Abstract
The prognosis of locally advanced (T3/T4 or N1) and metastatic disease urothelial carcinoma is poor. In this retrospective study, we reviewed data about patients receiving third-line chemotherapy for metastatic disease, in view of the lack of data in this setting.
We retrospectively analyzed medical records of patients with a pathologic diagnosis of urothelial carcinoma treated with systemic chemotherapy for metastatic disease at 4 participating Institutions between January, 2010, and January, 2015. Cox proportional hazards regression was used to evaluate the association of the chemotherapy agent used versus others with overall survival, adjusted for 5 externally validated prognostic factors in advanced urothelial carcinoma.
Of 182 patients that received first-line chemotherapy/adjuvant chemotherapy as defined above, 116 patients (63.73%) received second-line salvage treatment. Fifty-two patients were finally included in this analysis, whereas 9 were excluded due to missing data. Third-line chemotherapy was based on cyclophosphamide, platinum, vinflunine, taxanes, and gemcitabine in 16, 12, 11, 10, and 3 patients, respectively. Median PFS (progression-free survival) and OS (overall survival) of the population were 13 (10–17) and 31 (28–36) weeks. Single-agent cyclophosphamide was associated with a PFS of 18 (13–22) and an OS of 38 (33–41) weeks, whereas platinum-based combinations were associated with a PFS of 5 weeks and an OS of 8 weeks. Multivariate analysis showed improved survival in patients treated with cyclophosphamide (hazard ratio (HR) = 0.42; 95% CI: 0.20–0.89; P = 0.025) and a worse survival in those treated with platinum-based regimens (HR: 4.37; 95% CI = 1.95–9.77; P < 0.01).
We observed a significantly longer overall survival in patients receiving single-agent cyclophosphamide, with few grade 3 to 4 toxicities. Further studies should assess the efficacy of metronomic single-agent cyclophosphamide in advanced lines of treatment, as it may yield a survival benefit with low costs and no detrimental effects on quality of life.
INTRODUCTION
In 2012, an estimated 429,800 new cases of urothelial bladder cancer and 165,100 related deaths were reported worldwide.1 Bladder carcinoma is associated with multiple risk factors, which include smoking, occupational exposure, and diabetes, whereas fruit consumption has been associated with a decreased risk of having the disease.2,3 The prognosis of locally advanced (T3/T4 or N1)4 and metastatic disease5,6 is poor, with few approved therapeutic options. Platinum-based chemotherapy is the standard first-line treatment in patients with recurrent disease and is associated with a median survival of 14 to 15 months.5 In spite of a wealth of systemic options that showed activity after cisplatin failure,7 there is no universal consensus about the optimal salvage agent in this setting. Vinflunine is approved in Europe but not in the US on the basis of the results of a phase 3 trial that demonstrated its limited efficacy versus best supportive care alone after cisplatin failure with a significant survival improvement of 2.5 months in the eligible, but not in the intention-to-treat population (6.8 vs 4.3).6 Taxanes are also commonly used in cisplatin-pretreated patients, either alone or in combination with a variety of agents, such as gemcitabine, cyclophosphamide, ifosfamide, and epirubicin, with a meta-analysis indicating better efficacy of taxane-based combination chemotherapy versus single-agent taxane.8
In this retrospective, multi-institutional study, we observed patients at the time they received third-line systemic treatment for metastatic disease. Patients that were eligible for this study included (1) those that had relapsed < 12 months since the end of adjuvant chemotherapy and had then received a single line of treatment for metastatic disease; (2) patients who had relapsed > 12 months since the end of adjuvant chemotherapy and had received 2 lines of treatment for metastatic disease and (3) patients who had not received adjuvant chemotherapy and had received 2 lines of treatment for metastatic disease. The primary objective of our analysis was to assess the overall survival of the population and the survival differences in patients receiving different third-line treatments. The proportion of patients receiving third-line systemic treatment with respect to those treated with first-line therapy was also assessed, along with measures of third-line chemotherapy toxicity and efficacy.
PATIENTS AND METHODS
Inclusion Criteria
We retrospectively analyzed medical records of patients with a pathologic diagnosis of urothelial carcinoma treated with systemic chemotherapy for metastatic disease at 4 participating Italian Institutions between January, 2010, and January, 2015. Patients receiving at least 1 cycle of first-, second- and third-line systemic treatment for metastatic disease were included in this analysis. Salvage treatment administered if a patient had relapsed < 12 months since the end of adjuvant chemotherapy was considered as second-line treatment, whereas systemic treatment administered to a patient with metastatic disease who had relapsed > 12 months since the end of adjuvant chemotherapy was considered as first-line treatment, as discussed by Sonpavde et al.8 In order to avoid biases due to data incompleteness, the following data were required for inclusion in this retrospective study: overall survival, albumin levels, hemoglobin, performance status, time from prior chemotherapy, third-line chemotherapy received, and presence of liver metastasis. The protocol was notified to the Internal Institutional Review Board of the participating Institutions. Approval of retrospective observational studies by the ethics committee is not required according to the Italian law. This study was undertaken in accordance with principles of the Declaration of Helsinki and Good Clinical Practice guidelines.
