Supplemental Digital Content is available in the text
Abstract
Mucinous adenocarcinoma (MC) is a special histology subtype of colorectal adenocarcinoma. The survival of MC is controversial and the prognostic biomarkers of MC remain unclear. To analyze prognostic significance and molecular features of colorectal MC. This study included 755,682 and 1001 colorectal cancer (CRC) patients from Surveillance, Epidemiology, and End Results program (SEER, 1973–2011), and Linköping Cancer (LC, 1972–2009) databases. We investigated independently the clinicopathological characteristics, survival, and variety of molecular features from these 2 databases. MC was found in 9.3% and 9.8% patients in SEER and LC, respectively. MC was more frequently localized in the right colon compared with nonmucinous adenocarcinoma (NMC) in both SEER (57.7% vs 37.2%, P < 0.001) and LC (46.9% vs 27.7%, P < 0.001). Colorectal MC patients had significantly worse cancer-specific survival (CSS) than NMC patients (SEER, P < 0.001; LC, P = 0.026), prominently in stage III (SEER, P < 0.001; LC, P = 0.023). The multivariate survival analysis showed that MC was independently related to poor prognosis in rectal cancer patients (SEER, hazard ratios [HR], 1.076; 95% confidence intervals [CI], 1.057–1.096; P < 0.001). In LC, the integrated analysis of genetic and epigenetic features showed that that strong expression of PINCH (HR, 3.954; 95% CI, 1.493–10.47; P = 0.013) and weak expression of RAD50 (HR 0.348, 95% CI, 0.106–1.192; P = 0.026) were significantly associated with poor CSS of colorectal MC patients. In conclusion, the colorectal MC patients had significantly worse CSS than NMC patients, prominently in stage III. MC was an independent prognostic factor associated with worse survival in rectal cancer patients. The PINCH and RAD50 were prognostic biomarkers for colorectal MC patients.
INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer and a major cause of cancer mortality worldwide.1 As a special histology subtype of adenocarcinoma, mucinous adenocarcinoma (MC) is defined by the World Health Organization (WHO) containing more than 50% extracellular mucin within the tumor and accounts for 1.6% to 25.4% CRCs.2,3 There were several consistent findings concerning differences between MC and nonmucinous adenocarcinoma (NMC) in the colorectum. Most notable of these differences were that MC more often affected young and female patients,4,5 frequently located on the proximal colon4,6 and had more later-stage presentations.4–8
However, the findings about the survival of colorectal MC patients were constantly inconsistent. Some studies reported that MC had a poorer prognosis compared with NMC, while others did contradict results.7,9–12 Parts of results were not stratified analyzed stage by stage. A recent meta-analysis of 44 articles showed a 2% to 8% significantly increased hazard of death of MC compared with NMC in the colorectum, which persisted after correction for stage. Nevertheless, they did not further analyze the prognostic impact of MC on different tumor locations.13
Furthermore, there were a limited number of literatures on molecular features showing that MC is associated with increased BRAF mutation rate,14,15 microsatellite instability (MSI),14,15 CpG island methylator phenotype,15,16 decreased APC mutation rate,17 and p53 expression.18,19 The prognostic biomarkers of MC remain unclear. One of the important reasons for these controversies was that signet-ring cell carcinoma (SRCC) as confounding factor existed in some studies.11 Despite sharing common feature of overproduction of mucin (intracellular) with MC (extracellular), SRCC had different biological behaviors and survival outcomes.10
In the present study, we analyzed clinicopathological characteristics and their survival significance of colorectal MC patients with exclusion of SRCC from 2 databases in the United States and Sweden. Furthermore, we assessed the molecular features of MC and their prognostic value of the patients from Sweden.
