Supplemental Digital Content is available in the text
Abstract
Currently, there is a paucity of study investigating postoperative ileus in gastric cancer surgery. This prospective study aims to identify the risk factors for prolonged postoperative ileus (PPOI) and to use these risk factors to generate a risk stratification scoring system for the occurrence of PPOI.
Patients who underwent radical gastrectomy for gastric cancer were included in this study. A multivariate logistic analysis was applied to identify independent risk factors for PPOI and to generate the scoring system. A receiver operating characteristic curve was generated and the area under the curve was calculated to demonstrate the predictive power of the scoring system.
Finally, 296 patients were included and analyzed, of whom 96 (32.4%) developed PPOI. The multivariate analysis showed that age ≥65 years, operative duration ≥4 hours, tumor–node–metastasis (TNM) stage = III, open/converted operative technique, and total postoperative opiates dose (TOD) ≥0.3 mg/kg were independent risk factors for PPOI. Based on these factors, a risk stratification scoring system was generated, classified by low-risk (score 0–2), moderate-risk (score 3–4), and high-risk (score 5–6) groups. The incidence of PPOI increased by 7.5-fold from low-risk to high-risk group. The area under the curve of the scoring system was 0.841 (95% CI, 0.793–0.890), indicating a good predictive capability for the occurrence of PPOI.
We have identified independent risk factors for the occurrence of PPOI and used these factors to construct a risk stratification scoring system.
INTRODUCTION
Postoperative ileus (POI) is defined as a temporary impairment in gastrointestinal motility following surgery.1 It is characterized by nausea, vomiting, abdominal pain, and distension, a delayed passage of flatus and stool, and the inability to tolerate oral diet.2 “Normal” or “uncomplicated” POI is generally identified as an inevitable process after surgery, which typically resolves within 3 days.1,2 When this situation extends beyond the expected duration, it is defined as “paralytic” or “prolonged” postoperative ileus (PPOI).1,2 In addition to the features mentioned above, PPOI could further result in a range of significant consequences, including continuing discomfort, pain, compromised postoperative nutrition and the need for parenteral nutrition, and the elevated risk of other postoperative complications.3,4 Moreover, PPOI was an important contributor to the increased length of postoperative hospital stay (LOS) and the excessive hospitalization costs.5
Gastric cancer remains the third common cause of cancer death worldwide, and is among the most prevalent types of malignancy in Eastern Asia, including Japan, Korea, and China.6 Radical gastrectomy with lymph node dissection is the mainstay of therapy in the management of patients with resectable gastric cancer, and complete resection (R0) brings the most favorable survival benefit in gastric cancer.7,8 Nevertheless, the patients who underwent gastrectomy always have to bear a relatively slow postoperative recovery process.9 To accelerate the postoperative recovery process after gastrectomy, Enhanced Recovery after Surgery (ERAS) programs have been proposed.10 Prevention of postoperative ileus is an important item of ERAS10 since PPOI is one of the major causes for the delayed postoperative recovery.5,11 However, there is still a lack of effective therapeutic method for postoperative ileus. Therefore, it is necessary to investigate the risk factors for PPOI and develop a risk stratification system to better predict and prevent the occurrence of PPOI. To our knowledge, there is a paucity of study investigating PPOI in patients undergoing radical gastrectomy. Radical gastrectomy differed from other types of surgery in terms of surgical site, extent of organ and tissue resection, and the reconstruction of gastrointestinal tract. Moreover, total gastrectomy results in resection of vagus nerve, and importantly, vagus nerves participate in gastrointestinal motor activity.12 Therefore, the results of studies in the setting of other types of surgery may not be generalized to the patients undergoing radical gastrectomy.
The objective of this prospective study was to investigate the risk factors for PPOI and to generate a simplified and clinical practical score-based risk stratification system for the occurrence of PPOI in a consecutive series of patients who underwent radical gastrectomy for gastric cancer.
METHODS
Patients
From August 2014 to May 2015, consecutive patients who underwent elective radical gastrectomy for gastric cancer at the Gastrointestinal Surgical Department, The First Affiliated Hospital of Wenzhou Medical University were included in this prospective study. The inclusion criteria included patients who were over 18 years old, were scheduled to receive elective radical gastrectomy for gastric cancer, had the American Society of Anesthesiologists (ASA) grade ≤III, without evidence of metastatic disease, and agreed to take part in the study and signed the informed consent. All of the included patients had a relatively accurate preoperative diagnosis of gastric adenocarcinoma on the basis of the result of abdominal computed tomography (CT), gastroscopy and histological evidence. The exclusion criteria included patients who received a palliative surgery, had anastomotic leakage after surgery (considered a cause of postoperative ileus),3 had gastroparesis after surgery, and had early postoperative small-bowel obstruction (EPSBO). Anastomotic leakage was identified if one or more of the following 2 criteria are met: methylene blue discharge from the peritoneal drains after an oral intake of methylene blue, or extravasation of contrast material after an oral intake of water-soluble contrast material on CT scans.13 Meanwhile, EPSBO was diagnosed if one or more of the following 2 criteria are met within the first 30 days: patients developed signs, symptoms, and radiographic evidence of small-bowel obstruction after evidence of return of gastrointestinal function, or mechanical intestinal obstruction was definitively confirmed by relaparotomy or contrast study.14 Additionally, gastroparesis, a syndrome characterized by delayed gastric emptying in the absence of mechanical obstruction,15 was diagnosed by radiographic contrast techniques. Little or no emptying of barium within 30 minutes and retention of gastric barium at 6 hours lead to a diagnosis of gastroparesis.16
All of the included patients were scheduled to receive gastrectomy with curative intent according to Japanese gastric cancer treatment guidelines 2010 (ver. 3).17 Surgical specimens were staged according to the 7th edition of the International Union Against Cancer (UICC) tumor–node–metastasis (TNM) classification of malignant tumors.18
All patients were treated with a standard general anesthesia regimen, using propofol, sulfentanyl, and rocuronium. Epidural anesthesia was applied to all patients unless contraindicated, using 0.5% ropivacaine and 1% lidocaine. For patients who received epidural anesthesia, epidural analgesia was used after surgery, using 0.15% ropivacaine and morphine 3 mg. Otherwise, intravenous patient control analgesia was used, using sulfentanyl 0.1 mg and flurbiprofen axetil 100 mg. For additional postoperative pain, a stepwise analgesia regimen was adopted. In brief, transanal indometacin or intravenous parecoxib sodium was used at the beginning, if the pain still cannot be controlled, intramuscular injections of pethidine were administered.
