Abstract
Alcohol use disorder (AUD) is considered a possible risk factor for irritable bowel syndrome (IBS); however, previous studies investigating the association between AUD and IBS have yielded inconsistent results. The study investigated whether AUD increases the risk of IBS by using a population-based database in Taiwan.
This retrospective matched-cohort study included the health insurance claims data of 56,355 AUD inpatients and 225,420 randomly selected controls by frequency-matched for sex, age, and index year. Cox proportional hazards regression analysis was performed to measure the risk of IBS among AUD patients compared with non-AUD patients.
During the follow-up period, the incidence rate ratio (IRR) of IBS had 12.3-fold (95% CI: 11.9–12.7) in the AUD patients than non-AUD patients and the adjusted hazard ratio (aHR) for IBS in the AUD patients was 5.51 (95% CI: 4.36–6.96). For several comorbidities, the risk of IBS was significantly higher in the AUD patients than in non-AUD patients, with aHRs of 2.14 (95% confidence interval [CI]: 1.19–3.84), 2.05 (95% CI: 1.06–3.96), and 2.91 (95% CI: 1.26–6.72) for sleep disorders, acute pancreatitis, and hepatitis B, respectively. When we stratified the severity of AUD according to the length of hospital stay, the aHRs exhibited a significant correlation (P < 0.001) with severity, yielding aHRs of 3.24 (95% CI: 2.49–4.22), 11.9 (95% CI: 8.96–15.9), and 26.1 (95% CI: 19.4–35.2) for mild, moderate, and severe AUD, respectively.
The risk of IBS was higher among AUD patients, and increased with the length of hospital stay.
INTRODUCTION
Irritable bowel syndrome (IBS) is a worldwide public health problem. Although IBS is not life-threatening, it is one of the most diagnosed gastrointestinal (GI) disorders, with prevalence rates between 7% and 21% in the general population.1 Annually, IBS is estimated to cost the United States an average of US$1.35 billion in direct medical expenses, and US$205 million more in indirect costs.2 No consensus regarding the pathophysiology of IBS exists, because numerous factors, such as visceral hypersensitivity,3 psychological disorders,4 infections,5 and food allergies,6 are considered possible causes.
Alcohol use disorder (AUD) is also a severe public health problem. Approximately 3.8% of all global deaths and 4.6% of global disability-adjusted life-years are attributed to alcohol.7 According to recent reports, AUD has a lifetime prevalence of about 30%8 and increasing rates according to the new diagnostic criteria.9 Alcohol causes a substantial number of physical and psychiatric disorders. In the digestive system, alcohol causes adverse effects such as gastritis, hepatitis, cirrhosis, and pancreatitis.10 However, the association between AUD and IBS remains unclear. Previous studies examining AUD and IBS have yielded conflicting results; some reported that alcohol consumption is more prevalent in patients with IBS, whereas others observed no such association.11–16
Elucidating the possible association between AUD and IBS would aid in preventing and treating IBS. Therefore, we conducted a retrospective matched-cohort study to determine whether patients with AUD are at an increased risk of developing IBS and whether other comorbidities increase this risk.
MATERIALS AND METHODS
Study Design
By using the health insurance claims data from the National Health Insurance Research Database (NHIRD) of Taiwan, we conducted a nationwide retrospective matched-cohort study. This database was established in 1995 upon the launch of the National Health Insurance program. The National Health Insurance covers approximately 99.9% of Taiwan's population. The National Health Research Institutes manages various insurance databases, including the registration files of insurees and claims data for reimbursement. Our data source was NHIRD inpatient claims data from 1996 to 2011. Diseases were diagnosed according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). Before release for research, the personal identification numbers in the database are encrypted to protect patient privacy. The Institutional Review Board of China Medical University approved this study (CMU-REC-101-012).
Data Availability Statement
All data and related metadata were deposited in an appropriate public repository. The data on the study population that were obtained from the NHIRD (http://w3.nhri.org.tw/nhird//date_01.html) are maintained in the NHIRD (http://nhird.nhri.org.tw/ ). The NHRI is a nonprofit foundation established by the government.
