Abstract
The risk of peptic ulcer disease (PUD) among patients with depression has raised concern. This study determined the association between depression and the subsequent development of PUD using claims data.
Patients newly diagnosed with depression in 2000 to 2010 were identified as depression cohort from the Taiwan National Health Insurance Research Database. The comparison cohort was randomly selected from subjects without depression, frequency matched by age and gender and diagnosis date, with a size 2-fold of the size of the depression cohort. The incidence of PUD was evaluated for both cohorts by the end of 2011. We calculated the hazard ratios (HRs) and 95% confidence intervals (CIs) of PUD using the Cox proportional hazards regression model.
The depression cohort consisted of 23,536 subjects (129,751 person-years), and the comparison cohort consisted of 47,069 subjects (285,592 person-years). The incidence of PUD was 2-fold higher in the depression cohort than in the comparison cohort (33.2 vs 16.8 per 1000 person-years) with an age adjusted HR of 1.97 (95% CI = 1.89–2.06) or a multivariable adjusted HR of 1.35 (95% CI = 1.29–1.42).
Depression might increase the risk of developing PUD. Prospective clinical studies of the relationship between depression and PUD are warranted.
INTRODUCTION
Depression, a type of mood disorder, is characterized by emotional dysregulation and depressive cognition, which induces distress in patients.1 In patients with depression, stress not only plays a major role in pathogenesis but also is associated with the disturbance of the hypothalamus-pituitary-adrenal (HPA) axis.2 Additionally, patients with depression have been reported to experience somatic consequences associated with HPA axis dysregulation.3 Regarding the gastrointestinal (GI) system, evidences have shown that depression is associated with irritable bowel syndrome, ulcerative colitis, dyspepsia, and gastroesophageal reflux disease.4–6 However, few studies have reported evidence regarding the relationship between peptic ulcer disease (PUD) and depression.
PUD, including gastric and peptic ulcers, is a prevalent GI disease with a high mortality.7 Evidence has shown that both physical stress and psychological stress are closely related to PUD.8,9 Notably, PUD risk among schizophrenia or anxiety disorder patients has been documented,10,11 but not for depression patients. To make the diagnosis of unipolar depression, patient should not have mania or hypomania, or their diagnosis should be bipolar disorders (BD) instead.12 However, evidence had shown that some patients with BD have unipolar depression as their initial presentation, before mania or hypomania.13 Even though the differential diagnosis between BD and unipolar depression was challenging, they shared the common manifestation of having depressive episode.14 Particularly, we have previously demonstrated a positive association between BD and PUD.15 However, we could not identify whether the depression, mania, or hypomania predisposed the patients having subsequent PUD. Therefore, we investigated the association between depression and PUD. We hypothesized that if depression was associated with subsequent PUD, more concern would be raised among patients with unipolar depression or depressive episode of BD.
To test the study hypothesis, we designed a nationwide population-based study to compare the incidence raes of PUD between cohorts with and without depression using longitudinal insurance data.
METHODS
Data Source
Outpatient and inpatient claims data documented in the Longitudinal Health Insurance Database (LHID) of Taiwan were used in this study. The LHID containing annual claims data from 1 million randomly selected insurants based on the Taiwan National Health Insurance (NHI) program established in 1996 and updated annually by the National Health Research Institutes (NHRI) thereafter. The NHI program is a universal single-payer system that covers >99% of the 23.74 million residents of Taiwan. The NHRI provided scrambled identification numbers that were used to connect each person's relevant claims information, including sex and date of birth, registry for medical services, and medication prescriptions. Disease definition in the LHID is based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). In accordance with the Personal Information Protection Act, identification of beneficiaries was recoded by a computer. This study was approved to follow the condition for exemption by the Institutional Review Board (IRB) of China Medical University (CMUH-104-REC2-115). The IRB had specifically waived the consent requirement.
Study Cohorts Selection
This was a retrospective cohort study using the population-based insurance claims data to establish study cohorts. Patients aged 20 years and older with newly diagnosed with depression (ICD-9 codes 296.2, 296.3, 300.4, 311) from 2000 to 2010 and free of PUD (ICD-9-CM codes 531–533) were identified and selected into the depression cohort (Figure 1). The index date was used and defined as the first date on which depression was diagnosed from the medical records in LHID. The insured population without the history of depression and PUD in the LHID were randomly selected into the comparison cohort frequency matched by age (within 5 years), gender, and index date. The enrollment date for the subject in the comparison cohort was matched with the same year and month of the subject in the depression cohort, whereas the day was randomly assigned. The sample size of the comparison cohort was 2-fold of the depression cohort. Subjects without demographic information at the baseline were excluded from the study cohorts.
FIGURE 1.