The total postoperative opiate dose (TOD) for each patient was calculated by converting the dose of all opiates medications into milligram equivalents of parenteral morphine.19 To allow comparisons between patients, total equivalent dose was then divided by weight (kg) of the patient, presented as “mg/kg.”
A similar perioperative care program was applied to all the included patients. In brief, patients were fasted for 8 hours before surgery; nasogastric tubes, urinary catheters, and peritoneal drains were routinely used after surgery, nasogastric tubes were removed after the passage of flatus, urinary catheter and peritoneal drains were removed at postoperative day (POD) 3 or 4; the maintenance of water and electrolyte balance was based on blood biochemical examination; a clear fluid oral diet was administered to patients after the passage of flatus and progressively evolved into a solid diet as tolerated.
PPOI was diagnosed if 2 or more of the following 5 criteria are met on or after POD 4 without prior resolution of “postoperative ileus”: nausea or vomiting, inability to tolerate oral diet over 24 hours, absence of flatus over 24 hours, abdominal distension, and radiologic confirmation of ileus.2 All patients were given written informed consent for participation in this study and the study was approved by the ethics committee of The First Affiliated Hospital of Wenzhou Medical University.
Data Collection
The clinical data of all patients were collected prospectively, and patients were followed up for 30 days by phone calls every 10 days after discharge. The patient preoperative demographics and clinical data were obtained, including sex, age, body mass index (BMI), ASA grade, history of smoking, and alcohol using; comorbidities including cardiovascular and cerebrovascular diseases, chronic obstructive pulmonary disease (COPD), renal insufficiency, diabetes, hypertension and anemia (defined as male <120 g/L and female <110 g/L); preoperative plasma albumin level (hypoalbuminemia was defined as <35 g/L); history of previous abdominal surgery. Preoperative nutritional risk was assessed by nutritional risk screening (NRS) 2002 within 24 hours of admission, patients with a total score of 3 or more were considered at nutritional risk.20
Intraoperative data collected including type of anesthesia (with or without epidural anesthesia); type of surgery (open/converted or laparoscopic operation, total or subtotal gastrectomy, combined or not combined with multiorgan resection); type of gastrointestinal tract reconstruction (Billroth I, Billroth II, Roux-en-Y); duration of surgery and tumor–node–metastasis (TNM) stage of tumor.
Postoperative outcomes included postoperative complications observed during hospitalization and within the 30-day follow-up period. The postoperative blood leukocyte and neutrophils count were tested at POD 3. TOD was calculated and recorded for each patient. Furthermore, LOS and economic costs were also calculated accurately to evaluate medical resources and economy burden resulting from the treatment.
Statistical Analysis
