Abstract
Sleep-related movement disorders (SRMD) are sleep disorders. As poor sleep quality is associated with cognitive impairment, we hypothesized that SRMD patients were exposed to a great risk for developing dementia.
The present study was aimed to retrospectively examine the association of SRMD and dementia risk.
A retrospective longitudinal study was conducted using the data obtained from the Longitudinal Health Insurance Database (LHID) in Taiwan. The study cohort enrolled 604 patients with SRMD who were initially diagnosed and 2416 patients who were randomly selected and age/gender matched with the study group. SRMD, dementia, and other confounding factors were defined according to International Classification of Diseases Clinical Modification Codes. Cox proportional-hazards regressions were employed to examine adjusted hazard ratios (HR) after adjusting with confounding factors.
Our data revealed that patients with SRMD had a 3.952 times (95% CI = 1.124–4.767) higher risk to develop all-cause dementia compared with individuals without SRMD. The results showed that SRMD patients aged 45 to 64 exhibited highest risk of developing all-cause dementia (HR: 5.320, 95% CI = 1.770–5.991), followed by patients age ≥65 (HR: 4.123, 95% CI = 2.066–6.972) and <45 (HR: 3.170, 95% CI = 1.050–4.128), respectively. Females with SRMD were at greater risk to develop all-cause dementia (HR: 4.372, 95% CI = 1.175–5.624). The impact of SRMD on dementia risk was progressively increased by various follow-up time intervals (<1 year, 1–2 years, and ≥2 years).
The results suggest that SRMD is linked to an increased risk for dementia with gender-dependent and time-dependent characteristics.
INTRODUCTION
Sleep is a sophisticated process involving a complex interaction of various neurotransmitter systems and an oscillation of homeostatic and circadian systems to initiate and maintain the activity. Insufficient sleep increases a risk for developing various chronic disease and conditions including obesity, diabetes, and cardiovascular diseases.1,2 Sleep disturbance is a common symptom in patients with neurodegenerative disorders with diverse etiology such as Parkinson disease.3–5 Recent studies have demonstrated that disordered sleep is associated with cognitive impairment and memory loss.6–8 Moreover, poor sleep quality has been associated with brain atrophy.7,9–12 As the nature of sleep disturbance is diverse, the neurocognitive consequences of sleep disturbance after onset over time remain to be determined.
Sleep-related movement disorders (SRMD) are considered as a class of sleep disorders, which is characterized by simple, stereotyped repetitive movements during sleep. Patients with SRMD are reported to experience fragmented sleep, disturbance of sleep initiation, and excessive daytime sleepiness, resulting in decreased quality of life.13,14 Among SRMD, periodic limb movement disorder (PLMD) and restless legs syndrome (RLS) are most common sleep complaints, which involve nocturnal involuntary limb movements. The prevalence of PLMD and RLS has been reported to be 3 to 10% of general population, increasing with age.15 Both conditions have been reported to be associated with several physical disorders and mental abnormalities. They have been linked to poor quality of life through fatigue, compromised work performance, and impaired social and family life.16,17 It has been suggested that SRMD is a common complication and comorbidity of neurodegenerative disorders such as Parkinson disease. However, the relation of SRMD such as PLMD and RLS with cognitive impairments like dementia remains sketchy.
Researches investigating relationship between sleep disorders and cognitive illness have predominantly focused on sleep behavior disorders and degenerative dementia. The present study was aimed to ascertain whether patients with SRMD had an elevated risk of developing all-cause dementia during more than 5 years of follow-up, using data from the National Health Insurance Research Database.
METHODS
Database
This retrospective study was conducted with an aim to investigate the association of SRMD and dementia risk using the data retrieved from Longitudinal Health Insurance Database (LHID) released by the Taiwan National Health Research Institute. LHID contains all original claims data of 1 million beneficiaries, randomly sampled from the registry for Beneficiaries of National Health Insurance Research Database with more than 23 million individuals enrolled into the National Health Insurance (NHI) program, the universal payer for healthcare in Taiwan. The LHID contains records on inpatient, outpatient, and ambulatory care services, covering the period from 1997 to 2010. The diagnostic coding system for disease accepted by the NHI is the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). NHI has a rigorous monitoring system to ensure that the claims for reimbursement for healthcare are based on valid diagnoses. NHI randomly audits the healthcare records to validate medical claims for diagnosis and treatment. Previous reports have shown the reliability of the diagnosis coding in the LHID. This study protocol was approved by Institutional Review Board of Tri-Service General Hospital (TSGHIRB No.: 2-104-05-045). Because data in the LHID are deidentified, the signed informed consent of participants was waived.
