Abstract
The association between vitamin D receptor (VDR) FokI polymorphism and tuberculosis (TB) risk remains a matter of debate. Potential selection bias exists in most studies using HIV-positive TB patients.
An update meta-analysis was carried out to derive a more reliable assessment of the association between FokI polymorphisms and TB risk, especially in HIV-negative TB patients. All major databases from inception to June 2015 were searched for all publications that studied the association between FokI polymorphism and TB risk. The odds ratios (ORs) and the corresponding 95% confidence intervals (CIs) were calculated according to the frequencies of genotypes.
In total, 32 studies with 4894 cases and 5319 controls were included in this meta-analysis. In the overall analysis, the estimated OR was 1.34 (95% CI=1.091–1.646, P = 0.005) in the best genetic model (recessive model, ff vs fF+FF) with moderate heterogeneity (I2 = 32.2%, P = 0.043). In the subgroup analysis stratified by HIV status, significant associations were found only in the HIV-negative TB group (OR = 1.60, 95% CI = 1.180–2.077, P = 0.002; I2 = 29.5%, and P = 0.141 for heterogeneity). In the subgroup analysis stratified by ethnicity, significant associations were found in the Asian group (OR = 1.65, 95% CI = 1.205–2.261, P = 0.002; I2 = 43.9%, and P = 0.024 for heterogeneity), but not in the Caucasian group (OR = 1.09, 95% CI = 0.762–1.547, P = 0.649; I2 = 0.0%, and P = 0.740 for heterogeneity) and African group (OR = 0.99, 95% CI = 0.726–1.341, P = 0.934; I2 = 43.9%, and P = 0.024 for heterogeneity).
This meta-analysis confirms that VDR FokI polymorphism contributes to the risk of TB, especially in HIV-negative TB patients and in the Asian group. Further studies are required to clarify the role of the FokI polymorphism in HIV-positive TB and in other ethnic groups.
INTRODUCTION
Tuberculosis (TB) is a global public health problem and remains a great burden throughout the world.1 The risk of developing TB ranges from 5% to 10% after infection by Mycobacterium tuberculosis (MTB) for individuals, and only a minority of individuals develops clinical disease, even though infected with virulent mycobacteria. Other factors, such as environmental and genetic factors, HIV infection, and diabetes, also play important roles in the process.2–5 Likewise, genetic factors are important in determining susceptibility and resistance to MTB and are considered related to the susceptibility to TB.5,6
Vitamin D is now considered to be a key factor in the body's defense against TB, mediated by binding to the vitamin D receptor (VDR) in monocytes, macrophages, and lymphocytes.7,8 The VDR gene is located in the chromosomal 12q13 region, and there are 4 classically typed single-nucleotide polymorphisms (SNPs), FokI, BsmI, ApaI and TaqI, which were studied intensively for association with various human traits and were reported to affect risk of variousdiseases.9 The FokI restriction site defines an SNP (rs10735810, C to T) in the first of 2 potential translations—initiation start sites for VDR mRNA. The VDR protein synthesizes full-length (427 amino acids) in the alternate allele form (ATG) (designated f) and has 3 more amino acids than the VDR encoded by the common allele form (ACG) (designated F). The FokI restriction site is a functional polymorphism of the VDR gene.10 The polymorphisms of FokI can alter the amount of VDR produced 9,11 and are related to plasma vitamin D levels in TB patients.12
To date, the polymorphisms of FokI have been studied in relation to the risk of TB in many populations; however, the results remained contradictory.10,13–15 Recently, Chen et al16 and Sun and Cai17 carried out meta-analyses focusing on the associations between FokI polymorphisms and TB risk; these 2 meta-analyses missed many studies.12,18–23 Moreover, HIV infection status should be adjusted in studies focused on genetic susceptibility to TB since TB is the frequent major opportunistic infection in HIV-infected patients.24 Thus, we carried out an update meta-analysis to derive a more reliable assessment on the association between FokI polymorphisms and TB risk, especially in HIV-negative TB patients.
METHODS
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement was used in the process of the meta-analysis (Table S1).25
Search Strategy and Study Selection
A search of the medical literature was conducted using the Embase, PubMed, and Cochrane Library databases through June 30, 2015. The search terms were used as follows: vitamin D receptor or VDR in combination with polymorphism, polymorphisms, and mutation or variant in combination with tuberculosis or TB. Two investigators (LH and XY) conducted an extensive literature search independently for all publications. Articles in reference lists were also hand-searched and authors of trial reports published only as abstracts were contacted and asked to contribute full datasets or completed papers. There were no language restrictions and only human studies were searched.
Case-control studies with enough data to calculate odds ratio (OR) were included in our study. We excluded duplicate studies or studies containing overlapping data. Family-based studies were also excluded.
Data Extraction
All data were extracted independently by 2 investigators (LH and XY). The following clinical data were extracted from eligible studies: the baseline characteristics, such as the first author's name, publication year, country, ethnicity, total sample size, genotyping method, and source of control group, and details of TB types and genotype frequencies of cases and controls. Hardy-Weinberg equilibrium (HWE) was calculated from genotype frequencies of controls. Investigators would try to contact the author to get the original data if the literature could not provide sufficient data. A third reviewer (JC) resolved any discrepancies when the abovementioned reviewers disagreed.
