Abstract
Let-7 family plays a key role in the progression of atherosclerosis and intracranial aneurysm (IA). We hypothesized that rs10877887 and rs13293512 polymorphisms in the promoters of let-7 family may be associated with the susceptibility of IA. We genotyped the 2 single nucleotide polymorphisms (SNPs) in 305 patients with IA and 401 healthy controls. The rs10877887 was analyzed using a polymerase chain reaction-restriction fragment length polymorphism assay, and the rs13293512 was analyzed using a TaqMan SNP genotyping method. The relative expression of let-7 family was measured in plasma of cases and controls using real-time PCR. We found that the rs13293512CT genotype was associated with a significantly increased risk of developing IA in a heterozygote comparison (adjusted OR = 1.43, 95% CI, 1.00–2.05, P = 0.048) and dominant comparison (adjusted OR = 1.44, 95% CI, 1.02–2.03, P = 0.04). Combined analysis showed that the rs10877887TT and rs13293512CC/CT genotypes had a significantly increased risk of IA (OR = 1.67, 95% CI, 1.04–2.68, P = 0.03). Moreover, the levels of let-7a, let-7d, and let-7f were downregulated in IA patients, and patients with the rs13293512CC/CT genotypes had a lower level of let-7a than those with rs13293512TT genotype (P = 0.03). These findings indicate that the rs13293512CC/CT is a risk factor for the development of IA, possibly because of the genotypes resulting in a lower level of let-7a.
INTRODUCTION
Intracranial aneurysm (IA) is a localized dilation or ballooning of cerebral arteries and affects 2% to 5% of the general population.1 Although IA is usually asymptomatic, its rupture can cause spontaneous subarachnoid hemorrhage (SAH) and substantial morbidity and mortality. Previous reports have shown that IA accounts for 85% of SAH, which contributes for ∼5% to 15% of stroke cases.2
Intracranial aneurysm is a complex disease with some known risk factors, including female gender, increasing age, hypertension, cigarette smoking, and alcohol abuse.3–5 Beside these environmental risk factors, genetic risk factors may contribute to the pathogenesis of IA.6 Familial clustering of IA is reported with a 7-fold increased risk of IA rupture in first-degree relatives compared to second-degree relatives.7–11 Moreover, the occurrence of IA in monozygotic twins is higher than that in general population and dizygotic twins.12,13 Our previous work showed that pri-miR-34b/c rs4938723 and NFKB1-94 insertion/deletion ATTG polymorphisms were susceptibility genes for the development of IA.14,15
miRNAs are noncoding molecules with a length of ∼22 nucleotides, which function as pre- and post-transcriptional regulators of gene expression.16,17 In both human and animal models of IA, miRNAs are differentially expression, including let-7,18–21 indicating that let-7 may play an important role in the progression of IA. Let-7 family has several members, including let-7a-1, let-7d, let-7f-1, let-7i, and others. In human genome, let-7a-1/let-7f-1/let-7d forms a cluster which maps in chromosome 9, and let-7i locates in chromosome 12. Recently, Xie et al discovered 2 potentially functional polymorphisms (ie, rs10877887 and rs13293512) in the promoter regions of let-7 family, which might affect binding affinity of a predicted transcription factor.22 More recently, these single nucleotide polymorphisms (SNPs) were reported to be associated with the risk of major depressive disorder,23 lung cancer,24 and survival of hepatocellular carcinoma.22 To date, little is known about the polymorphisms with IA risk.
Based on this background, we hypothesized that genetic variants in the promoters of let-7 family might be susceptibility genes for the development of IA. To test this hypothesis, we conducted a case-control study to evaluate the association of the rs10877887 and rs13293512 polymorphisms with IA risk in a Chinese Han population. We also detected the expression of let-7 family in plasma of IA patients and controls. We found that the rs13293512CC/CT is a risk factor for the development of IA, possibly because of the genotypes resulting in a lower level of let-7a.
