Choice of contraception after venous thromboembolism (VTE) is challenging because hormonal contraception may increase the risk of recurrent VTE. Estrogen contraception is usually contraindicated in women with a personal history of VTE (category 4, unacceptable health risk according to the World Health Organization).1 On the other hand, interruption of oral contraception in young women after a VTE event may lead to unintended pregnancies, and possibly to an increased rate of abortion.2 The use of hormonal, non-estrogen contraception, i.e. progestin-only contraception (POC), is the subject of debate in women at high risk for VTE, and especially in those with prior VTE. In recent observational studies in a general population, the use of POC was not associated with increased risk of first VTE when compared with non-users of hormonal contraceptives, except for injectable medroxyprogesterone acetate (DMPA).3–6 POC might also be a good option in women after a first VTE. In France, following the publication of a cohort study by Conard et al. in 2004, it has become common practice to give POC to women with a past history of VTE.7 To our knowledge, only three studies, which included a total of 117 women, assessed the risk of VTE recurrence associated with POC exposure.2,7,8 Since the results of these studies were not consistent, we aimed to determine recurrence risk associated with POC exposure in our cohort of pre-menopausal women with VTE.
All patients with documented symptomatic VTE seen between January 1992 and December 2013 at Brest University Hospital, France, were enrolled in a prospective cohort study. For the purpose of this analysis, we selected women aged 50 years or under following the diagnosis of a first VTE. We excluded women with active cancer or on hormone replacement therapy (HRT), and women who were still on anticoagulant treatment at the time of the last follow up (FU). The study was approved by our hospital scientific and ethics boards. Patients’ written consent for participation in the study and for DNA analysis was obtained. Eligible patients were identified through daily collection of positive diagnosis of VTE by imaging units. Diagnosis of deep venous thrombosis (DVT) was carried out in the absence of full compressibility of a proximal (involving the popliteal vein or above) or distal vein of the deep lower limb on compression ultrasonography (CUS). Diagnosis of pulmonary embolism (PE) was made by: 1) a segmental or larger artery filling defect on chest computed tomography (CT) scan; or 2) the combination of high pre-test clinical probability of PE with high probability ventilation-perfusion (V/Q) lung scan according to PIOPED criteria; or 3) proximal DVT on CUS in a patient with suspected PE. Initial VTE was classified as being provoked only in case of a major risk factor: surgery or trauma in the past three months or immobilization for more than three days. All included patients underwent thrombophilia testing (Factor V G1691A gene mutation, prothrombin G20210A gene variation).
Users of estrogen contraception were advised to stop using this method. An alternative contraception method, e.g. a POC or non-hormonal contraception, was recommended to all patients. Follow-up visits were planned annually. Data were recorded on provoking factors of VTE and hormone exposure. The primary end point of the study was recurrence of symptomatic VTE confirmed by venous CUS, V/Q lung scanning or chest CT scan. Diagnosis of recurrence was made by an independent adjudication committee using previously described criteria.9
Characteristics of the women exposed to POC at some point during FU and women not exposed to POC were compared using Student’s t-test, Mann–Whitney-Wilcoxon test, χ2 test or Fisher’s exact depending on data characteristics. Follow-up time was calculated as the time interval between cessation of initial anticoagulation and end of FU, e.g. recurrence, death or last visit, whichever occurred first. The total FU time for each woman was divided into periods of exposure to POC, estrogens [combined hormonal contraception (CHC), HRT or pregnancy] or without hormonal exposure. For the purpose of this analysis, only periods of exposure to POC and periods without hormones were considered. Generalized linear model, using a Poisson probability distribution, a log link function and an FU time set as an offset variable, was used to estimate the incidence rate (IR) of recurrent VTE (expressed per 1000 women-years) with 95% confidence interval (CI). Crude and age-adjusted incidence rate ratios (IRRs) were calculated using a Poisson regression model to compare the rates of recurrent VTE during periods with POC and without hormones.