Study Samples
The study group and a control cohort were selected from the LHID. The diagnoses of SRMD and dementia were the basis for claims for reimbursement for relevant services rendered by the hospitals and physicians. NHI maintains stringent regulations and makes periodic auditing of claims field for reimbursement. The study group comprised all patients who had been diagnosed for SRMD based on ICD-9-CM codes 327.5 or 333.9 for the first time from 2000 to 2005 (N = 604). Patients diagnosed of SRMD before 2000 were excluded to increase the likelihood of identifying new cases. From the beginning of 2000 to the end of 2005 during which a patient was first diagnosed with SRMD was set as the index date. We randomly selected 2416 subjects (a sample size 4-fold that of the SRMD group), frequency matched with the study cohort in terms of age, gender, and comorbidities including hypertension, diabetes, ischaemic heart diseases, hyperlipidemia, smoking, alcoholism, obesity, atrial fibrillation, Parkinson disease, cerebrovascular accident, major depression, chronic kidney disease, and index date. Each patient was then followed up from the index date until the occurrence of dementia. For those who did not have dementia, the last day of follow-up was defined as the date of insurance withdrawal or the last day of the study period (December 31, 2010).
Definitions of Dementia Subtypes by ICD Classification
Dementia subtypes were classified into all-cause dementia (ICD-9-CM codes 290-294, 330.0, 331.0), Alzheimer disease (AD, ICD-9-CM codes 290.1, 331.0), and vascular dementia (ICD-9-CM code 290.4). Comorbidities that are also dementia risk factors were established before the index date based on outpatient data with the following ICD codes: hypertension (ICD-9-CM codes 401-405), diabetes (ICD-9-CM code 250), ischaemic heart diseases (ICD-9-CM codes 410-414), hyperlipidemia (ICD-9-CM code 272), tobacco use disorder (ICD-9-CM code 305.1), alcoholism (ICD-9-CM codes 291, 303.9, 334.4, 980.0), obesity (ICD-9-CM codes 278, 649.1, 783.1), atrial fibrillation (ICD-9-CM code 427.3), Parkinson disease (ICD-9-CM code 332.0), cerebral vascular accident (ICD-9-CM codes 430-432, 433-437), major depression (ICD-9-CM code 296), and chronic kidney disease (ICD-9-CM code 585).
Statistical Analysis
Continuous variables were presented as mean ± SD and categorical variables as frequencies and percentages. Differences between study group and control cohort in the distribution of demographic characteristics (age and gender) and comorbidities (hypertension, diabetes, ischaemic heart diseases, hyperlipidemia, smoking, alcoholism, obesity, atrial fibrillation, Parkinson disease, cerebrovascular accident, major depression, and chronic kidney disease) were examined by Chi-square/Fisher exact test. Cox proportional hazard regression analysis was performed to calculate adjusted hazard ratios (HR), with 95% confidence intervals (CIs), for the impact of SRMD on developing dementia. To investigate the interaction of covariates in relation to the association of SRMD and dementia, we also calculated adjusted HR stratified by age (<45, 45–64, ≥65 years), gender, and follow-up time. All statistical analyses were performed with SPSS software version 22.0. A 2-tailed P value less than 0.05 was considered statistically significant.
RESULTS
A total of 604 patients diagnosed with SRMD and 2416 gender- and age-matched controls for comparison were included in this cohort study. Demographic characteristics of both groups were presented in Table 1. There were no significant differences in distribution of age, gender, and comorbidities between the study group with SRMD and the control cohort. Our data showed that over 5-year follow-up, 12.7% of SRMD patients (n = 77) developed all-cause dementia with an overall rate of 34.44 cases per 1000 person-years, whereas 89 non-SRMD individuals (3.7%) had dementia. The results revealed that patients with SRMD had a 3.952 times (95% CI = 1.124–4.767) higher risk to develop all-cause dementia compared with individuals without SRMD (Table 2). To explore whether SRMD is an age-dependent risk factor for all-cause dementia, patients were divided into 3 groups according to age, namely <45, 45 to 64, and ≥65 years. The results showed that in comparison with age/gender matched controls, SRMD patients aged 45 to 64 exhibited highest risk of developing all-cause dementia (HR: 5.320, 95% CI = 1.770–5.991), followed by patients age ≥65 (HR: 4.123, 95% CI = 2.066–6.972), and <45 (HR: 3.170, 95% CI = 1.050–4.128), respectively. We also examined if SRMD is a sex-dependent risk factor for developing dementia. The Cox regression analysis revealed that female SRMD patients had greater risk to develop all-cause dementia (HR: 4.372, 95% CI = 1.175–5.624). We next analyzed the incidence of dementia and dementia subtypes using multivariate Cox proportional hazards regression analysis based on time intervals. Our data showed that patients with SRMD were likely to develop dementia within a year after diagnosis with time-dependent characteristic (Table 3). Kaplan–Meier analysis showed that, compared to the matched controls, patients with SRMD had significantly higher incidence of all-cause dementia (log-rank test P < 0.001), AD (log-rank test P = 0.032), and vascular dementia (log-rank test P = 0.003) (Fig. 1).