MATERIALS AND METHODS
Study Population
The study protocol was approved by the Institutional Review Board of West China Hospital of Sichuan University. Patients were recruited consecutively from the department of neurosurgery of the hospital between January 2008 and April 2014. The diagnosis of IA was confirmed by digital subtraction angiography. During the same period, healthy volunteers after physical examination were identified as controls. The controls were unrelated Chinese Han population who live in Sichuan province or surrounding area. We excluded individuals with nervous system diseases or other diseases being risk factors for IA, including hypertension, head trauma, and intracranial atherosclerosis. The control subjects were frequency-matched to the cases by age (±5 years) and gender.
After an informed consent form was signed, demographic data and clinical information were collected. Each subject donated 5 mL of ethylenediaminetetraacetic acid (EDTA)-blood, which was centrifuged at 1600 g for 10 min at 4°C. Plasma was isolated and further centrifuged at 16,000 g for 10 min at 4°C. Blood cells and plasma were stored at −80°C until analysis.
Genotyping
Genomic DNA was isolated from leukocytes using a commercial kit (Bioteke, Beijing, China). The rs10877887 polymorphism was genotyped using a polymerase chain reaction–restriction fragment length polymorphism assay, and the rs13293512 polymorphism was genotyped using a TaqMan SNP genotyping method. Detailed information was presented as previously described.23 To improve the accuracy of the genotyping methods, positive control (heterozygote genotype) and negative control (double distilled water) were used in each experiment. All the results were verified by DNA sequencing.
Quantitative Measurement of Expression of Let-7 Family
Total RNA was extracted from plasma of 56 IA patients and 56 controls using a QIAamp circulating nucleic acid kit (Qiagen, Hilden, Germany) according to the manufacturer's protocol. RNA quality and concentration were determined using Nucleic Acid/Protein Analyzer (DU730, Beckman Coulter, Inc). After reverse transcription of cDNA, real-time PCR was done in Mastercycler ep realplex (Eppendorf, Hamburg, Germany) using a QuantiFast SYBR Green PCR Kit (Qiagen, Hilden, Germany). All primers were synthesized by Ribobio Corp. (Guangzhou, China). U6 was used to normalize the let-7 level in both cases and controls. Each sample was analyzed in triplicate. The relative expression of let-7 family was described using the 2–ΔCt method.25
Statistical Analyses
The frequencies of the rs10877887 and rs13293512 polymorphisms were calculated by the gene-counting method, and the χ2 test was used to identify the departure from Hardy–Weinberg equilibrium. The association of the rs10877887 and rs13293512 polymorphisms with IA risk was estimated using the χ2 test. Logistic regression was performed to compute adjusted and crude odds ratios (OR) and 95% confidence intervals (CI), with and without adjustment for the age and the gender. Relative expression of let-7 family and their association with the polymorphisms were compared using the Mann–Whitney U test. Statistical analysis was carried out using the SPSS software, version 11.5 (SPSS Inc, Chicago, IL), and a 2-tailed P value of 0.05 was the criteria for significance.
RESULTS
Characteristics of the Study Population
A total of 706 subjects were included in this study, including 305 cases and 401 controls. The mean age of the cases was 52.4 ± 12.5 years and the mean age of the controls was 50.2 ± 10.1 years. The gender in the cases was frequency matched to the controls (P = 0.43). Most of the cases had single intracranial aneurysm and ruptured aneurysm, with frequencies of 86.6 and 85.9%, respectively (Table 1).
TABLE 1.