During the study period, 502 women aged 50 years or younger were included for a first VTE. Eighteen women were excluded because of active cancer (n=10) or VTE occurring while on HRT (n=8). Sixty-five women were still on ongoing anticoagulant therapy at the time of the last FU. Thus, our final sample was made up of 419 women. Of these, 163 women (38.9%) were exposed to POC at some time point during FU.
Women exposed to POC during FU were significantly younger at first VTE event than women not exposed to POC during FU [mean (standard deviation: SD): 30.0 years (8.1) vs. 35.18 years (9.0); P<0.001]. There were significantly more women with a first VTE while on CHC in the group of women exposed to POC during FU [121 (74.2%) vs. 139 (54.5%); P<0.001]. Median duration of FU after discontinuation of anticoagulation was 4.4 years (IQR: 1.6–8.5): 5.1 years (IQR: 2.1–8.4) in women exposed to progestin during FU, and 3.8 years (IQR: 1.2–8.6) in unexposed women (P=0.082).
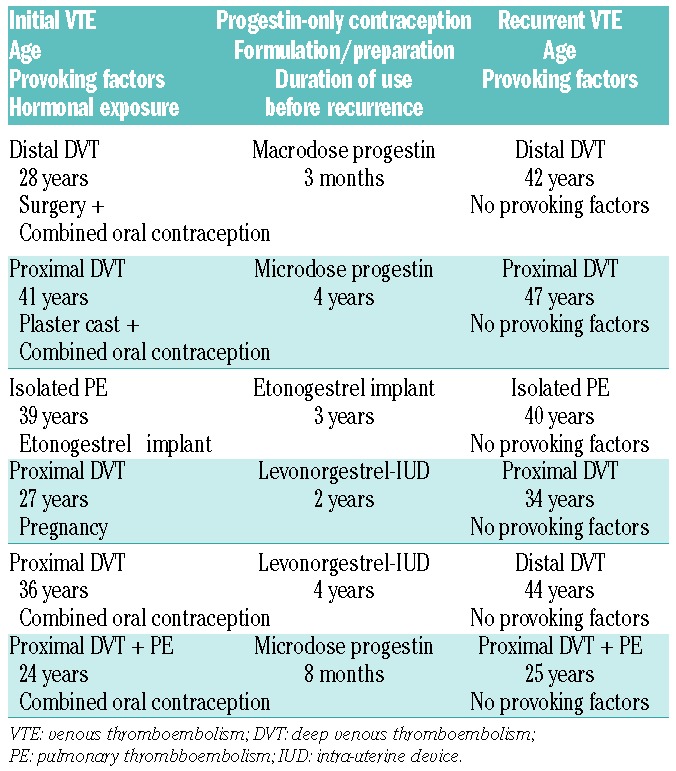
Median duration of exposure to POC was 2.5 years (IQR: 0.7–5.5). Progestins were mainly oral levonorgestrel 30 μg/day or desogestrel 75 μg/day (43%, 242.4 women-years) and levonorgestrel intra-uterine device (LNG-IUD) (49%, 278.7 women-years). No woman used injectable DMPA. During FU, there were 35 adjudicated recurrent VTE; of these 6 occurred while on POC. Characteristics of recurrent VTE on POC are presented in Table 1.
Table 1.
Characteristics of recurrent venous thromboembolism events during progestin-only contraception.