TABLE 1.
Baseline Demographic Status and Comorbidities Compared Between Comparison and SRMD Group
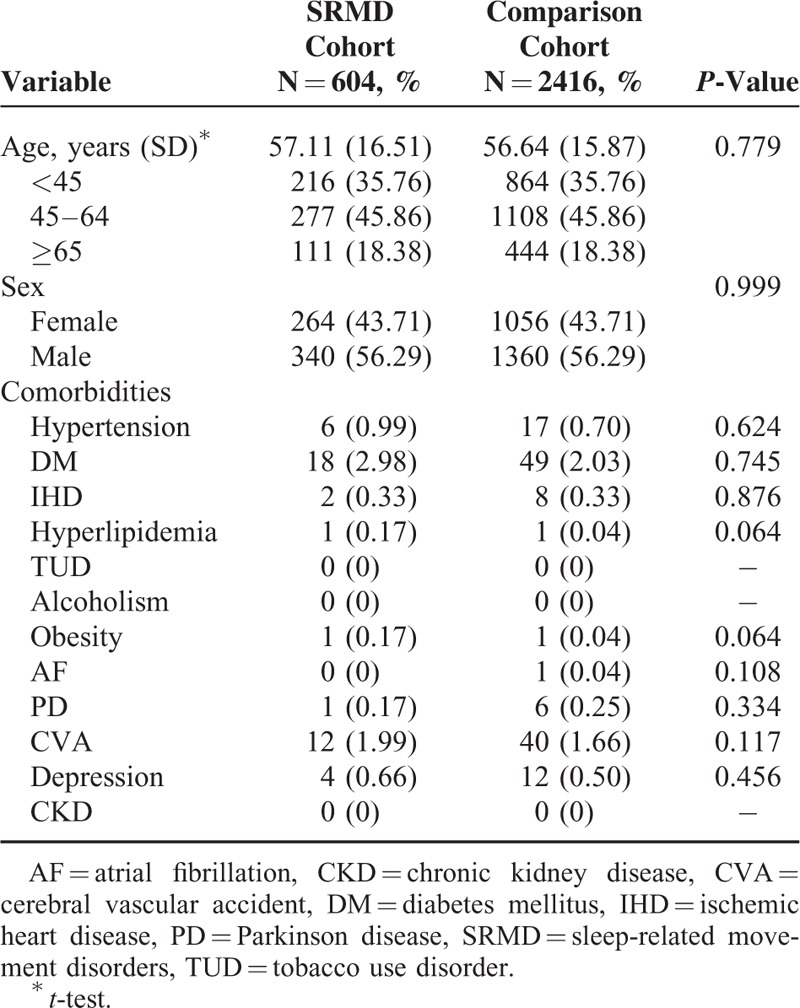
TABLE 2.