The continuous data which subjected to normal distribution were presented as mean and standard deviation (SD). The nonnormally continuous distributed data were presented as median and range. The Student t test and nonparametric test (Mann–Whitney U test) were used for normally and nonnormally distributed continuous data respectively. Meanwhile, categorical data were assessed for their association with PPOI by χ2 test. Spearman rank correlation and Kruskal–Wallis test were used to assess the correlation between ranked data and other factors. For all analyses, a P <0.05 was considered to be statistically significant.
To evaluate the risk factors for PPOI, all variables associated with PPOI with P < 0.1 from the univariate analyses were selected as potential parameters, and then a forward stepwise variable selection was used to establish a multivariable logistic regression by individual sequential exclusion of variables with P >0.05. Variables identified as independent predictors of PPOI on the multivariate analysis were then used to generate a score-based risk stratification system. The statistical analysis was performed using a standard statistical software package, SPSS statistics version 22.0. (IBM, New York).
RESULTS
During the study period, 330 patients met the inclusion criteria initially. Nineteen patients were excluded for receiving a palliative operation. Moreover, anastomotic leakage, gastroparesis, and EPSBO occurred in 5, 7, and 3 patients, respectively, and these patients were also excluded. Finally, 296 patients remained to be included in the final analysis, of whom 96 (32.4%) developed PPOI. Patient characteristics are summarized in Table 1, the mean age of all the patients was 64.6 years, and the majority of patients (n = 218, 73.6%) were men. All patients were followed up for 30 days after discharge and no one lost to the follow-up.
TABLE 1.
Characteristics of Patients, Operation, and Tumor
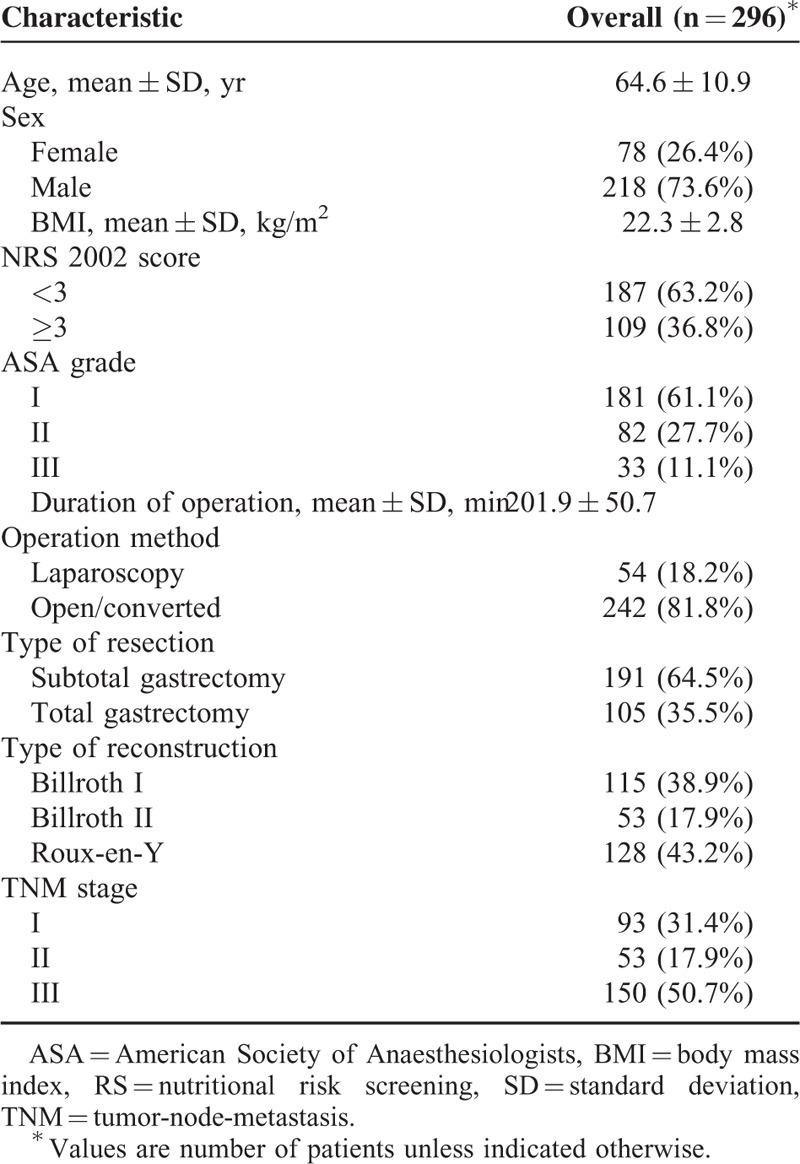
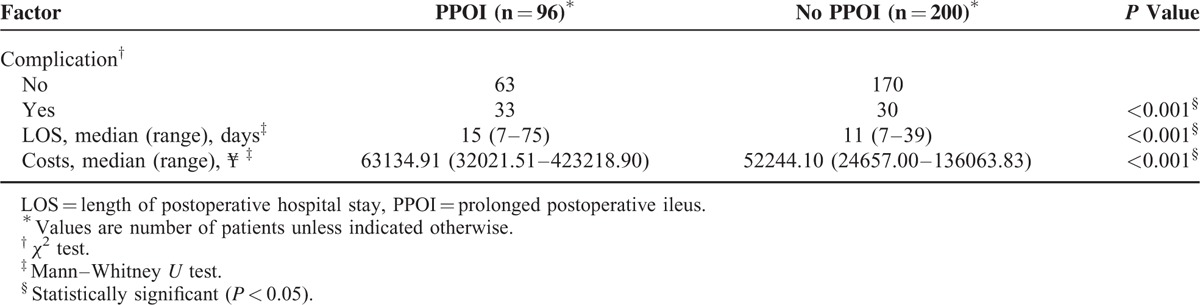
Table 2 shows that the patients with PPOI were more likely to suffer from postoperative complications than the patients without PPOI (P < 0.001). Moreover, patients with PPOI had a longer postoperative hospitalization stay (P < 0.001), as well as a significant more hospital costs than the patients without PPOI (P < 0.001).
TABLE 2.
Comparison of Short-Term Clinical Outcomes Between Groups With or Without Prolonged Postoperative Ileus
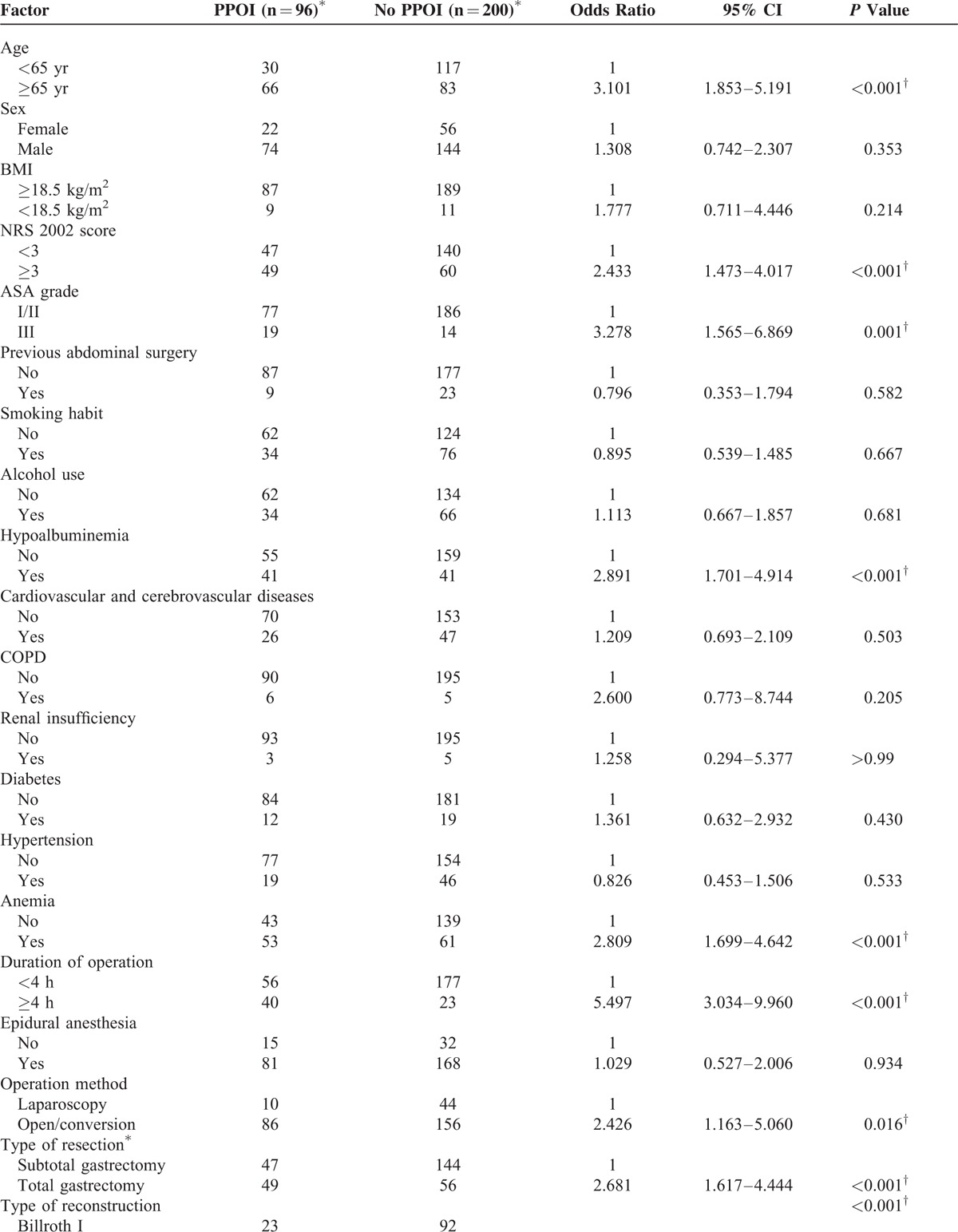
The results of the univariate analysis are presented in Table 3 in detail. As for preoperative factors, PPOI was associated with age ≥65 years (P < 0.001), preoperative anemia (P < 0.001), preoperative hypoalbuminemia (P < 0.001), ASA grade = III (P = 0.001), and NRS 2002 score ≥3 (P < 0.001). As for operative factors, duration of operation ≥4 hours (P < 0.001), open/converted operative technique (P = 0.016), total gastrectomy (P < 0.001), combined organ and tissue resection (P = 0.035), types of reconstruction (P < 0.001), and TNM stage = III (P < 0.001) were associated with PPOI. As for postoperative factors, TOD ≥0.3 mg/kg (P < 0.001) was associated with PPOI. The other factors were not significantly associated with PPOI, including sex; BMI, history of smoking, alcohol using, prior abdominal operation history, cardiovascular and cerebrovascular diseases; COPD; renal insufficiency; diabetes; hypertension, epidural anesthesia, blood leukocyte count and neutrophils count.