Demographics of Controls and Patients With Intracranial Aneurysm
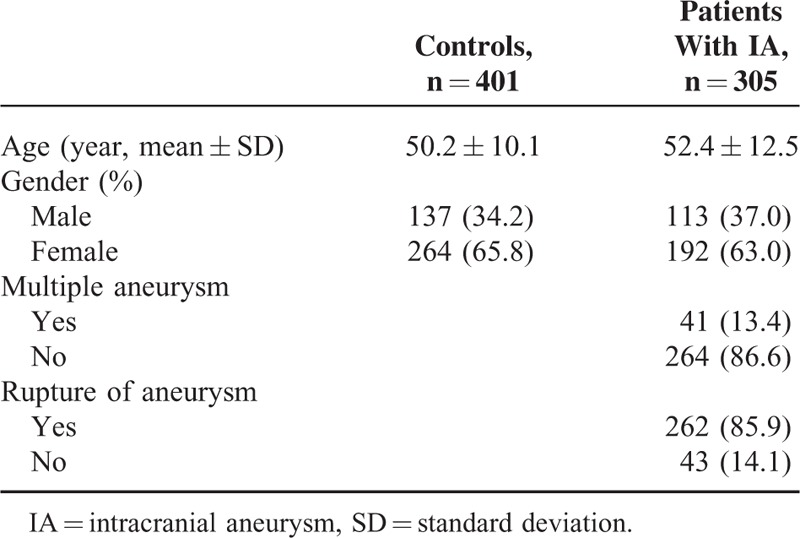
Association of the rs10877887 and rs13293512 Polymorphisms and IA Risk
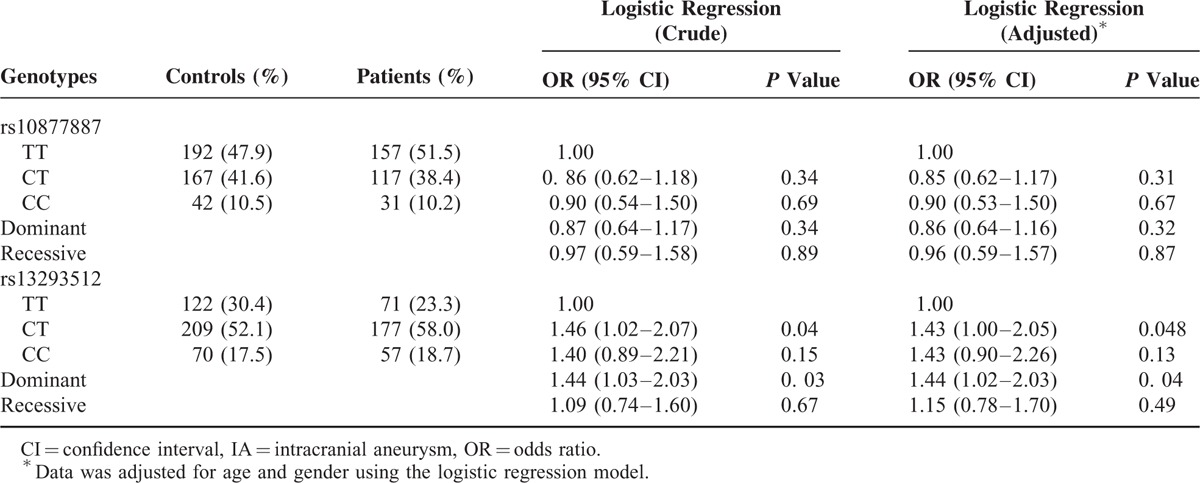
The distributions of the rs10877887 and rs13293512 polymorphisms in the study population are shown in Table 2. The frequencies of 2 SNPs were in Hardy–Weinberg equilibrium in controls (P = 0.53 and 0.23). The rs13293512CT genotype was observed in 58.0% of cases and 52.1% of controls, whereas the rs13293512TT genotype was observed in 23.3% of cases and 30.4% of controls. Compared with the rs13293512TT genotype, the rs13293512CT genotype was associated with a significantly increased risk of developing IA in a heterozygote comparison (adjusted OR = 1.43, 95% CI, 1.00–2.05, P = 0.048) and dominant comparison (adjusted OR = 1.44, 95% CI, 1.02–2.03, P = 0.04). However, the genotype frequencies of the rs10877887 were not statistically different between cases and controls.
TABLE 2.