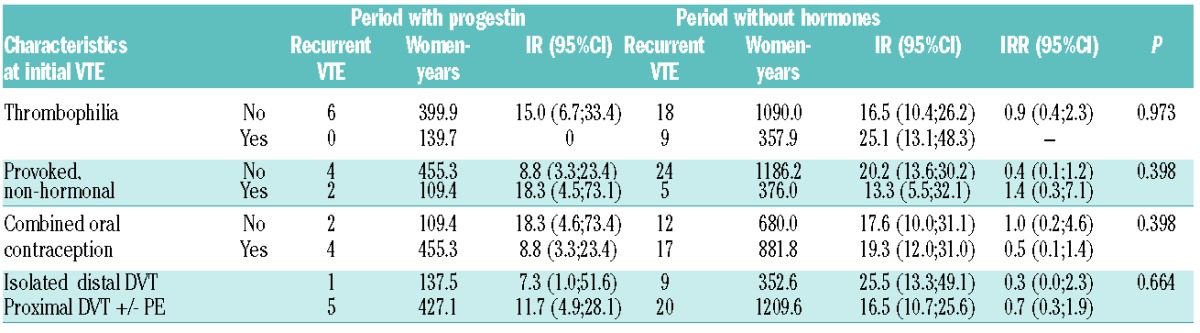
Incidence rate of recurrent VTE according to progestin exposure during FU is shown in Table 2. There was no statistical difference in incidence rate of recurrent VTE according to exposure to progestin: 10.6/1000 women-years (95%CI: 4.8–23.7) while on POC versus 18.6/1000 women-years (95%CI: 12.9–26.7) without hormones. The age-adjusted incidence rate ratio of recurrent VTE during exposure to POC compared to recurrent VTE during period without hormones was 0.6 (95%CI: 0.3–1.5). When restricting the analysis to those women who were exposed to POC at any time during FU (i.e. excluding women who never took progestin during FU), there was no statistical difference in incidence rates between periods of exposure to POC and periods without hormone: age-adjusted-IRR was 1.6 (95%CI: 0.3–7.8). IRRs in subgroups of women according to characteristics at initial VTE are described in Table 3.
Table 2.
Risk of recurrent venous thromboembolism according to progestin-only contraception exposure during follow up.
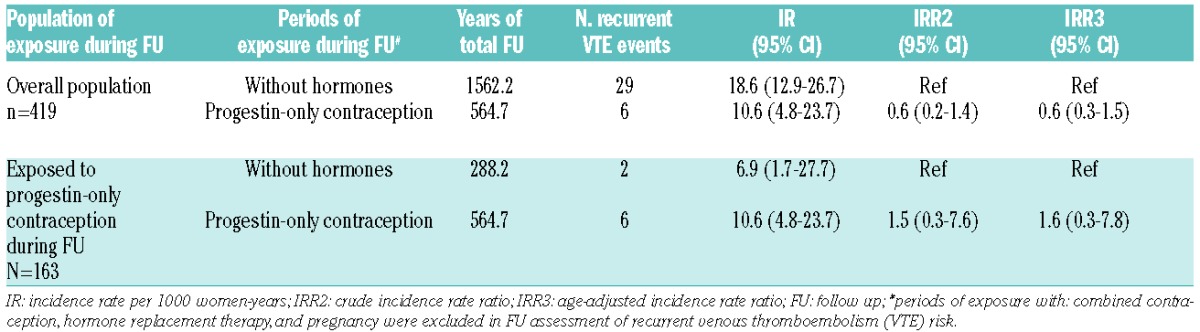
Table 3.
Risk of recurrent venous thromboembolism in subgroups of women according to characteristics at initial VTE.
In this cohort, POC did not appear to be associated with increased risk of recurrent VTE in women after a first VTE. By contrast, in the Leiden Thrombophilia Study (LETS), 133 women aged 16–48 years with hormonal risk factors at the time of their first event were followed. In the subgroup of 12 women exposed to POC during FU, there were 2 recurrent VTE, corresponding to an incidence of 38.4/1000 women-years, as compared with 10.5/1000 women-years in non-users.2 This discrepancy with our results may be due to differences in preparation for POC (i.e. recurrences occurred while on DMPA in LETS study vs. oral progestin, LNG-IUD and implant in our study). This hypothesis is supported by the literature regarding the effect of DMPA on coagulation, through its glucocorticoid-like effect on thrombin receptor.10–12
The non-randomized design of our study prevents any formal conclusion to be drawn. Non-explicit criteria might have led to selection of women for treatment or no treatment with POC. Women exposed to POC during FU were significantly younger than women not exposed to progestin. Initial VTE while on estrogen was significantly more frequent in women exposed to POC during FU. However, adjustment for age and subgroup analysis did not alter our results. We included women with provoked and unprovoked VTE. This was deliberate, since in clinical practice, both clinical presentations are considered to be a contraindication for the later use of estrogen contraception. A limitation of our study was that we observed a small number of recurrences and consequently had wide confidence intervals around our estimates. For the same reason, we were unable to analyze the specific effect of LNG-IUD. Another limitation is the single center design of our study; however, our patients’ characteristics were similar to those of related literature.13 The strengths of our study were that we included a large number of young women exposed to POC and followed them for an extended period. This study had a prospective design and all VTE events were documented and adjudicated. FU time was divided into periods with POC exposure and without hormones in order to identify the duration of exposure and the specific incidence rate for each period.