METHODS
SEER Database
In this study, 2 databases from the United States and Sweden were used. The Surveillance, Epidemiology, and End Results (SEER) 1973 to 2011 database collected patients from 18 population-based cancer registries representing approximately 28% of the U.S. population (http://seer.cancer.gov/about/overview.html). Characteristics recorded for each patient included sex, age at diagnosis, tumor location, tumor numbers, TNM stage, histological type, histological grade, and receipt of surgery and radiation therapy. The tumor location included the appendix C18.1, right colon (C18.0, C18.2–C18.4), left colon (C18.5–C18.7), large intestine NOS (C18.8–C18.9, C26.0), and rectum (C19.9 and C20.9). All TNM classification was restaged according to the criteria described in the American Joint Committee on Cancer (AJCC) Cancer Staging Manual, 7th edition, 2010 (Stages I, II, III, and IV). According to the International Classification of Diseases for Oncology (third edition, ICD-O-3) coding schema, the tumor histological subtypes were identified as MC (8480, 8481), SRCC (8490), and NMC (8010, 8140–8141, 8144–8145, 8210–8211, 8220–8221, 8230–8231, 8260–8263). Histological grade was classified as well differentiated (G1), moderately differentiated (G2), poorly differentiated (G3), and undifferentiated (G4). The cancer-specific survival (CSS) time was calculated from the date of diagnosis to the date of cancer-specific death or the end of follow-up (cutoff date: December 2011). Deaths attributed to the cancer of interest (CRC) were treated as events, and deaths from other causes are treated as censored observation. From an initial cohort of 866,626 patients, the following exclusions were carried out: tumors located on appendix C18.1 (n = 8800), tumors with in situ stage (n = 57,194), SRCC (n = 6435), and other histological type (n = 38,515). The final sample size was 755,682 (survival information available, n = 616,547) patients.
LC Database
The Linköping Cancer (LC) 1972 to 2009 database included CRC patients from the Southeast Swedish Health Care region including hospitals in Linköping, Norrköping, Motala, Jönköping, Kalmar, Oskarshamn, Västervik, Eksjö, and Värnamo. The clinicopathological information for each patient was recorded, including sex, age at diagnosis, tumor location, tumor numbers, TNM stage, growth pattern, histological type, histological grade, surgical type, receipt of radiation therapy and chemotherapy. The surgical specimens once obtained were divided into 2 parts. One part was snap-frozen immediately in dry ice and stored at −80°C freezer until further biomarker analysis. Another part was fixed in formalin and made to 5-ìm section routinely stained with hematoxylin and eosin. MC and SRCC were identified according to the WHO definition.20 NMC was defined as adenocarcinoma other than MC and SRCC. The histopathological characteristics, inflammatory infiltration, necrosis, and fibrosis were also evaluated. The follow-up was performed by matching all patients against the Swedish Cancer Register and the Cause of Death Register until July 2013. Data including local/distant recurrence, disease-free survival (DFS) time, survival time, and death causes were collected. All patients gave the required informed consent. Excluding SRCC (n = 13), a total of 1001 patients were included in this study.
Molecular Feature Analysis
The molecular features analysis was performed at surgical specimens of LC Database from the following aspects: immunohistochemistry for astrocyte elevated gene-1 (AEG-1), basic transcription factor 3 (BTF3), c-erbB-2, D2-40, deleted in colorectal cancer gene (DCC), FXYD-3, heat shock proteins (HSP), Ki-67, legumain, meningioma associated protein 3 (MAC30), Mre11, mismatch repair (MMR) proteins (including MLH1, MSH2, MSH3, and MSH6), NBS1, nuclear factor-kappaB p65 (NF-êB p65), Nup88, p27, p53, p73, proliferating cell nuclear antigen (PCNA), particularly interesting new cysteine-histidine-rich protein (PINCH), RAD50, ras, SPARCL1, stromelysin-3/MMP11; microsatellite status analysis; TUNEL assay for apoptotic cells; flow cytometry for DNA content and S-phase fraction (SPF); Mutation Analysis of adenomatous polyposis coli (APC), glutathione S-transferase T1 (GSTT1) and M1 (GSTM1), K-ras, MYH and peroxisome proliferator-activated receptor delta (PPARD); and DNA methylation analysis of O6-MGMT, p14ARF, p16INK4a, RASSF1A, and APC1A. For details, see supplementary materials and methods.
Statistical Analysis
All statistical analyses were conducted with R version 3.1.2 (http://www.R-project.org/). The t test or χ2 test was used to compare clinicopathological characteristics and biomarkers. Survival curves were based on Kaplan–Meier method. The differences between the curves were analyzed by log-rank test. Univariate and multivariate survival analyses were examined by the Cox proportional hazard models. The data were presented as hazard ratios (HR) with 95% confidence intervals (CI). All tests were 2-sided, and P of less than 0.05 was considered to indicate statistical significance.