Retrieved Data
Demographic data of eligible patients were retrieved along with clinical and histologic characteristics at the time of the third-line chemotherapy such as albumin levels, hemoglobin, performance status, prior chemotherapy, time from prior chemotherapy received, presence of liver metastasis. The following data were extracted about previous chemotherapy: duration of treatment, number of cycles, time from treatment initiation to third-line chemotherapy. Albumin levels, hemoglobin, and performance status (PS) were required to be assessed within 15 days before third-line chemotherapy initiation. The presence of liver metastasis had to be assessed with CT scan within 30 days before third-line treatment initiation. Data about overall survival, progression, response, grade 3 to 4 toxicity, and treatment suspension/interruption associated with third-line treatment were collected. Patients alive as of June 1, 2015, or who were lost at follow-up were censored from the survival analysis. Toxicity severity was defined according to the National Cancer Institute common toxicity criteria (version 4.0), if applicable. Response associated with treatment was assessed by the treating physician using the RECIST criteria 1.0 according to Institutional policy and was retrieved by the reviewed charts.
Data Analysis
Descriptive statistics and frequency counts were used to summarize characteristics of the study population. Median numbers were presented with interquartile ranges, unless specified otherwise (IQR). Progression was defined as objective tumor progression or death from any cause and was calculated using the Kaplan–Meier method. Progression-free survival (PFS) was calculated from the date of study entry until progression or death. Overall survival (OS) was calculated from the date of study entry until death from any cause and was calculated using the Kaplan–Meier method. Comparison of continuous variables between independent groups was performed by the use of Mann–Whitney U test. Cox proportional hazards regression was used to evaluate the association of the chemotherapy agent used versus others with overall survival, adjusted for 5 externally validated prognostic factors in advanced urothelial carcinoma.9 Internal validation was performed using bootstrap methods, with 95% bias-corrected and accelerated (BCa) confidence intervals (CIs) and P values calculated. All tests were 2-sided, and a value of P ≤0.05 was considered statistically significant. All calculations were performed using SPSS IBM v. 23.