TABLE 3.
Univariate Analysis of Factors Associated With Prolonged Postoperative Ileus
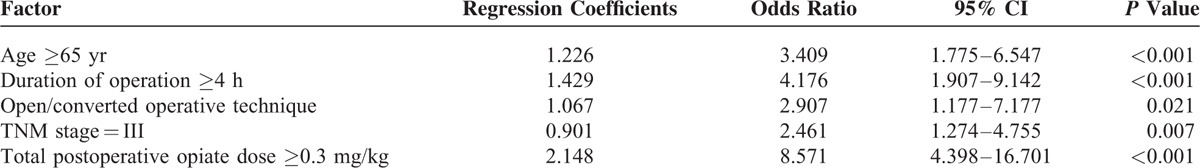
A multivariate forward logistic regression analysis was applied to identify independent risk factors for PPOI. Five variables were independently associated with PPOI, including age ≥65 years, operative duration ≥4 hours, TNM stage = III, open/converted operative technique, and TOD ≥0.3 mg/kg (Table 4). To illustrate the relationship between the independent risk factors and other variables, we further did several analyses. The results showed that elderly patients had a higher NRS 2002 score, a higher ASA score, and a higher prevalence of anemia and hypoalbuminemia (Table 5). Moreover, total gastrectomy cost a longer operative time compared with subtotal gastrectomy (P < 0.001).
TABLE 3 (Continued).
Univariate Analysis of Factors Associated With Prolonged Postoperative Ileus
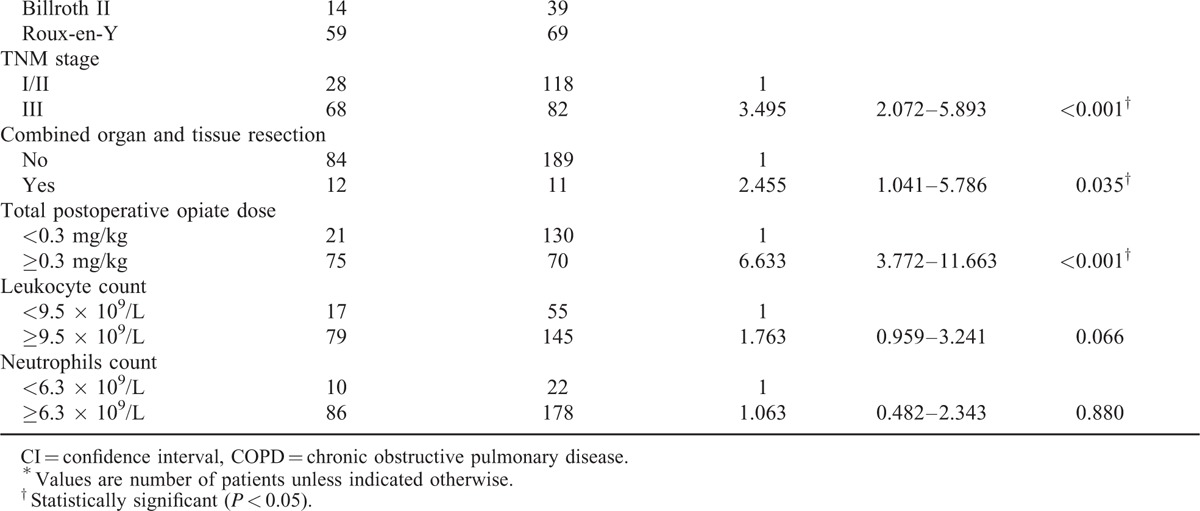
TABLE 4.
Multivariate Logistic Regression Analysis of Factors Associated With Prolonged Postoperative Ileus
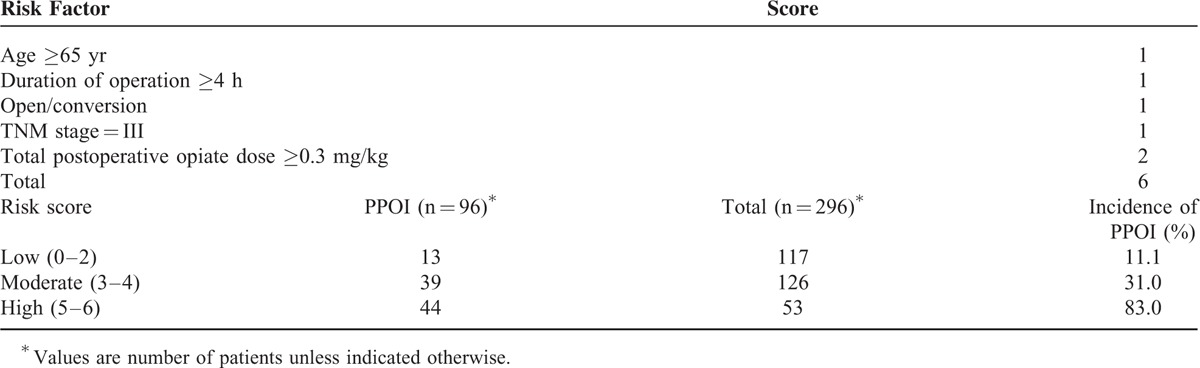
The risk stratification scoring system was constructed using the independent risk factors based on the results of logistic regression (Table 6). To simplify the scoring system for the facilitation of practical use, 1 point was assigned for age ≥65 years, operative duration ≥4 hours, TNM stage = III, and open/converted operative technique, despite the differences in the regression coefficients, which ranged from 0.901 to 1.429. Particularly, 2 points were assigned for TOD ≥0.3 mg/kg, which has a regression coefficient of 2.148. The scoring system was classified by low-risk (score 0–2), moderate-risk (score 3–4), and high-risk (score 5–6) groups. The incidence of PPOI was 11.1%, 31.0%, and 83.0% for the low-risk, moderate-risk, and high-risk groups, respectively (P < 0.001). A receiver operating characteristic (ROC) curve was generated and the area under the curve (AUC) was calculated to demonstrate the predictive power of the scoring system. The incidence of PPOI the AUC for the scoring system was 0.841 (95% CI, 0.793–0.890), indicating a good discrimination capability at predicting PPOI21 (Figure 1). The individual data points behind means, medians, and variance measures presented in the results are available in Supplemental Digital Content 1 (see Table, Supplemental Digital Content 1, which contains the raw data of this study).