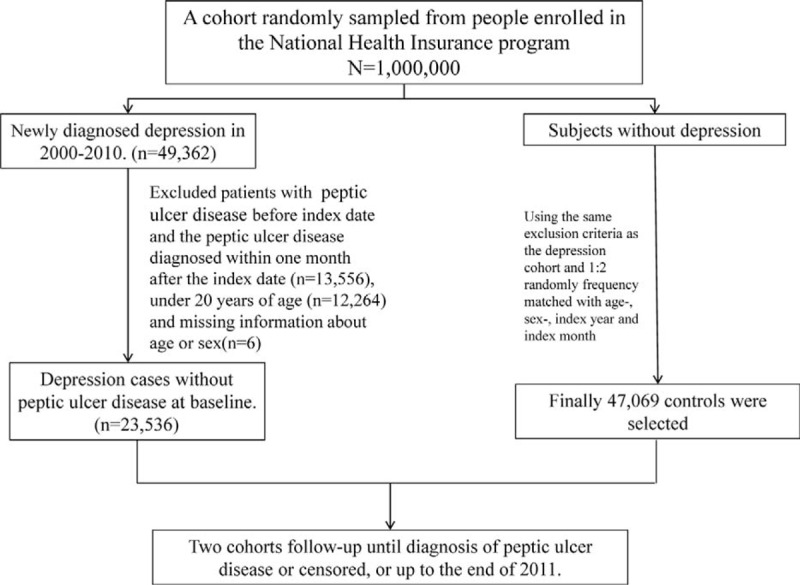
Flowchart of establishing the study cohorts.
Endpoint of Study
The major endpoint in this study was the development of PUD (ICD-9-CM codes 531–533). Person-years of follow-up were calculated for each person until PUD was diagnosed or censored for death (119 and 129, respectively) and withdrawing from the insurance system due to the loss of follow-up, moving abroad or imprisonment, and so on, (2170 and 3205, respectively), or the end of 2011.
Comorbidities and Medications
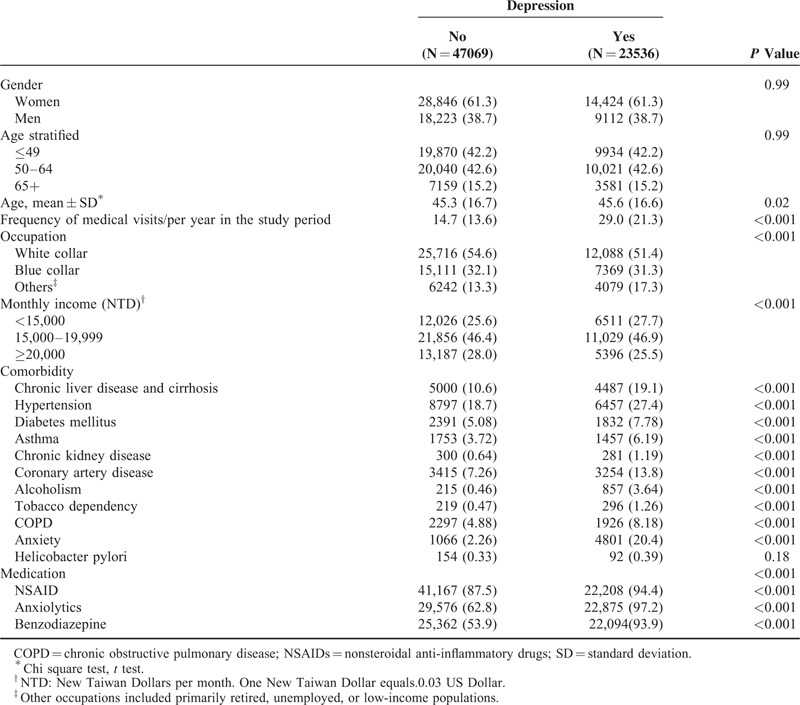
Comorbidities and medications were considered as possible confounding factors. The examined comorbidities were chronic liver disease and cirrhosis (ICD-9-CM code 571), hypertension (HTN) (ICD-9-CM code 401–405), diabetes mellitus (DM) (ICD-9-CM code 250), asthma (ICD-9-CM code 493), chronic kidney disease (CKD) (ICD-9-CM code 585), coronary artery disease (CAD) (ICD-9-CM codes 410–414), alcoholism (ICD-9-CM codes 291, 303, 305.00, 305.01, 305.02, 305.03, 790.3, and V11.3), tobacco dependency (ICD-9-CM code 305.1), chronic obstructive pulmonary disease (COPD) (ICD-9-CM codes 490, 491, 496), anxiety (ICD-9-CM code 300.0), and helicobacter pylori (ICD-9-CM code 041.86), identified before the index date according to inpatient and outpatient files. And the examined medications were nonsteroidal anti-inflammatory drugs (NSAIDs), anxiolytics, and benzodiazepines (BZDs) that were used before the index date according to inpatient and outpatient files. Available antidepressants were divided into 2 groups, selective serotonin reuptake inhibitors (SSRIs) and non-SSRIs.
Statistical Analysis
The distributions of these 2 cohorts were presented by the mean and standard deviation (SD) for age and frequency of medical visits (per year during the study period) and numbers and proportions for gender, occupation, monthly income (NTD), comorbidities, and medication uses. Student's t test and chi square test were applied to test differences in the continuous and categorical variables between the depression and comparison cohorts. The cumulative incidence curves of PUD were estimated for the 2 cohorts using the Kaplan–Meier method, and the log-rank test was used to evaluate the difference between the curves. The sex-, age-, tobacco dependency-, NSAID-, and comorbidity-specific incidences of PUD per 1000 person-years of follow-up for each cohort were calculated. We used Cox proportional hazards regression models to examine the hazard ratios (HRs) with 95% confidence intervals (CIs) of depression patients developing PUD relative to the comparison cohort. We first adjusted for age in the Cox model. The multivariable Cox model was then used controlling for age, sex, and comorbidities of cirrhosis, HTN, DM, asthma, CKD, CAD, alcoholism, COPD, and anxiety as well as the use of NSAIDs, anxiolytics, and BZDs. The proportional hazard model assumption was also examined using a test of scaled Schoenfeld residuals. In the model evaluating the PUD risk throughout overall follow-up period, results of the test revealed a significant relationship between Schoenfeld residuals for depression and follow-up time, suggesting that the proportionality assumption was violated (P value = 0.002). In the subsequent analyses, we stratified the follow-up duration to deal with the violation of proportional hazard assumption. Data management and statistical analysis were performed using SAS 9.3 (SAS Institute, Cary, NC). The statistical significance was defined as a (2-sided) P value < 0.05.