Incidence of Dementia and Dementia Subtypes and Multivariate Cox Proportional Hazards Regression Analysis Measured Hazard Ratio for Study Cohort
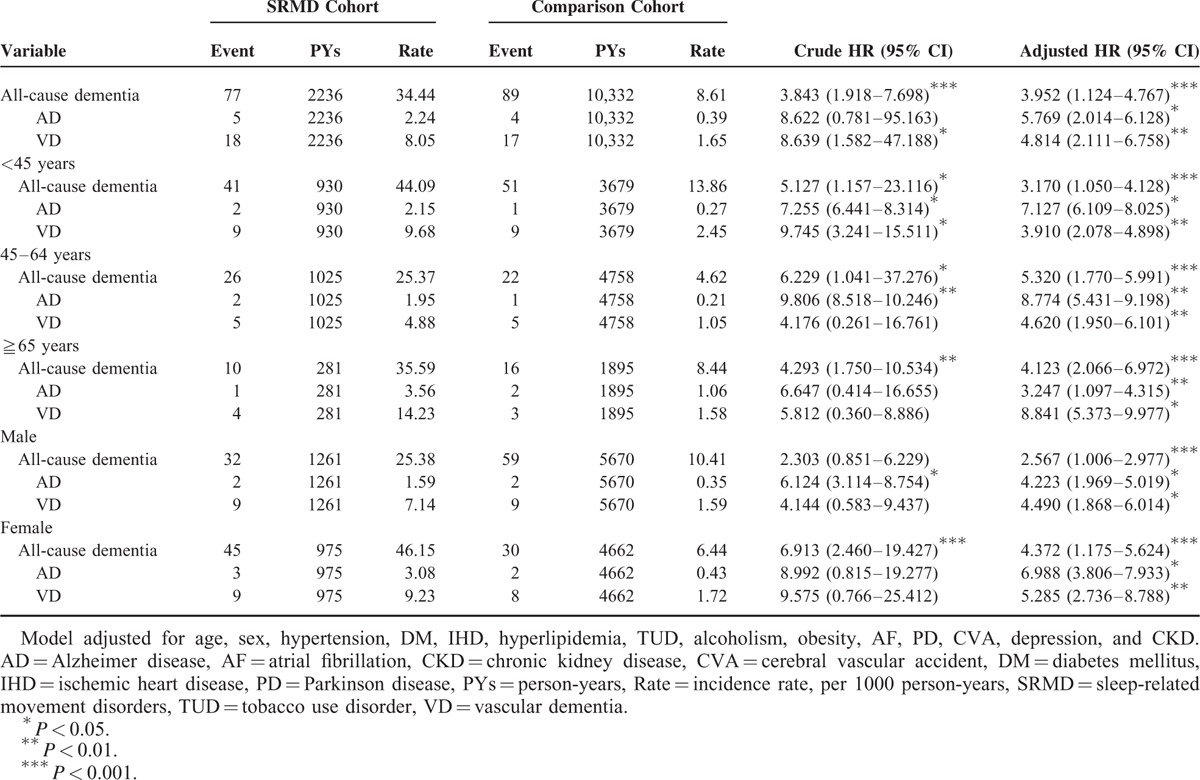
TABLE 3.
Incidence of Dementia and Dementia Subtypes and Multivariate Cox Proportional Hazards Regression Analysis Measured Hazard Ratio for Study Cohort by Various Time Intervals
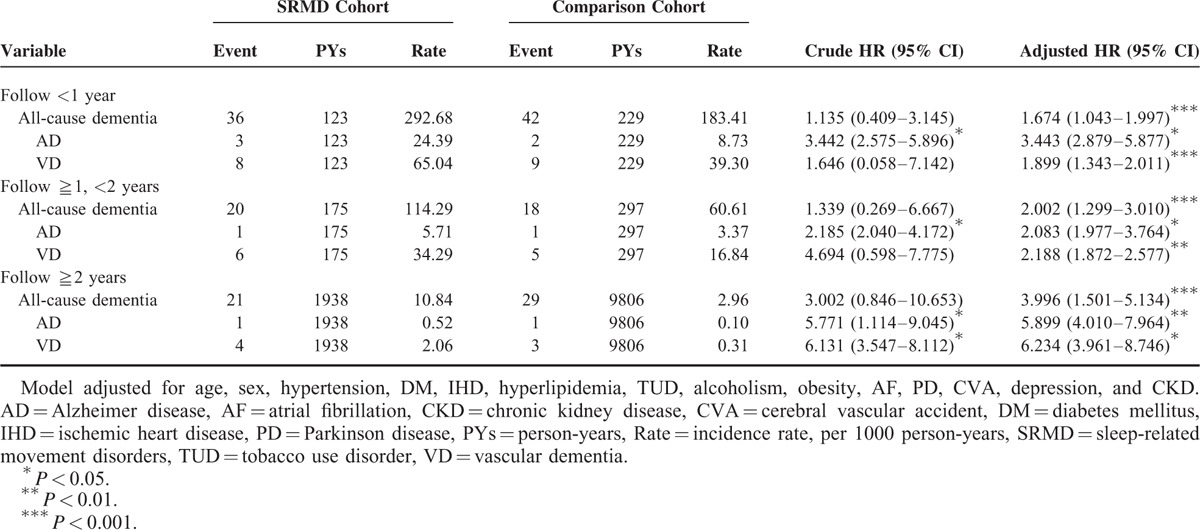
FIGURE 1.