TABLE 5.
Relationship Between Age and Other Factors
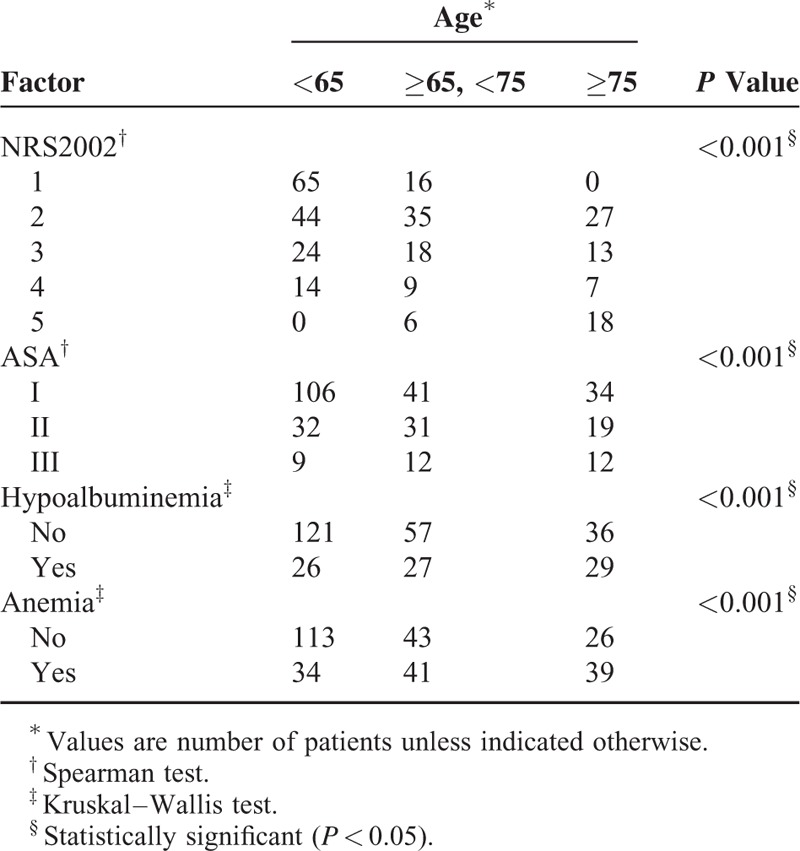
FIGURE 1.
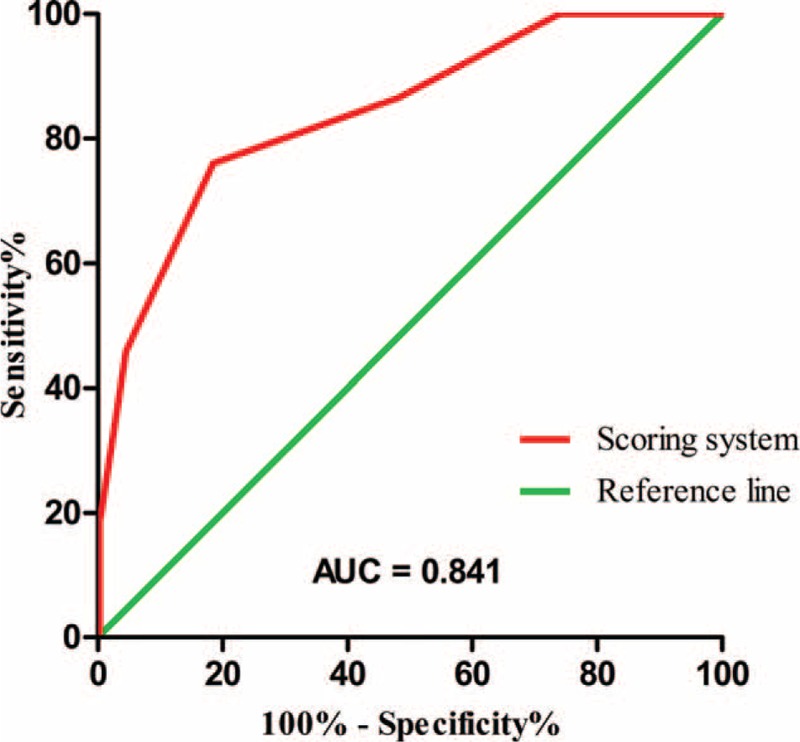
Receiver operating characteristic (ROC) curve for the scoring system to predict the occurrence of prolonged postoperative ileus (PPOI). The area under the curve (AUC) was 0.841 (95% CI, 0.793–0.890).
TABLE 6.
Scoring System for the Occurrence of Prolonged Postoperative Ileus
DISCUSSION
This prospective study is the first study that investigates the risk factors and generates a risk stratification scoring system for PPOI in gastric cancer surgery. Multivariate analysis showed that age ≥65 years, operative duration ≥4 hours, TNM stage = III, open/converted operative technique, and TOD ≥0.3 mg/kg were independent risk factors for PPOI (Table 4). Furthermore, a risk stratification scoring system was constructed using these factors to predict the occurrence of PPOI (Table 6).
The incidence of PPOI in patients undergoing radical gastrectomy is 32.4% in this study. Actually, the incidence rates of PPOI varied in the literature, depending on the surgery types or due to the different diagnostic criteria.3–5,22,23 The cut-off day of distinguishing between PPOI and normal POI varied from 3 days to 6 days in previous studies,3–5,22,23 because there had been a lack of an internationally accepted standardized clinical definition for PPOI.2 In this study, we selected POD 4 to be the most suitable cut-off point as suggested by Vather et al2 based on a systematic review and global survey.
