Abstract
Background
In the absence of a feasible, noninvasive gold standard, iron deficiency (ID) is best measured by the use of multiple indicators. However, the choice of an appropriate single iron biomarker to replace the multiple-criteria model for screening for ID at the population level continues to be debated.
Objective
We compared ID defined as ≥2 of 3 abnormal ferritin (<12 μg/L), soluble transferrin receptor (TfR; >8.3 mg/L), or zinc protoporphyrin (ZP; >80 μmol/mol) concentrations (ie, multiple-criteria model) with ID defined by abnormal concentrations of any of the independent candidate iron biomarkers (ferritin alone, TfR alone, or ZP alone) and TfR/ferritin index (ID, >500). Values either were adjusted for inflammation [as measured by C-reactive protein (>5 mg/L) and α1-acid glycoprotein (>1 g/L) before applying cutoffs for ID] or were unadjusted.
Design
In this community-based cluster survey, capillary blood was obtained from 680 children (aged 6–35 mo) for measurement of iron status by using ferritin, TfR, and ZP.
Results
On the basis of the multiple-criteria model, the mean (±SE) prevalence of ID was 61.9 ± 2.2%, whereas the prevalences based on abnormal ferritin, TfR, or ZP concentrations or an abnormal TfR/ferritin index were 26.9 ± 1.7%, 60.9 ± 2.2%, 82.8 ± 1.6%, and 43.1 ± 2.3%, respectively, for unadjusted values. The prevalences of ID were higher for adjusted values only for low ferritin and an elevated TfR/ferritin index compared with the unadjusted values. The κ statistics for agreement between the multiple-criteria model and the other iron indicators ranged from 0.35 to 0.88; TfR had the best agreement (κ = 0.88) with the multiple-criteria model. Positive predictive values of ID based on the other iron indicators in predicting ID based on the multiple-criteria model were highest for ferritin and TfR. Receiver operating characteristic curve analysis indicated that TfR (AUC = 0.94) was superior to the other indicators in diagnosing ID based on the multiple-criteria model (P < 0.001). The inflammation effect did not appear to alter these observations appreciably.
Conclusion
TfR better estimates the prevalence of ID in preschoolers than do ferritin, ZP, and the TfR/ferritin index on the basis of multiple indexes in a high inflammation, resource-poor setting. This trial was registered at clinicaltrials.gov as NCT101088958.
INTRODUCTION
Anemia, mainly caused by ID7 (iron deficiency), globally affects up to 60% of children aged <48 mo, with the highest prevalence found in developing countries (1, 2). Several adverse effects on health and development have been attributed to ID without anemia, including reduced cognitive, mental, and physical functions in children and increased perinatal and maternal mortality (3–7). In children, ID can be prevented and treated with an increased intake of iron. This may include consumption of high-iron-content foods, fortification of common staple and complementary foods, or provision of therapeutic doses of iron in supplements. As a consequence of the WHO iron-supplementation guidelines, a noninvasive assessment tool for ID is urgently needed, especially in settings with inadequate malaria control (8–10). The use of feasible approaches for assessing ID at the population level in high-inflammation, resource-poor, and remote field settings continues to be a critical need (11, 12).
Three stages are normally used to characterize ID anemia: depletion of iron stores reflected by a fall in serum ferritin concentration, iron-deficient erythropoiesis reflected through increased TfR (soluble transferrin receptor) and ZP (zinc protoporphyrin), and anemia reflected by a reduction in hemoglobin due to a restricted supply of iron to the bone marrow for erythropoiesis (13). In the absence of a noninvasive or feasible gold standard, these stages are best characterized by the use of multiple-criteria indicators and the TfR/ferritin index (14–16). Iron-status estimates are potentially improved through the use of a combination of several (≥3) tests of iron status rather than a single indicator. The presence of ≥2 abnormal values indicates impaired iron status (13, 14). In developing country field settings, however, multiple-criteria models may be problematic because of the need for venous blood collection and the cost of analyzing multiple indicators (12). Some workers have thus investigated the feasibility of capillary blood for assessing iron status in field studies for many biomarkers, such as ferritin (17), ZP (18), and TfR (12). Measures of inflammation from capillary blood are also required to assess their effect on these iron biomarkers. The use of a combination of 3 biomarkers (ferritin, transferrin saturation, and red blood cell protoporphyrin/ZP) has long been suggested for estimating population ID prevalence (16). However, transferrin saturation has some important limitations, including diurnal variations in values, results being affected by inflammation, and the method is not suitable for field trials (15). In our multiple-criteria model, we therefore replaced transferrin saturation with TfR, which is less affected by inflammation and is suitable for field assays (15).
The goal of this study was to identify a better biomarker to determine ID as compared with the multiple-criteria model (ferritin, TfR, and ZP). This single biomarker must approximate the multiple-criteria model in its ability to detect modifications in iron status of preschool children in areas of high infection burden if it is to replace the multiple-criteria model. A further desirable characteristic is that the biomarker be the least affected by inflammation.
SUBJECTS AND METHODS
Study area and study population
The data for the current study are from the NICHE (Nyando Integrated Child Health and Education) project (19, 20). The data were obtained by a cross-sectional survey of children 6–35 mo of age in 60 randomly selected villages from Nyando Division (population 80,000) in the Nyanza Province of western Kenya from March to May 2009. After developing community maps, a complete household census was carried out in 60 selected villages. Children were selected by simple random sampling from each village, after the census. Children were selected if they were 6–35 mo of age at the time of enrollment and lived within the catchment area of the study. Children with hemoglobin <70 g/L (n = 14) were referred to the nearest clinic for treatment of severe anemia but were still approached for enrollment.