Study Population
Investigating the association between AUD and IBS was the study objective. A cohort of patients newly diagnosed with AUD (ICD-9-CM: 303, 3050, and V113) from 2000 to 2009 (N = 56,355) was established and compared with a cohort of non-AUD patients. The index date was defined as the date of AUD diagnosis. For selecting controls, inpatient claims data and a 1:4 ratio of AUD-to-comparison patients were used, and 225,420 non-AUD patients were selected and frequency-matched by sex, age, and index year. The main outcome was IBS (ICD-9-CM: 5641). Patients with IBS diagnosis before the index date or missing sex or age information were excluded. Follow-up was terminated after an IBS event or on December 31, 2011 (the end of the evaluation period). During the study period, the data of patients with comorbidities before the end date were estimated after adjustment for hypertension (ICD-9-CM: 401-405), hyperlipidemia (ICD-9-CM: 272), diabetes (ICD-9-CM: 250), depression (ICD-9-CM: 2962, 2963, 29682, 3004, and 311), anxiety (ICD-9-CM: 30000), fibromyalgia (ICD-9-CM: 7291), sleep disorder (ICD-9-CM: 3074 and 7805), acute pancreatitis (ICD-9-CM: 5770), cirrhosis (ICD-9-CM: 5712, 5715, and 5716), hepatitis B (ICD-9-CM: V0261, 07020, 07022, 07030, and 07032), and hepatitis C (ICD-9-CM: V0262, 07041, 07044, 07051, and 07054).
Statistical Analysis
For comparing differences in the distributions of demographic variables and comorbidities between the AUD and non-AUD patient groups, Chi-square and Student t-tests were used for the categorical and continuous variables, respectively. The IBS incidence rate was calculated for both groups. The Kaplan–Meier method was then used for estimating the event survival functions for IBS between the AUD and non-AUD patient groups during the follow-up period, and the log-rank test was used for assessing the differences between both curves. The incidence rate ratios and 95% confidence intervals (CIs) of IBS between the AUD and non-AUD patient groups were calculated using the Poisson regression model. The Cox proportional hazards regression model was used for measuring the risk of IBS in the AUD patients compared with the non-AUD patients and presented using adjusted hazard ratios (aHRs) and 95% CIs. We presented the aHR of IBS between AUD and non-AUD groups stratified by sex (women/men), age, and different comorbidity types (yes/no) by Cox proportional hazards regression model too. The mean of age was chosen as an age stratified analysis cut-point and then presented the relative risk of IBS between both groups in <45 years old and >45 years old. We further examined the association between the severity of AUD and IBS according to severity, which was defined as the total length of hospital stay caused by AUD in the follow-up period to the length of follow-up period. The AUD severity was classified by the tertile method, including mild (the 1st tertile in the AUD severity, T1), moderate (the 2nd tertile in the AUD severity, T2), and severe (the 3rd tertile in the AUD severity, T3).
All statistical analyses were performed using the SAS statistical package, version 9.4 (SAS Institute Inc., NC). A 2-tailed P value of <0.05 was considered significant. Survival curves were graphed using R program (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Table 1 presents the demographic and comorbidity data of the AUD and non-AUD patient groups. Age and sex did not differ significantly between the groups. Most of the patients were aged 35 to 65 years (69.9%) and were male (90.1%). After the frequency-matched, the mean of age was similar in AUD (44.5; standard deviation [SD]: 12.5) and non-AUD (44.4; SD: 12.6) group (Student's t-test P-value = 0.49). The proportion of patients with comorbidities was higher in the AUD patient group than in the non-AUD patient group (P < 0.0001, Chi-square test). The mean of follow-up were presented 5.53 and 6.84 in AUD (SD: 3.26) and non-AUD (SD: 2.98) groups, respectively. Toward the end of the follow-up period, the cumulative incidence of IBS was higher in the AUD patient group than in the non-AUD patient group (log-rank test P < 0.0001; Fig. 1).
TABLE 1.