Statistical Analysis
In this study, we considered f is the increasing or risk allele; therefore, an allelic model (f vs F), a codominant model (ff vs FF, fF vs FF), a dominant model (ff+fF vs FF), and a recessive model (ff vs fF+FF) are accessed by calculating the unadjusted odds ratios (ORs) and the corresponding 95% confidence intervals (CIs) according to the frequencies of genotypes. To avoid the problem of multiple comparisons, we applied the method for meta-analysis of molecular association studies to dictate the best genetic model.26
Heterogeneity was assessed with a χ2Q test and I2 statistics. The heterogeneity was significant if PQ < 0.1 or I2 > 50%, and a random-effects model was conducted using the DerSimonian and Laird method. Otherwise, the fixed-effects model (the Mantel-Haenszel method) was performed.27,28 A subgroup analysis of ethnicity was carried out considering that the same gene polymorphism plays different roles in the risk of diseases among different ethnic subpopulations. HIV-negative TB patients who were studied were also considered a subgroup and pooled in this meta-analysis. Galbraith plots analysis was performed for further exploration of the heterogeneity.
HWE in the controls was tested with the χ2 test for goodness of fit, and a P value <0.05 was considered out of HWE. Sensitivity analysis was conducted to examine such influence by removing studies one by one and by recalculating the pooled OR and 95% CI. The Begg rank correlation method and the Egger weighted regression method were used to statistically assess publication bias.
Ethical approval was not necessary, as this study is a meta-analysis, which is based on the published data.
All the tests in this meta-analysis were conducted with STATA software (version 12.0; Stata Corporation, College Station, TX); P <0.05 indicated that the result was statistically significant.
RESULTS
Study Excluded and Characteristics of Included Studies
Thirty-eight articles were initially evaluated for the meta-analysis, of which 8 studies were excluded. Two studies were excluded because, even though an attempt was made to contact the study authors, no sufficient data were obtained.29,30 Four studies were excluded for not focusing on FokI polymorphism.31–34 In addition, a meeting abstract 35 and a study about nontuberculous mycobacterial lung disease 36 were also excluded. The study by Alagarasu et al13 was separated into 3 studies for different TB types and HIV status. Finally, 32 studies with 4894 cases and 5319 controls met inclusion criteria. Details of the study flow are documented in Figure 1.
FIGURE 1.
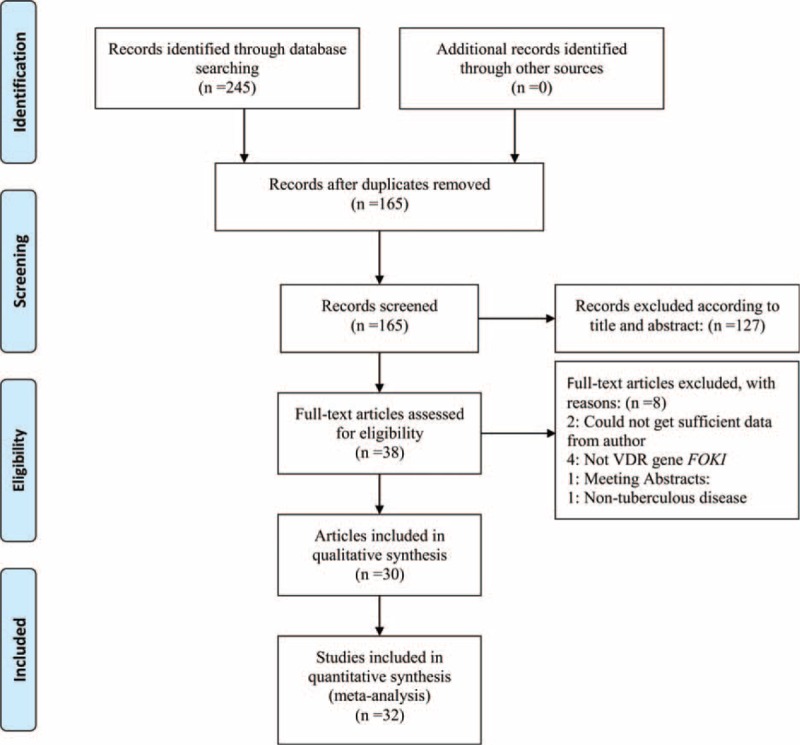
Flow diagram of included studies for this meta-analysis.
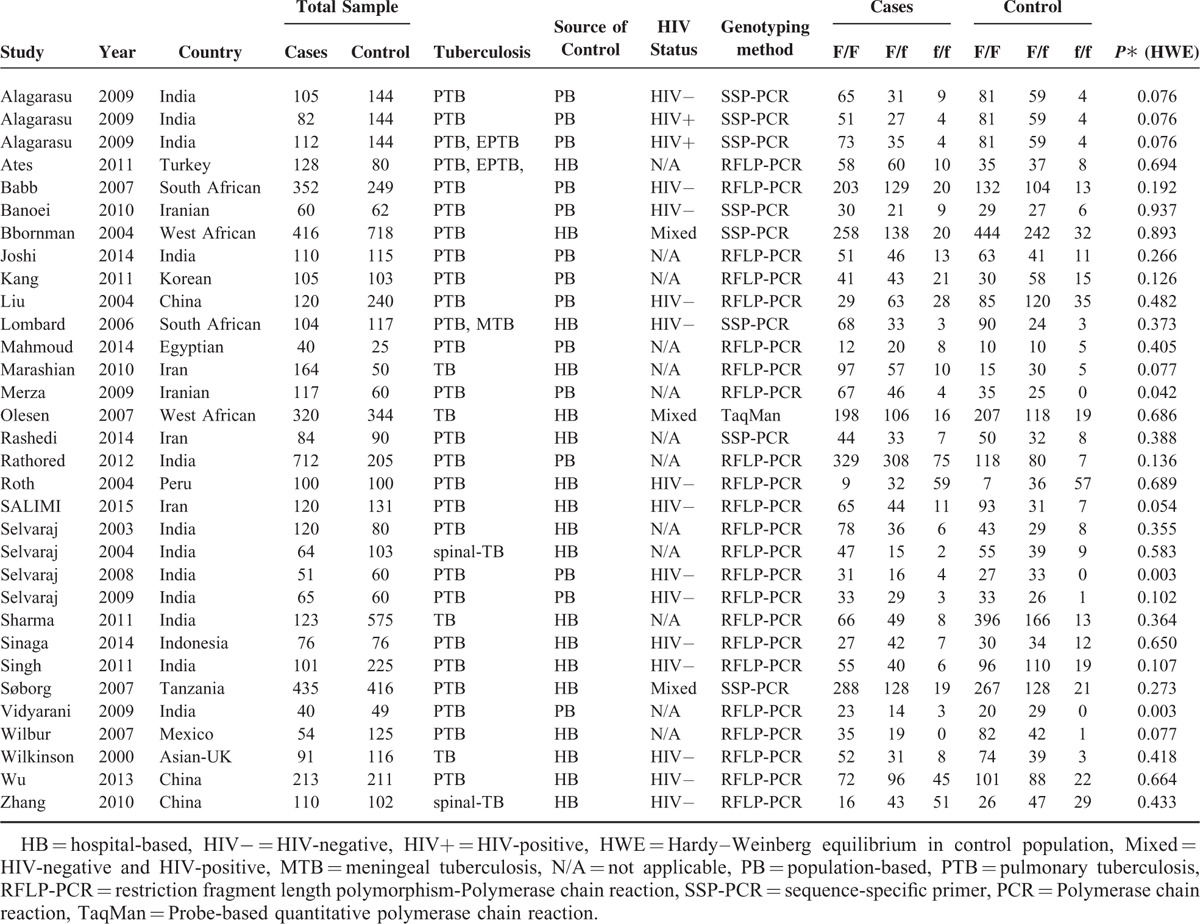