Association of the rs10877887 and rs13293512 Polymorphisms and IA Risk
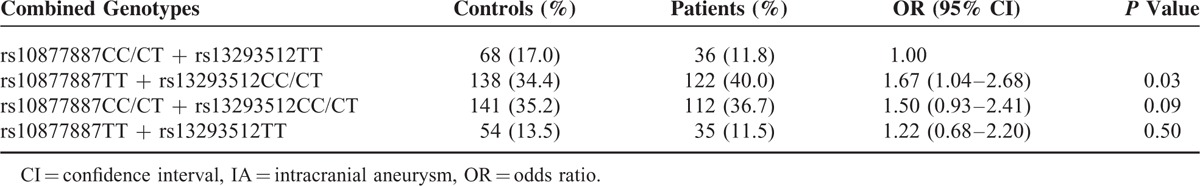
Combined analysis was also performed to evaluate the effect of the rs10877887- rs13293512 on IA risk. As shown in Table 3, the combined genotypes of rs10877887TT and rs13293512CC/CT were found in 40.0% of cases and 34.4% of controls, whereas the combined genotypes of rs10877887CC/CT and rs13293512TT were found in 11.8% of cases and 17.0% of controls. The rs10877887TT and rs13293512CC/CT genotypes, compared with the rs10877887CC/CT and rs13293512TT genotypes, had a significantly increased risk of IA (OR = 1.67, 95% CI, 1.04–2.68, P = 0.03).
TABLE 3.
The Combined Effects of the rs10877887 and rs13293512 on IA Risk
Association of the 2 Polymorphisms and Relative Expression of Let-7 family
Relative expressions of let-7a, let-7d, let-7f, and let-7i were detected in plasma of controls and IA patients using real-time PCR. As shown in Figure 1, the levels of let-7a, let-7d, and let-7f were downregulated in IA patients compared with the controls (P = 0.01, 0.03, and 0.04, respectively). However, there was no significant difference of let-7i between cases and controls (P > 0.05). To examine whether the rs10877887 and rs13293512 polymorphisms influence the expression of let-7, we analyzed the expression of let-7 in 56 IA patients. We found that patients with the rs13293512CC/CT genotypes had a lower level of let-7a than those with rs13293512TT genotype (P = 0.03) (Figure 2).
FIGURE 1.
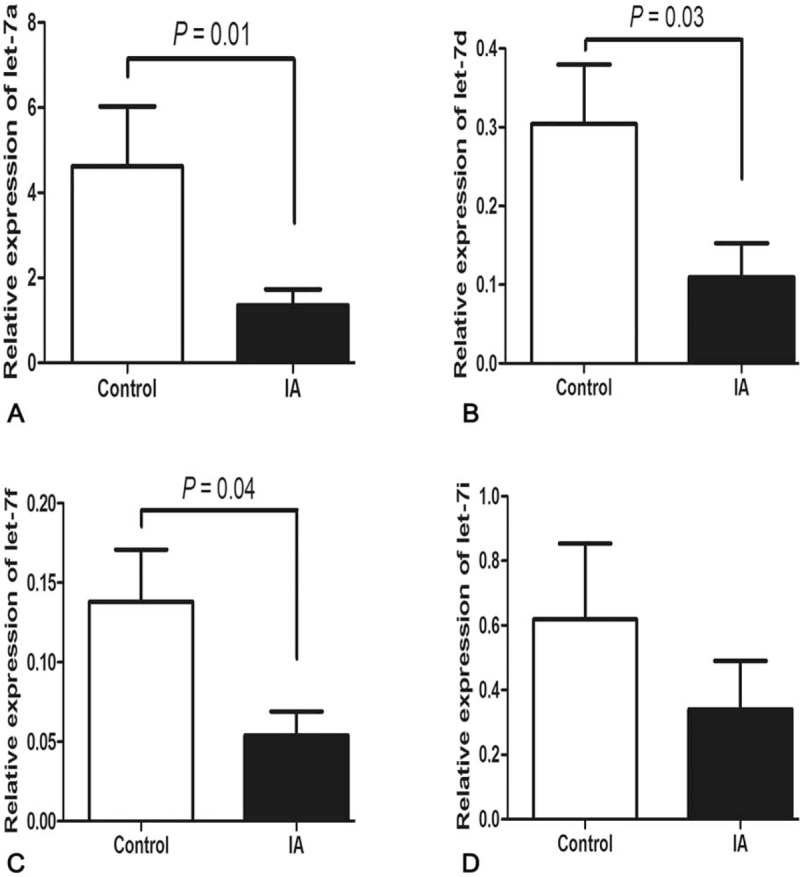
Relative expression of let-7 family in controls and IA patients. Using U6 as an internal control, let-7a (A), let-7d (B), and let-7f (C) were downregulated in IA patients. No significant differentiation of let-7i between controls and IA group (D). Data are presented as mean ± SEM. IA = intracranial aneurysm, SEM = standard error of the mean.