Comparison of Demographics and History of Comorbidity Between AUD and Non-AUD Groups
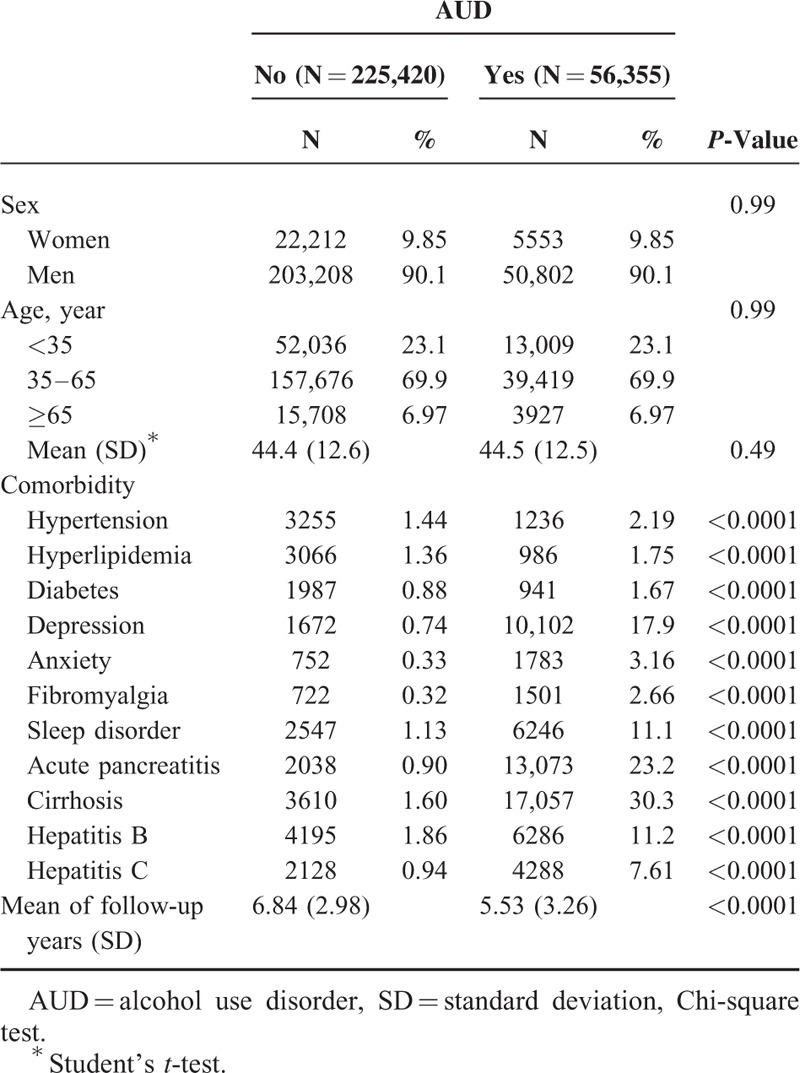
FIGURE 1.
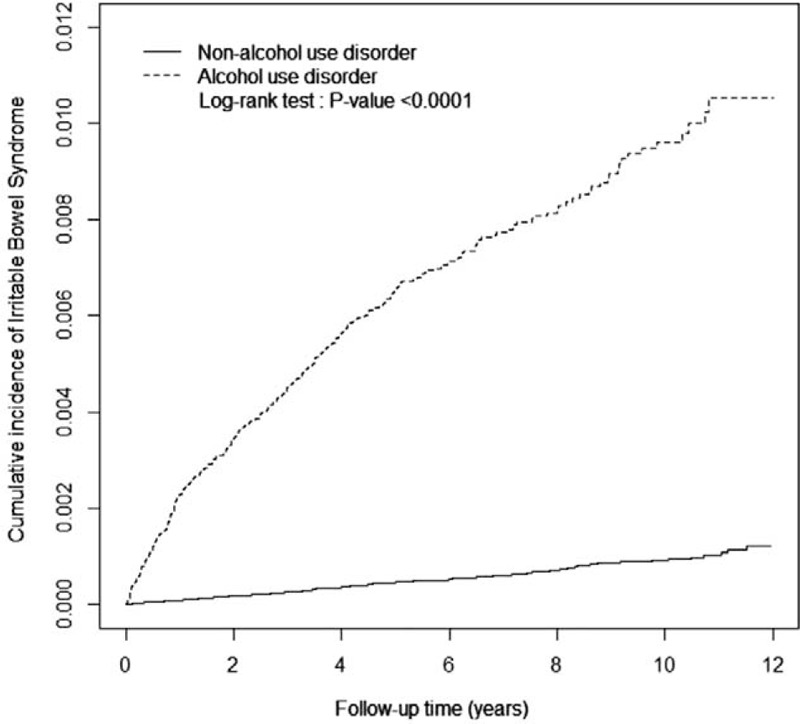
Cumulative incidence of irritable bowel syndrome between the alcohol use disorder (dashed line) and comparison (solid line) patient groups.
The incidence of IBS in the AUD patient group was 11.5 per 10,000 person-years, which was nearly 12.3-fold (95% CI: 11.9–12.7) higher than that in the non-AUD patient group (0.93 per 10,000 person-years; Table 2). After adjustment for potential risk factors, the AUD patient group exhibited a 5.51-fold (95% CI: 4.36–6.96) higher risk of developing IBS than the non-AUD patient group did.
TABLE 2.