Table 2 shows a summary of the characteristics of the included studies. There were 18 studies involving Asians,13–15,19,21–23,37–45 8 studies involving Caucasians,12,18,43,46–50 and 6 studies involving Africans.20,51–55 Fourteen studies included HIV-negative TB patients,10,13–15,19,22,37,39,45,47,50,51,53,56 but only the study by Alagarasu et al13 included HIV-positive TB patients, and the other 16 studies did not offer detailed information. The genotype distributions among the controls of all studies were consistent with HWE, with the exception of 3 studies.39,44,49 TB types, genotyping methods, and genotype numbers are shown in Table 2.
TABLE 2.
Study Characteristics
Quantitative Data Synthesis
The evaluations of the association of FokI polymorphisms and TB risk are shown in Table 1. According to the method for dictating the best genetic model,26 the estimated OR1(ff vs FF), OR2(fF vs FF), and OR3(ff vs fF) were 1.34 (95% CI = 1.036–1.730), 0.96 (95% CI = 0.827–1.110), and 1.34 (95% CI = 1.122–1.599). These indicated that OR1 and OR3 were significant (P < 0.05) and OR2 was not significant (P = 0.566); the genetic model was most likely recessive.
TABLE 1.
Meta-Analysis of FokI Polymorphism and TB Risk
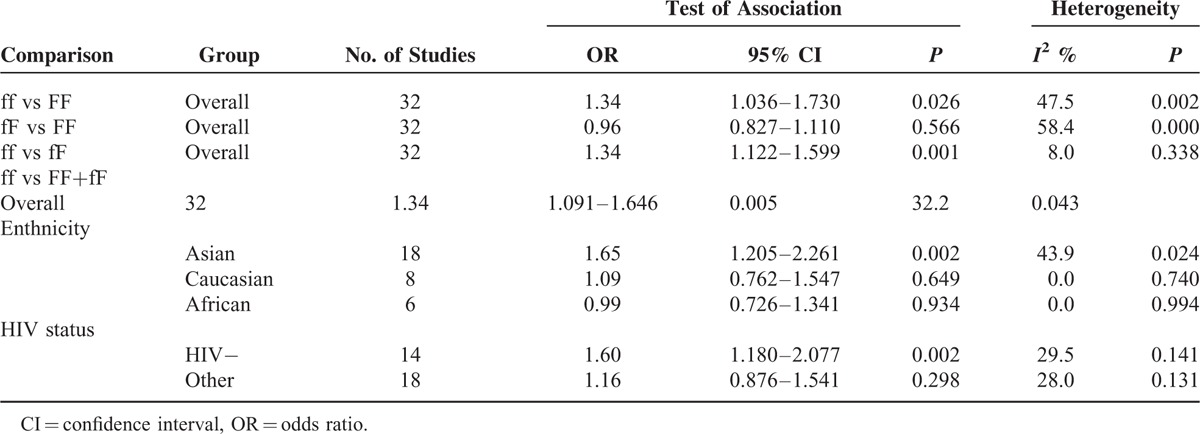
Using a recessive model, data for the fF and FF group were collapsed and compared to the ff group (ff vs fF+FF). The estimated OR was 1.34 (95% CI = 1.091–1.646, P = 0.005). There was moderate heterogeneity in the pooled results (I2 = 32.2%, P = 0.043). Therefore, we performed subgroup analysis according to ethnicity and HIV status. In the subgroup analysis by ethnicity (Fig. 2 and Table 1), significant associations were found in the Asian group (OR = 1.65, 95% CI = 1.205–2.261, P = 0.002; I2 = 43.9%, and P = 0.024 for heterogeneity), but not in the Caucasian group (OR = 1.09, 95% CI = 0.762–1.547, P = 0.649; I2 = 0.0%, and P = 0.740 for heterogeneity), and the African group (OR = 0.99, 95% CI = 0.726–1.341, P = 0.934; I2 = 43.9%, and P = 0.024 for heterogeneity). The HIV status was stratified as the HIV-negative TB group and the other group (HIV-positive or no information). As shown in Figure 3 and Table 1, significant associations were found in the HIV-negative TB group (OR = 1.60, 95% CI = 1.180–2.077, P = 0.002; I2 = 29.5%, and P = 0.141 for heterogeneity). To further explore the sources of heterogeneity, we carried out a Galbraith plot analysis to confirm the outliers that might cause the heterogeneity (Fig. 4). The results showed that Rathored et al38 and Wu et al22 were the outlier studies. Therefore, we excluded these 2 studies and reran the meta-analysis; the heterogeneity decreased significantly in the recessive model, but the pooled results were not changed significantly (OR = 1.24, 95% CI = 1.016–1.509, P = 0.034; I2 = 19.7%, and P = 0.170 for heterogeneity).