Children were excluded if they were unavailable for enrollment at 3 separate household visits or if their parents refused to give informed consent. Data were recorded in the field by using Dell Axim PDA (DDH Software) and downloaded into an Access 2007 (Microsoft Corp) database on a daily basis. Reports of fever 24 h before the interview were obtained through caregiver recall. The apparent health of children at the time of enrollment was determined by questionnaires to participants’ mothers asking about their children’s morbidity status (eg, diarrhea, fever, and cough) during the previous 24 h. Recent illness was not a criterion for exclusion. All children who provided consent were enrolled. Referral criteria included severe anemia and clinical malaria (fever with positive malaria smear, n = 42).
Written informed consent was obtained from all participating households. The Ethics Committee of KEMRI (Kenyan Medical Research Institute) in Nairobi, Kenya (protocol 1176), and the Institutional Review Board of the CDC in Atlanta, GA (protocol 5039), approved the study.
Laboratory analysis
Capillary blood was obtained from children aged 6–35 mo by trained laboratory technicians using single-use sterile microlancets (Becton Dickinson) into a purple-top microtainer capillary blood collector with EDTA (Becton Dickinson) to prevent clotting. Hemoglobin was measured within 3 min of blood collection by using the HemoCue B-Hemoglobin machine (Ängelholm). A single drop of blood was placed on a microscope slide (Thermo Fisher Scientific Inc) for thick and thin malaria smears for detection of malaria parasitemia. An additional 400–500 μL capillary blood was collected into a microtainer with EDTA (Becton Dickinson). The blood was transported on ice to the KEMRI/CDC laboratory in Kisian, Kenya, where a drop of whole blood was transferred from the microcontainers to a disposable glass cover slip (Aviv Biomedical) to assess ZP in duplicate. The remaining blood was centrifuged at 1500 × g for 5 min at 27°C, and the plasma was removed and stored in cryovials (Thermo Fisher Scientific Inc) at −20°C within 12 h of blood collection. The plasma samples were transported to Germany for subsequent laboratory analysis of ferritin, TfR, AGP (α1-acid glycoprotein), and CRP (C-reactive protein) by using a simple sandwich ELISA technique (21). The acute phase proteins AGP and CRP were used to identify children with infection and inflammation, which could confound measures of iron status, especially ferritin and ZP (12, 22, 23). All indicators were measured twice, and the average of the duplicate measures was used; the intra- and interassay CVs were <10%.
Anemia was defined according to the WHO threshold as hemoglobin <110 g/ L for children aged 0.5–4.99 y (1). The HemoCue machines were calibrated and checked for accuracy twice daily (morning before fieldwork and evening after field-work) by using Hemocontrols and control cuvettes, respectively, the records of which were kept in a secure place. ZP was measured at the laboratory of the KEMRI/CDC in Kisian, Kenya, with the Aviv ZP Hematofluorometer (Aviv Biomedical). The hematofluorometer was standardized daily with control solutions provided by Aviv Biomedical. We applied a correction factor to the results of the ZP on the advice of the manufacturers and the CDC quality-assurance laboratory. A cutoff value of 80 μmol heme/mol was used to indicate elevated ZP concentrations and hence iron deficiency (24).
The thresholds for defining abnormal values for the above biochemical indicators were as follows (25): ferritin, <12 μg/L; TfR, > 8.3 mg/L (Ramco Laboratories Inc); CRP, >5 mg/L; and AGP, >1.0 g/L. For the multiple-criteria model, ID was considered present if individuals had ≥2 abnormal values from among ferritin, TfR, and ZP (13, 14). The ratio of TfR to ferritin (TfR/ferritin index) is being advocated as the indicator of choice by the international community for estimating ID prevalence (15). The TfR/ferritin index was calculated by dividing TfR (μg/L) by ferritin (μg/L), with elevated TfR/ ferritin index defined as >500 (26, 27).
Statistical analysis
We used SAS 9.2 (SAS Institute Inc) for all statistical analyses. Statistical significance was defined as P < 0.05. Plasma ferritin, TfR, and ZP were found to have non-Gaussian distributions when they were assessed for normality by using plots and Kolmogorov-Smirnov tests and were therefore log-transformed and reported as geometric means and SDs or as medians and IQRs (28). The analyses for this study were carried out for 680 children who had all biochemical data available. No statistically significant differences in demographic characteristics were found between excluded (n = 32) and included children (P < 0.05) for these analyses.
To identify the best single estimate of ID, the proportion of children who were iron deficient based on the multiple-criteria model (defined as ≥2 of 3 abnormal values in ferritin, TfR, or ZP), the standard, was compared by using chi-square and Fisher tests to the proportion of children with abnormal values for each of the 3 independent tests (ie, ferritin alone, TfR alone, or ZP alone) and the TfR/ferritin index.
The κ statistic was computed to assess the extent of agreement between estimates of ID prevalence on the basis of the multiple-criteria model and each of the 3 independent tests and the TfR/ferritin index by using PROC FREQ with the AGREE option in SAS 9.2 (SAS Institute Inc). We characterized κ > 0.75 as indicating excellent agreement, 0.40–0.75 as fair to good agreement, and <0.40 as poor agreement (29).