Patients with EPSBO and gastroparesis were excluded in this study, both of which have a similar symptom as postoperative ileus. However, their pathophysiology and treatment are different from postoperative ileus.24,25
The present study confirmed the results of previous studies1,5,26 that PPOI lead to a higher incidence of postoperative complications, a longer length of postoperative hospital stay and more hospital costs (Table 2).
Advanced age (age ≥65 years) was identified as an independent risk factor for PPOI in this study (Table 4), consistent with the finding of several previous studies.22,27 A previous mechanism research has demonstrated that imbalances between pro- and anti-inflammatory mechanisms may be the underlying pathophysiology for the increased susceptibility to POI and the increased severity and duration of POI observed in the elderly.28 Moreover, elderly patients generally have a decreased nutritional and functional condition, as reflected by a higher NRS 2002 score, a higher ASA score, and a higher prevalence of anemia and hypoalbuminemia in our study (Table 5). Preoperative hypoalbuminemia and comorbidities were reported to be independent risk factors for PPOI in several previous studies,4,22,23 indicating that the decreased nutritional and functional status may play a role in the development of PPOI. In our study, these factors were associated with PPOI in the univariate analysis (Table 3 ), but when they were included in the multivariate analysis, these associations became not significant, which can be explained by their connections with advanced age (Table 5). This result suggested that advanced age can reflect a more comprehensive body functional and nutritional status, serving as an independent risk factor for PPOI.
A duration of operation over 3 hours was associated with PPOI in colorectal cancer surgery as reported by Chapuis et al.4 In the present study, we found that a duration of operation over 4 hours was an independent risk factor for PPOI (Table 4). A longer operative duration is always associated with a higher operative difficulty and a more intense handling of the bowel, both of which have been identified to be independent predictors for PPOI in colorectal cancer surgery.23 Moreover, a longer operative duration always accompanies with a greater degree of surgical trauma and tissue damage. A previous mechanism research has demonstrated that the release of tissue damage mediators and proinflammatory cytokines into the systemic circulation is the most possibly pathogenesis for the impaired motility of the intestine.29
An important difference between gastrectomy and other abdominal surgery is the management of vagus nerve. Vagectomy was executed in total gastrectomy, whereas celiac branch of vagus nerve was preserved in subtotal gastrectomy in the practice of our department. It has been demonstrated that the preservation of the celiac branch of the vagus nerve results in a faster recovery of gastrointestinal motility in an animal model.12 This finding was consistent with our result, which showed a higher incidence of PPOI after total gastrectomy than after subtotal gastrectomy (Table 3 ). However, total gastrectomy was not identified to be an independent risk factor, possible due to its connection with a longer operative duration (P < 0.001). A longer operative duration can influence PPOI from several aspects and was independently associated with PPOI (Table 4). Therefore, the effect of total gastrectomy may be offset by the inclusion of operative duration in the multivariate analysis.
Advanced tumor stage (TNM = III) was identified to be independently associated with PPOI after gastrectomy in this study (Table 4). This finding may be probably due to the fact that advanced tumor stage was associated with an elevated systemic and surgical site inflammatory response,30,31 which may result in a more pronounced POI.32–34
Opiates have been widely reported to be independently associated with POI after colorectal surgery.1,22 This conclusion was also confirmed in gastric cancer surgery by our study. It has been demonstrated that the inhibitory effect of opiates on postoperative gastrointestinal motility was mediated by peripheral μ-opioid receptors.35 Postoperative opiates dose is one of the most important modifiable risk factors for POI. Therefore, various measures should be adopted to reduce the usage of opiates, including using nonsteroidal anti-inflammatory drugs as alternatives to opiate analgesics and using thoracic epidural analgesia.
In the present study, we identified the open/converted operative technique as an independent risk factor for PPOI (Table 4), which was consistent with the finding of a previous study in colorectal surgery.23 Thus, laparoscopic surgery is a protective factor for PPOI. A previous meta-analysis of randomized controlled trials has demonstrated that laparoscopic surgery leads to a faster recovery of gastrointestinal function after gastric cancer surgery.36 Laparoscopic surgery is less traumatic compared with the open surgery, which may lead to a faster recovery of postoperative bowel function.32,33 Moreover, the level of systemic inflammation was lower after laparoscopic surgery than after open surgery, as measured by circulating levels of cytokines (interleukin-1 beta and interleukin-6) and C-reactive protein.37 Thus, the decreased inflammatory response of laparoscopic surgery may also contribute to its advantage on the postoperative gastrointestinal function. Recent studies have tended to conclude that the long-term survival outcome of laparoscopic surgery is comparable to that of open surgery in gastric cancer patients, even in patients with advanced tumor stage.38,39 Therefore, laparoscopic gastrectomy should be recommended in patients with gastric cancer to reduce POI.
To make the plan of this prospective study, we had performed preliminary analysis to identify suitable factors to be included in the final study. Estimated blood loss (EBL) had been included in the preliminary analysis. However, our preliminary analysis did not show a relationship between EBL and PPOI. Considering the imprecise estimation of blood loss, we ultimately decided not to include the data in the final analysis. Blood transfusion should have been included as an alternative to EBL since it reflects the intraoperative blood loss. Regrettably, we did not include blood transfusion in the final analysis and this is a limitation of this study. Despite this, we could retrieve the data of EBL and blood transfusion from our prospectively maintained database. Retrospective univariate and multivariate analyses including EBL and blood transfusion showed no connections between these 2 factors and PPOI. Including the 2 factors into analysis did not change the results of this study (see Tables, Supplemental Digital Content 2, which show the results of univariate and multivariate analyses after including the EBL and blood transfusion). Increased blood loss can potentially result in a greater traumatic sympathetic and endocrine stress response, which may in turn impair gastrointestinal motility.1 In addition, blood loss may also serve as an indicator of bowel injury or an elevated inflammatory response, both of which are closely related to PPOI.1 However, the association of EBL and/or blood transfusion with PPOI has not been well established.22,23 More high-quality studies are needed to obtain a valid conclusion regarding this issue in the future.