FIGURE 2.
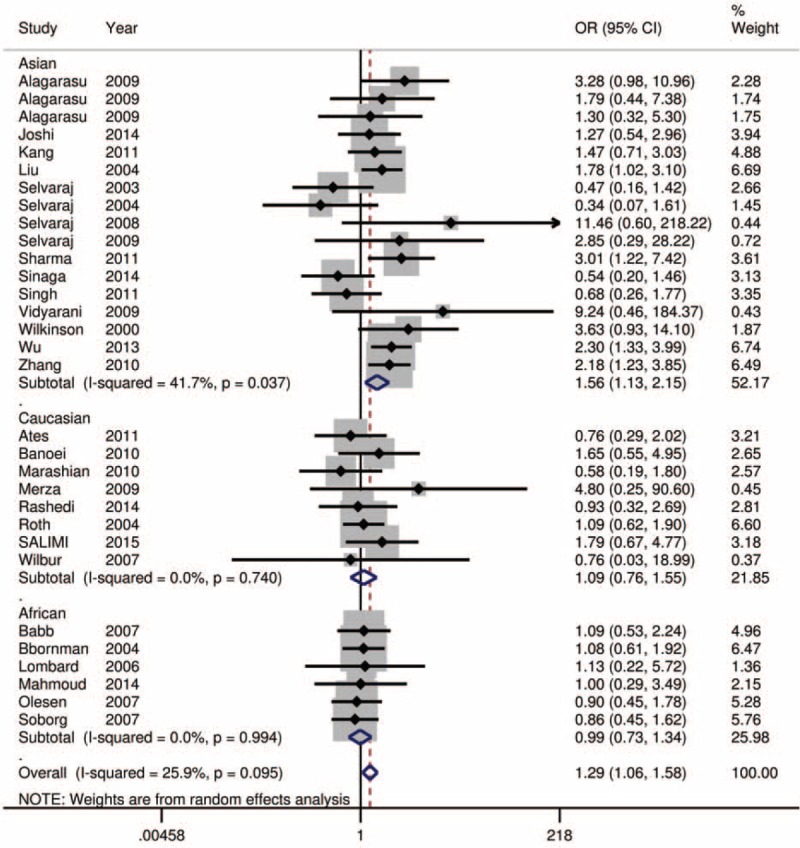
Forest plot for the association between FokI polymorphisms and TB risk stratified by ethnicity in recessive model (ff vs fF+FF).
FIGURE 3.
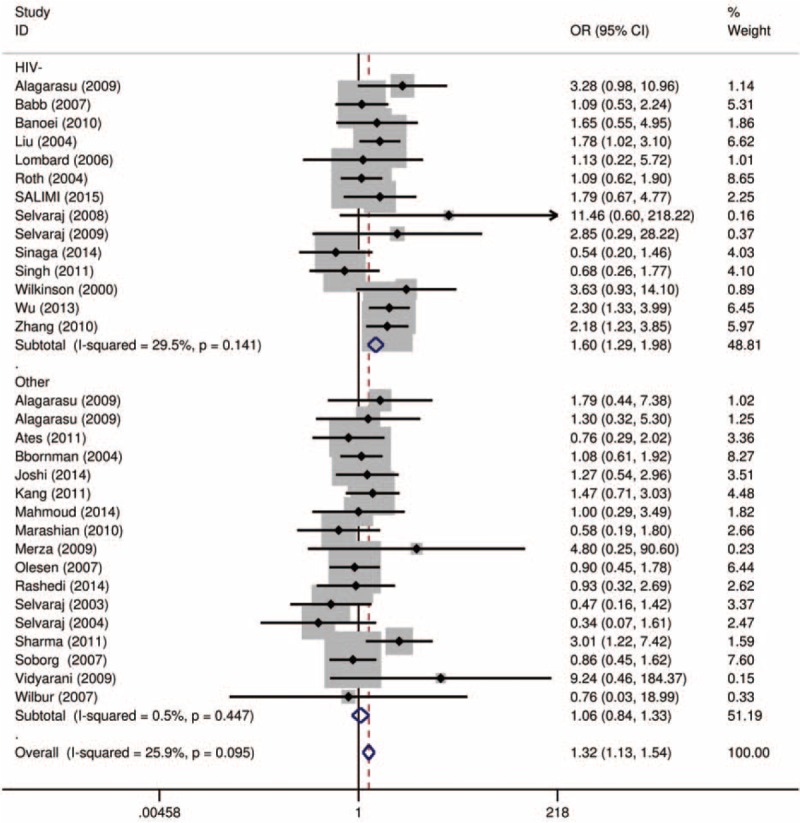
Forest plot for the association between FokI polymorphisms and TB riskstratified by HIV status in recessive model (ff vs fF+FF).
FIGURE 4.