RESULTS
Study Population
Of 182 patients that received first-line chemotherapy/adjuvant chemotherapy as defined above, 116 patients (63.73%) received second-line salvage treatment and 61 (33.5%) received third-line chemotherapy for metastatic disease. Fifty-two patients were finally included in this analysis, whereas 9 were excluded due to missing data (PS in 2 cases, albumin in 5 cases, overall survival in 3 cases, hemoglobin in 1 case, and presence of liver metastasis in 6 cases). Of the 34 patients who had received prior adjuvant chemotherapy, 7 had a recurrence within 12 months since the last cycle of adjuvant chemotherapy and were treated with second-line chemotherapy, whereas the remaining had a recurrence > 12 after the last cycle of adjuvant chemotherapy and were treated with first-line chemotherapy(Table 1). First-line chemotherapy was administered in 45 patients, who received a median of 5 cycles (interquartile range, 5–6), with a median treatment duration of 18 weeks (16–21,3). First-line treatment was platinum-based in the majority of cases. Median time from the beginning of first-line treatment to the beginning of third-line treatment was 47 weeks (range: 39–51). Seven patients did not receive first-line treatment. These patients received a median of 6 cycles of adjuvant chemotherapy (range: 5–6; median treatment duration: 17; range 15–18). In these 7 patients, median time from the beginning of adjuvant chemotherapy to the beginning of third-line chemotherapy was 74 weeks (range: 67–74,5). All patients received second-line chemotherapy (median number of cycles, 4, range: 3–5; median treatment duration 15.5 weeks; range: 12.8–18). The median time from the beginning of second-line treatment to the beginning of third-line treatment was 21.5 weeks (range 17.8–24). Second-line treatment was based on vinflunine or docetaxel/paclitaxel. Doses and schedules used for chemotherapy before third-line treatment are detailed in Table 2.
TABLE 1.
Characteristics of the Patients’ Population (N = 52)
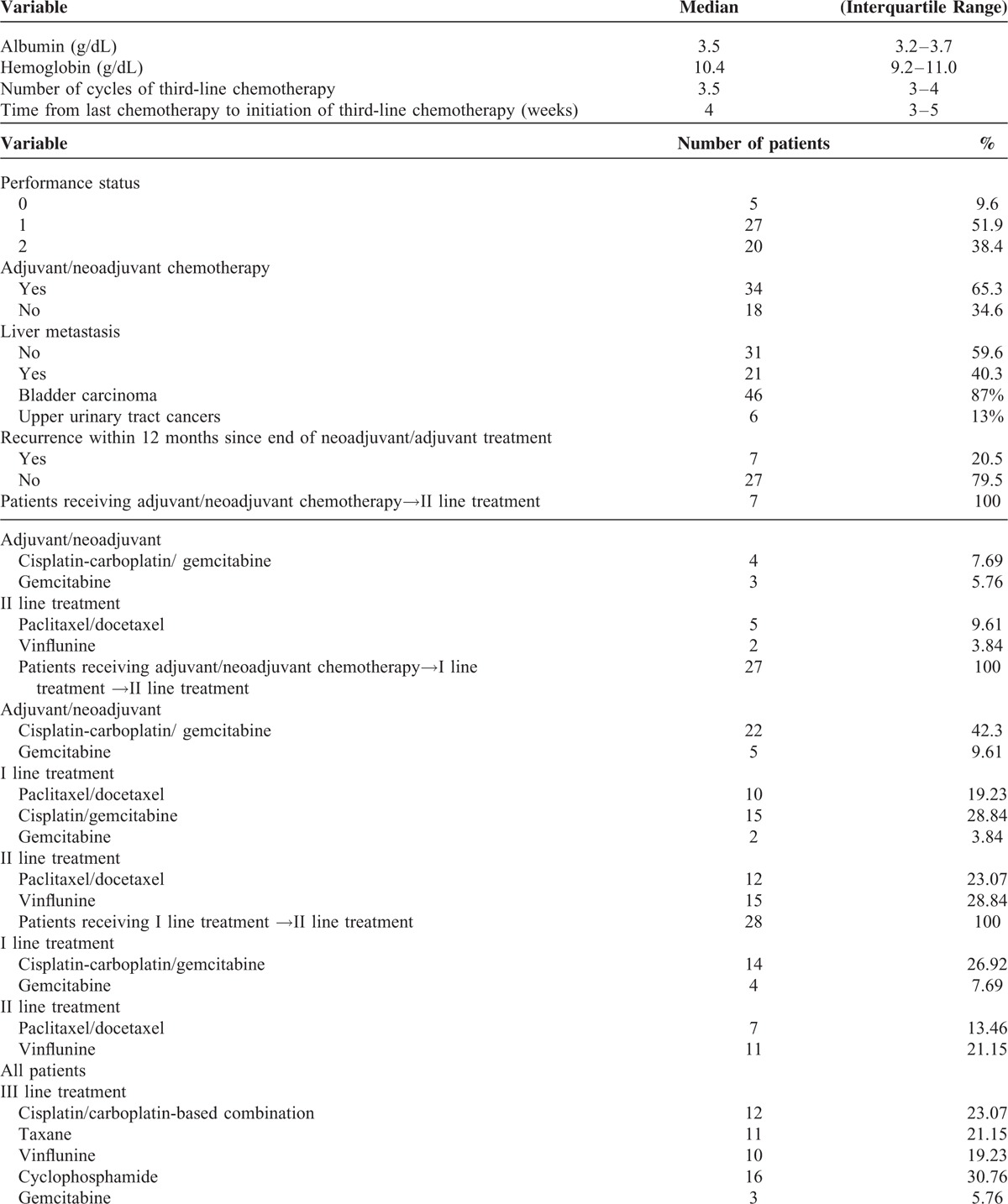
TABLE 2.
Regimens Used Before Third-Line Chemotherapy
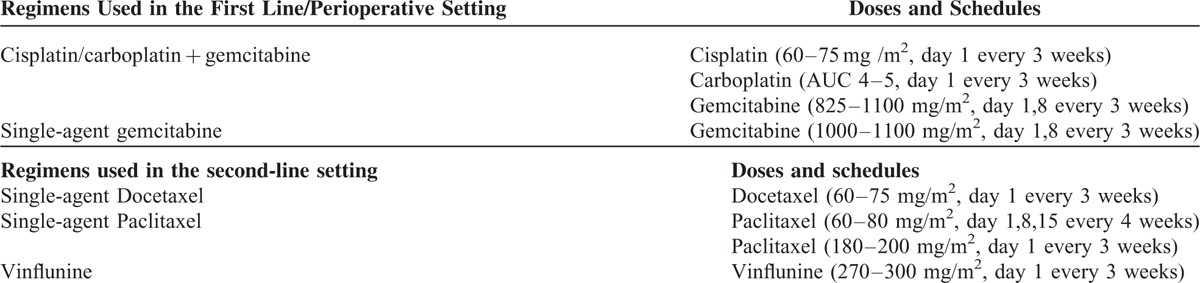
Third-Line Chemotherapy
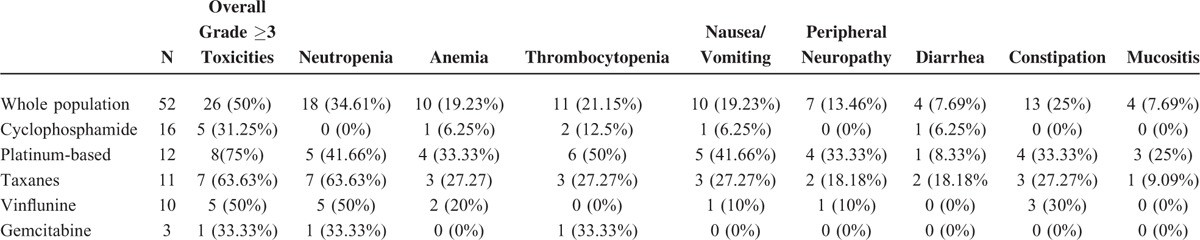