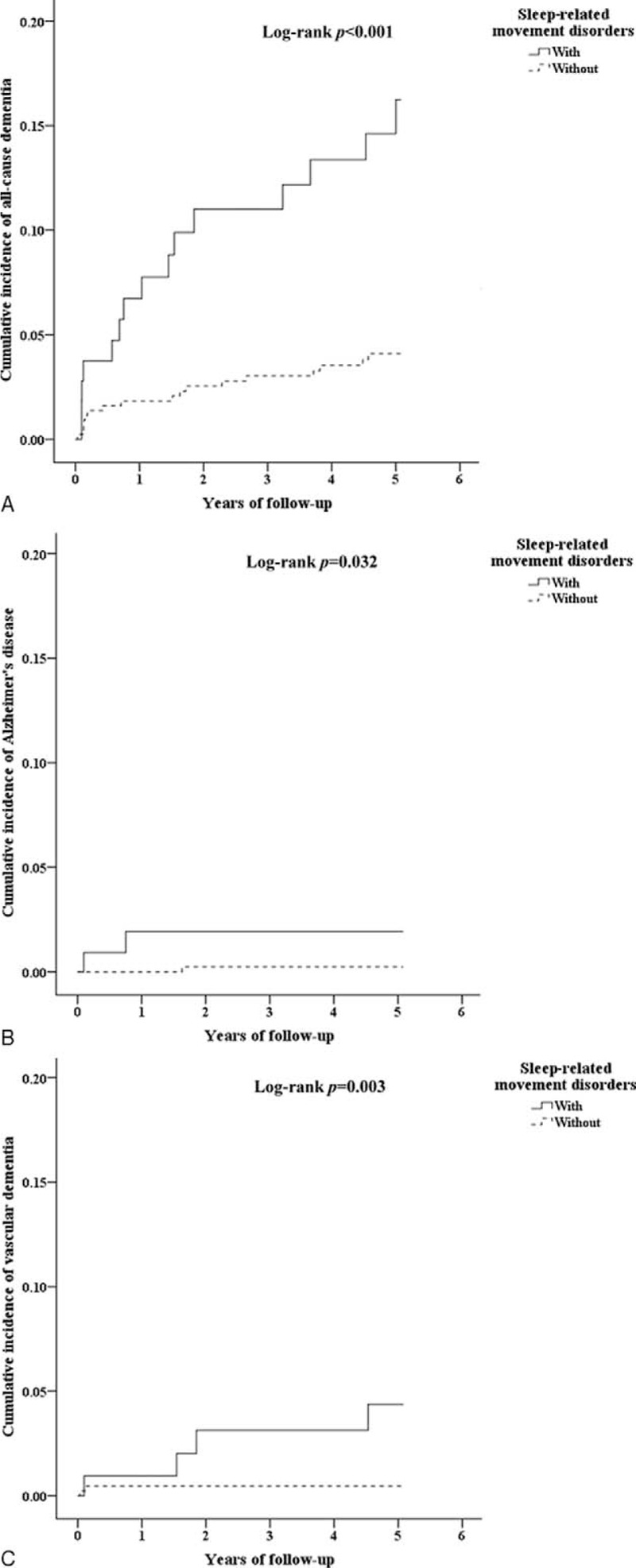
(A) The cumulative incidence curves of all-cause dementia for the individual with and without sleep-related movement disorders (log-rank P value <0.001). (B) The cumulative incidence curves of Alzheimer disease for the individual with and without sleep-related movement disorders (log-rank P value = 0.032). (C) The cumulative incidence curves of vascular dementia for the individual with and without sleep-related movement disorders (log-rank P value = 0.003).
DISCUSSION
In this longitudinal study using nationwide database, we demonstrated that SRMD were associated with an increased risk to develop all-cause dementia in initially cognitively healthy individuals without comorbid neurodegenerative disorders. We found that female SRMD patients with normal cognition are more likely to develop dementia. The results indicate that increasing age was not a risk factor for developing dementia in SRMD patients. Furthermore, the impact of SRMD on dementia risk was progressively increased by various follow-up time intervals with time-dependent characteristic.