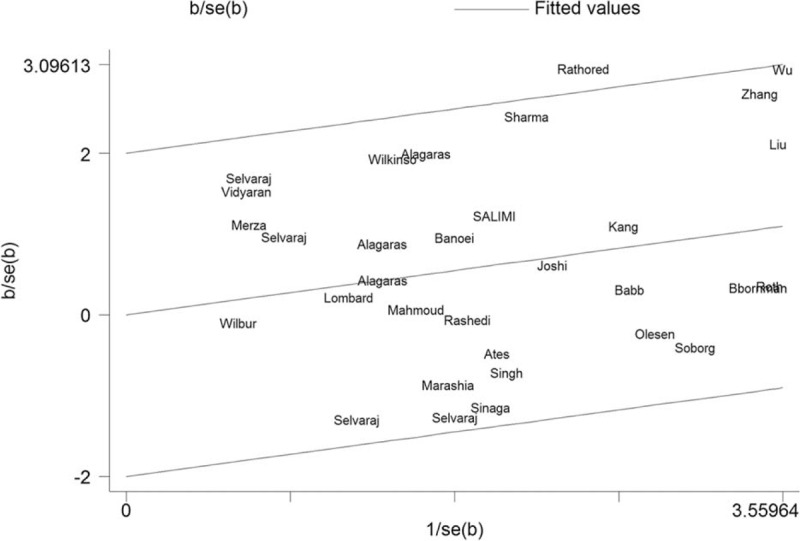
Galbraith plot analysis to evaluate heterogeneity: Rathored et al and Wu et al were the outlier studies in recessive model (ff vs fF+FF).
Sensitivity Analysis
First, sensitivity analysis was performed by omitting 1 study at a time, and there were no statistically significant changes in all ORs. We then omitted the 3 studies, which were out of HWE, and the statistical significance of the pooled result did not change (OR = 1.31, 95% CI = 1.068–1.604, P = 0.010).
Publication Bias
As shown in Figure 5, the funnel plot was symmetrical. The Begg's funnel plot and the Egger test also confirmed the absence of publication bias among the included studies (PEggertest = 0. 841).
FIGURE 5.
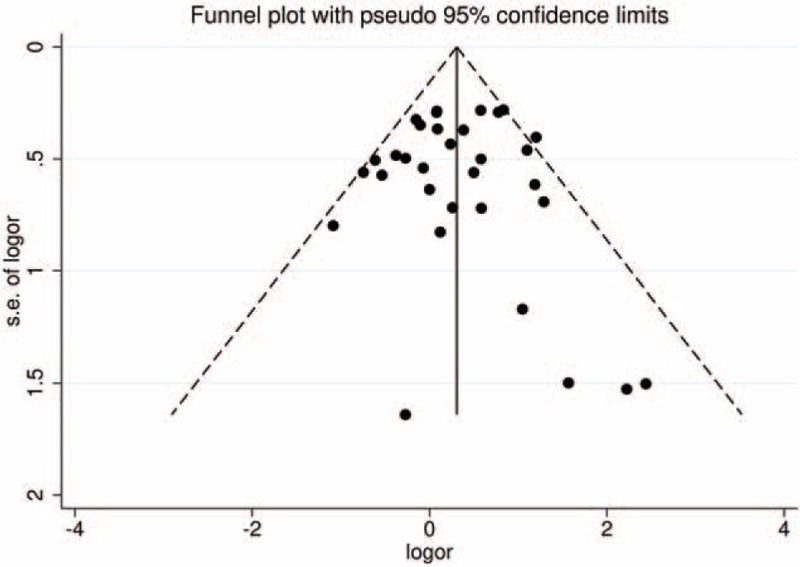
Funnel plot for studies of the association between in recessive model (ff vs fF+FF). The horizontal and vertical axes correspond to the OR and 95% CI. CI = confidence interval; OR = odds ratio.
DISCUSSION
This meta-analysis with 32 case-control studies indicates that VDR FokI polymorphism contributes to the risk of TB. The results suggest that people who had genotype ff had a 34% higher risk of developing TB than people who had genotypes fF/FF, and the risk effect was confirmed in HIV-negative TB patients (OR = 1.60). In addition, results from subgroup analysis stratified by ethnicity indicate that TB risk was increased in Asians with ff genotype (OR = 1.65), but not in Caucasians and Africans.
The results of the present meta-analysis are consistent with a similar meta-analysis performed by Chen et al16 in 2013. Compared with the previous study, our meta-analysis included 7 additional studies on the FokI polymorphism.12,18–23 Recently, a meta-analysis was performed by Sun et al. However, this meta-analysis missed 11 studies12,18–20,22,23,39,42–44,47 according to the specific combinations of search terms and their inclusion and exclusion criteria. In addition, some comparison genetic models in this study were incorrect (eg, the recessive model should be ff vs FF+fF but not ff+fF vs FF). Therefore, this update meta-analysis has more statistical power than the 2 previous studies. Likewise, considering TB is the frequent major opportunistic infection in HIV-infected patients, we carried out a subgroup analysis stratified by HIV status. Interestingly, the risk effect was found only in HIV-negative TB patients. As expected, the heterogeneity decreased significantly, which not only strongly confirms the conclusion that FokI polymorphism contributes to the risk of TB, but also indicates that HIV status was the main source of heterogeneity in the previous meta-analysis. This may be a reason for controversial results from previous studies. Indeed, HIV infection is associated with a greater risk for disease than HIV-negative individuals.57 Of note, a study by Xu et al58 also focused on this topic; nevertheless, our study is more comprehensive than this study and we found the risk effect only in HIV-negative TB patients but not observed in HIV-positive or not clearly identified group. Therefore, our results suggest it is crucial to avoid selection bias in such genotype association studies.
Our results are also consistent with the functional studies on the VDR genepolymorphisms59; the active form of vitamin D (1,25(OH)2D3) is an important immunoregulatory hormone and moves into the nucleus by binding to the VDR complex.60 Low vitamin D levels have been found to contribute to the risk of TB infection.61 VDR gene polymorphisms are related to vitamin D-related disease,11 and significant interaction between vitamin D status and VDR gene polymorphisms has also been observed.10 Indeed, VDR polymorphism may influence susceptibility to infectious diseases, such as hepatitis B virus infection62 and leprosy.63 With respect to FokI polymorphisms, the short 424 amino acid VDR protein variant (corresponding with the C-allele or “big F” allele) has been found to be more active than the long 427 ff variant.59 Hence, the f allele of FokI might decrease the activity of the VDR protein, and then block the binding of active vitamin D and VDR. In summary, VDR polymorphism may influence the function of vitamin D and, therefore, contribute to the susceptibility to TB infection.