The relative accuracy of the 3 individual tests and the TfR/ ferritin index at predicting ID based on the multiple-criteria model was assessed by examining the sensitivity, specificity, and positive and negative predictive values of the various indicators. Also, we applied receiver operating characteristic curves for the diagnosis of ID based on the multiple-criteria model by using the 3 different independent tests and the TfR/ferritin index by using SAS 9.2 (SAS Institute Inc).
RESULTS
Approximately equal proportions of boys and girls, with a mean (±SD) age of 21.1 ± 9.2 mo, were enrolled in this study (Table 1). The prevalence of malaria parasitemia was lower than expected, whereas the prevalence of reported fever within 24 h was high. Mean concentrations of ferritin and the TfR/ferritin index were within normal ranges; the mean hemoglobin concentration was lower and concentrations of ZP and TfR were higher than normal values. The prevalence of ID as assessed by the multiple-criteria model was significantly different from that estimated separately by ferritin, TfR, ZP, and the TfR/ferritin index (P < 0.001). Elevated CRP and AGP were present in 23% and 46% of the participants, respectively. The concentrations of ferritin and ZP and the TfR/ferritin index were different between the unadjusted and adjusted values for the effect of inflammation; unadjusted ferritin and ZP concentrations were higher than the adjusted values, whereas the adjusted TfR/ferritn index was higher than the unadjusted value. However, differences in the prevalence of ID were observed only for ferritin and the TfR/ ferritin index (Table 1), with adjusted values showing a higher proportion of both low ferritin and elevated TfR/ferritin index compared with the unadjusted values.
TABLE 1.
Demographic, infection, and biochemical characteristics in a sample of Kenyan children aged 6–35 mo (n = 680)1
| Characteristic | Unadjusted value | Adjusted value2 |
|---|---|---|
| Sex (% male) | 51.4 | — |
| Age (mo)3 | 21.1 ± 9.2 | — |
| Recent fever (%) | 28.0 | — |
| Malaria parasitemia (%) | 12.4 | — |
| CRP >5 mg/L (%) | 23.0 | — |
| AGP >1 g/L (%) | 46.0 | — |
| Hemoglobin (g/L)3 | 108.6 ± 15.4 | 113.0 ± 17.5 |
| Ferritin (μg/L)4 | 21.9 ± 2.6 | 14.9 ± 2.3 |
| TfR (mg/L)4 | 9.6 ± 1.5 | 9.3 ± 1.5 |
| ZP (μmol/mol heme)4 | 135.0 ± 1.7 | 122.7 ± 1.7 |
| TfR/ferritin index4 | 437.1 ± 3.0 | 635.3 ± 2.8 |
| Prevalence of anemia and ID (%)5 | ||
| Anemia6 | 45.9 ± 2.2 | 42.8 ± 2.3 |
| Multiple-criteria model7 | 61.9 ± 2.2 | 62.1 ± 1.9 |
| Low ferritin, <12 μg/L | 26.9 ± 1.7 | 40.7 ± 1.9 |
| Elevated TfR, >8.3 mg/L | 60.9 ± 2.2 | 56.6 ± 1.9 |
| Elevated ZP, >80 μmol/mol | 82.8 ± 1.6 | 79.9 ± 1.6 |
| TfR/ferritin index, >500 | 43.1 ± 2.3 | 55.9 ± 1.7 |
AGP, plasma α1-acid glycoprotein; CRP, plasma C-reactive protein; ID, iron deficiency; TfR, plasma soluble transferrin receptor; TfR/ferritin index, ratio of plasma soluble transferrin receptor to plasma ferritin index; ZP, whole-blood zinc protoporphyrin.
Values adjusted for inflammation by using group-specific correction factors estimated from the ratios of geometric means for the various iron indicators (30).
Values are arithmetic means ± SDs.
Values are geometric means ± SDs.
Values are prevalence ± SE. Significant differences were observed between the combined model and each of the other indicators in assessing the proportions of children with ID (chi-square tests, P < 0.001).
Anemia defined as hemoglobin <110 g/L (1).
The κ statistics for agreement between the multiple-criteria model and the other iron indicators in the estimation of ID prevalence among the preschool children ranged from 0.35 to 0.88, ie, fair to excellent agreement with the multiple-criteria model (Table 2).
TABLE 2.
Agreement between the multiple-criteria model and other indicators used to define iron deficiency in a sample of Kenyan children aged 6–35 mo1
| Iron deficiency | Iron deficiency by multiple-criteria model2
|
κ Statistic (95% CI) | |
|---|---|---|---|
| Yes | No | ||
| % | % | ||
| By low ferritin, <12 μg/L | |||
| Yes | 26.3 ± 1.7 (179) | 0.6 ± 0.3 (4) | 0.35 (0.30, 0.40) |
| No | 35.6 ± 2.0 (242) | 37.5 ± 2.1 (255) | — |
| By elevated TfR, >8.3 mg/L | |||
| Yes | 58.5 ± 2.2 (398) | 2.4 ± 0.6 (16) | 0.88 (0.84, 0.92) |
| No | 3.4 ± 0.7 (23) | 35.7 ± 2.1 (243) | — |
| By elevated ZP, >80 μmol/mol | |||
| Yes | 61.5 ± 2.2 (418) | 21.3 ± 1.6 (145) | 0.48 (0.42, 0.55) |
| No | 0.4 ± 0.3 (3) | 16.8 ± 1.6 (114) | — |
| By TfR/ferritin index, >500 | |||
| Yes | 38.8 ± 2.2 (264) | 4.3 ± 0.8 (29) | 0.81 (0.76, 0.85) |
| No | 23.1 ± 1.9 (157) | 33.8 ± 2.1 (230) | — |
Iron deficiency was considered present if individuals had ≥2 abnormal values from among ferritin (<12 μg/L), TfR (>8.3 mg/L), and ZP (>80 μmol/mol) (13, 14, 16). TfR, plasma soluble transferrin receptor; TfR/ferritin index, ratio of plasma soluble transferrin receptor to plasma ferritin index; ZP, whole-blood zinc protoporphyrin.