Sleep disturbance is considered as a common manifestation in demented patients. Increasing evidence has highlighted the link between sleep problem and incidence of dementia. Poor sleep quality has been shown to be a risk factor for dementia in old patients.18,19 It has been reported that daytime sleepiness is associated with cognitive impairment.20,21 Sleep fragmentation has been linked to an increased risk of AD in elderly population.22 Recent researches have demonstrated the association of sleep disturbance with incident dementia and mortality.23–25 In this longitudinal study, we found that patients with SRMD are at high risk for dementia. RLS and PLMD, 2 common types of SRMD, have been shown to be causes of chronic daytime sleepiness and sleep loss. They have been suggested to have impact on cognitive function associated with sleep deprivation.13,26–28 Our finding that SRMD as a risk factor for developing dementia is postulated to be attributed to the chronic poor quality of sleep. AD has been considered as the most common cause of dementia. In contrast, our results showed that SRMD patients exhibit a higher risk of vascular dementia compared to AD. It is in an agreement with previous study that sleep disturbance and daytime sleepiness are considered as predictor for vascular dementia.29 Sleep quality has been linked to an increased risk of cardiovascular diseases.29–31 RLS and PLMD have been associated with increased risk for cardiovascular disease in the general population.15,32,33 Age is generally acknowledged as the most important risk factor for dementia. We showed that patients with SRMD are likely to develop dementia before 65. This is agreed with the results of a French population-based survey that the prevalence of RLS was 11.3% for participants between 50 and 64 years of age.34 It is generally accepted that women have slightly greater probability to develop dementia than men.35 The gender difference in the prevalence of dementia among SRMD patients was found in this population-based longitudinal study.
Dementia is caused when nerve cells in the brain are damaged by diseases. Several types of dementia have been attributed to brain atrophy such as AD, frontotemporal dementia, and Lewy body dementia, as well as vascular dementia. Sleep problem is considered to be a cause of neurologic disorder. Imaging studies have revealed that poor sleep quality is associated with an increased brain atrophy.10,36 Short sleep duration has been reported to contribute to age-related changes in brain structure and cognitive performance.7 Deposition of amyloid, a protein normally found in brain, is known to play a role in pathogenesis of AD. Serum concentration of amyloid rises during wakefulness and drops during sleep. Recent studies have reported that nondemented individuals reporting sleep problems exhibited greater amyloid burden.24,37,38 It is suggested that sleep disorders have effects on brain health, leading to an increased risk to dementia. In addition to sleep-associated brain damage, RLS is a neurologic disorder leading to inability to sleep during the night, whereas PLMD is motor disorder diagnosed by polysomnography. It has reported to lead to insomnia, sleepiness, and problems with concentration, cognition, motivation, anxiety, and depression. RLS has been reported to be attributed to brain dopaminergic system dysfunction, leading to a motor abnormality.39 Dopaminergic deficit has been related to cognitive impairment in movement disorder patients such as Parkinson disease. It is implied that cognitive decline may develop with concomitant dopamine deficiency and lead to dementia in a long-term setting. Nevertheless, the correlation of RLS-related dopaminergic dysregulation with dementia requires further studies to elucidate.
In this study, we found no statistically significant association between the risk of dementia and those commonly acceptable factors such as diabetes and hypertension in SRMD population. It might be explained by the nature of dementia which is acknowledged as a multifactorial disease and contribution of a single risk factor can be relatively slight and insignificant. There are several limitations to the present study. First, in this cohort study, there was an inability to validate diagnoses and objective measure of SRMD. In addition, coding error might occur in the database. We focused on the cases with clear ICD-9 definition. Second, as a retrospective longitudinal study, we had no access to the data of medication that might have effects on SRMD and/or dementia. Third, as baseline risk factor was used, changes in risk factor profile during follow-up may need to be considered for the effect on association. In addition, we were unable to distinguish the causal relationship between SRMD and dementia based on the study population. Although the data collection was extensive, lack of information on some adaptable risk factors, such as physical activity, dietary habits, depression, education, and social engagement might lead to a misinterpretation of the results.
In conclusion, we provided subjective evidence supporting the hypothesis that patients with SRMD are at relatively high risk of developing dementia. SRMD is suggested to be a gender-dependent and time-dependent risk factor of dementia. Further studies are required to elucidate the mechanism underlying the association revealed in this study.