RESULTS
The depression cohort consisted of 23,536 subjects (129,751 person-years), and the control cohort consisted of 47,069 subjects without depression (285,592 person-years). (Table 1). The distributions of gender and age were similar in both cohorts. Of the study patients, 61.3% were women, with 42.2% being ≤ 49 years old. The mean age of the depression cohort was 45.6 (SD = 16.6) years, and the mean age of the comparison cohort was 45.3 (SD = 16.8) years. The prevalence rates of comorbidities and medications were greater in the depression cohort than in the comparison cohort (all P values < 0.001). The mean follow-up time was 5.51 (SD = 3.31) years for the depression cohort and 6.07(SD = 3.23) years for the comparison cohort.
TABLE 1.
Comparisons in Demographic Characteristics and Comorbidities in Patient With and Without Depression
After a 12-year follow-up, the Kaplan–Meier method estimated cumulative incidence of PUD was 9.47% higher in the depression cohort than that in the comparison cohort (27.0% vs 17.53%; P < 0.001) (Figure 2).
FIGURE 2.
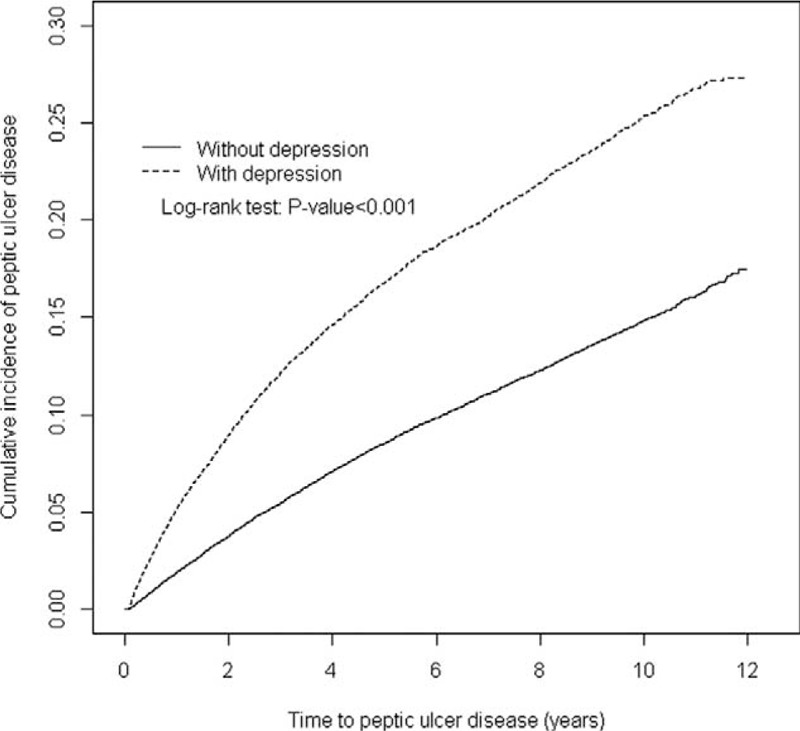
Cummulative incidence of peptic ulcer disease in depression and comparison cohorts.
The incidence of PUD was 2-fold higher in the depression cohort than in the comparison cohort (33.2 vs 16.8 per 1000 person-years) with an age adjusted HR of 1.97 (95% CI = 1.89, 2.06) or a multivariable adjusted HR of 1.35 (95% CI = 1.29–1.42) (Table 2). The incidence rate of PUD was consistently higher in the depression cohort than in the comparison cohort in each stratum by gender, age, NSAID use, comorbidity, and follow-up year, except tobacco dependency. The PUD incidence increased with age and decreased with follow-up year. However, the depression cohort to the comparison cohort relative HR decreased with age and follow-up year. For those with tobacco dependency, the PUD incidence was lower in the depression cohort than in controls, but not significant.
TABLE 2.