FIGURE 2.
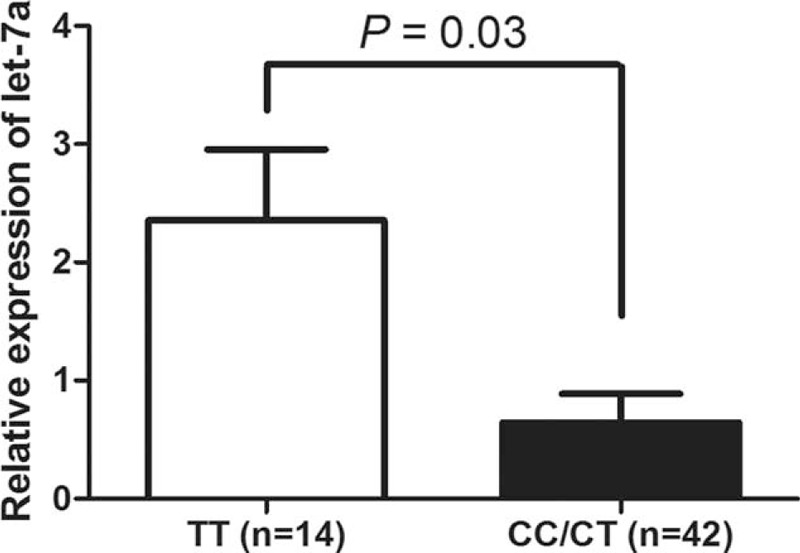
The expression of let-7a is associated with rs13293512 genotypes. Compared with the let-7a level in IA patient with rs13293512TT genotype, it was lower in patients with rs13293512CC/CT genotypes. Data are presented as mean ± SEM. IA = intracranial aneurysm, SEM = standard error of the mean.
DISCUSSION
To identify whether the rs10877887 and rs13293512 polymorphisms in the promoters of let-7 were associated with IA in Chinese population, we performed a case-control study with a total of 305 IA cases and 401 controls. We demonstrated that the rs13293512CT and CT/CC genotypes had a 1.43- and 1.44-fold increased risk of IA, respectively. The combined genotypes of rs10877887TT and rs13293512CC/CT had a 1.67-fold increased risk of IA. Moreover, we found that the expression of let-7a, let-7d, and let-7f was downregulated in plasma of IA patients and the rs13293512CC/CT genotypes correspond to a lower level of let-7a. The evidence of the findings supported the idea that the rs10877887 and rs13293512 polymorphisms may be related to the etiology of IA. Although the sample size is relatively small, our study has >80% power under a dominant model, suggesting that the results were statistically reliable.
It is well known that hypertension, structural proteins, angiogenesis factors, and atherosclerosis are important factors in initiating the development of IA.26–28 Li et al reported that let-7e expression in plasma samples of hypertensive patients was upregulated compared to control subjects. Similarly increasing expression of let-7 was also observed in endothelial cells. Interestingly, the expression of let-7 in endothelial progenitor cells was opposite to that in patients’ plasma samples, suggesting that plasma let-7e may originate partially from endothelial progenitor cells rather than endothelial cells.29 Kuehbacher et al reported that knockdown of Drosha and Dicer induced a downregulation of let-7a, let-7b, let-7c, let-7f, and let-7 g, and the reduction of let-7f impairs the sprout formation, indicating that let-7f promotes angiogenesis.30 Hulsmans et al reported that miRNA-containing microvesicles can regulate inflammation in association with atherosclerotic disease. These miRNAs include let-7 family.31 Moreover, let-7 family was found to be upregulated in atherosclerotic abdominal aortic aneurysm32 and downregulated in IA.19 In this study, we found an association of the rs13293512 polymorphism in the promoter region of let-7 with IA. Taken together, these findings indicate that let-7 family may have a pathogenic role in the development of IA.