Incidence and Adjusted Hazard Ratio of Irritable Bowel Syndrome Stratified by Sex, Age, and Comorbidity (Yes/No) Between 2 Groups
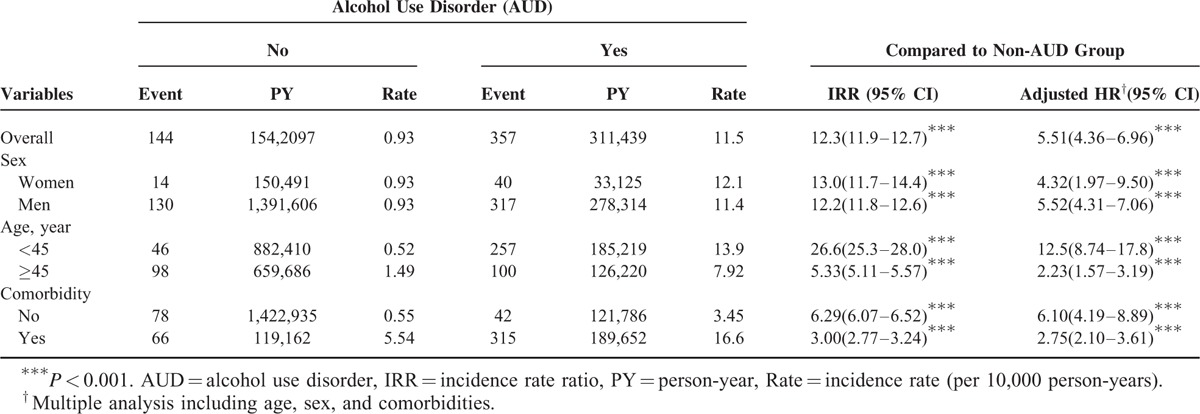
The incidence rates and aHRs of IBS were stratified according to sex, age (<45 and ≥45 years), and comorbidity (no or yes). The risk of IBS was higher in the AUD patients than in the non-AUD patients, regardless of sex (females, aHR: 4.32, 95% CI: 1.97–9.50; males, aHR: 5.52, 95% CI: 4.31–7.06). After adjustment for sex and each comorbidity, the AUD patients aged <45 years exhibited a 12.5-fold (95% CI: 8.74–17.8) higher risk of developing IBS than the non-AUD patients did. The incidence of IBS was increased in subjects with comorbidities. The aHR of IBS in the AUD patient group was 2.75-fold (95% CI: 2.10–3.61) higher than that in the non-AUD patient group.
Table 3 presents the risk of IBS of both groups stratified according to comorbidities. For several comorbidities, the AUD patients had a significantly higher risk of IBS than the non-AUD patients did, specifically for sleep disorder (aHR: 2.14, 95% CI: 1.19–3.84), acute pancreatitis (aHR: 2.05, 95% CI: 1.06–3.96), and hepatitis B (aHR: 2.91, 95% CI: 1.26–6.72).
TABLE 3.
Incidence and Adjusted Hazard Ratio of Irritable Bowel Syndrome Stratified by Comorbidity Between AUD and Non-AUD Groups
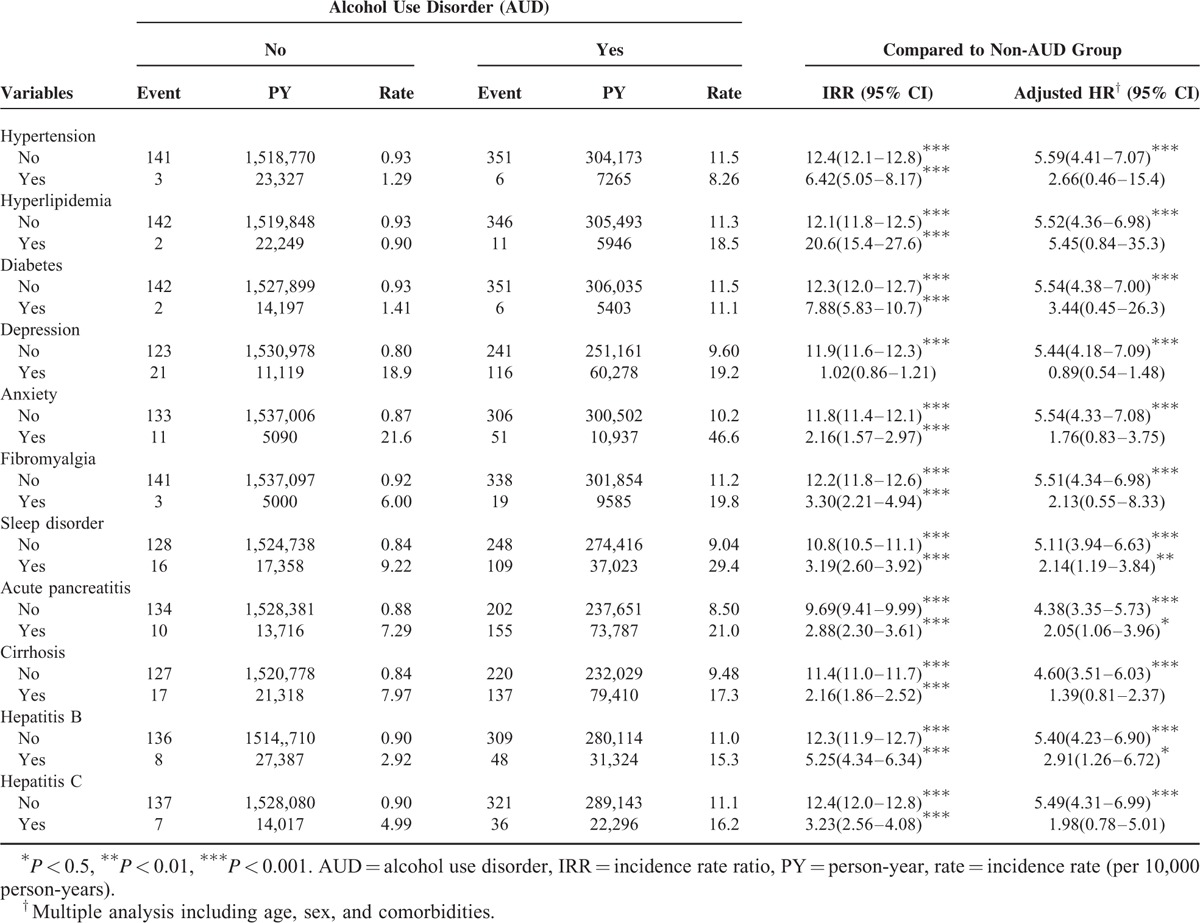
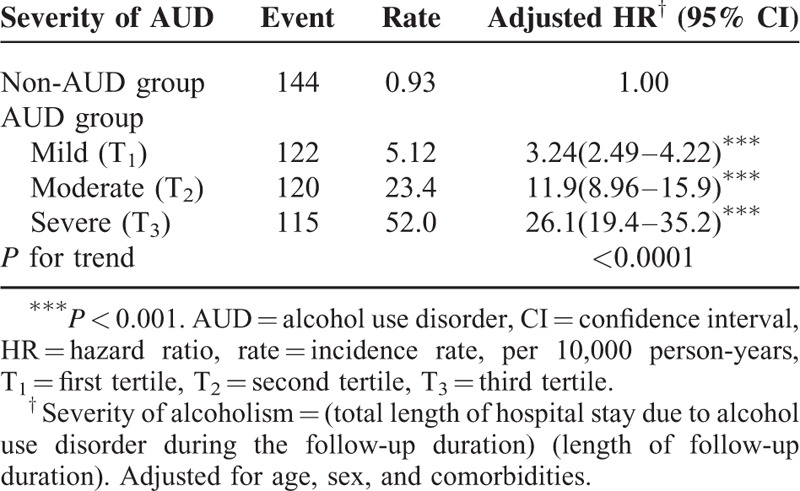
Compared with the non-AUD patient group, an increased risk of IBS stratified according to AUD severity was observed, with aHRs of 3.24 (95% CI: 2.49–4.22), 11.9 (95% CI: 8.96–15.9), and 26.1 (95% CI: 19.4–35.2) for mild, moderate, and severe AUD, respectively (Table 4).