Third-line chemotherapy was based on cyclophosphamide, platinum, vinflunine, taxanes, and gemcitabine in 16, 12, 11, 10, and 3 patients, respectively. Cyclophosphamide was delivered orally as a single agent at 100 mg daily, with no interruption in all patients, and it was cycled every 28 days. Four patients received MVAC, consisting of methotrexate 20 to 30 mg/m2 on day 1 or 2, vinblastine 1.5 to 2 mg/m2 on day 2, doxorubicin 20 to 30 mg/m2 on day 2, and cisplatin 40 to 50 mg/m2 or carboplatin AUC 3 to 4 on day 2, every 21 days. Alternatively, patients received cisplatin 40 to 50 mg/m2 /carboplatin AUC 3 to 4 in combination with either methotrexate 20 mg/m2 (5 patients) or gemcitabine (800 mg/m2) (3 patients). Of these, 4 patients had been treated with 2 prior lines of platinum-based chemotherapy. Vinflunine was delivered at 200 to 250 mg/m2 every 3 weeks. Taxanes included single-agent paclitaxel (150–170 mg/2 3 weekly) (8 patients) or 3 weekly docetaxel 50 to 60 mg/m2 (3 patients). Gemcitabine was administered as a single-agent treatment at the dose of 800 to 1000 mg/m2. No significant differences were found between patients receiving different third-line treatments with respect to previous first- and second-line treatment received (data not shown). Median PFS and OS of the population were 13 (10–17) and 31 (28–36) weeks. Single-agent cyclophosphamide was associated with a PFS of 18 (13–22) and an OS of 38 (33–41) weeks, whereas platinum-based combination was associated with a PFS of 5 weeks and an OS of 8 weeks. Ten patients were judged to have a radiological response by their treating physician. Only 1 patient reported a complete response, which was associated with single-agent cyclophosphamide (Fig. 1). This patient was an 82-year old man with a poor PS and lymphnode-only disease, who was treated with single-agent cyclophosphamide for 56 weeks and went on to receive fourth-line treatment after progression. This patient had previously received 6 cycles of platinum-based treatment as first-line therapy for metastatic disease, but he had received only 4 administrations of weekly paclitaxel as second-line treatment. Anecdotally, this patient also consumed quercetin, provided as a courtesy of Quercegen Pharmaceuticals, during the entire treatment duration. Outcomes and toxicity associated with third-line chemotherapy are detailed in Tables 3 and 4, respectively.
FIGURE 1.
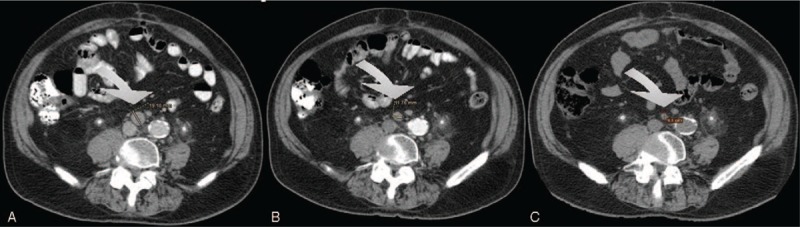
Complete radiologic response in a patient treated with 100 mg cyclosphosphamide daily. (A = baseline; B = after 2 months; C = after 4 months).
TABLE 3.
Outcome Associated With the Third-Line Regimen Used (N = 52)
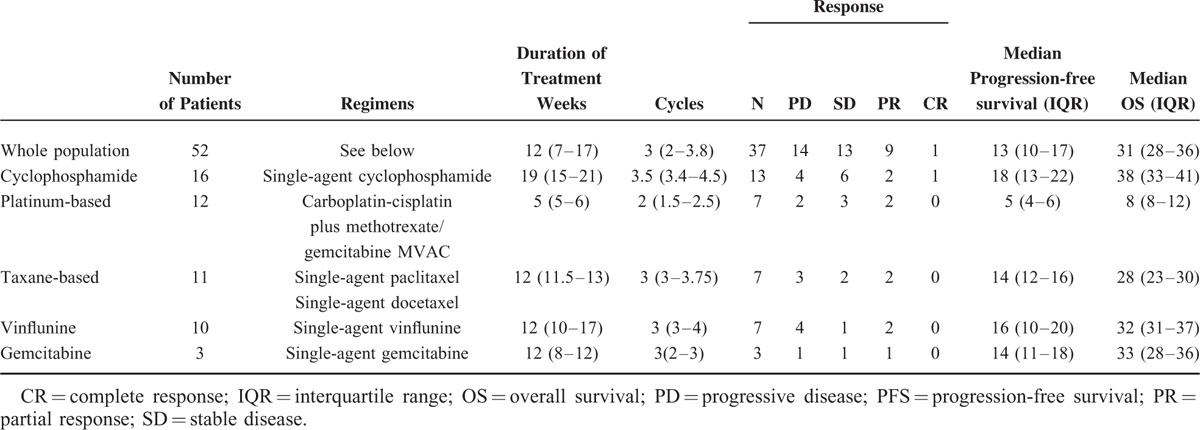
TABLE 4.