Enhanced Recovery after Surgery (ERAS) principles have been proposed since the mid-1990s, aiming to attenuate the surgical stress response, reduce complications, and shorten hospital stays.11 Currently, ERAS programs have been widely implemented in colorectal surgery, especially in the Western countries.11 However, in the field of gastrectomy, the development of ERAS programs is far behind that in the field of colorectal surgery. ERAS programs in gastrectomy surgery were first reported in the literature in 2010,40 and the international working group within the Enhanced Recovery After Surgery Society first published the consensus guidelines for enhanced recovery after gastrectomy in 2014.10 According to the consensus guidelines, the ERAS programs for gastrectomy contain 25 items, including epidural analgesia, no use of nasogastric tubes, early oral feeding, early mobilization, etc.10 Postoperative hospital stays after gastrectomy could be reduced to 5 to 7 days in the context of ERAS programs.41 However, not all patients are suitable for the ERAS programs. For example, early oral feeding and no use of nasogastric tubes would cause abdominal distention, nausea, and vomiting in patients with PPOI. Therefore, these ERAS items may not be suitable for patients with PPOI. The present study provided information for the prediction of PPOI and may help in identifying patients who may not be suitable for some ERAS items, which may contribute to the optimization of ERAS programs based on the characteristic of individual patients.
Regrettably, this study was not conducted in the context of ERAS programs since ERAS programs are still not well implemented in the field of gastrectomy.40 However, the absence of ERAS programs probably makes the results of this study more suitable to be generalized to the many centers where ERAS programs are still not well implemented, especially in China. Despite this, the ERAS programs are actually being implemented in more and more hospitals and have been proven to accelerate the recovery of the gastrointestinal function and shorten the postoperative hospital stays after gastrectomy for gastric cancer.41 Therefore, further studies are necessary to identify the incidence and risk factors for PPOI after radical gastrectomy in the context of ERAS programs.
There are several other limitations in this study. First, our current study is a single-center study. However, we optimized the study design to minimize possible bias. Second, there was a lack of robust external validation of the scoring system. Therefore, whether the proposed scoring system will retain its predictive capability in an independent dataset is yet to be determined. A prospective multiple-center study is required to provide evidence for the validation of the scoring system in the future.
Two previous studies have proposed scoring systems for PPOI in colorectal cancer surgery. One study included 3 variables in the scoring system but did not construct a ROC curve to show its the predictive power.22 Another study did not identify opiates consumption as a predictive factor.23 However, the impact of opiates on the gastrointestinal function has been well elucidated,1,32,35 probably making that study underpowered.
The present study was strengthened by the prospective study design, the strict inclusion and exclusion criteria, and a standard surgical and perioperative management protocol. The scoring system proposed by this study demonstrated a good predictive capability, with an AUC of 0.841 (95% CI, 0.793–0.890), higher than the 0.742 (95% CI, 0.684–0.799) that was previously reported,23 indicating the relative high quality of this study.
In conclusion, the incidence of PPOI was 32.4% in the present study. PPOI played a crucial role in the process of recovery after radical gastrectomy for gastric cancer. A risk stratification scoring system was constructed using the results of multivariate analysis for the prediction of PPOI. This scoring system demonstrated a good predictive capability on the basis of the result of ROC analysis. A prospective multiple-center study is required to provide evidence for the validation of the scoring system in the future.
Supplementary Material
Supplementary Material
Acknowledgment
This work was supported by the National Natural Science Foundation of China (No. 81171857) and the clinical nutriology of medical supporting discipline of Zhejiang Province (11-ZC24).
Footnotes
Abbreviations: ASA = American Society of Anesthesiologists, AUC = area under the curve, BMI = body mass index, COPD = chronic obstructive pulmonary disease, CT = computed tomography, EPSBO = early postoperative small-bowel obstruction, LOS = length of postoperative hospital stay, NRS = nutritional risk screening, POD = postoperative day, POI = postoperative ileus, PPOI = prolonged postoperative ileus, ROC = receiver operating characteristic, SD = standard deviation, TNM = tumor–node–metastasis, TOD = total postoperative opiate dose, UICC = International Union Against Cancer.
D-DH and C-LZ contributed equally to this work.
This work was supported by the National Natural Science Foundation of China (No. 81171857) and the clinical nutriology of medical supporting discipline of Zhejiang Province (11-ZC24).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Artinyan A, Nunoo-Mensah JW, Balasubramaniam S, et al. Prolonged postoperative ileus-definition, risk factors, and predictors after surgery. World J Surg 2008; 32:1495–1500. [DOI] [PubMed] [Google Scholar]
- 2.Vather R, Trivedi S, Bissett I. Defining postoperative ileus: results of a systematic review and global survey. J Gastrointest Surg 2013; 17:962–972. [DOI] [PubMed] [Google Scholar]
- 3.Millan M, Biondo S, Fraccalvieri D, et al. Risk factors for prolonged postoperative ileus after colorectal cancer surgery. World J Surg 2012; 36:179–185. [DOI] [PubMed] [Google Scholar]
- 4.Chapuis PH, Bokey L, Keshava A, et al. Risk factors for prolonged ileus after resection of colorectal cancer: an observational study of 2400 consecutive patients. Ann Surg 2013; 257:909–915. [DOI] [PubMed] [Google Scholar]
- 5.Iyer S, Saunders WB, Stemkowski S. Economic burden of postoperative ileus associated with colectomy in the United States. J Manag Care Pharm 2009; 15:485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65:87–108. [DOI] [PubMed] [Google Scholar]
- 7.Korenaga D, Okamura T, Baba H, et al. Results of resection of gastric cancer extending to adjacent organs. Br J Surg 1988; 75:12–15. [DOI] [PubMed] [Google Scholar]