Acknowledgments
The authors thank Tri-Service General Hospital (TSGH-C104-084; TSGH-C105-084; TSGH-C100-101; TSGH-C101-080; TSGH-C103-085; TSGH-C104-083; TSGH-C105-085), Ministry of Science and Technology (MOST104-2314-B-016-017-MY3), and Teh-Tzer Study Group for Human Medical Research Foundation (A1031031) for the support. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Abbreviations: CI = confidence interval, HR = hazard ratios, LHID = Longitudinal Health Insurance Database, PLMD = periodic limb movement disorder, RLS = restless legs syndrome, SRMD = sleep-related movement disorders.
This study was supported in part by grants from the Tri-Service General Hospital (TSGH-C104-084; TSGH-C105-084; TSGH-C100-101; TSGH-C101-080; TSGH-C103-085; TSGH-C104-083; TSGH-C105-085), Ministry of Science and Technology (MOST104-2314-B-016-017-MY3), Teh-Tzer Study Group for Human Medical Research Foundation (A1031031).
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Badran M, Yassin BA, Fox N, et al. Epidemiology of sleep disturbances and cardiovascular consequences. Can J Cardiol 2015; 31:873. [DOI] [PubMed] [Google Scholar]
- 2.Larcher S, Benhamou PY, Pepin JL, et al. Sleep habits and diabetes. Diabetes Metab 2015; 41:263. [DOI] [PubMed] [Google Scholar]
- 3.Gama RL, Tavora DG, Bomfim RC, et al. Sleep disturbances and brain MRI morphometry in Parkinson's disease, multiple system atrophy and progressive supranuclear palsy – a comparative study. Parkinsonism Relat Disord 2010; 16:275. [DOI] [PubMed] [Google Scholar]
- 4.Rye DB. Excessive daytime sleepiness and unintended sleep in Parkinson's disease. Curr Neurol Neurosci Rep 2006; 6:169. [DOI] [PubMed] [Google Scholar]
- 5.Zucconi M, Ferini-Strambi L. Epidemiology and clinical findings of restless legs syndrome. Sleep Med 2004; 5:293. [DOI] [PubMed] [Google Scholar]
- 6.Chen JC, Espeland MA, Brunner RL, et al. Sleep duration, cognitive decline, and dementia risk in older women. Alzheimers Dement 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lo JC, Loh KK, Zheng H, et al. Sleep duration and age-related changes in brain structure and cognitive performance. Sleep 2014; 37:1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee D, Heo SH, Yoon SS, et al. Sleep disturbances and predictive factors in caregivers of patients with mild cognitive impairment and dementia. J Clin Neurol 2014; 10:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grant MD, Marbella A, Wang AT, et al. Menopausal Symptoms: comparative effectiveness of therapies. Rockville (MD) 2015. [PubMed] [Google Scholar]
- 10.Sexton CE, Storsve AB, Walhovd KB, et al. Poor sleep quality is associated with increased cortical atrophy in community-dwelling adults. Neurology 2014; 83:967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinkerton JV, Pickar JH, Racketa J, et al. Bazedoxifene/conjugated estrogens for menopausal symptom treatment and osteoporosis prevention. Climacteric 2012; 15:411. [DOI] [PubMed] [Google Scholar]
- 12.Giannaki CD, Sakkas GK, Karatzaferi C, et al. Evidence of increased muscle atrophy and impaired quality of life parameters in patients with uremic restless legs syndrome. PLoS One 2011; 6:e25180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pigeon WR, Yurcheshen M. Behavioral sleep medicine interventions for restless legs syndrome and periodic limb movement disorder. Sleep Med Clin 2009; 4:487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Ad B, Korczyn AD. Disorders of excessive daytime sleepiness – an update. J Neurol Sci 1998; 153:192. [DOI] [PubMed] [Google Scholar]
- 15.Ohayon MM, Roth T. Prevalence of restless legs syndrome and periodic limb movement disorder in the general population. J Psychosom Res 2002; 53:547. [DOI] [PubMed] [Google Scholar]
- 16.Earley CJ, Silber MH. Restless legs syndrome: understanding its consequences and the need for better treatment. Sleep Med 2010; 11:807. [DOI] [PubMed] [Google Scholar]
- 17.Vetrugno R, Provini F, Montagna P. Restless legs syndrome and periodic limb movements. Rev Neurol Dis 2006; 3:61. [PubMed] [Google Scholar]