Grade 3–4 Toxicities of Associated With Third-Line Treatments
Univariate and Multivariate Analysis for OS
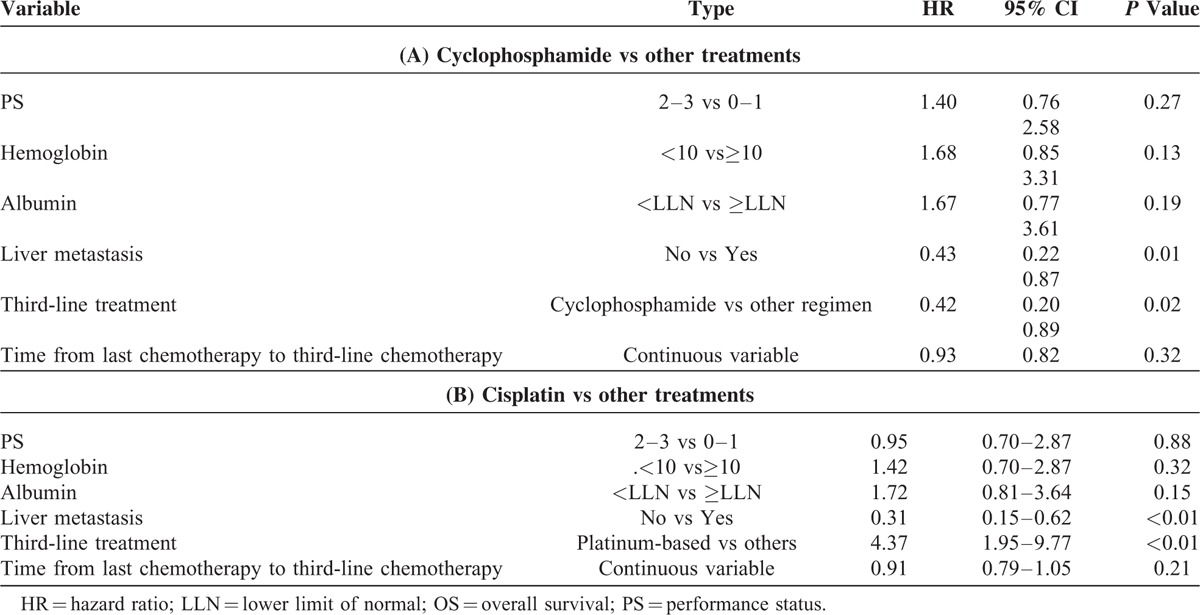
Univariate analysis showed that PS (2–3 vs 0–1), albumin levels ≤ULN vs ≥ULN, presence of liver metastasis (yes vs no), use of taxanes and platinum compounds versus other agents were associated with a significantly worse survival in this patient population. Lower hemoglobin levels and shorter time from prior chemotherapy (continuous variable) were only marginally significant. At univariate analysis, we observed that single-agent cyclophosphamide was associated with longer OS (HR = 0.3), whereas platinum- and taxane-based chemotherapy were associated with shorter OS (HR = 4.7 and HR = 2.5, respectively) (Table 5 and Fig. 2). The better survival associated with cyclophosphamide was also confirmed at multivariate analysis (HR = 0.42; 95% CI: 0.20–0.89; P = 0.025) (Table 6A). Platinum-based (Table 6B) but not taxane-based (data not shown) chemotherapy was associated with a worse survival at multivariate analysis. Bootstrapping performed to internally validate the results showed that the 95% BCa CI for the HR for OS of cyclophosphamide versus other agents was 0.13 to 0.82 (P = .07), while bootstrapped 95% BCa CI for the HR for the use of cisplatin was (1.94–59.73; P = 0.01).
TABLE 5.
Univariable Analyses for Association of Variables With OS
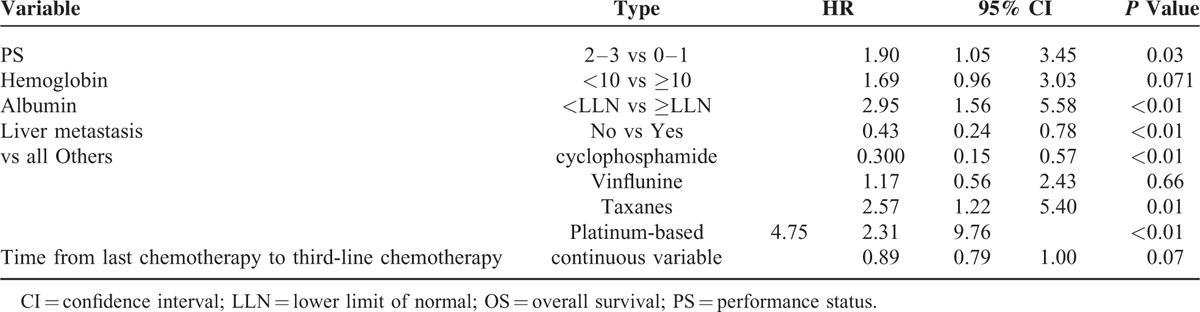
FIGURE 2.
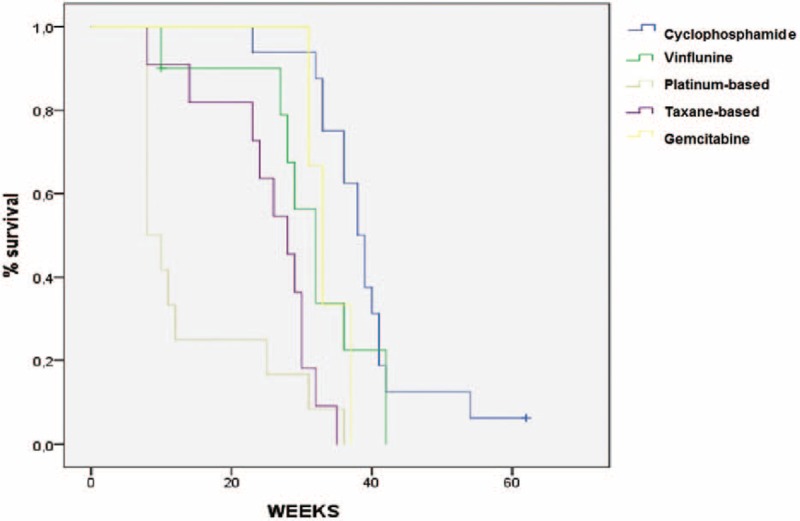
Overall survival in patients treated with different third-line regimens.