- 8.Martin 2nd RC, Jaques DP, Brennan MF, Karpeh M. Achieving RO resection for locally advanced gastric cancer: is it worth the risk of multiorgan resection? J Am Coll Surg 2002; 194:568–577. [DOI] [PubMed] [Google Scholar]
- 9.Liedman B. Symptoms after total gastrectomy on food intake, body composition, bone metabolism, and quality of life in gastric cancer patients: is reconstruction with a reservoir worthwhile? Nutrition 1999; 15:677–682. [DOI] [PubMed] [Google Scholar]
- 10.Mortensen K, Nilsson M, Slim K, et al. Consensus guidelines for enhanced recovery after gastrectomy: Enhanced Recovery After Surgery (ERAS(R)) Society recommendations. Br J Surg 2014; 101:1209–1229. [DOI] [PubMed] [Google Scholar]
- 11.Kehlet H. Fast-track colorectal surgery. Lancet 2008; 371:791–793. [DOI] [PubMed] [Google Scholar]
- 12.Ando H, Mochiki E, Ohno T, et al. Effect of distal subtotal gastrectomy with preservation of the celiac branch of the vagus nerve to gastrointestinal function: an experimental study in conscious dogs. An Surg 2008; 247:976–986. [DOI] [PubMed] [Google Scholar]
- 13.Marquez MF, Ayza MF, Lozano RB, et al. Gastric leak after laparoscopic sleeve gastrectomy. Obes Surg 2010; 20:1306–1311. [DOI] [PubMed] [Google Scholar]
- 14.Ellozy SH, Harris MT, Bauer JJ, et al. Early postoperative small-bowel obstruction: a prospective evaluation in 242 consecutive abdominal operations. Dis Colon Rectum 2002; 45:1214–1217. [DOI] [PubMed] [Google Scholar]
- 15.Tack J, Carbone F, Rotondo A. Gastroparesis. Curr Opin Gastroenterol 2015; 31:499–505. [DOI] [PubMed] [Google Scholar]
- 16.Malagelada JR, Rees WD, Mazzotta LJ, et al. Gastric motor abnormalities in diabetic and postvagotomy gastroparesis: effect of metoclopramide and bethanechol. Gastroenterology 1980; 78:286–293. [PubMed] [Google Scholar]
- 17.Japanese Gastric Cancer A. Japanese gastric cancer treatment guidelines 2010 (ver 3). Gastric Cancer 2011; 14:113–123. [DOI] [PubMed] [Google Scholar]
- 18.Sobin LH, Gospodarowicz MK, Wittekind C. International Union Against Cancer (UICC) TNM Classification of Malignant Tumors. 7th edNew York: Wiley-Liss; 2010. [Google Scholar]
- 19.Foley KM. The treatment of cancer pain. N Engl J Med 1985; 313:84–95. [DOI] [PubMed] [Google Scholar]
- 20.Kondrup J, Rasmussen HH, Hamberg O, et al. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr 2003; 22:321–336. [DOI] [PubMed] [Google Scholar]
- 21.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982; 143:29–36. [DOI] [PubMed] [Google Scholar]
- 22.Kronberg U, Kiran RP, Soliman MS, et al. A characterization of factors determining postoperative ileus after laparoscopic colectomy enables the generation of a novel predictive score. Ann Surg 2011; 253:78–81. [DOI] [PubMed] [Google Scholar]
- 23.Vather R, Josephson R, Jaung R, et al. Development of a risk stratification system for the occurrence of prolonged postoperative ileus after colorectal surgery: a prospective risk factor analysis. Surgery 2015; 157:764–773. [DOI] [PubMed] [Google Scholar]
- 24.Sajja SB, Schein M. Early postoperative small bowel obstruction. Br J Surg 2004; 91:683–691. [DOI] [PubMed] [Google Scholar]
- 25.Pasricha PJ. Future directions in the treatment of gastroparesis. Gastroenterol Clin North Am 2015; 44:185–189. [DOI] [PubMed] [Google Scholar]
- 26.Senagore AJ. Pathogenesis and clinical and economic consequences of postoperative ileus. Am J Health Syst Pharm 2007; 64 (20 Suppl 13):S3–7. [DOI] [PubMed] [Google Scholar]
- 27.Svatek RS, Fisher MB, Williams MB, et al. Age and body mass index are independent risk factors for the development of postoperative paralytic ileus after radical cystectomy. Urology 2010; 76:1419–1424. [DOI] [PubMed] [Google Scholar]
- 28.Moore BA, Albers KM, Davis BM, et al. Altered inflammatory gene expression underlies increased susceptibility to murine postoperative ileus with advancing age. Am J Physiol Gastrointest Liver Physiol 2007; 292:G1650–1659. [DOI] [PubMed] [Google Scholar]
- 29.van Bree SH, Cailotto C, Di Giovangiulio M, et al. Systemic inflammation with enhanced brain activation contributes to more severe delay in postoperative ileus. Neurogastroenterol Motil 2013; 25:e540–e549. [DOI] [PubMed] [Google Scholar]
- 30.Kersten C, Louhimo J, Algars A, et al. Increased C-reactive protein implies a poorer stage-specific prognosis in colon cancer. Acta Oncol 2013; 52:1691–1698. [DOI] [PubMed] [Google Scholar]
- 31.Kuman NK, Yaman E, Karul A, et al. Bronchial cytokine level changes in lung cancer operations. Turk Gogus Kalp Damar Cerrahisi Dergisi 2014; 22:382–388. [Google Scholar]
- 32.Baig MK, Wexner SD. Postoperative ileus: a review. Dis Colon Rectum 2004; 47:516–526. [DOI] [PubMed] [Google Scholar]
- 33.Wehner S, Vilz TO, Stoffels B, et al. Immune mediators of postoperative ileus. Langenbecks Arch Surg 2012; 397:591–601. [DOI] [PubMed] [Google Scholar]
- 34.Boeckxstaens GE, de Jonge WJ. Neuroimmune mechanisms in postoperative ileus. Gut 2009; 58:1300–1311. [DOI] [PubMed] [Google Scholar]
- 35.Kaufman PN, Krevsky B, Malmud LS, et al. Role of opiate receptors in the regulation of colonic transit. Gastroenterology 1988; 94:1351–1356. [DOI] [PubMed] [Google Scholar]
- 36.Jiang L, Yang KH, Guan QL, et al. Laparoscopy-assisted gastrectomy versus open gastrectomy for resectable gastric cancer: an update meta-analysis based on randomized controlled trials. Surg Endosc 2013; 27:2466–2480. [DOI] [PubMed] [Google Scholar]
- 37.Leung KL, Lai PB, Ho RL, et al. Systemic cytokine response after laparoscopic-assisted resection of rectosigmoid carcinoma: a prospective randomized trial. Ann Surg 2000; 231:506–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen XZ, Wen L, Rui YY, et al. Long-term survival outcomes of laparoscopic versus open gastrectomy for gastric cancer: a systematic review and meta-analysis. Medicine (Baltimore) 2015; 94:e454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim HH, Han SU, Kim MC, et al. Long-term results of laparoscopic gastrectomy for gastric cancer: a large-scale case-control and case-matched Korean multicenter study. J Clin Oncol 2014; 32:627–633. [DOI] [PubMed] [Google Scholar]
- 40.Liu XX, Jiang ZW, Wang ZM, et al. Multimodal optimization of surgical care shows beneficial outcome in gastrectomy surgery. JPEN J Parenter Enteral Nutr 2010; 34:313–321. [DOI] [PubMed] [Google Scholar]
- 41.Yu Z, Zhuang CL, Ye XZ, et al. Fast-track surgery in gastrectomy for gastric cancer: a systematic review and meta-analysis. Langenbecks Arch Surg 2014; 399:85–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