Values are means ± SEs; n in parentheses.
The sensitivity for identifying ID (proportion ± SE), as defined by the multiple-criteria model, was greater for ZP (0.99 ± 0.01) and TfR (0.95 ± 0.01), but lower for ferritin (0.43 ± 0.02) and the TfR/ferritin index (0.63 ± 0.02) (Table 3). However, the specificity for identifying ID was higher for TfR and ferritin and the TfR/ferritin index and lowest for ZP. Consequently, TfR least misclassified children as iron deficient on the basis of the multiple-criteria model compared with the other iron indicators. This observation was independent of the effect of inflammation.
TABLE 3.
Accuracy and predictability of iron deficiency based on the multiple-criteria model by using the TfR/ferritin index and 3 different independent tests in a sample of Kenyan children aged 6–35 mo1
| Iron deficiency | Combined model2
|
Accuracy and predictive values3 | κ Percentage misclassified4 | |
|---|---|---|---|---|
| Yes5 | No5 | |||
| % | % | |||
| Defined by low ferritin, 12 μg/L | ||||
| Yes | 26.3 ± 1.7 (179) | 0.6 ± 0.3 (4) | Ŝe = 0.43 ± 0.02 (421) | 36.2 |
| No | 35.6 ± 2.0 (242) | 37.5 ± 2.1 (255) | ŜP = 0.98 ± 0.01 (259) | |
| PPV = 0.98 ± 0.01 (183) | ||||
| NPV = 0.51 ± 0.02 (497) | ||||
| Defined by elevated TfR, >8.3 mg/L | ||||
| Yes | 58.5 ± 2.2 (398) | 2.4 ± 0.6 (16) | Ŝe = 0.95 ± 0.01 (421) | 5.7 |
| No | 3.4 ± 0.7 (23) | 35.7 ± 2.1 (243) | ŜP = 0.94 ± 0.01 (259) | |
| PPV = 0.96 ± 0.01 (414) | ||||
| NPV = 0.91 ± 0.02 (266) | ||||
| Defined by elevated ZP, >80 μmol/mol | ||||
| Yes | 61.5 ± 2.2 (418) | 21.3 ± 1.6 (145) | Ŝe = 0.99 ± 0.01 (421) | 21.8 |
| No | 0.4 ± 0.3 (3) | 16.8 ± 1.6 (114) | ŜP = 0.44 ± 0.03 (259) | |
| PPV = 0.74 ± 0.02 (563) | ||||
| NPV = 0.97 ± 0.02 (117) | ||||
| Defined by TfR/ferritin index, >500 | ||||
| Yes | 38.8 ± 2.2 (264) | 4.3 ± 0.8 (29) | Ŝe = 0.63 ± 0.02 (421) | 24.4 |
| No | 23.1 ± 1.9 (157) | 33.8 ± 2.1 (230) | ŜP = 0.89 ± 0.02 (259) | |
| PPV = 0.90 ± 0.02 (293) | ||||
| NPV = 0.59 ± 0.02 (387) | ||||
NPV, negative predictive value = [children without iron deficiency as defined by both the single iron indicator test and multiple-criteria model (true-negative results)/children without iron deficiency as defined by the single iron indicator test alone (true-negative results + false-negative results)]; PPV, positive predictive value = [children with iron deficiency as defined by the single iron indicator test and multiple-criteria model (true-positive results)/children with iron deficiency as defined by the single iron indicator alone (true-positive results + false-positive results)]; Ŝe, sensitivity = [children with iron deficiency as defined by the single iron indicator test and multiple-criteria model (true-positive results)/children with iron deficiency as defined by the single iron indicator test (true-positive results + false-negative results)]; Ŝp, specificity = [children without iron deficiency as defined by both the single iron indicator test and the multiple-criteria model (true-negative results)/children without iron deficiency as defined by the single iron indicator test alone (true-negative results + false-positive results)] (14); TfR, plasma soluble transferrin receptor; TfR/ferritin index, ratio of plasma soluble transferrin receptor to plasma ferritin index; ZP, whole-blood zinc protoporphyrin.
Iron deficiency was considered present if individuals had ≥2 abnormal values from among ferritin (<12 μg/L), TfR (>8.3 mg/L), and ZP (>80 μmol/ mol) (13, 14).
Values are proportions ± SDs; n in parentheses.
Percentage misclassification = (1 − sensitivity) × (percentage iron-deficient children) + (1 − specificity) × (percentage iron-replete children) (41).
Values are means ± SEs; n in parentheses.