TABLE 6.
A Multivariable Analyses for association of variables with OS: A (cyclophosphamide vs other treatments); B: (cisplatin vs other treatments)
DISCUSSION
The setting of patients treated with third-line chemotherapy for metastatic disease has been poorly explored in urothelial carcinoma. One meta-analysis including 731 patients7 enrolled in several phase II trials showed that progression-free survival and overall survival were not influenced by the number of prior treatment lines, which were calculated by including both adjuvant and metastatic treatments. This analysis was not conceived to capture the potential prognostic differences of patients receiving prior treatment in the adjuvant versus the metastatic setting or with different progression-free survival times after adjuvant treatment, so it cannot be concluded that these patients should be grouped together for prognostic purposes. As at the participating Institutions patients relapsing < 12 months after finishing adjuvant chemotherapy generally receive second-line treatment, whereas patients relapsing >1 year after receiving adjuvant treatment are generally candidates for a first-line platinum-based regimen, we used a similar approach to define the third-line setting in this study. This approach was consistent with the findings of a retrospective analysis of 41 patients receiving cisplatin-based first-line therapy for advanced urothelial carcinoma following previous perioperative cisplatin-based therapy. In this study, the median overall survival was 68 weeks, with patients relapsing <52 weeks, between 52 and 104 weeks, and after 104 since cisplatin completion showing a median survival of 42, 70, and 162 weeks, respectively.10 Furthermore, patients relapsing <12 months after completion of adjuvant treatment are considered to be eligible for inclusion in a second-line treatment salvage trial.11 In our retrospective study, we found that approximately half of the patients treated with second-line chemotherapy went on to receive third-line treatment, which underlines the clinical meaningfulness of this setting, and that third-line treatment was associated with a median PFS of 13 weeks and a median OS of 31 weeks, which is line with the median PFS of 2 to 4 months and the median OS of 6 to 9 months reported in other published series.12–15 Only a few studies have been specifically conducted in the third-line setting in patients with urothelial carcinoma. Matsumoto16 et al evaluated the efficacy of gemcitabine plus nedaplatin in 10 patients with metastatic urothelial carcinoma who had been previously treated with methotrexate, vinblastine, doxorubicin, and cisplatin, followed by gemcitabine and paclitaxel. The median overall survival was 8.8 months, whereas the median progression-free survival was 5.0 months. Similarly, the use of pegylated liposomal doxorubicin as a third-line chemotherapy was associated with a median progression free survival and overall survival of 4.1 and 6.3 months in 23 patients with advanced urothelial carcinoma, whereas median time to progression and overall survival were 2 and 7.3 months, respectively, in 13 patients treated with third-line gemcitabine.17,18 These results are consistent with those obtained in the overall population of patients treated with third-line chemotherapy. In our study, substantial heterogeneity in outcome was reported in patients treated with different agents and regimens. Surprisingly, patients receiving platinum-based combinations showed a median survival of only 8 weeks (8–12). A detrimental effect of platinum-based treatment was confirmed after controlling for known prognostic factors, with an HR for death of 4.3 (95% 1.95–9.77). In 45 patients receiving accelerated MVAC (methotrexate, vinblastine, adriamycin, and cisplatin) after platinum-gemcitabine chemotherapy, median time to progression and median overall survival were 5.8 and 14.2 months, respectively.19 In the subset of 12 patients treated with platinum-based chemotherapy analyzed in our work only 4 patients received MVAC, whereas the remaining patients received a combination of a platinum agent with gemcitabine or methotrexate. Platinum-based chemotherapy was poorly tolerated, with grade 3 to 4 hematologic toxicities reported in ∼50% of patients, whereas grade 3 to 4 nausea/vomiting, peripheral neuropathy, and diarrhea/constipation were reported in 30 to 40% of patients. The heterogeneity of the platinum-based chemotherapy delivered along with the prior exposure to platinum agents can explain the poor outcome observed in this subset of patients. Conversely, patients treated with single-agent cyclophosphamide showed a median OS of 38 (33–41) weeks, which was coupled with a median progression-free survival of 18 weeks. Furthermore, patients treated with cyclophosphamide received a median of 3.5 cycles and showed a very low rate of grade 3 to 4 toxicities. Multivariate analysis of the use of cyclophosphamide versus other agents showed a substantial reduction of the risk of death, with an HR of 0.42; 95% CI: 0.20 to 0.89, after controlling for established prognostic factors. To the best of our knowledge, there are no previous reports on the use of single-agent cyclophosphamide in urothelial carcinoma. In a phase I/II trial on the combined use of paclitaxel with cyclophosphamide, Di Lorenzo et al that showed that the maximum tolerated daily dose of cyclophosphamide administered on days 1 to 7 in combination with 175 mg/m(2) of paclitaxel administered on day 1 was only 50 mg. This regimen was associated with a median time to progression of 5 months (95% CI, 2 months–7.5 months) and a median OS of 8 months (95% CI, 4 months–14 months). In the 32 patients enrolled in the phase II part of the trial, grade 1/2 vomiting, peripheral neuropathy, and neutropenia were reported in 34%, 