia, we acknowledge that our findings have potential biases inherent in post hoc analyses. Also, multiple comparisons that were performed in our analyses create the chance of a Type 1 error in our conclusions. Nonetheless, our findings are consistent with the results of a pivotal RCT that included a 14-day oral iron run-in phase prior to randomization of IDA subjects with various etiologies to additional oral iron versus IV ferric carboxymaltose.9 In this study, 143 of 374 subjects (38.4%) who completed the prospective oral iron run-in study phase achieved Hb of 1 g/dL or more. Future clinical trials that examine the utility of day 14 Hb in this regard are needed to confirm these findings. Given that iron deficiency and iron-deficiency anemia are recognized common medical conditions, further efforts to characterize medication adherence and treatment effectiveness will optimize and promote global health.1
Acknowledgments
The authors would like to thank David Morris, PhD for his statistical support and Aesculapius Consulting, Inc. for their editorial support.
Footnotes
Funding: Luitpold Pharmaceuticals Inc., supported this study.
Information on authorship, contributions and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org
References
- 1.Camaschella C. Iron-deficiency anemia. N Engl J Med. 2015; 372(19):1832–1843. [DOI] [PubMed] [Google Scholar]
- 2.Carlson KJ, Miller BA, Fowler FJ., Jr The Maine Women's Health Study: II. Outcomes of nonsurgical management of leiomyomas, abnormal bleeding, and chronic pelvic pain. Obstet Gynecol. 1994; 83(4):566–572. [DOI] [PubMed] [Google Scholar]
- 3.Varner RE, Ireland CC, Summitt RL, Jr, et al. Medicine or Surgery (Ms): a randomized clinical trial comparing hysterectomy and medical treatment in premenopausal women with abnormal uterine bleeding. Control Clin Trials. 2004;25(1):104–118. [DOI] [PubMed] [Google Scholar]
- 4.Van Wyck DB, Mangione A, Morrison J, Hadley PE, Jehle JA, Goodnough LT. Large-dose intravenous ferric carboxymaltose injection for iron deficiency anemia in heavy uterine bleeding: a randomized, controlled trial. Transfusion. 2009;49(12):2719–2728. [DOI] [PubMed] [Google Scholar]
- 5.Van Wyck DB, Martens MG, Seid MH, Baker JB, Mangione A. Intravenous ferric carboxymaltose compared with oral iron in the treatment of postpartum anemia: a randomized controlled trial. Obstet Gynecol. 2007;110(2 Pt 1):267–278. [DOI] [PubMed] [Google Scholar]
- 6.Seid MH, Derman RJ, Baker JB, Banach W, Goldberg C, Rogers R. Ferric carboxymaltose injection in the treatment of postpartum iron deficiency anemia: a randomized controlled clinical trial. Am J Obstet Gynecol. 2008;199(4):435 e431–437. [DOI] [PubMed] [Google Scholar]
- 7.Kulnigg S, Stoinov S, Simanenkov V, et al. A novel intravenous iron formulation for treatment of anemia in inflammatory bowel disease: the ferric carboxymaltose (FERINJECT) randomized controlled trial. Am J Gastroenterol. 2008;103(5):1182–1192. [DOI] [PubMed] [Google Scholar]
- 8.Barish CF, Koch T, Butcher A, Morris D, Bregman DB. Safety and Efficacy of Intravenous Ferric Carboxymaltose (750 mg) in the Treatment of Iron Deficiency Anemia: Two Randomized, Controlled Trials. Anemia. 2012;2012:172104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Onken JE, Bregman DB, Harrington RA, et al. A multicenter, randomized, active-controlled study to investigate the efficacy and safety of intravenous ferric carboxymaltose in patients with iron deficiency anemia. Transfusion. 2014;54(2):306–315. [DOI] [PubMed] [Google Scholar]


