Systemic anaplastic large cell lymphoma (ALCL) is a subtype of peripheral T-cell lymphoma. It is divided into cases with and without translocation of the anaplastic lymphoma kinase gene (ALK), which most commonly involves chromosomes 2 and 5 and leads to ALK overexpression. Most patients with ALK translocations (ALK+) are potentially curable with doxorubicin-based chemotherapy, whereas patients without a translocation (ALK−) have a significantly lower survival. We tested dose-adjusted infusional etoposide, vincristine and doxorubicin with prednisone and cyclophosphamide (DA-EPOCH) in 24 patients with untreated ALK + (n=15) and ALK− (n=9) ALCL. Patients’ characteristics were similar between the two groups. At a median potential followup time of 14 years, the event-free survival (EFS) in ALK+ and ALK− ALCL was 72.0% and 62.5% (P=0.54), respectively, and overall survival (OS) was 78.0% and 87.5% (P=0.83), respectively. DA-EPOCH had a favorable outcome in systemic ALCL and was independent of ALK status.
Anaplastic large cell lymphoma comprises around 6% of non-Hodgkin lymphomas. It is pathologically characterized by a broad morphological spectrum but usually contains large pleomorphic cells with strong CD30 expression and a cohesive growth pattern. ALCL is divided into systemic and cutaneous types based on clinical and immunophenotypic criteria. Furthermore, within systemic ALCL, there is biological heterogeneity in expression of the anaplastic lymphoma kinase (ALK) protein with positive cases usually harboring a translocation between the ALK gene on chromosome 2 and the nucleophosmin (NPM) gene chromosome 5, and ALK negative cases lacking a translocation.1,2 This translocation can be detected in over 80% of ALK positive cases, although variant translocations involving ALK and other partner genes also occur. A recent study demonstrated that ALK negative ALCLs have chromosomal rearrangements of DUSP22 or TP63 in 30% and 8% of cases, respectively.3
The clinical characteristics and outcome of systemic ALK positive and negative ALCL are significantly different. ALK positive cases are characterized by a younger median age, occur more commonly in pediatric patients, and are more likely to present in extranodal sites, compared with ALK negative cases. Importantly, ALK positive ALCL has a significantly better survival compared to ALK negative cases following treatment with doxorubicin-based therapy.4–6 Based on the efficacy of infusional DA-EPOCH in aggressive B-cell lymphomas, we investigated its activity in adults with ALK positive and negative ALCL.7–9 In a previous short report, we had investigated serum soluble interleukin-2 levels in response to treatment in 9 patients with ALCL.9
Twenty-four patients with newly diagnosed ALCL were prospectively enrolled on a study of DA-EPOCH between September 1993 and December 2009. Patients with primary cutaneous ALCL were excluded. The primary objectives of the study included time to disease progression, overall survival and treatment toxicity. Patients were eligible if: 1) they had not received prior systemic chemotherapy; 2) had any stage of disease; 3) had a negative pregnancy test in women of child bearing potential; and 4) had adequate major organ function, unless it was related to the lymphoma. Evaluation included standard laboratory studies, whole body computed tomography scans, and a bone marrow aspirate and biopsy. Patients received 6–8 cycles of DA-EPOCH with prophylactic filgrastim on all cycles from 24 h after the last dose of chemotherapy through the neutrophil nadir (until recovery of ≥5000 cells/μl), as previously described.10 Disease sites were evaluated after cycles 4, 6 and 8. Standard criteria were used to assess tumor response.11 The study was approved by the National Cancer Institute Institutional Review Board and all patients provided informed consent. The trial was registered at clinicaltrials.gov (clinicaltrials.gov identifier:00001337).
The pathology of all cases was confirmed at the National Cancer Institute by ESJ and/or SP in compliance with the 4th edition of the WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Standard immunohistochemical studies for diagnosis as well as immunohistochemistry for ALK protein overexpression were conducted on 5 µm formalin-fixed, paraffin-embedded tissue sections using monoclonal and polyclonal antibodies. The panel included CD20, CD3, CD30, and TIA-1 and also CD4, CD8, CD5, granzyme B and perforin if sufficient material was available.
Overall survival was calculated from on-study date until death or last follow up and EFS was calculated from on-study date until the date of progression, death or last follow up using the Kaplan-Meier method. Significance of the difference between pairs of Kaplan-Meier curves was determined by the log rank test. The median potential follow up was calculated between the on-study date and April 1st 2015.