25%, and 31% of cases, respectively, whereas grade 3 to 4 neutropenia was the most common severe toxicity, occurring in 34.5% of patients. Another phase II trial conducted in 46 patients with bladder or upper urinary tract cancer treated with paclitaxel + cyclophosphamide with the same doses and schedule used by Di Lorenzo et al showed a median time to progression of 3.0 months (95% CI 1.7–4.3 months), an objective response rate of 33.3%, and a median OS of 6.3 months (95% CI 4.6–8.0 months). Differently from the results by Di Lorenzo et al, grade ≥ 3 neutropenia occurred only in 2 patients (6%), with one of them developing febrile neutropenia. Interestingly, cyclophosphamide was daily administered without interruption after the first cycle, with no apparent increased toxicity. This finding is consistent with the results obtained in our series, which showed an excellent safety and efficacy profile for continuous metronomic cyclophosphamide. The absence of any concomitant treatment allowed us to double the dose (100 mg) used with respect to published trials. Metronomic cyclophosphamide, which has been tested in several solid tumors, including breast, prostate, and bladder cancer, exerts a potent anti-angiogenic activity in preclinical models.20,21 Furthermore, it may also contribute to controlling tumor progression through an immunostimulatory effect, which induces a reduction in circulating regulatory T cells (Treg), leading to restoration of natural killer cell cytotoxicity, and peripheral T-cell proliferation.22 Of note, we were intrigued by a complete and durable response achieved with single-agent cyclophosphamide in an 82-year old man, who also consumed quercetin supplements. Although quercetin has been extensively investigated in preclinical models of various types of cancer (eg leukemia),23 and also in murine models of breast cancer in combination with cyclophosphamide,24 there is a lack of clinical data about its effects if used in combination with chemotherapy agents, and additional studies are required to define its potential role in cancer patients. The major strength of our study includes the selection of patients treated in a poorly explored setting.
Limitations typical of retrospective studies apply to our work. Chemotherapy regimens were not administered according to a protocol defining doses, schedules, dose reduction algorithms, and so on. Analyzing differences in outcomes associated with distinct treatments in a nonrandomized study is biased by a number of imbalances between the subgroups considered that can only be partially taken into account by using multivariate analysis. In this regard, it must be noted that the accuracy of the model9 based on the 5 prognostic variables that were included in the multivariate analysis of this work does not exceed 62%, so the apparently improved prognosis of patients treated with cyclophosphamide may be due to more favorable baseline characteristics. We therefore believe that no conclusion can be made on the grounds of the data presented here about differences of the various treatments administered in the third-line setting in our patient population, although a number of hypotheses can be generated. In our series, besides being associated with an unfavorable effect on survival with respect to other treatments, taxane- and cisplatin-based chemotherapy was associated with a 63% and 75% overall incidence of grade 3 to 4 toxicity, respectively. In the study mentioned above involving 45 patients receiving accelerated MVAC after platinum-gemcitabine regimen, the median time to progression and overall survival, respectively, were 9.6 and 16.5 months when gemcitabine-platinum was given in the adjuvant setting (40% of patients), but only 4.4 and 5.7 months when gemcitabine-platinum was administered in the metastatic settings (60% of patients). Of note, grade 3 to 4 toxicities were reported in 31 patients (69%), with 4 sepsis-related deaths.19 Consistently with the results described in our series, these findings suggest that platinum-based chemotherapy should be administered early throughout the course of advanced bladder cancer, whereas vinflunine and taxane-based chemotherapy are valuable second-line treatment option, although only vinflunine has been tested in a phase III trial. Due to the radiologic activity observed coupled with its excellent safety profile, metronomic cyclophosphamide may be an option in the third-line setting, if supported by adequate evidence provided in prospective clinical trials. Patients who are not considered fit to receive intravenous chemotherapy may tolerate single-agent metronomic cyclophosphamide.
CONCLUSIONS
In the patient population of 52 patients of this retrospective study, we showed that the median overall survival associated with the use of third-line chemotherapy was 31 (28–36) weeks, which suggests that third-line chemotherapy can be administered with benefit to suitable patients. Unexpectedly, we observed a significantly longer overall survival in patients receiving single-agent cyclophosphamide, with few grade 3 to 4 toxicities, and a shorter survival in patients treated with platinum-based combinations, with a high rate of grade 3 to 4 toxicities. Given the lack of any approved treatment in heavily pretreated patients with urothelial carcinoma, further studies should explore the efficacy of metronomic single-agent cyclophosphamide, which may yield a survival benefit with low costs and no detrimental effect on quality of life, due to its oral route of administration and low toxicity.
Footnotes
Abbreviations: BCA = bias-corrected and accelerated, HR = hazard ratio, IQR = interquartile range, MVAC = methotrexate, vinblastine, adriamycin, and cisplatin, OS = overall survival, PFS = progression-free survival.
GDL and CB equally contributed to this study.