TABLE 4.
Incidence Rate and Hazard Ratio for Irritable Bowel Syndrome Stratified by Severity of AUD
DISCUSSION
Previous studies investigating the association between IBS and AUD have yielded conflicting results. Masand et al11 reported that IBS was more prevalent among patients with AUD than among controls in a general physician's clinic for other medical illnesses (41.9% vs 2.5%, respectively). However, this study had a small sample size (71 patients) and did not consider other possible risk factors that may influence AUD and IBS development.
A study in Japan analyzed 2717 completed self-report questionnaires and revealed that alcohol consumption was only associated with diarrhea-predominant IBS (adjusted odds ratio [OR]: 2.15, 95% CI: 1.40–3.31),12 and another nationally representative study of 98,411 Japanese adolescents reported an association between AUD and IBS (adjusted OR: 1.21, 95% CI: 1.17–1.26).13 Similarly, a study involving 340 Chinese nurses indicated that alcohol consumption is a risk factor for IBS (OR: 2.611, 95% CI: 1.25–5.43).14
By contrast, a questionnaire study of 3022 participants in the United States15 and an unmatched case–control study involving 316 patients in Iran16 both observed no association between AUD and IBS. The researchers ascribed their conflicting results to the study design, diagnostic criteria, cultural differences, environmental factors, and sample size of their and the previous studies.