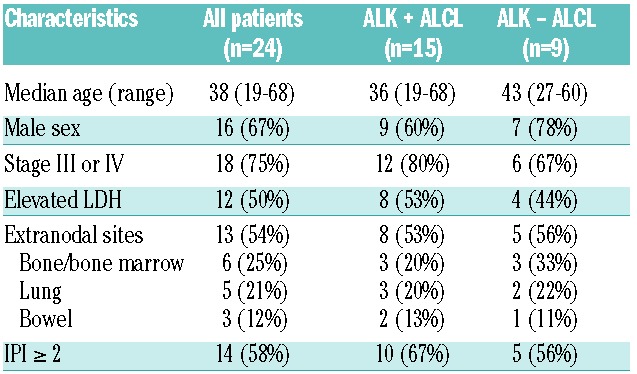
Twenty-four patients with newly diagnosed ALCL were enrolled (Table 1). Fifteen patients were ALK positive and 9 ALK negative according to ALK immunohistochemistry; clinical characteristics of the 2 groups were similar. Median patient age was 38 years (range 19–68) and most patients were male (67%). Indices of advanced disease included stage III or IV disease in 75% and an elevated lactate dehydrogenase in 50%. Fifty-four per cent of patients had extra-nodal disease and 25% of patients had peripheral blood or bone marrow involvement.
Table 1.
Patients’ characteristics.
At a median potential follow up of 14.4 years, ALK positive and negative patients had EFS probabilities of 72% (95%CI: 45.9–88.6%) and 62.5% (95%C: 30.6–86.3%) (P=0.54) and OS probabilities of 78% (95%CI: 51.0–92.3%) and 87.5% (95%C: 52.9–97.8%) (P=0.83), respectively (Figure 1). Toxicity was assessed on all 141 cycles. The ANC pharmacodynamic target of less than 500 cells/mm3 occurred on 35% of cycles and an ANC less than 100 cells/mm3 on 17% of cycles. Thrombocytopenia less than 25,000/mm3 and fever and neutropenia occurred on 10% of cycles, respectively. Non-hematopoietic toxicities were similar to prior reports.12
Figure 1.
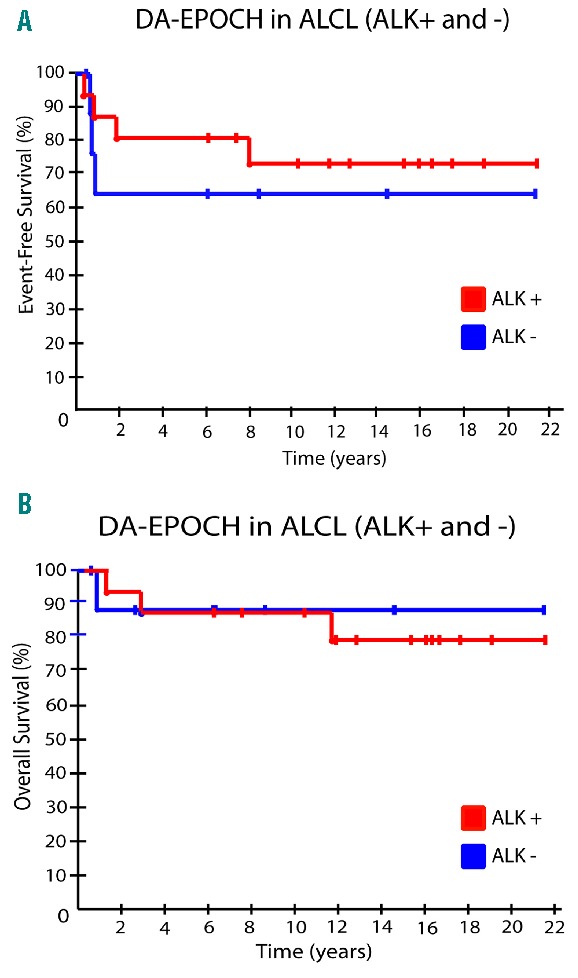
Outcome of 24 patients (15 ALK positive and 9 ALK negative) following DA-EPOCH. (A) Event-free survival (EFS) of ALK positive versus ALK negative patients. (B) Overall survival of ALK positive versus negative cases.