Regarding the mechanism of the rs13293512 polymorphism in the onset of IA, we hypothesized that the polymorphism may regulate the expression of let-7 because it locates in the promoter region of let-7 family. We thus measured the expression of let-7a, let-7d, let-7f, and let-7i in plasma of IA patients. We found that let-7a expression level is significantly lower in the rs13293512CC/CT genotypes than in the rs13293512TT genotype. These findings indicate that rs13293512CC/CT genotypes may cause a lower expression of let-7a, and finally increase the susceptibility to IA.
Although this study provides evidence of the rs13293512CC/CT genotypes with IA risk, some limitations cannot be ruled out. In the study design, we do not take environmental factors into consideration, and thus gene–environment interaction analysis cannot be evaluated. Follow-up data was not available in this study. We cannot assess whether the 2 polymorphisms are related to prognosis and survival of IA.
We conclude for the first time that the rs13293512CC/CT were risk genotypes for IA pathogenesis in the Chinese population. Further studies with larger sample sizes are needed to confirm these findings. Additionally, gene–environment interaction analysis is helpful to understand phenotypic variation in the pathogenesis of IA. Once accomplished, it is of potential value in the etiology and treatment of IA.
Footnotes
Abbreviations: CI = confidence interval, EDTA = ethylenediaminetetraacetic acid, IA = intracranial aneurysm, OR = odds ratio, PCR = polymerase chain reaction, SAH = subarachnoid hemorrhage, SNP = single nucleotide polymorphism.
Funding: this work was supported by the Project of the National Science & Technology Pillar Program (2011BAI08B05). XS and HS have equally contributed to this study.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Rinkel GJ, Djibuti M, Algra A, et al. Prevalence and risk of rupture of intracranial aneurysms: a systematic review. Stroke 1998; 29:251–256. [DOI] [PubMed] [Google Scholar]
- 2.Bederson JB, Awad IA, Wiebers DO, et al. Recommendations for the management of patients with unruptured intracranial aneurysms: a statement for healthcare professionals from the Stroke Council of the American Heart Association. Circulation 2000; 102:2300–2308. [DOI] [PubMed] [Google Scholar]
- 3.Teunissen LL, Rinkel GJ, Algra A, et al. Risk factors for subarachnoid hemorrhage: a systematic review. Stroke 1996; 27:544–549. [DOI] [PubMed] [Google Scholar]
- 4.Inagawa T. Risk factors for the formation and rupture of intracranial saccular aneurysms in Shimane, Japan. World Neurosurg 2010; 73:155–164.discussion e123. [DOI] [PubMed] [Google Scholar]
- 5.Gu YX, Chen XC, Song DL, et al. Risk factors for intracranial aneurysm in a Chinese ethnic population. Chin Med J (Engl) 2006; 119:1359–1364. [PubMed] [Google Scholar]
- 6.Bilguvar K, Yasuno K, Niemela M, et al. Susceptibility loci for intracranial aneurysm in European and Japanese populations. Nat Genet 2008; 40:1472–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim DH, Van Ginhoven G, Milewicz DM. Incidence of familial intracranial aneurysms in 200 patients: comparison among Caucasian, African-American, and Hispanic populations. Neurosurgery 2003; 53:302–308. [DOI] [PubMed] [Google Scholar]
- 8.Wills S, Ronkainen A, Van Der Voet M, et al. Familial intracranial aneurysms: an analysis of 346 multiplex Finnish families. Stroke 2003; 34:1370–1374. [DOI] [PubMed] [Google Scholar]
- 9.Kojima M, Nagasawa S, Lee YE, et al. Asymptomatic familial cerebral aneurysms. Neurosurgery 1998; 43:776–781. [DOI] [PubMed] [Google Scholar]
- 10.Bromberg JE, Rinkel GJ, Algra A, et al. Subarachnoid haemorrhage in first and second degree relatives of patients with subarachnoid haemorrhage. BMJ 1995; 311:288–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schievink WI, Schaid DJ, Michels VV, et al. Familial aneurysmal subarachnoid hemorrhage: a community-based study. J Neurosurg 1995; 83:426–429. [DOI] [PubMed] [Google Scholar]