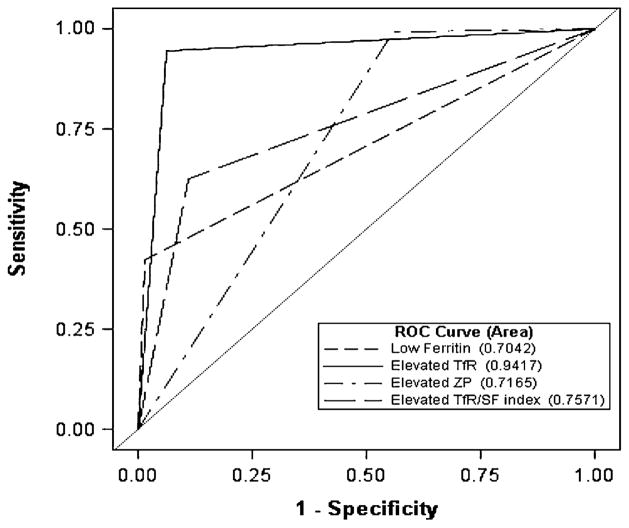
The receiver operating characteristic curves for the various iron indicators were used to diagnose ID as defined by the multiple-criteria model (Figure 1). The AUC values (and 95% CIs) indicated that the diagnostic accuracy of TfR (0.942; 95% CI: 0.923, 0.960) in predicting iron deficiency was superior to that of the other iron-status indicators (P < 0.001).
FIGURE 1.
ROC curve analysis for the diagnosis of ID (based on the multiple-criteria model) by using the TfR/ferritin index and 3 different independent tests in a sample of Kenyan children aged 6–35 mo. ID was defined per the multiple-criteria model as ≥2 abnormal values from among ferritin (<12 μg/L), TfR (>8.3 mg/L), and ZP (>80 μmol/mol) (13, 14). AUC values (and 95% CIs) for low ferritin (<12 μg/L), elevated TfR (>8.3 mg/L), elevated ZP (>80 μmol/mol), and an elevated TfR/ferritin index (>500) were as follows: 0.704 (0.679, 0.729), 0.942 (0.923, 0.960), 0.717 (0.686, 0.747), and 0.757 (0.727, 0.787), respectively. TfR was the most accurate estimator of ID (P < 0.0001; n = 679). ID, iron deficiency; ROC, receiver operating characteristic; TfR, plasma soluble transferrin receptor; TfR/SF index, ratio of plasma soluble transferrin receptor to plasma ferritin index; ZP, whole-blood zinc protoporphyrin.
DISCUSSION
Multiple criteria indicators that use a combination of ≥3 tests of iron status have been used to estimate the prevalence of nutritional iron deficiency in populations (13, 14, 16). Recent assay techniques allow for the measurement of these indicators by using small quantities of blood from capillary finger-stick samples (12, 17, 18, 21). The costs of laboratory assessments are similar for ferritin, TfR, and to some extent ZP and are therefore largely a function of the number of individuals and indicators measured. Costs per test are ~US$8–26 for ferritin, US$8–30 for TfR, US$1–6 for ZP, US$6–22 for CRP, and US$2–10 for AGP. Venous compared with capillary blood testing cost differences are minimal. Thus, it is more expensive to measure all 3 indicators required for the multiple-criteria model (US$24–90) compared with just one indicator (US$1–30) (21). Therefore, the identification of a single iron indicator that provides similar information as the multiple-criteria model will be cost-effective and, hence, is of importance. The sandwich ELISA technique that was used in this study was, however, considerably cheaper (US$12 for 5 combined analytes) than other techniques (21).
The prevalence of ID in this preschool population ranged from 27% to 83% based on the different iron indicators used. The prevalence of ID was highest for ZP and least for ferritin and the TfR/ferritin index. The high prevalence of ID by ZP may have been due to interfering substances in the plasma produced by hemolysis and inflammation, which can increase ZP concentrations 3–4-fold in the absence of ID (31). Furthermore, the specificity of ZP in identifying ID may be limited by increased blood lead concentrations, hemolytic anemias, malaria, or hemoglobinopathies (24, 31).
The prevalence of anemia was 46% compared with 69% reported in the same setting among 2–36-mo-old children by Verhoef et al (22). Although similar methods for obtaining blood as well as similar cutoffs for hemoglobin were applied in both studies, the children were younger and the prevalence of malaria was greater than in our study (18% compared with 12%). The lower prevalence of malaria, and consequently of anemia, in our study was probably due to the existence of an active malaria-control program (22).
The κ statistics for agreement between the multiple-criteria model and the other iron indicators for ID was fair to excellent; TfR had the best agreement with the multiple-criteria model, whereas ferritin had the least agreement with the multiple-criteria model. This confirms results from earlier studies that indicated TfR to be a more sensitive index of ID than ZP or ferritin (12, 22, 23, 32, 33), because it is less influenced by malaria or inflammation (12, 22, 23).
The trans-membrane glycoprotein TfR is expressed on cell surfaces and regulated by posttranscriptional regulation of the iron-mediated iron-reactive element. It is important for iron uptake of the cell; expression of TfR concentrations increases during iron deficiency and decreases when there is iron overload. Early measure of suboptimal iron supply can thus be reflected by an increased TfR cellular uptake of iron (15, 34, 35). Transferrin receptor as an indicator has many strengths; it appears to be a specific indicator of iron deficiency erythropoeisis not significantly confounded by inflammation, although it may be influenced by malaria, age, and ethnicity (31). Additionally, it has been shown to be more sensitive than ZP in detecting functional ID (34), with greater changes being observed earlier in TfR concentrations than in other iron indicators (36), and more reliable in reflecting early-stage tissue ID (37). However, lack of international cutoffs and its assessment by different assays inhibits direct comparison of TfR values between studies (31), although a recent report identifies a radiolabeled assay for assessing TfR for diagnostic purposes (38).