- 18.Spira AP, Chen-Edinboro LP, Wu MN, et al. Impact of sleep on the risk of cognitive decline and dementia. Curr Opin Psychiatry 2014; 27:478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crowley K. Sleep and sleep disorders in older adults. Neuropsychol Rev 2011; 21:41. [DOI] [PubMed] [Google Scholar]
- 20.Jaussent I, Bouyer J, Ancelin ML, et al. Excessive sleepiness is predictive of cognitive decline in the elderly. Sleep 2012; 35:1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merlino G, Piani A, Gigli GL, et al. Daytime sleepiness is associated with dementia and cognitive decline in older Italian adults: a population-based study. Sleep Med 2010; 11:372. [DOI] [PubMed] [Google Scholar]
- 22.Lim AS, Kowgier M, Yu L, et al. Sleep Fragmentation and the risk of Incident Alzheimer's disease and cognitive decline in older persons. Sleep 2013; 36:1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yaffe K, Nettiksimmons J, Yesavage J, et al. Sleep quality and risk of dementia among older male veterans. Am J Geriatr Psychiatry 2015; 23:651. [DOI] [PubMed] [Google Scholar]
- 24.Benedict C, Byberg L, Cedernaes J, et al. Self-reported sleep disturbance is associated with Alzheimer's disease risk in men. Alzheimers Dement 2014. [DOI] [PubMed] [Google Scholar]
- 25.Sterniczuk R, Theou O, Rusak B, et al. Sleep disturbance is associated with incident dementia and mortality. Curr Alzheimer Res 2013; 10:767. [DOI] [PubMed] [Google Scholar]
- 26.Gamaldo CE, Benbrook AR, Allen RP, et al. A further evaluation of the cognitive deficits associated with restless legs syndrome (RLS). Sleep Med 2008; 9:500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pearson VE, Allen RP, Dean T, et al. Cognitive deficits associated with restless legs syndrome (RLS). Sleep Med 2006; 7:25. [DOI] [PubMed] [Google Scholar]
- 28.Edinger JD, Fins AI, Sullivan RJ, et al. Comparison of cognitive-behavioral therapy and clonazepam for treating periodic limb movement disorder. Sleep 1996; 19:442. [PubMed] [Google Scholar]
- 29.Osonoi Y, Mita T, Osonoi T, et al. Poor sleep quality is associated with increased arterial stiffness in Japanese patients with type 2 diabetes mellitus. BMC Endocr Disord 2015; 15:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jackson CL, Redline S, Emmons KM. Sleep as a potential fundamental contributor to disparities in cardiovascular health. Annu Rev Public Health 2015; 36:417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Del Brutto OH, Mera RM, Zambrano M, et al. Association between sleep quality and cardiovascular health: a door-to-door survey in rural Ecuador. Environ Health Prev Med 2014; 19:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cuellar NG. The effects of periodic limb movements in sleep (PLMS) on cardiovascular disease. Heart Lung 2013; 42:353. [DOI] [PubMed] [Google Scholar]
- 33.Van Den Eeden SK, Albers KB, Davidson JE, et al. Risk of cardiovascular disease associated with a restless legs syndrome diagnosis in a retrospective cohort study from Kaiser Permanente Northern California. Sleep 2015; 38:1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tison F, Crochard A, Leger D, et al. Epidemiology of restless legs syndrome in French adults: a nationwide survey: the INSTANT Study. Neurology 2005; 65:239. [DOI] [PubMed] [Google Scholar]
- 35.Guilbert JJ. The world health report 2002 – reducing risks, promoting healthy life. Educ Health (Abingdon) 2003; 16:230. [DOI] [PubMed] [Google Scholar]
- 36.Joo EY, Kim H, Suh S, et al. Hippocampal substructural vulnerability to sleep disturbance and cognitive impairment in patients with chronic primary insomnia: magnetic resonance imaging morphometry. Sleep 2014; 37:1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sprecher KE, Bendlin BB, Racine AM, et al. Amyloid burden is associated with self-reported sleep in nondemented late middle-aged adults. Neurobiol Aging 2015; 36:2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spira AP, Gamaldo AA, An Y, et al. Self-reported sleep and beta-amyloid deposition in community-dwelling older adults. JAMA Neurol 2013; 70:1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin CC, Fan YM, Lin GY, et al. 99mTc-TRODAT-1 SPECT as a potential neuroimaging biomarker in patients with restless legs syndrome. Clin Nucl Med 2015. [DOI] [PubMed] [Google Scholar]