While the outcome of ALK positive ALCL with anthracycline-based therapy is excellent, particularly in pediatric populations, ALK negative cases have a significantly lower survival.4 In the largest experience of outcome of PTCL to date (the retrospective International PTCL Project), the 5-year failure-free survival and OS for ALK negative ALCL was 36% and 49%, respectively, compared to 60% and 70% for ALK positive cases. The German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL) retrospectively analyzed the outcome of 191 ALCL patients treated with CHOP or CHOEP and reported a 3-year EFS of 76% and 46% for ALK positive and negative ALCL, respectively.13 Recently, the Nordic Lymphoma Group evaluated the role of up-front autologous stem cell transplantation after CHOEP in an intent-to-transplant study in those with chemotherapy-sensitive disease and of PTCL subtypes (ALK positive cases were excluded), ALK negative ALCL had the best outcome with a 5-year PFS and OS of 61% and 70%.14 Interestingly, a recent analysis of three GELA studies demonstrated no difference in outcome of ALK negative and positive ALCL in younger patients, but found that ALK negative ALCL had a significantly worse outcome in patients who were 40 years old or older.15
We tested DA-EPOCH in ALCL based on its promising activity in aggressive B-cell lymphoma. While we recognize that our series is small, the long EFS of most patients reflects cure in the majority. The clinical characteristics of the patients in our series are similar to those of the International Peripheral T-Cell Study (IPTCS); our ALK positive and negative cases had median ages of 36 (compared to 34) and 43 (compared to 58) years, respectively.4 In both the IPTCS and our series, most patients were male and the majority in both ALK positive and negative groups had stage III or IV disease. Our results using DA-EPOCH in ALCL compare favorably with the DSHNHL and Nordic studies and may represent a less toxic approach; we observed no treatment-related deaths and fever/neutropenia in our study occurred on just 10% of cycles.14 While the GELA study demonstrated equivalent survivals in ALK negative and positive patients under 40 years of age, the majority of patients received intensive therapy, with or without autologous transplantation, and older ALK negative patients performed poorly. Although our numbers of older ALK negative patients are small, 80% of those 40 years and older are alive despite poor clinical prognostic features at diagnosis.15 The incorporation of etoposide, infusional scheduling and dose adjustment may play important roles in ALCL therapeutics. Based on these results, DA-EPOCH should be considered as a reasonable first-line approach for ALCL, particularly for older patients in whom approaches such as transplantation may not be feasible.
Footnotes
Funding: this work was funded by the Intramural Program at the National Cancer Institute. We wish to acknowledge Nicole Grant and Therese White for research nurse support.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Lamant L, Meggetto F, al Saati T, et al. High incidence of the t(2;5)(p23;q35) translocation in anaplastic large cell lymphoma and its lack of detection in Hodgkin’s disease. Comparison of cytogenetic analysis, reverse transcriptase-polymerase chain reaction, and P-80 immunostaining. Blood. 1996;87(1):284–291. [PubMed] [Google Scholar]
- 2.Dunleavy K, Piekarz RL, Zain J, et al. New strategies in peripheral T-cell lymphoma: understanding tumor biology and developing novel therapies. Clin Cancer Res. 2010;16(23):5608–5617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parrilla Castellar ER, Jaffe ES, Said JW, et al. ALK-negative anaplastic large cell lymphoma is a genetically heterogeneous disease with widely disparate clinical outcomes. Blood. 2014;124(9):1473–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vose J, Armitage J, Weisenburger D, International TCLP. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124–4130. [DOI] [PubMed] [Google Scholar]
- 5.Savage KJ, Harris NL, Vose JM, et al. ALK− anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood. 2008;111(12):5496–5504. [DOI] [PubMed] [Google Scholar]
- 6.Foss FM, Zinzani PL, Vose JM, Gascoyne RD, Rosen ST, Tobinai K. Peripheral T-cell lymphoma. Blood. 2011;117(25):6756–6767. [DOI] [PubMed] [Google Scholar]
- 7.Wilson WH, Dunleavy K, Pittaluga S, et al. Phase II study of dose-adjusted EPOCH and rituximab in untreated diffuse large B-cell lymphoma with analysis of germinal center and post-germinal center biomarkers. J Clin Oncol. 2008;26(16):2717–2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunleavy K, Pittaluga S, Shovlin M, et al. Low-intensity therapy in adults with Burkitt’s lymphoma. N Engl J Med. 2013;369(20):1915–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janik JE, Morris JC, Pittaluga S, et al. Elevated serum-soluble interleukin-2 receptor levels in patients with anaplastic large cell lymphoma. Blood. 2004;104(10):3355–3357. [DOI] [PubMed] [Google Scholar]
- 10.Dunleavy K, Pittaluga S, Maeda LS, et al. Dose-adjusted EPOCH-rituximab therapy in primary mediastinal B-cell lymphoma. New Engl J Med. 2013;368(15):1408–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–586. [DOI] [PubMed] [Google Scholar]
- 12.Wilson WH, Jung SH, Porcu P, et al. A Cancer and Leukemia Group B multi-center study of DA-EPOCH-rituximab in untreated diffuse large B-cell lymphoma with analysis of outcome by molecular subtype. Haematologica. 2012;97(5):758–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmitz N, Trumper L, Ziepert M, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116(18):3418–3425. [DOI] [PubMed] [Google Scholar]
- 14.d’Amore F, Relander T, Lauritzsen GF, et al. Up-front autologous stem-cell transplantation in peripheral T-cell lymphoma: NLG-T-01. J Clin Oncol. 2012;30(25):3093–3099. [DOI] [PubMed] [Google Scholar]
- 15.Sibon D, Fournier M, Briere J, et al. Long-term outcome of adults with systemic anaplastic large-cell lymphoma treated within the Groupe d’Etude des Lymphomes de l’Adulte trials. J Clin Oncol. 2012;30(32):3939–3946. [DOI] [PubMed] [Google Scholar]