Plasma ferritin, an indicator of iron stores in healthy individuals, is an acute phase reactant and is affected by inflammation. The low prevalence of ID based on ferritin in this study may have been found in children with depleted iron stores who were yet to progress to iron-deficient erythropoiesis (39).
The results of the current study support the finding that TfR, compared with ZP, ferritin, and the TfR/ferritin index, is more strongly associated with the multiple-criteria model as an indicator of ID. Fewer children were misdiagnosed as iron deficient when TfR was used than when ZP, ferritin, and the TfR/ferritin index were used.
The results presented were obtained by using iron-status indicators that were unadjusted for the influence of inflammation. An approach has been proposed to improve the estimation of iron deficiency in populations exposed to frequent infections (30, 40). However, there is no consensus on this approach, and most previous studies used unadjusted values. We repeated the analyses using cutoff values adjusted for the influence of inflammation based on CRP (<5 mg/L) and AGP (<1 g/L) concentrations (30) to compare the results; the results and conclusions of our study did not change appreciably with respect to the best indicator for assessing iron deficiency based on the multiple-criteria model, which was still TfR. However, iron-status levels and the prevalence of ID were different between the unadjusted and adjusted iron indicators (Table 1). The TfR/ferritin index and concentrations of ferritin and ZP and were higher when not adjusted for inflammation. This difference was not obvious for TfR, an indication that this indicator is less influenced by inflammation.
A limitation of our study was that blood lead and hemoglobinopathies (eg, sickle cell anemia, thalassemias) were not measured, which may have confounded the ZP concentrations. Additionally, we tested the performance of an indicator against other indicators, which were partly based on it and hence not statistically independent. This lack of independence may have affected the interpretation of the κ and the AUC statistics. Last, our 3-factor outcome model may not necessarily be the gold standard for estimating ID. The main strength of the study was the use of field-friendly capillary blood methods to minimize discomfort among the children and to measure multiple iron-status indicators in capillary blood by using the sandwich ELISA technique, which requires only a small volume of plasma.
Our study showed that plasma TfR, obtained from capillary blood, can accurately estimate ID based on the multiple-criteria model when assessing ID in preschool children at the population level in high-inflammation settings.
In conclusion, in this high-inflammation setting, our study indicated excellent agreement between TfR and the multiple-criteria model for identifying the prevalence of ID in pre-schoolers. Furthermore, compared with the other indicators (ferritin, ZP, and the TfR/ferritin index), TfR misdiagnosed the least percentage of children as iron deficient based on the multiple-criteria model. However, our reference ID model (the 3-factor multiple-criteria model) may not be the gold standard for estimating ID, and further studies are needed to compare the diagnostic ability of the candidate iron-status indicators with the known gold standard—stainable bone marrow iron—to confirm our findings.
Acknowledgments
We thank Juergen Erhardt for analyzing the samples in his laboratory, the study participants, Alie Eleveld and the staff of the Safe Water and AIDS Project, Vincent Were and the staff of the Kenya Medical Research Institute and CDC offices based in Kenya, and Cliff Ochieng and Jared Oremo and the staff of the Nyando Integrated Child Health and Education Project study team.
Footnotes
The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the CDC.
Presented at the annual meeting of Experimental Biology 2011, in Washington, DC.
This study is dedicated to the memory of Alfredo Obure, the Nyando Integrated Child Health and Education Project study coordinator, who died in 2010.
Supported in part by an Ellison Medical Foundation/Nevin Scrimshaw International Nutrition Foundation fellowship (FKEG) and the CDC (PSS).
Abbreviations used: AGP, α1-acid glycoprotein; CRP, C-reactive protein; ID, iron deficiency; KEMRI, Kenyan Medical Research Institute; NICHE, Nyando Integrated Child Health and Education project; TfR, soluble transferrin receptor; ZP, zinc protoporphyrin.
The authors’ responsibilities were as follows—FKEG, PSS, LJR, RF-A, and RM: designed the study; FKEG, LJR, and PSS: conducted the study and collected the data; FKEG, PSS, RF-A, CRC, UR, and RM: analyzed the data; and FKEG: wrote the initial manuscript. All authors were responsible for preparing the final manuscript. None of the authors had a personal or financial conflict of interest. The funding agencies had no role in the design or implementation of the study, the analysis and interpretation of the data, the preparation of the manuscript.