Comparison of Incidence Densities of Peptic Ulcer Disease Hazard Ratio Between With and Without Depression by Demographic Characteristics and Comorbidity
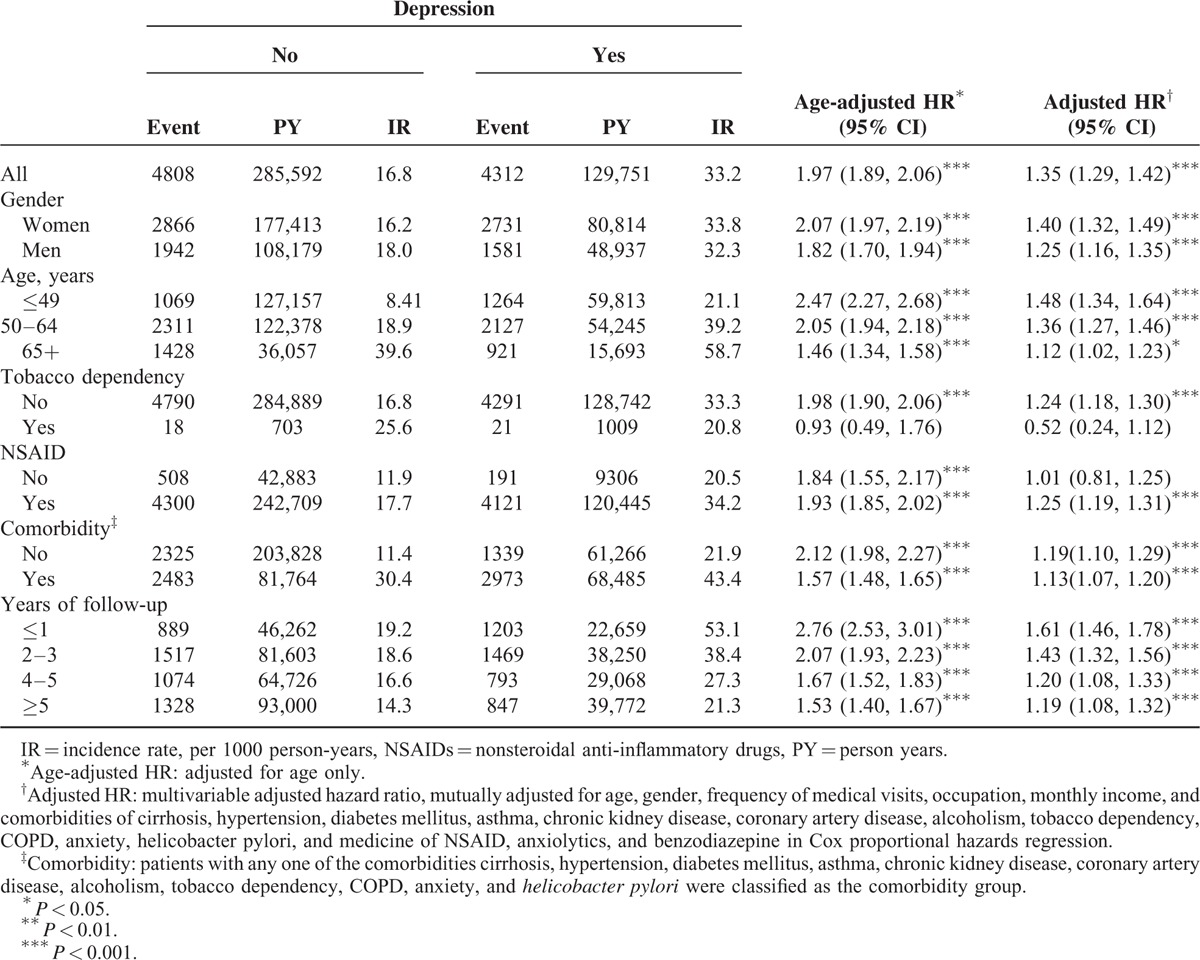
Table 3 shows the PUD associations with socioeconomic status. Subjects of nonwhite collar and middle income were at significantly higher hazard of PUD. Almost all comorbidity and drug use factors were related with greater PUD except tobacco dependency.
TABLE 3.
Hazard Ratios of Peptic Ulcer Disease in Association With Gender, Age, and Comorbidities in Univariable and Multivariable Cox Regression Models
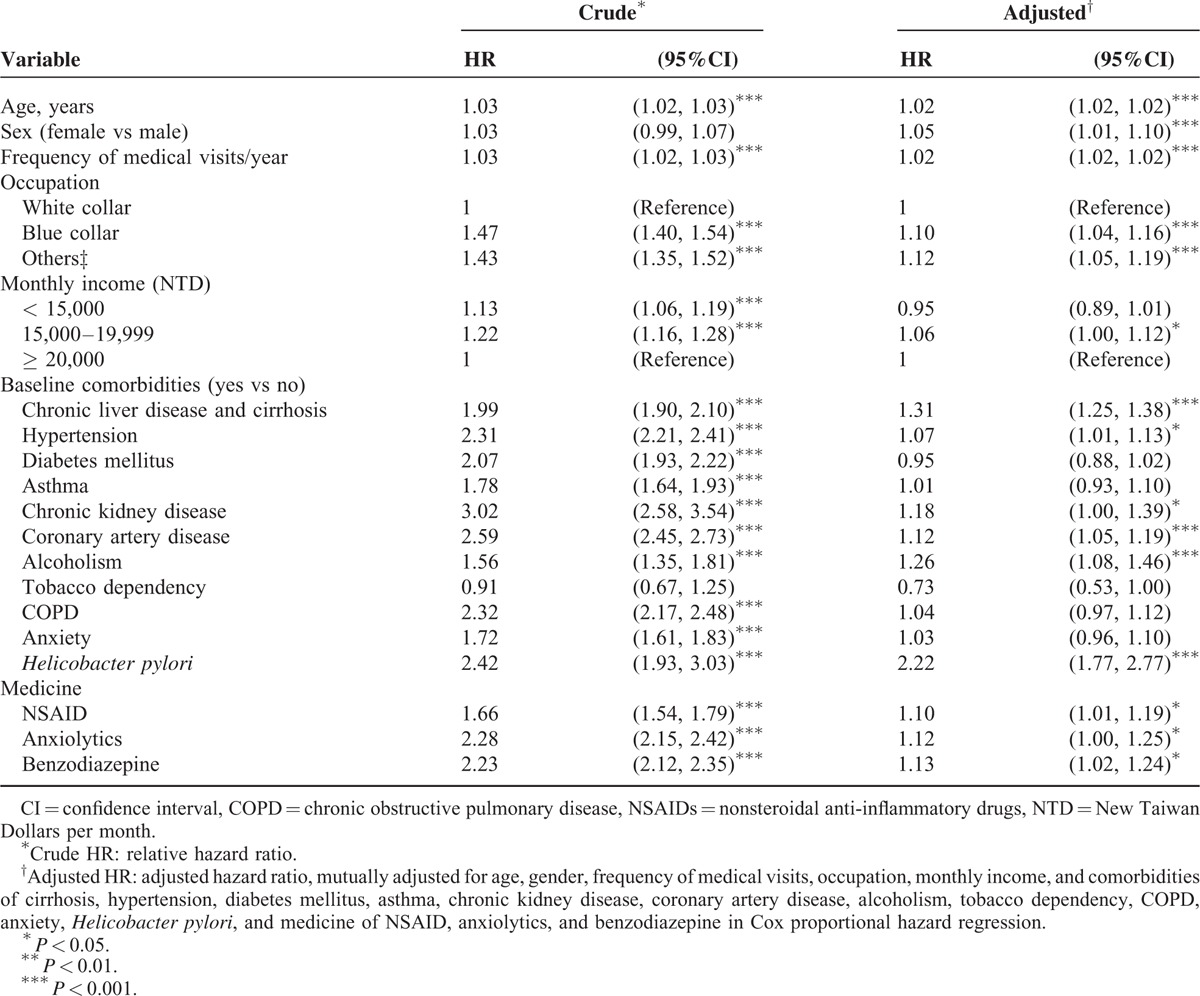