The present study has some advantages compared with previous studies. First, this update meta-analysis has more statistical power than the 2 previous studies. We also selected the best genetic model to avoid multiple comparisons. Second, we confirmed the conclusion in the HIV-negative TB group, which would further reveal the association between FokI polymorphism and TB. Likewise, our results were relatively reliable for no significant heterogeneity, and some results were given in the sensitivity analysis. However, having some limitations is a required consideration in this study. We should note the potential publication biases when explaining the results, although no significant publication biases were found in this study; positive results mainly come from the Asian region, especially China. In addition, we did not stratify or analyze the other factors, such as sex or clinical and environmental variables, because of a lack of original data from authors. Also, our HIV status-specific analysis included only 2 studies from HIV-positive TB patients, and HIV positive or no information were together as a subgroup in meta-analysis would represent a bias in the analysis and conclusions; additional studies are warranted to explore the relationship between HIV-positive TB and FokI polymorphisms.
CONCLUSIONS
In conclusion, this meta-analysis confirms that VDR FokI polymorphism contributes to the risk of TB, especially in HIV-negative TB patients and the Asian group. Further studies are required to clarify the role of the FokI polymorphism in HIV-positive TB and in other ethnic groups.
Acknowledgments
We thank Yifan Sun for assistance with the statistics analysis and valuable discussion. We acknowledge the editors and the anonymous reviewers for insightful suggestions on this work.
Footnotes
Abbreviations: CIs = confidence intervals, HIV = human immunodeficiency virus, HWE = Hardy–Weinberg equilibrium, MTB = mycobacterium tuberculosis, ORs = odds ratios, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-analyses, TB = tuberculosis, VDR = vitamin D receptor.
LH and CL contributed equally to the article, so they should be considered as the co-first authors.
JC and CL conceived and designed the experiments, LH and XY performed the experiments. GL and XT analyzed the data. LH and CJ contributed to the writing of the manuscript. CL revised the manuscript.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.WHO. Global Tuberculosis Report 2014[M]. World Health Organization, 2014. [Google Scholar]
- 2.Pawlowski A, Jansson M, Skold M, et al. Tuberculosis and HIV co-infection. PLoS Pathog 2012; 8:e1002464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frieden TR, Sterling TR, Munsiff SS, et al. Tuberculosis. Lancet (London, England) 2003; 362:887–899. [DOI] [PubMed] [Google Scholar]
- 4.Martinez N, Kornfeld H. Diabetes and immunity to tuberculosis. Eur J Immunol 2014; 44:617–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellamy R. Genetic susceptibility to tuberculosis. Clin Chest Med 2005; 26:233–246. [DOI] [PubMed] [Google Scholar]
- 6.Bellamy R. Susceptibility to mycobacterial infections: the importance of host genetics. Genes Immun 2003; 4:4–11. [DOI] [PubMed] [Google Scholar]
- 7.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006; 311:1770–1773. [DOI] [PubMed] [Google Scholar]
- 8.Deluca HF, Cantorna MT. Vitamin D: its role and uses in immunology. FASEB J 2001; 15:2579–2585. [DOI] [PubMed] [Google Scholar]
- 9.Nejentsev S, Godfrey L, Snook H, et al. Comparative high-resolution analysis of linkage disequilibrium and tag single nucleotide polymorphisms between populations in the vitamin D receptor gene. Hum Mol Genet 2004; 13:1633–1639. [DOI] [PubMed] [Google Scholar]
- 10.Wilkinson RJ, Llewelyn M, Toossi Z, et al. Influence of vitamin D deficiency and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west London: a case-control study. Lancet 2000; 355:618–621. [DOI] [PubMed] [Google Scholar]
- 11.Uitterlinden AG, Fang Y, van Meurs JB, et al. Vitamin D receptor gene polymorphisms in relation to Vitamin D related disease states. J Steroid Biochem Mol Biol 2004; 89:187–193. [DOI] [PubMed] [Google Scholar]
- 12.Rashedi J, Asgharzadeh M, Moaddab SR, et al. Vitamin d receptor gene polymorphism and vitamin d plasma concentration: correlation with susceptibility to tuberculosis. Adv Pharm Bull 2014; 4 Suppl 2:607–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alagarasu K, Selvaraj P, Swaminathan S, et al. 5′ regulatory and 3′ untranslated region polymorphisms of vitamin D receptor gene in south Indian HIV and HIV-TB patients. J Clin Immunol 2009; 29:196–204. [DOI] [PubMed] [Google Scholar]
- 14.Selvaraj P, Prabhu Anand S, Harishankar M, et al. Plasma 1,25 dihydroxy vitamin D3 level and expression of vitamin d receptor and cathelicidin in pulmonary tuberculosis. J Clin Immunol 2009; 29:470–478. [DOI] [PubMed] [Google Scholar]
- 15.Singh A, Gaughan JP, Kashyap VK. SLC11A1 and VDR gene variants and susceptibility to tuberculosis and disease progression in East India. Int J Tuberc Lung Dis 2011; 15:1468–1474. [DOI] [PubMed] [Google Scholar]