References
- 1.McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr. 2009;12:444–54. doi: 10.1017/S1368980008002401. [DOI] [PubMed] [Google Scholar]
- 2.Majid Ezzati SVH, Lopez AD, Danaei G, Rodgers A, Mathers CD, Murray CJL. Global burden of disease and risk factors. In: Lopez AD, Ezzati M, Jamison DT, Murray CJL, editors. Global burden of disease and risk factors. Washington, DC: World Bank; 2006. [PubMed] [Google Scholar]
- 3.Haas JD, Brownlie TT. Iron deficiency and reduced work capacity: a critical review of the research to determine a causal relationship. J Nutr. 2001;131:676S–90S. doi: 10.1093/jn/131.2.676S. [DOI] [PubMed] [Google Scholar]
- 4.Brabin BJ, Hakimi M, Pelletier D. An analysis of anemia and pregnancy-related maternal mortality. J Nutr. 2001;131:604S–15S. doi: 10.1093/jn/131.2.604S. [DOI] [PubMed] [Google Scholar]
- 5.Brabin BJ, Premji Z, Verhoeff F. An analysis of anemia and child mortality. J Nutr. 2001;131:636S–48S. doi: 10.1093/jn/131.2.636S. [DOI] [PubMed] [Google Scholar]
- 6.Sachdev H, Gera T, Nestel P. Effect of iron supplementation on mental and motor development in children: systematic review of randomised controlled trials. Public Health Nutr. 2005;8:117–32. doi: 10.1079/phn2004677. [DOI] [PubMed] [Google Scholar]
- 7.Murray-Kolb LE, Beard JL. Iron treatment normalizes cognitive functioning in young women. Am J Clin Nutr. 2007;85:778–87. doi: 10.1093/ajcn/85.3.778. [DOI] [PubMed] [Google Scholar]
- 8.Suchdev PS, Leeds IL, McFarland DA, Flores R. Is it time to change guidelines for iron supplementation in malarial areas? J Nutr. 2010;140:875–6. doi: 10.3945/jn.109.118638. [DOI] [PubMed] [Google Scholar]
- 9.Allen L, Black RE, Brandes N, Brittenham G, Chazot G, Chunming C, Crawley J, de Benoist B, Dalmiya N, Darnto-Hill I, et al. Conclusions and recommendations of a WHO expert consultation meeting on iron supplementation for infants and young children in malaria endemic areas. Med Trop (Mars) 2008;68:182–8. in French. [PMC free article] [PubMed] [Google Scholar]
- 10.WHO. Conclusions and recommendations of the WHO Consultation on prevention and control of iron deficiency in infants and young children in malaria endemic areas. Food Nutr Bull. 2007;28:S621–7. doi: 10.1177/15648265070284s414. [DOI] [PubMed] [Google Scholar]
- 11.Stoltzfus R. Defining iron-deficiency anemia in public health terms: a time for reflection. J Nutr. 2001;131:565S–7S. doi: 10.1093/jn/131.2.565S. [DOI] [PubMed] [Google Scholar]
- 12.Shell-Duncan B, McDade T. Use of combined measures from capillary blood to assess iron deficiency in rural Kenyan children. J Nutr. 2004;134:384–7. doi: 10.1093/jn/134.2.384. [DOI] [PubMed] [Google Scholar]
- 13.Gibson RS. Principles of nutritional assessment. 2. New York, NY: Oxford University Press, Inc; 2005. [Google Scholar]
- 14.Cogswell ME, Looker AC, Pfeiffer CM, Cook JD, Lacher DA, Beard JL, Lynch SR, Grummer-Strawn LM. Assessment of iron deficiency in US preschool children and nonpregnant females of childbearing age: National Health and Nutrition Examination Survey 2003–2006. Am J Clin Nutr. 2009;89:1334–42. doi: 10.3945/ajcn.2008.27151. [DOI] [PubMed] [Google Scholar]
- 15.Lynch S. Case studies: iron. Am J Clin Nutr. 2011;94(suppl):673S–8S. doi: 10.3945/ajcn.110.005959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cook JD, Finch CA, Smith NJ. Evaluation of the iron status of a population. Blood. 1976;48:449–55. [PubMed] [Google Scholar]
- 17.Lu Y, Lynch SR, Cook JD, Madan N, Bayer WL. Use of capillary blood for the evaluation of iron status. Am J Hematol. 1987;24:365–74. doi: 10.1002/ajh.2830240406. [DOI] [PubMed] [Google Scholar]
- 18.Labbé RF, Rettmer RL, Shah AG, Turnlund JR. Zinc protoporphyrin. Past, present, and future. Ann N Y Acad Sci. 1987;514:7–14. doi: 10.1111/j.1749-6632.1987.tb48755.x. [DOI] [PubMed] [Google Scholar]
- 19.CDC. Baseline data from the Nyando integrated Child Health and Education Project—Kenya. MMWR Morb Mortal Wkly Rep. 2007;56:1109–13. [PubMed] [Google Scholar]
- 20.Suchdev PS, Ruth L, Obure A, Were V, Ochieng C, Ogange L, Owuor M, Ngure F, Quick R, Juliao P, et al. Monitoring the marketing, distribution, and use of Sprinkles micronutrient powders in rural western Kenya. Food Nutr Bull. 2010;31:S168–78. doi: 10.1177/15648265100312S209. [DOI] [PubMed] [Google Scholar]
- 21.Erhardt JG, Estes JE, Pfeiffer CM, Biesalski HK, Craft NE. Combined measurement of ferritin, soluble transferrin receptor, retinol binding protein, and C-reactive protein by an inexpensive, sensitive, and simple sandwich enzyme-linked immunosorbent assay technique. J Nutr. 2004;134:3127–32. doi: 10.1093/jn/134.11.3127. [DOI] [PubMed] [Google Scholar]