Halder et al17 reported that alcohol consumption (>7 drinks per week) was associated with increased odds of dyspepsia (OR: 2.3; 95% CI: 1.1–5.0) and frequent abdominal pain (OR: 1.5; 95% CI: 1.1–2.0) but not IBS. However, in females with a low somatic symptom checklist score, excessive alcohol consumption (≥7 drinks per week) increased the odds of IBS more than moderate alcohol consumption did. Reding et al18 demonstrated that for IBS patients, the strongest related associations were between binge drinking and GI symptoms the following day. The authors suggested that different drinking patterns may partly explain the inconsistent findings between AUD and IBS symptoms. In summary, most of the aforementioned studies have agreed that people with AUD, particularly binge or heavy drinkers, have a higher risk of IBS than non-AUD people do, which is consistent with our results. In this study, the aHRs of IBS also increased with the severity of AUD. When the patients with AUD were stratified into tertiles as mild, moderate, or severe AUD according to their length of hospital stay, the incidence rates of IBS per 10,000 person-years were 5.12, 23.4, and 52.0, respectively, with aHRs of 3.24, 11.9, and 26.1, respectively (Table 4).
Compared with previous study results, our results showed a higher risk of IBS in the AUD patients than in the non-AUD patients. Unlike the questionnaire methods used in previous studies, we analyzed inpatient claims data from a national database and conducted a 10-year follow-up. Self-reports of alcohol consumption in questionnaire studies often have inaccuracies because patients frequently underestimate their alcohol intake.17 Moreover, previous studies have included participants with AUD mainly from outpatient clinics, community centers, and schools, whereas we included inpatient participants with AUD; therefore, our patient group experienced more severe IBS. This finding may explain our markedly higher HR results.
The association between AUD and IBS may be of psychiatric origin. Several psychiatric disorders, such as anxiety and sleep disorder, and related somatic symptoms are independent risk factors for IBS development.19 Patients with AUD have a high risk to envelope mood disorders and anxiety disorders that are risk factors for IBS. And also anxious patients often use alcohol like therapy.20 The role of stress and psychosocial dysfunction in IBS is associated with alterations in central corticotropin-releasing factor (CRF) signaling pathways.21 Many studies have indicated that the CRF system is influenced by alcohol addiction, as is IBS.22,23 Furthermore, in animal experiments, some CRF antagonists have exhibited therapeutic effects on both IBS and AUD.24–26 Recently, in human studies, pexacerfont, a CRF antagonist, has been demonstrated to be effective for both IBS27 and AUD.28 This evidence indicated that IBS and AUD have the same central neuropsychiatric dysfunction.
Aside from the possible central cause of CRF pathway dysfunction, alcohol itself may also directly influence IBS development. The alcohol metabolism process affects the bloodstream, motility, and digestive functions of the GI tract.29 In addition, alcohol exerts a toxic effect on autonomic nerves,30 and a subgroup of IBS patients showed various autonomic abnormalities.31 Thus, AUD may trigger or aggravate IBS.
It has been reported that incidence of IBS ranged between 2 per 1000 person-years and 67 per 1000 person-years with female predominance.32 However, IBS incidence rates in our study is significantly lower (11.5 and 0.93 per 10,000 person years in the AUD and comparison groups, respectively). This may be because that our cohort is not the same with normal population in sex and age, even the comparison group is a 4-folds of AUD patients selected and frequency-matched by sex, age, and index year. AUD is a male predominant disorder as our data revealed (male 90.1%) and therefore the 2 groups both contained a majority of male subjects who are less susceptible to IBS in the follow-up period. Besides, 50% of patients with IBS report having first had symptoms before the age of 35 years,33 and prevalence is 25% lower in those aged over 50 years than in those who are younger,34 while the mean age in our cohorts is older than normal population (44.5 and 44.4 years in AUD and comparison groups, respectively. The median age of general population in Taiwan in 2015 is 39.7 years).35 These disparities of sex ratio and age may explain the lower incidence rates of IBS in our study.
This study had several limitations. First, the NHIRD does not include patients’ personal histories, including smoking, diet, occupational exposure, and family disease information. Second, accurate diagnosis depends on ICD-9 coding by the treating physician in this study. However, NHIRD covers a highly representative sample of Taiwan's general population because the reimbursement policy is universal and operated by a single-buyer, the government in Taiwan. All insurance claims should be scrutinized by medical reimbursement specialists and peer review according to the standard diagnosed criteria in the study. If these doctors or hospitals make wrong diagnoses or coding, they will be punished with a lot of penalties. Therefore, the diagnoses of the diseases based on ICD-9 codes in this study were highly reliable. Finally, data on alcohol consumption frequency per week and IBS subtypes were not available.