- 16.Chen C, Liu Q, Zhu L, et al. Vitamin D receptor gene polymorphisms on the risk of tuberculosis, a meta-analysis of 29 case-control studies. PLoS One 2013; 8:e83843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun YP, Cai QS. Vitamin D receptor FokI gene polymorphism and tuberculosis susceptibility: a meta-analysis. Genet Mol Res 2015; 14:6156–6163. [DOI] [PubMed] [Google Scholar]
- 18.Salimi S, Farajian-Mashhadi F, Alavi-Naini R, et al. Association between vitamin D receptor polymorphisms and haplotypes with pulmonary tuberculosis. Biomed Rep 2015; 3:189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sinaga BY, Amin M, Siregar Y, et al. Correlation between Vitamin D receptor gene FOKI and BSMI polymorphisms and the susceptibility to pulmonary tuberculosis in an Indonesian Batak-ethnic population. Acta Med Indones 2014; 46:275–282. [PubMed] [Google Scholar]
- 20.Mahmoud AA, Ali AHK. Vitamin D receptor gene polymorphism and 25 hydroxy vitamin D levels in Egyptian patients with pulmonary tuberculosis. Egyptian J Chest Dis Tuberculosis 2014; 63:651–655. [Google Scholar]
- 21.Joshi L, Ponnana M, Penmetsa SR, et al. Serum vitamin D levels and VDR polymorphisms (BsmI and FokI) in patients and their household contacts susceptible to tuberculosis. Scand J Immunol 2014; 79:113–119. [DOI] [PubMed] [Google Scholar]
- 22.Wu F, Zhang W, Zhang L, et al. NRAMP1, VDR, HLA-DRB1, and HLA-DQB1 gene polymorphisms in susceptibility to tuberculosis among the Chinese Kazakh population: a case-control study. BioMed Res Int 2013; 2013:484535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang TJ, Jin SH, Yeum CE, et al. Vitamin D receptor gene TaqI, BsmI and FokI polymorphisms in Korean patients with tuberculosis. Immune Netw 2011; 11:253–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raghavan S, Alagarasu K, Selvaraj P. Immunogenetics of HIV and HIV associated tuberculosis. Tuberculosis (Edinb) 2012; 92:18–30. [DOI] [PubMed] [Google Scholar]
- 25.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Internal Med 2009; 151:264–269. [DOI] [PubMed] [Google Scholar]
- 26.Thakkinstian A, McElduff P, D’Este C, et al. A method for meta-analysis of molecular association studies. Stat Med 2005; 24:1291–1306. [DOI] [PubMed] [Google Scholar]
- 27.Higgins J, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 28.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arji N, Busson M, Iraqi G, et al. Genetic diversity of TLR2, TLR4, and VDR loci and pulmonary tuberculosis in Moroccan patients. J Infect Dev Ctries 2014; 8:430–440. [DOI] [PubMed] [Google Scholar]
- 30.Motsinger-Reif AA, Antas PRZ, Oki NO, et al. Polymorphisms in IL-1(beta), vitamin D receptor Fok1, and Toll-like receptor 2 are associated with extrapulmonary tuberculosis. BMC Med Genet 2010; 11:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bellamy R. Identifying genetic susceptibility factors for tuberculosis in Africans: a combined approach using a candidate gene study and a genome-wide screen. Clin Sci (Lond) 2000; 98:245–250. [PubMed] [Google Scholar]
- 32.Bellamy R, Ruwende C, Corrah T, et al. Tuberculosis and chronic hepatitis B virus infection in Africans and variation in the vitamin D receptor gene. J Infect Dis 1999; 179:721–724. [DOI] [PubMed] [Google Scholar]
- 33.Delgado JC, Baena A, Thim S, et al. Ethnic-specific genetic associations with pulmonary tuberculosis. J Infect Dis 2002; 186:1463–1468. [DOI] [PubMed] [Google Scholar]
- 34.Ahmad Z, Arrahmanda K, Ergan D, et al. Effect of Taqi vitamin D receptor gene polymorphism on the incidence of pulmonary tuberculosis. Respirology 2011; 16:78.20946335 [Google Scholar]
- 35.Kim J, Ahn JH, Park CK, et al. Influence of vitamin d receptor polymorphism on tuberculosis among south Korean. CHEST Journal 2011; 140:780A–780A. [Google Scholar]
- 36.Park S, Kim EJ, Lee SH, et al. Vitamin D-receptor polymorphisms and non-tuberculous mycobacterial lung disease in Korean patients. Int J Tuberc Lung Dis 2008; 12:698–700. [PubMed] [Google Scholar]
- 37.Liu W, Cao WC, Zhang CY, et al. VDR and NRAMP1 gene polymorphisms in susceptibility to pulmonary tuberculosis among the Chinese Han population: a case-control study. Int J Tuberc Lung Dis 2004; 8:428–434. [PubMed] [Google Scholar]
- 38.Rathored J, Sharma SK, Singh B, et al. Risk and outcome of multidrug-resistant tuberculosis: vitamin D receptor polymorphisms and serum 25(OH)D. Int J Tuberc Lung Dis 2012; 16:1522–1528. [DOI] [PubMed] [Google Scholar]
- 39.Selvaraj P, Vidyarani M, Alagarasu K, et al. Regulatory role of promoter and 3′ UTR variants of vitamin D receptor gene on cytokine response in pulmonary tuberculosis. J Clin Immunol 2008; 28:306–313. [DOI] [PubMed] [Google Scholar]
- 40.Selvaraj P, Chandra G, Jawahar MS, et al. Regulatory role of vitamin D receptor gene variants of Bsm I, Apa I, Taq I, and FokI polymorphisms on macrophage phagocytosis and lymphoproliferative response to mycobacterium tuberculosis antigen in pulmonary tuberculosis. J Clin Immunol 2004; 24:523–532. [DOI] [PubMed] [Google Scholar]