- 22.Verhoef H, West CE, Ndeto P, Burema J, Beguin Y, Kok FJ. Serum transferrin receptor concentration indicates increased erythropoiesis in Kenyan children with asymptomatic malaria. Am J Clin Nutr. 2001;74:767–75. doi: 10.1093/ajcn/74.6.767. [DOI] [PubMed] [Google Scholar]
- 23.Asobayire FS, Adou P, Davidsson L, Cook JD, Hurrell RF. Prevalence of iron deficiency with and without concurrent anemia in population groups with high prevalences of malaria and other infections: a study in Cote d’Ivoire. Am J Clin Nutr. 2001;74:776–82. doi: 10.1093/ajcn/74.6.776. [DOI] [PubMed] [Google Scholar]
- 24.Labbé RF, Dewanji A, McLaughlin K. Observations on the zinc protoporphyrin/heme ratio in whole blood. Clin Chem. 1999;45:146–8. [PubMed] [Google Scholar]
- 25.WHO/CDC. Assessing the iron status of populations: report of a Joint World Health Organization/Centers for Disease Control and Prevention Technical Consultation on the assessment of iron status at the population level. Geneva, Switzerland: Apr 6–8, 2004. WHO Library Cataloguing-in-Publication Data 2007. Available from: http://whqlib-doc.who.int/publications/2004/9241593156_eng.pdf (cited 20 September 2011) [Google Scholar]
- 26.Ronnenberg AG, Wood RJ, Wang X, Xing H, Chen C, Chen D, Guang W, Huang A, Wang L, Xu X. Preconception hemoglobin and ferritin concentrations are associated with pregnancy outcome in a prospective cohort of Chinese women. J Nutr. 2004;134:2586–91. doi: 10.1093/jn/134.10.2586. [DOI] [PubMed] [Google Scholar]
- 27.Akesson A, Bjellerup P, Berglund M, Bremme K, Vahter M. Soluble transferrin receptor: longitudinal assessment from pregnancy to post-lactation. Obstet Gynecol. 2002;99:260–6. doi: 10.1016/s0029-7844(01)01684-2. [DOI] [PubMed] [Google Scholar]
- 28.Weisberg S. Applied linear regression. New York, NY: John Wiley & Sons; 1985. [Google Scholar]
- 29.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. [PubMed] [Google Scholar]
- 30.Grant FK, Suchdev PS, Flores-Ayala R, Cole CR, Ramakrishnan U, Ruth LJ, Martorell R. Correcting for inflammation changes estimates of iron deficiency among rural Kenyan preschool children. J Nutr. 2012;142:105–11. doi: 10.3945/jn.111.146316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zimmermann MB. Methods to assess iron and iodine status. Br J Nutr. 2008;99(suppl 3):S2–9. doi: 10.1017/S000711450800679X. [DOI] [PubMed] [Google Scholar]
- 32.Brugnara C, Zurakowski D, DiCanzio J, Boyd T, Platt O. Reticulocyte hemoglobin content to diagnose iron deficiency in children. JAMA. 1999;281:2225–30. doi: 10.1001/jama.281.23.2225. [DOI] [PubMed] [Google Scholar]
- 33.Baker RD, Greer FR. Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0–3 years of age) Pediatrics. 2010;126:1040–50. doi: 10.1542/peds.2010-2576. [DOI] [PubMed] [Google Scholar]
- 34.Lin XM, Zhang J, Zou ZY, Long Z, Tian W. Evaluation of serum transferrin receptor for iron deficiency in women of child-bearing age. Br J Nutr. 2008;100:1104–8. doi: 10.1017/S0007114508966101. [DOI] [PubMed] [Google Scholar]
- 35.Lynch S. Improving the assessment of iron status. Am J Clin Nutr. 2011;93:1188–9. doi: 10.3945/ajcn.111.015214. [DOI] [PubMed] [Google Scholar]
- 36.Flowers CH, Skikne BS, Covell AM, Cook JD. The clinical measurement of serum transferrin receptor. J Lab Clin Med. 1989;114:368–77. [PubMed] [Google Scholar]
- 37.Cook JD, Baynes RD, Skikne BS. The physiological significance of circulating transferrin receptors. Adv Exp Med Biol. 1994;352:119–26. doi: 10.1007/978-1-4899-2575-6_9. [DOI] [PubMed] [Google Scholar]
- 38.Magro G, Cataldo I, Amico P, Torrisi A, Vecchio GM, Parenti R, Asioli S, Recupero D, D’Agata V, Mucignat MT, et al. Aberrant expression of TfR1/CD71 in thyroid carcinomas identifies a novel potential diagnostic marker and therapeutic target. Thyroid. 2011;21:267–77. doi: 10.1089/thy.2010.0173. [DOI] [PubMed] [Google Scholar]
- 39.Zimmermann MB, Molinari L, Staubli-Asobayire F, Hess SY, Chaouki N, Adou P, Hurrell RF. Serum transferrin receptor and zinc protoporphyrin as indicators of iron status in African children. Am J Clin Nutr. 2005;81:615–23. doi: 10.1093/ajcn/81.3.615. [DOI] [PubMed] [Google Scholar]
- 40.Thurnham DI, McCabe LD, Haldar S, Wieringa FT, Northrop-Clewes CA, McCabe GP. Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: a meta-analysis. Am J Clin Nutr. 2010;92:546–55. doi: 10.3945/ajcn.2010.29284. [DOI] [PubMed] [Google Scholar]
- 41.Schneider JM, Fujii ML, Lamp CL, Lonnerdal B, Dewey KG, Zidenberg-Cherr S. Anemia, iron deficiency, and iron deficiency anemia in 12–36-mo-old children from low-income families. Am J Clin Nutr. 2005;82:1269–75. doi: 10.1093/ajcn/82.6.1269. [DOI] [PubMed] [Google Scholar]