Funding: this work was partially supported by LILT (Lega Italiana alla Lotta ai Tumori) - Sez. Napoli.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65:87–108. [DOI] [PubMed] [Google Scholar]
- 2.Chen HF, Chen SW, Chang YH, Li CY. Risk of malignant neoplasms of kidney and bladder in a cohort study of the diabetic population in Taiwan with age, sex, and geographic area stratifications. Medicine (Baltimore) 2015; 94:e1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu C, Zeng XT, Liu TZ, et al. Fruits and vegetables intake and risk of bladder cancer: a PRISMA-compliant systematic review and dose-response meta-analysis of prospective cohort studies. Medicine (Baltimore) 2015; 94:e759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferro M, De Cobelli O, Buonerba C, et al. Modified Glasgow prognostic sccore is associated with risk of recurrence in bladder cancer patients after radical cystectomy: a multicenter experience. Medicine (Baltimore) 2015; 94:e1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol 2005; 23:4602–4608. [DOI] [PubMed] [Google Scholar]
- 6.Bellmunt J, Theodore C, Demkov T, et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol 2009; 27:4454–4461. [DOI] [PubMed] [Google Scholar]
- 7.Pond GR, Bellmunt J, Rosenberg JE, et al. Impact of the number of prior lines of therapy and prior perioperative chemotherapy in patients receiving salvage therapy for advanced urothelial carcinoma: implications for trial design. Clin Genitourin Cancer 2015; 13:71–79. [DOI] [PubMed] [Google Scholar]
- 8.Sonpavde G, Pond GR, Choueiri TK, et al. Single-agent taxane versus taxane-containing combination chemotherapy as salvage therapy for advanced urothelial carcinoma. Eur Urol [DOI] [PubMed] [Google Scholar]
- 9.Sonpavde G. Externally validated improved 5-factor prognostic model in patients (pts) receiving salvage systemic therapy for advanced urothelial carcinoma (UC). J Clin Oncol 2015; 33 (suppl): abstr 4527. [Google Scholar]
- 10.Necchi A, Pond GR, Giannatempo P, et al. Cisplatin-based first-line therapy for advanced urothelial carcinoma after previous perioperative cisplatin-based therapy. Clin Genitourin Cancer 2015; 13:178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sonpavde G, Bellmunt J, Rosenberg JE, et al. Patient eligibility and trial design for the salvage therapy of advanced urothelial carcinoma. Clin Genitourin Cancer 2014; 12:395–398. [DOI] [PubMed] [Google Scholar]
- 12.Choueiri TK, Ross RW, Jacobus S, et al. Double-blind, randomized trial of docetaxel plus vandetanib versus docetaxel plus placebo in platinum-pretreated metastatic urothelial cancer. J Clin Oncol 2012; 30:507–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albers P, Park SI, Niegisch G, et al. Randomized phase III trial of 2nd line gemcitabine and paclitaxel chemotherapy in patients with advanced bladder cancer: short-term versus prolonged treatment [German Association of Urological Oncology (AUO) trial AB 20/99]. Ann Oncol 2011; 22:288–294. [DOI] [PubMed] [Google Scholar]
- 14.Di Lorenzo G, Montesarchio V, Autorino R, et al. Phase 1/2 study of intravenous paclitaxel and oral cyclophosphamide in pretreated metastatic urothelial bladder cancer patients. Cancer 2009; 115:517–523. [DOI] [PubMed] [Google Scholar]
- 15.Necchi A, Mariani L, Zaffaroni N, et al. Pazopanib in advanced and platinum-resistant urothelial cancer: an open-label, single group, phase 2 trial. Lancet Oncol 2012; 13:810–816. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto K, Mochizuki K, Hirayama T, et al. Gemcitabine plus nedaplatin as salvage therapy is a favorable option for patients with progressive metastatic urothelial carcinoma after two lines of chemotherapy. Asian Pac J Cancer Prev 2015; 16:2483–2487. [DOI] [PubMed] [Google Scholar]
- 17.Rozzi A, Santini D, Salerno M, et al. Pegylated liposomal doxorubicin as third-line chemotherapy in patients with metastatic transitional cell carcinoma of urothelial tract: results of a phase II study. Med Oncol 2013; 30:407. [DOI] [PubMed] [Google Scholar]
- 18.Soga N, Kise H, Arima K, Sugimura Y. Third-line gemcitabine monotherapy for platinum-resistant advanced urothelial cancer. Int J Clin Oncol 2010; 15:376–381. [DOI] [PubMed] [Google Scholar]
- 19.Edeline J, Loriot Y, Culine S, et al. Accelerated MVAC chemotherapy in patients with advanced bladder cancer previously treated with a platinum-gemcitabine regimen. Eur J Cancer 2012; 48:1141–1146. [DOI] [PubMed] [Google Scholar]
- 20.Gnoni A, Silvestris N, Licchetta A, et al. Metronomic chemotherapy from rationale to clinical studies: a dream or reality? Crit Rev Oncol Hematol 2015; 95:46–61. [DOI] [PubMed] [Google Scholar]
- 21.Penel N, Adenis A, Bocci G. Cyclophosphamide-based metronomic chemotherapy: after 10 years of experience, where do we stand and where are we going? Crit Rev Oncol Hematol 2012; 82:40–50. [DOI] [PubMed] [Google Scholar]
- 22.Le DT, Jaffee EM. Regulatory T-cell modulation using cyclophosphamide in vaccine approaches: a current perspective. Cancer Res 2012; 72:3439–3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russo GL, Russo M, Spagnuolo C, et al. Quercetin: a pleiotropic kinase inhibitor against cancer. Cancer Treat Res 2014; 159:185–205. [DOI] [PubMed] [Google Scholar]
- 24.Indap MA BSC, Shinde AD, Barkume MS. Tumour response to quercetin, a bioflavonoid with some promises in therapies. Indian J Pharm Sci 2006; 68:570–574. [Google Scholar]