Table 4 shows that among 23,536 depression cases, those who did not receive any treatment had the highest incidence of PUD, 55.2 per 1000 person-years with an adjusted HR of 2.27(95% CI 2.07, 2.48) for PUD compared to the comparison cohort. Treatment reduced the incidence to 31.0 per 1000 person-years for those took SSRI.
TABLE 4.
Incidence Rate and Hazard Ratio of Peptic Ulcer Disease Between Treatments for Depression
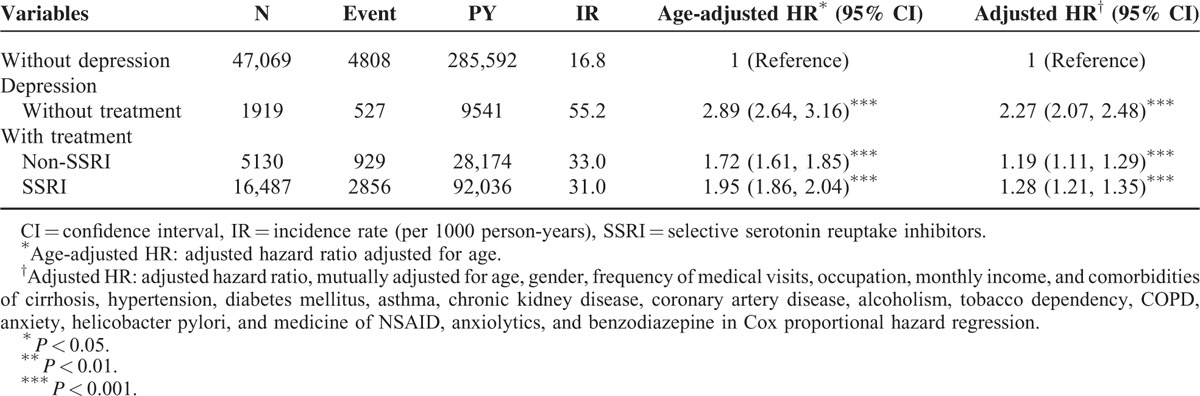
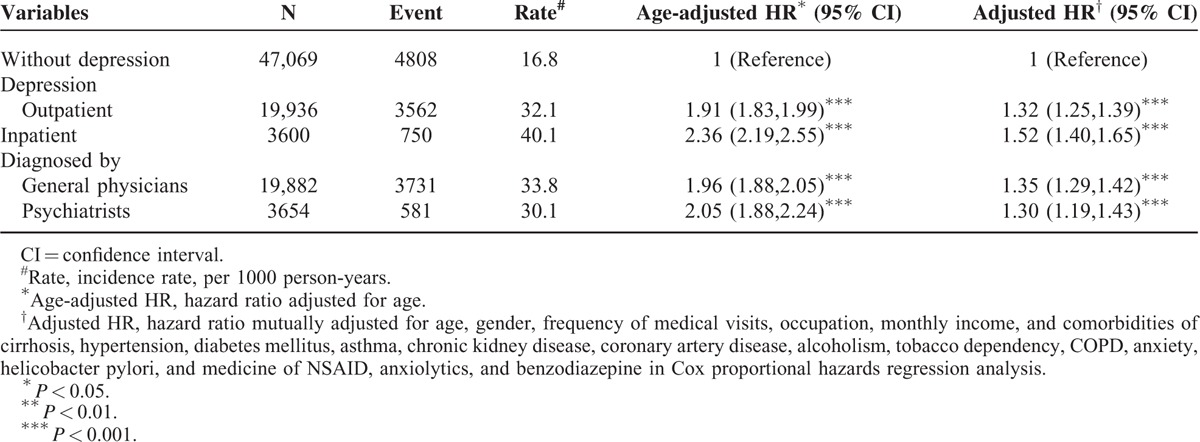
Table 5 shows that depression patients were diagnosed mainly in outpatient setting (84.70%) and by general physicians (84.47%). The PUD incidence was higher in those to be diagnosed as inpatients than as outpatients. Patients cared by psychiatrists were associated with a lower risk of PUD.