To conclude, the choice of a contraception method remains a challenge in young women after a first VTE. We believe that our study reinforces current guidelines recommending the prescription of oral progestin-only contraceptives or LNG-IUD in women after a first VTE.
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Medical Eligibility Criteria for Contraceptive Use. Geneva, Switzerland: World Health Organization. World Health Organization; 2009. [Google Scholar]
- 2.Christiansen SC, Lijfering WM, Helmerhorst FM, Rosendaal FR, Cannegieter SC. Sex difference in risk of recurrent venous thrombosis and the risk profile for a second event. J Thromb Haemost. 2010; 8(10):2159–2168. [DOI] [PubMed] [Google Scholar]
- 3.Barsoum MK, Heit JA, Ashrani AA, Leibson CL, Petterson TM, Bailey KR. Is progestin an independent risk factor for incident venous thromboembolism? A population-based case-control study. Thromb Res. 2010;126(5):373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lidegaard O, Nielsen LH, Skovlund CW, Skjeldestad FE, Lokkegaard E. Risk of venous thromboembolism from use of oral contraceptives containing different progestogens and oestrogen doses: Danish cohort study, 2001–9. BMJ. 2011;343:d6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mantha S, Karp R, Raghavan V, Terrin N, Bauer KA, Zwicker JI. Assessing the risk of venous thromboembolic events in women taking progestin-only contraception: a meta-analysis. BMJ. 2012; 345:e4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Hylckama Vlieg A, Helmerhorst FM, Rosendaal FR. The risk of deep venous thrombosis associated with injectable depot-medrox-yprogesterone acetate contraceptives or a levonorgestrel intrauterine device. Arterioscler Thrombo Vasc Biol. 2010;30(11):2297–2300. [DOI] [PubMed] [Google Scholar]
- 7.Conard J, Plu-Bureau G, Bahi N, Horellou MH, Pelissier C, Thalabard JC. Progestogen-only contraception in women at high risk of venous thromboembolism. Contraception. 2004;70(6):437–441. [DOI] [PubMed] [Google Scholar]
- 8.Vaillant-Roussel H, Ouchchane L, Dauphin C, Philippe P, Ruivard M. Risk factors for recurrence of venous thromboembolism associated with the use of oral contraceptives. Contraception. 2011;84(5):e23–30. [DOI] [PubMed] [Google Scholar]
- 9.Delluc A, Tromeur C, Le Moigne E, et al. Lipid lowering drugs and the risk of recurrent venous thromboembolism. Thromb Res. 2012; 130(6):859–863. [DOI] [PubMed] [Google Scholar]
- 10.Diab KM, Zaki MM. Contraception in diabetic women: comparative metabolic study of Norplant, depot medroxyprogesterone acetate, low dose oral contraceptive pill and CuT380A. J Obstet Gynaecol Res. 2000;26(1):17–26. [DOI] [PubMed] [Google Scholar]
- 11.Herkert O, Kuhl H, Sandow J, Busse R, Schini-Kerth VB. Sex steroids used in hormonal treatment increase vascular procoagulant activity by inducing thrombin receptor (PAR-1) expression: role of the gluco-corticoid receptor. Circulation. 2001;104(23):2826–2831. [DOI] [PubMed] [Google Scholar]
- 12.Kuhl H. Mechanisms of sex steroids. Future developments. Maturitas. 2004;47(4):285–291. [DOI] [PubMed] [Google Scholar]
- 13.Blanco-Molina A, Trujillo-Santos J, Tirado R, et al. Venous thromboembolism in women using hormonal contraceptives. Findings from the RIETE Registry. Thromb Haemost. 2009;101(3):478–482. [PubMed] [Google Scholar]