- 12.Nakajima H, Kishi H, Yasui T, et al. Intracranial aneurysms in identical twins. Surg Neurol 1998; 49:306–308. [DOI] [PubMed] [Google Scholar]
- 13.Mackey J, Brown RD, Sauerbeck L, et al. Affected twins in the familial intracranial aneurysm study. Cerebrovasc Dis 2015; 39:82–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li L, Sima X, Bai P, et al. Interactions of miR-34b/c and TP53 polymorphisms on the risk of intracranial aneurysm. Clin Dev Immunol 2012; 2012:567586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sima X, Xu J, Li J, et al. Association between NFKB1-94 insertion/deletion ATTG polymorphism and risk of intracranial aneurysm. Genet Test Mol Biomarkers 2013; 17:620–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004; 116:281–297. [DOI] [PubMed] [Google Scholar]
- 17.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009; 136:215–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee HJ, Yi JS, Lee HJ, et al. Dysregulated expression profiles of microRNAs of experimentally induced cerebral aneurysms in rats. J Korean Neurosurg Soc 2013; 53:72–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu D, Han L, Wu X, et al. Genome-wide microRNA changes in human intracranial aneurysms. BMC Neurol 2014; 14:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holcomb M, Ding YH, Dai D, et al. RNA-sequencing analysis of messenger RNA/microRNA in a rabbit aneurysm model identifies pathways and genes of interest. Am J Neuroradiol 2015; 36:1710–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li P, Zhang Q, Wu X, et al. Circulating microRNAs serve as novel biological markers for intracranial aneurysms. J Am Heart Assoc 2014; 3:e000972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie K, Liu J, Zhu L, et al. A potentially functional polymorphism in the promoter region of let-7 family is associated with survival of hepatocellular carcinoma. Cancer Epidemiol 2013; 37:998–1002. [DOI] [PubMed] [Google Scholar]
- 23.Liang Y, Zhao G, Sun R, et al. Genetic variants in the promoters of let-7 family are associated with an increased risk of major depressive disorder. J Affect Disord 2015; 183:295–299. [DOI] [PubMed] [Google Scholar]
- 24.Shen LQ, Xie YZ, Qian XF, et al. A single nucleotide polymorphism in the promoter region of let-7 family is associated with lung cancer risk in Chinese. Genet Mol Res 2015; 14:4505–4512. [DOI] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001; 25:402–408. [DOI] [PubMed] [Google Scholar]
- 26.Skirgaudas M, Awad IA, Kim J, et al. Expression of angiogenesis factors and selected vascular wall matrix proteins in intracranial saccular aneurysms. Neurosurgery 1996; 39:537–545.discussion 545–537. [DOI] [PubMed] [Google Scholar]
- 27.Kilic T, Sohrabifar M, Kurtkaya O, et al. Expression of structural proteins and angiogenic factors in normal arterial and unruptured and ruptured aneurysm walls. Neurosurgery 2005; 57:997–1007.discussion 1997–1007. [DOI] [PubMed] [Google Scholar]
- 28.Inci S, Spetzler RF. Intracranial aneurysms and arterial hypertension: a review and hypothesis. Surg Neurol 2000; 53:530–540.discussion 540-532. [DOI] [PubMed] [Google Scholar]
- 29.Li S, Zhu J, Zhang W, et al. Signature microRNA expression profile of essential hypertension and its novel link to human cytomegalovirus infection. Circulation 2011; 124:175–184. [DOI] [PubMed] [Google Scholar]
- 30.Kuehbacher A, Urbich C, Zeiher AM, et al. Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circ Res 2007; 101:59–68. [DOI] [PubMed] [Google Scholar]
- 31.Hulsmans M, Holvoet P. MicroRNA-containing microvesicles regulating inflammation in association with atherosclerotic disease. Cardiovascular Res 2013; 100:7–18. [DOI] [PubMed] [Google Scholar]
- 32.Kin K, Miyagawa S, Fukushima S, et al. Tissue- and plasma-specific microRNA signatures for atherosclerotic abdominal aortic aneurysm. J Am Heart Assoc 2012; 1:e000745. [DOI] [PMC free article] [PubMed] [Google Scholar]