TABLE 5.
Incidence and Hazard Ratios of Peptic Ulcer Disease Measured for Outpatients and Inpatients and Patients Diagnosed by General Physicians and Psychiatrists
DISCUSSION
This was the first population-based study, which examined depression as a risk factor for PUD by using matched cohorts and a long-term (12-y) follow-up period. As expected, results of this study showed that patients with depression were at an elevated risk of developing PUD. We hypothesized that the possible mechanism may be associated with the HPA axis. The HPA axis is closely related to stress, and a disturbed HPA axis response has been noted in patients with depression, which seems to be a stress-related disorder.16 In addition, psychological stress has been considered as a risk factor for PUD.8 As a type of psychological stress, depression is characterized by a high risk of lifelong persistence and chronic course.17 Animal models have shown that corticosterone may be ulcerogenic under chronic stressful conditions.18 Consequently, depression may increase the risk of PUD.
In the analyzed of risk factors associated with subsequent PUD in patients with depression, we found that patients with comorbidities, except tobacco dependency, were at a risk of developing PUD. Studies have shown that there is a dose–dependent relationship between chronic illness and depression.19 In other words, the longer chronic comorbidities persist, the higher the psychological stress patients may experience. Consequently, patients with depression and the aforementioned comorbidities may have an elevated risk of subsequent PUD. On the other hand, patients with depression were reported having positive association with tobacco dependence.20 Tobacco use was seemed as self-medication for mood disturbance and negative affectivity in patients with depression.21 Although smoking was a risk factor for PUD,22 smoking was also reported slowing the progression of depression.23 Hence, further progression of the association between depression and PUD may be delayed under the concern of this paradoxical effect.
Alcohol use disorders have been reported as major problems in patients with depression,24 and alcoholism is an independent risk factor for PUD.25 In consideration of this close relationship, this study also showed that patients with depression and alcoholism had a higher risk of subsequent PUD than those without alcoholism.
Depression has been associated with a neuroinflammatory mechanism.26 However, the use of NSAIDs is a known risk factor for PUD.25 Furthermore, patients with depression at high stress levels are at a higher risk of inappropriate use of anxiolytics and BZDs.27 Also, in depression, antidepressants were used under the consideration of restoring the level of brain-derived neurotrophic factor and the prevention of further hippocampus damage caused by disturbed HPA axis.28 However, the animal model was reported that concurrent use of BZDs and antidepressants would impair the neurogenesis of hippocampus.29 As a result, the course of depression was affected, and the risk of subsequent PUD increased.
Our study is the first longitudinal study using population-based data to examine depression as a risk factor for the subsequent PUD occurrence. The strength of our study is the age- and gender-matched design cohorts with and without depression with a maximum of 12 years follow-up and adequate adjustments for comorbidities and drug uses in data analysis. However, our study has several limitations that are inherent in the use of claims databases. First, the diagnoses of depression in the NHIRD were based on ICD-9-CM codes. Thus, the severity of depression as a risk factor for subsequent PUD was not explored. Second, as mentioned above, unipolar depression still had the possibility to reach the diagnosis of BD, if patients had mania or hypomania after their depression.14 Hence, we could not exactly confirm this condition, especially beyond our follow-up years. Third, our further data analysis showed that PUD were diagnosed mainly based on clinical symptoms (79.4%) and few were diagnosed with oesophageal-gastro-duodenoscopy (OGD) (20.6%). However, the corresponding adjusted HR of PUD for patients with depression was slightly higher for those diagnosed with OGD than those diagnosed by clinical symptoms. Fourth, the associations were assessed by the chronological order in which these two diseases were diagnosed. However, the possibility that PUD causes depression cannot be entirely excluded. Finally, information regarding many demographic variables, such as education, lifestyle, and family history, was unavailable. We used alcoholism, tobacco dependency, and COPD to substitute the drinking and smoking behavior. Our results show that those with COPD were also at a higher hazard of PUD.
Depression is a disorder often unrecognized and untreated. The depression cohort identified in this study may not represent the average mild “depressive” persons and may reflect a group of persons with more severe depressive illness perhaps, or with some personal characteristics that make them more likely to seek medical attention and made claims. The findings thus may not be readily generalized to people with milder depression, depression uncomplicated by medical comorbidities, or the healthy population of smaller size. However, this study compares between 2 matched cohorts based on the same database and adjusts related factors, and the real relationship between depression and PUD risk is supposed to be revealed to a certain degree even though the generalization is limited.