- 41.Selvaraj P, Chandra G, Kurian SM, et al. Association of vitamin D receptor gene variants of BsmI, ApaI and FokI polymorphisms with susceptibility or resistance to pulmonary tuberculosis. Current Sci 2003; 84:1563–1564. [Google Scholar]
- 42.Sharma PR, Singh S, Jena M, et al. Coding and non-coding polymorphisms in VDR gene and susceptibility to pulmonary tuberculosis in tribes, castes and Muslims of Central India. Infect Genet Evol 2011; 11:1456–1461. [DOI] [PubMed] [Google Scholar]
- 43.Wilbur AK, Kubatko LS, Hurtado AM, et al. Vitamin D receptor gene polymorphisms and susceptibility M. tuberculosis in native Paraguayans. Tuberculosis (Edinb) 2007; 87:329–337. [DOI] [PubMed] [Google Scholar]
- 44.Vidyarani M, Selvaraj P, Raghavan S, et al. Regulatory role of 1, 25-dihydroxyvitamin D3 and vitamin D receptor gene variants on intracellular granzyme A expression in pulmonary tuberculosis. Exp Mol Pathol 2009; 86:69–73. [DOI] [PubMed] [Google Scholar]
- 45.Zhang HQ, Deng A, Guo CF, et al. Association between FokI polymorphism in vitamin D receptor gene and susceptibility to spinal tuberculosis in Chinese Han population. Arch Med Res 2010; 41:46–49. [DOI] [PubMed] [Google Scholar]
- 46.Ates O, Dolek B, Dalyan L, et al. The association between BsmI variant of vitamin D receptor gene and susceptibility to tuberculosis. Mol Biol Rep 2011; 38:2633–2636. [DOI] [PubMed] [Google Scholar]
- 47.Banoei MM, Mirsaeidi MS, Houshmand M, et al. Vitamin D receptor homozygote mutant tt and bb are associated with susceptibility to pulmonary tuberculosis in the Iranian population. Int J Infect Dis 2010; 14:e84–e85. [DOI] [PubMed] [Google Scholar]
- 48.Marashian SM, Farnia P, Seyf S, et al. Evaluating the role of vitamin D receptor polymorphisms on susceptibility to tuberculosis among iranian patients: a case-control study. Tuberk Toraks 2010; 58:147–153. [PubMed] [Google Scholar]
- 49.Merza M, Farnia P, Anoosheh S, et al. The NRAMPI, VDR and TNF-alpha gene polymorphisms in Iranian tuberculosis patients: the study on host susceptibility. Braz J Infect Dis 2009; 13:252–256. [DOI] [PubMed] [Google Scholar]
- 50.Roth DE, Soto G, Arenas F, et al. Association between vitamin D receptor gene polymorphisms and response to treatment of pulmonary tuberculosis. J Infect Dis 2004; 190:920–927. [DOI] [PubMed] [Google Scholar]
- 51.Babb C, van der Merwe L, Beyers N, et al. Vitamin D receptor gene polymorphisms and sputum conversion time in pulmonary tuberculosis patients. Tuberculosis (Edinbur) 2007; 87:295–302. [DOI] [PubMed] [Google Scholar]
- 52.Bornman L, Campbell SJ, Fielding K, et al. Vitamin D receptor polymorphisms and susceptibility to tuberculosis in West Africa: a case-control and family study. J Infect Dis 2004; 190:1631–1641. [DOI] [PubMed] [Google Scholar]
- 53.Lombard Z, Dalton DL, Venter PA, et al. Association of HLA-DR, -DQ, and vitamin D receptor alleles and haplotypes with tuberculosis in the Venda of South Africa. Hum Immunol 2006; 67:643–654. [DOI] [PubMed] [Google Scholar]
- 54.Olesen R, Wejse C, Velez DR, et al. DC-SIGN (CD209), pentraxin 3 and vitamin D receptor gene variants associate with pulmonary tuberculosis risk in West Africans. Genes Immun 2007; 8:456–467. [DOI] [PubMed] [Google Scholar]
- 55.Soborg C, Andersen AB, Range N, et al. Influence of candidate susceptibility genes on tuberculosis in a high endemic region. Mol Immunol 2007; 44:2213–2220. [DOI] [PubMed] [Google Scholar]
- 56.Salimi S, Farajian-Mashhadi F, Alavi-Naini R, et al. Association between vitamin D receptor polymorphisms and haplotypes with pulmonary tuberculosis. Biomed Rep 2015; 3:189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ciesla JA, Roberts JE. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. Am J Psychiatry 2001; 158:725–730. [DOI] [PubMed] [Google Scholar]
- 58.Xu C, Tang P, Ding C, et al. Vitamin D receptor gene FOKI polymorphism contributes to increasing the risk of HIV-negative tuberculosis: evidence from a meta-analysis. PLoS One 2015; 10:e0140634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arai H, Miyamoto KI, Taketani Y, et al. A vitamin D receptor gene polymorphism in the translation initiation codon: effect on protein activity and relation to bone mineral density in Japanese women. J Bone Miner Res 1997; 12:915–921. [DOI] [PubMed] [Google Scholar]
- 60.Manolagas SC, Yu XP, Girasole G, et al. Vitamin D and the hematolymphopoietic tissue: a 1994 update. Seminars in nephrology 1994; 14:129–143. [PubMed] [Google Scholar]
- 61.Facchini L, Venturini E, Galli L, et al. Vitamin D and tuberculosis: a review on a hot topic. J Chemother 2015; 27:128–138. [DOI] [PubMed] [Google Scholar]
- 62.Suneetha PV, Sarin SK, Goyal A, et al. Association between vitamin D receptor, CCR5, TNF-alpha and TNF-beta gene polymorphisms and HBV infection and severity of liver disease. J Hepatol 2006; 44:856–863. [DOI] [PubMed] [Google Scholar]
- 63.Neela VSK, Suryadevara NC, Shinde VG, et al. Association of Tag I, Fok I and Apa I polymorphisms in Vitamin D Receptor (VDR) gene with leprosy. Hum Immunol 2015; 76:402–405. [DOI] [PubMed] [Google Scholar]