In conclusion, by analyzing nationwide health insurance data, we demonstrated that AUD increased the risk of IBS. After adjustment for age, sex, and comorbidities, AUD was associated with a 5.51-fold increased risk of IBS. The risk increased with the length of hospital stay for AUD. CRF pathway dysfunction is likely the common pathophysiology in IBS and AUD, which itself may also trigger IBS by directly affecting the GI tract and autonomic nerves.
Acknowledgments
The authors thank Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002); China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM104010092); NRPB Stroke Clinical Trial Consortium (MOST 103–2325-B-039-006); Tseng-Lien Lin Foundation, Taichung, Taiwan; Taiwan Brain Disease Foundation, Taipei, Taiwan; Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and CMU under the Aim for Top University Plan of the Ministry of Education, Taiwan for the support. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
Footnotes
Abbreviations: aHR = adjusted hazard ratio, AUD = alcohol use disorder, CI = confidence interval, GI = gastrointestinal, IBS = irritable bowel syndrome, ICD-9-CM = International Classification of Diseases Ninth Revision Clinical Modification, NHIRD = National Health Insurance Research Database, SD = standard deviation.
All authors have contributed substantially to, and are in agreement with the content of, the manuscript: Conception/Design: T-YH, C-HK; Provision of study materials: C-HK; Collection and/or assembly of data, Data analysis and interpretation, Manuscript preparation, and Final approval of manuscript: All authors.
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002); China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM104010092); NRPB Stroke Clinical Trial Consortium (MOST 103–2325-B-039-006); Tseng-Lien Lin Foundation, Taichung, Taiwan; Taiwan Brain Disease Foundation, Taipei, Taiwan; Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and CMU under the Aim for Top University Plan of the Ministry of Education, Taiwan.
All authors have no conflicts of interest to disclose.
REFERENCES
- 1.Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA 2015; 313:949–958. [DOI] [PubMed] [Google Scholar]
- 2.Inadomi JM, Fennerty MB, Bjorkman D. Systematic review: the economic impact of irritable bowel syndrome. Aliment Pharmacol Ther 2003; 18:671–682. [DOI] [PubMed] [Google Scholar]
- 3.Moshiree B, Price DD, Robinson ME, et al. Thermal and visceral hypersensitivity in irritable bowel syndrome patients with and without fibromyalgia. Clin J Pain 2007; 23:323–330. [DOI] [PubMed] [Google Scholar]
- 4.Wood JD. Neuropathophysiology of irritable bowel syndrome. J Clin Gastroenterol 2002; 35:S11–22. [DOI] [PubMed] [Google Scholar]
- 5.Spiller R, Garsed K. Postinfectious irritable bowel syndrome. Gastroenterology 2009; 136:1979–1988. [DOI] [PubMed] [Google Scholar]
- 6.Park MI, Camilleri M. Is there a role of food allergy in irritable bowel syndrome and functional dyspepsia? A systematic review. Neurogastroenterol Motil 2006; 18:595–607. [DOI] [PubMed] [Google Scholar]
- 7.Rehm J, Mathers C, Popova S, et al. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet 2009; 373:2223–2233. [DOI] [PubMed] [Google Scholar]
- 8.Bartoli F, Carrà G, Crocamo C, et al. From DSM-IV to DSM-5 alcohol use disorder: an overview of epidemiological data. Addict Behav 2015; 41:46–50. [DOI] [PubMed] [Google Scholar]
- 9.Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry 2015; 72:757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molina PE, Gardner JD, Souza-Smith FM, et al. Alcohol abuse: critical pathophysiological processes and contribution to disease burden. Physiology (Bethesda) 2014; 29:203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masand PS, Sousou AJ, Gupta S, et al. Irritable bowel syndrome (IBS) and alcohol abuse or dependence. Am J Drug Alcohol Abuse 1998; 24:513–521. [DOI] [PubMed] [Google Scholar]
- 12.Kubo M, Fujiwara Y, Shiba M, et al. Differences between risk factors among irritable bowel syndrome subtypes in Japanese adults. Neurogastroenterol Motil 2011; 23:249–254. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto R, Kaneita Y, Osaki Y, et al. Irritable bowel syndrome among Japanese adolescents: a nationally representative survey. J Gastroenterol Hepatol 2015; 9:1354–1360. [DOI] [PubMed] [Google Scholar]
- 14.Liu L, Xiao QF, Zhang YL, et al. A cross-sectional study of irritable bowel syndrome in nurses in China: prevalence and associated psychological and lifestyle factors. J Zhejiang Univ Sci B 2014; 15:590–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Talley NJ, Zinsmeister AR, Melton LJ., 3rd Irritable bowel syndrome in a community: symptom subgroups, risk factors, and health care utilization. Am J Epidemiol 1995; 142:76–83. [DOI] [PubMed] [Google Scholar]