In conclusion, this study suggests that depression increases the risk of developing PUD. On the basis of our data, we suggest that greater attention should be focused on female and aging patients, particularly those with comorbidities. Additional prospective clinical studies on the relationship between depression and PUD are warranted.
Footnotes
Abbreviations: aHR = adjusted hazard ratio, BNHI = Bureau of National Health Insurance, CI = confidence interval, LHID 2000 = Longitudinal Health Insurance Database 2000, NERD = nonerosive reflux disease, NHI = National Health Insurance, NHIA = National Health Insurance Administration, NHIRD = National Health Insurance Research Database, NHRI = National Health Research Institutes, PUD = peptic ulcer disease (PUD).
Author contributions: All authors have contributed significantly, and that all authors are in agreement with the content of the manuscript. Conception/design: Chih-Chao Hsu, Yi-Chao Hsu, Chia-Hung Kao; collection and/or assembly of data: all authors; data analysis and interpretation: all authors; manuscript writing: all authors; final approval of manuscript: all authors.
Chih-Chao Hsu and Yi-Chao Hsu contributed equally to this study.
Fung-Chang Sung and Chia-Hung Kao contributed equally to this study.
Funding: this study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002); China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM104010092); NRPB Stroke Clinical Trial Consortium (MOST 103-2325-B-039-006); Tseng-Lien Lin Foundation, Taichung, Taiwan; Taiwan Brain Disease Foundation, Taipei, Taiwan; Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and CMU under the Aim for Top University Plan of the Ministry of Education, Taiwan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study. We appreciate the financial support by grants from Ministry of Science and Technology (MOST103-2314-B-715- 001-MY2, and MOST104-2314-B-715-003-MY3), Mackay Medical College (MMC 1012A10, RD1010061, RD1020038, RD1020047, RD1012B13, RD1031B05, RD1030053, RD1030076, RD1040109), and Mackay Memorial Hospital (MMH-MM-10304, MMH-MM-10405).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Gotlib IH, Joormann J. Cognition and depression: current status and future directions. Ann Rev Clin Psychol 2010; 6:285–312.PubMed PMID: 20192795 Pubmed Central PMCID: PMC2845726 Epub 2010/03/03 eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bao AM, Swaab DF. The stress systems in depression: a postmortem study. Eur J Psychotraumatol 2014; 5:26521.PubMed PMID: 25511726 Epub 2014/12/17 eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Penninx BW, Milaneschi Y, Lamers F, et al. Understanding the somatic consequences of depression: biological mechanisms and the role of depression symptom profile. BMC Med 2013; 11:129.PubMed PMID: 23672628 Pubmed Central PMCID: PMC3661358 Epub 2013/05/16 eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah E, Rezaie A, Riddle M, et al. Psychological disorders in gastrointestinal disease: epiphenomenon, cause or consequence? Ann Gastroenterol 2014; 27:224–230.PubMed PMID: 24974805 Pubmed Central PMCID: PMC4073018 Epub 2014/07/01 Eng. [PMC free article] [PubMed] [Google Scholar]
- 5.Fujiwara Y, Arakawa T. Overlap in patients with dyspepsia/functional dyspepsia. J Neurogastroenterol Motil 2014; 20:447–457.PubMed PMID: 25257470 Pubmed Central PMCID: PMC4204405 Epub 2014/09/27 eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chou PH, Lin CC, Lin CH, et al. Prevalence of gastroesophageal reflux disease in major depressive disorder: a population-based study. Psychosomatics 2014; 55:155–162.PubMed PMID: 23953172 Epub 2013/08/21 eng. [DOI] [PubMed] [Google Scholar]
- 7.Prabhu V, Shivani A. An overview of history, pathogenesis and treatment of perforated peptic ulcer disease with evaluation of prognostic scoring in adults. Ann Med Health Sci Res 2014; 4:22–29.PubMed PMID: 24669326 Pubmed Central PMCID: PMC3952291 Epub 2014/03/29 eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levenstein S, Rosenstock S, Jacobsen RK, et al. Psychological stress increases risk for peptic ulcer, regardless of Helicobacter pylori infection or use of nonsteroidal anti-inflammatory drugs. Clin Gastroenterol Hepatol 2014; PubMed PMID: 25111233 Epub 2014/08/12 Eng. [DOI] [PubMed] [Google Scholar]
- 9.Krag M, Perner A, Wetterslev J, et al. Stress ulcer prophylaxis in the intensive care unit: is it indicated? A topical systematic review. Acta Anaesthesiol Scand 2013; 57:835–847.PubMed PMID: 23495933 Epub 2013/03/19 eng. [DOI] [PubMed] [Google Scholar]
- 10.Liao CH, Chang CS, Chang SN, et al. The association of peptic ulcer and schizophrenia: a population-based study. J Psychosom Res 2014; 77:541–546.PubMed PMID: 25199406 Epub 2014/09/10 eng. [DOI] [PubMed] [Google Scholar]
- 11.Lim WY, Subramaniam M, Abdin E, et al. Peptic ulcer disease and mental illnesses. Gen Hosp Psychiatry 2014; 36:63–67.PubMed PMID: 24120385 Epub 2013/10/15 eng. [DOI] [PubMed] [Google Scholar]