- 16.Farzaneh N, Ghobaklou M, Moghimi-Dehkordi B, et al. Effects of demographic factors, body mass index, alcohol drinking and smoking habits on irritable bowel syndrome: a case control study. Ann Med Health Sci Res 2013; 3:391–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halder SL, Locke GR, 3rd, Schleck CD, et al. Influence of alcohol consumption on IBS and dyspepsia. Neurogastroenterol Motil 2006; 18:1001–1008. [DOI] [PubMed] [Google Scholar]
- 18.Reding KW, Cain KC, Jarrett ME, et al. Relationship between patterns of alcohol consumption and gastrointestinal symptoms among patients with irritable bowel syndrome. Am J Gastroenterol 2013; 108:270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicholl BI, Halder SL, Macfarlane GJ, et al. Psychosocial risk markers for new onset irritable bowel syndrome – results of a large prospective population-based study. Pain 2008; 137:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kushner MG, Abrams K, Borchardt C. The relationship between anxiety disorders and alcohol use disorders: a review of major perspectives and findings. Clin Psychol Rev 2000; 20:149–171. [DOI] [PubMed] [Google Scholar]
- 21.Fukudo S. Role of corticotropin-releasing hormone in irritable bowel syndrome and intestinal inflammation. J Gastroenterol 2007; 42 Suppl 17:48–51. [DOI] [PubMed] [Google Scholar]
- 22.Phillips TJ, Reed C, Pastor R. Preclinical evidence implicating corticotropin-releasing factor signaling in ethanol consumption and neuroadaptation. Genes Brain Behav 2015; 14:98–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zorrilla EP, Logrip ML, Koob GF. Corticotropin releasing factor: a key role in the neurobiology of addiction. Front Neuroendocrinol 2014; 35:234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Correia D, Martynhak BJ, Pereira M, et al. Reduction of ethanol intake by corticotropin-releasing factor receptor-1 antagonist in “heavy-drinking” mice in a free-choice paradigm. Psychopharmacology (Berl) 2015; 15:2731–2739. [DOI] [PubMed] [Google Scholar]
- 25.Saito-Nakaya K, Hasegawa R, Nagura Y, et al. Corticotropin-releasing hormone receptor 1 antagonist blocks colonic hypersensitivity induced by a combination of inflammation and repetitive colorectal distension. Neurogastroenterol Motil 2008; 20:1147–1156. [DOI] [PubMed] [Google Scholar]
- 26.Zorrilla EP, Heilig M, de Wit H, et al. Behavioral, biological, and chemical perspectives on targeting CRF(1) receptor antagonists to treat alcoholism. Drug Alcohol Depend 2013; 128:175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mozaffari S, Nikfar S, Abdollahi M. Metabolic and toxicological considerations for the latest drugs used to treat irritable bowel syndrome. Expert Opin Drug Metab Toxicol 2013; 9:403–421. [DOI] [PubMed] [Google Scholar]
- 28.Kwako LE, Spagnolo PA, Schwandt ML, et al. The corticotropin releasing hormone-1 (CRH1) receptor antagonist pexacerfont in alcohol dependence: a randomized controlled experimental medicine study. Neuropsychopharmacology 2015; 40:1053–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bujanda L. The effects of alcohol consumption upon the gastrointestinal tract. Am J Gastroenterol 2000; 95:3374–3382. [DOI] [PubMed] [Google Scholar]
- 30.Mellion M, Gilchrist JM, de la Monte S. Alcohol-related peripheral neuropathy: nutritional, toxic, or both? Muscle Nerve 2011; 43:309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tougas G. The autonomic nervous system in functional bowel disorders. Gut 2000; 47 Suppl 4:iv78–iv80.discussion iv87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol 2014; 6:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maxwell PR, Mendall MA, Kumar D. Irritable bowel syndrome. Lancet 1997; 350:1691–1695. [DOI] [PubMed] [Google Scholar]
- 34.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol 2012; 10:712–721.e4. [DOI] [PubMed] [Google Scholar]
- 35.CIA The World Factbook/Median Age 2015: https://www.cia.gov/library/publications/the-world-factbook/fields/2177.html Access date November 4th, 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and related metadata were deposited in an appropriate public repository. The data on the study population that were obtained from the NHIRD (http://w3.nhri.org.tw/nhird//date_01.html) are maintained in the NHIRD (http://nhird.nhri.org.tw/ ). The NHRI is a nonprofit foundation established by the government.