- 12.Hirschfeld RM. Differential diagnosis of bipolar disorder and major depressive disorder. J Affect Disord 2014; 169 Suppl 1:S12–S16.PubMed PMID: 25533909 Epub 2014/12/24 eng. [DOI] [PubMed] [Google Scholar]
- 13.Pfennig A, Ritter PS, Hofler M, et al. Symptom characteristics of depressive episodes prior to the onset of mania or hypomania. Acta Psychiatr Scand 2015; PubMed PMID: 26252885 Epub 2015/08/08 Eng. [DOI] [PubMed] [Google Scholar]
- 14.Phillips ML, Kupfer DJ. Bipolar disorder diagnosis: challenges and future directions. Lancet (London, England) 2013; 381:1663–1671.PubMed PMID: 23663952 Epub 2013/05/15 eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu YC, Hsu CC, Chang KH, et al. Increased subsequent risk of peptic ulcer diseases in patients with bipolar disorders. Medicine 2015; 94:e1203.PubMed PMID: 26200637 Epub 2015/07/23 eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hohne N, Poidinger M, Merz F, et al. Increased HPA axis response to psychosocial stress in remitted depression is influenced by coping style. Biol Psychol 2014; 103:267–275.PubMed PMID: 25263610 Epub 2014/09/30 Eng. [DOI] [PubMed] [Google Scholar]
- 17.Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Ann Rev Public Health 2013; 34:119–138.PubMed PMID: 23514317 Pubmed Central PMCID: PMC4100461 Epub 2013/03/22 eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang S, Xu Z, Gao Y, et al. Bidirectional crosstalk between stress-induced gastric ulcer and depression under chronic stress. PloS One 2012; 7:e51148.PubMed PMID: 23251441 Pubmed Central PMCID: PMC3521024 Epub 2012/12/20 eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gunn JM, Ayton DR, Densley K, et al. The association between chronic illness, multimorbidity and depressive symptoms in an Australian primary care cohort. Soc Psychiatry Psychiatr Epidemiol 2012; 47:175–184.PubMed PMID: 21184214 Epub 2010/12/25 eng. [DOI] [PubMed] [Google Scholar]
- 20.Edwards AC, Maes HH, Pedersen NL, et al. A population-based twin study of the genetic and environmental relationship of major depression, regular tobacco use and nicotine dependence. Psychol Med 2011; 41:395–405.PubMed PMID: 20406522 Pubmed Central PMCID: PMC3016459 Epub 2010/04/22 eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carceller-Maicas N, Ariste S, Martinez-Hernaez A, et al. Smoking as a form of self-medication for depression or anxiety in young adults: results of a mixed-methods study. Adicciones 2014; 26:34–45.PubMed PMID: 24652397 Epub 2014/03/22 El consumo de tabaco como automedicacion de depresion/ansiedad entre los jovenes: resultados de un estudio con metodo mixto spa. [PubMed] [Google Scholar]
- 22.Li LF, Chan RL, Lu L, et al. Cigarette smoking and gastrointestinal diseases: the causal relationship and underlying molecular mechanisms (review). Int J Mol Med 2014; 34:372–380.PubMed PMID: 24859303 Epub 2014/05/27 eng. [DOI] [PubMed] [Google Scholar]
- 23.Chaiton M, Cohen J, O’Loughlin J, et al. Use of cigarettes to improve affect and depressive symptoms in a longitudinal study of adolescents. Addict Behav 2010; 35:1054–1060.PubMed PMID: 20691543 Epub 2010/08/10 eng. [DOI] [PubMed] [Google Scholar]
- 24.Boschloo L, Vogelzangs N, van den Brink W, et al. Depressive and anxiety disorders predicting first incidence of alcohol use disorders: results of the Netherlands Study of Depression and Anxiety (NESDA). J Clin Psychiatry 2013; 74:1233–1240.PubMed PMID: 24434092 Epub 2014/01/18 eng. [DOI] [PubMed] [Google Scholar]
- 25.Nagata N, Niikura R, Sekine K, et al. Risk of peptic ulcer bleeding associated with Helicobacter pylori infection, NSAIDs, low-dose aspirin, and antihypertensive drugs: a case-control study. J Gastroenterol Hepatol 2015; 30:292–298.PubMed PMID: 25339607 Epub 2014/10/24 Eng. [DOI] [PubMed] [Google Scholar]
- 26.Andrade C. Antidepressant augmentation with anti-inflammatory agents. J Clin Psychiatry 2014; 75:975–977.PubMed PMID: 25295422 Epub 2014/10/09 eng. [DOI] [PubMed] [Google Scholar]
- 27.Manthey L, van Veen T, Giltay EJ, et al. Correlates of (inappropriate) benzodiazepine use: the Netherlands Study of Depression and Anxiety (NESDA). Br J Clin Pharmacol 2011; 71:263–272.PubMed PMID: 21219408 Pubmed Central PMCID: PMC3040548 Epub 2011/01/12 eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masi G, Brovedani P. The hippocampus, neurotrophic factors and depression: possible implications for the pharmacotherapy of depression. CNS Drugs 2011; 25:913–931.PubMed PMID: 22054117 Epub 2011/11/08 eng. [DOI] [PubMed] [Google Scholar]
- 29.Sun Y, Evans J, Russell B, et al. A benzodiazepine impairs the neurogenic and behavioural effects of fluoxetine in a rodent model of chronic stress. Neuropharmacology 2013; 72:20–28.PubMed PMID: 23639432 Epub 2013/05/04 eng. [DOI] [PubMed] [Google Scholar]