RESULTS
Clinicopathological Characteristics in MC and NMC Patients
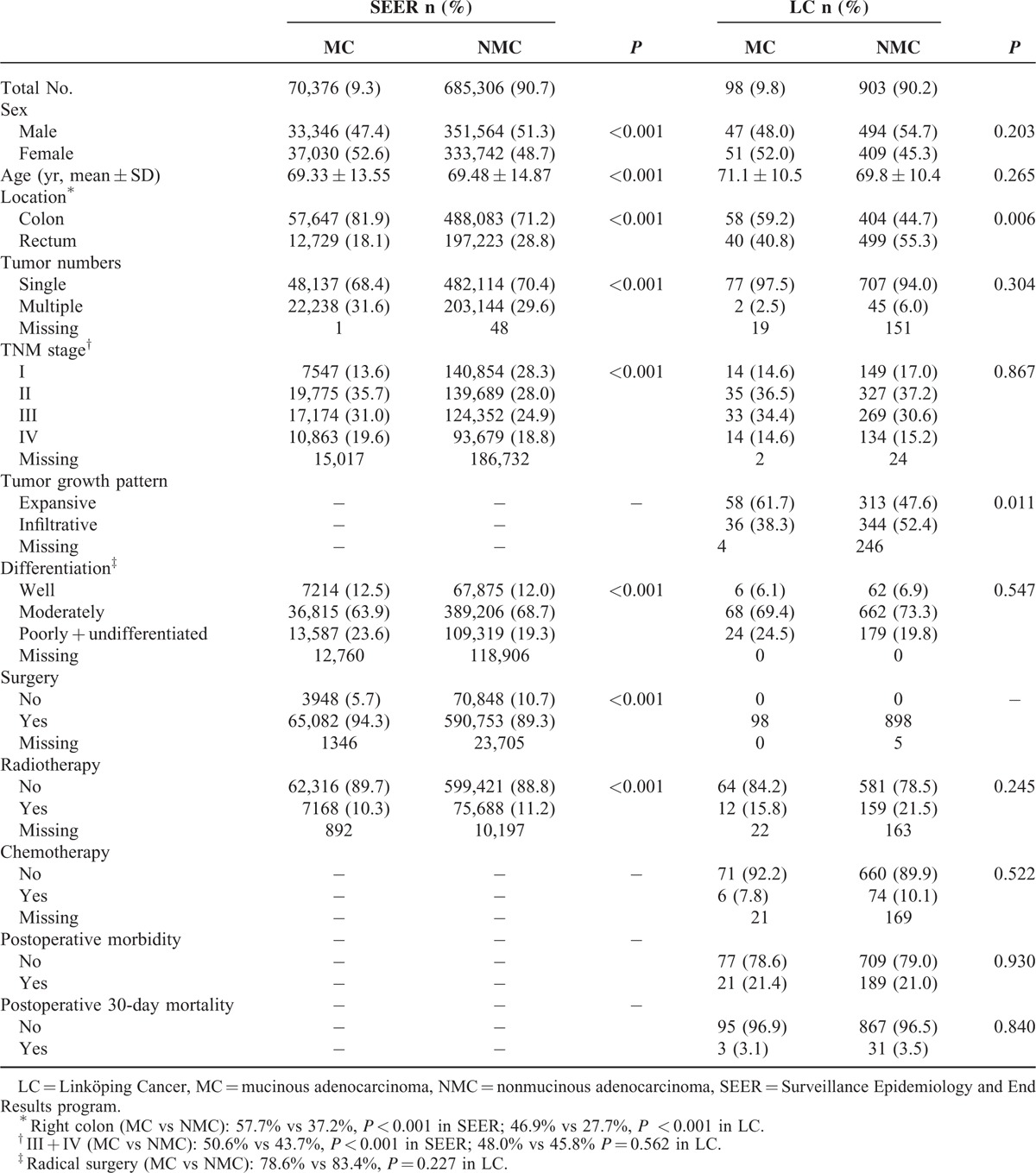
In total, 755,682 (SEER) and 1001 (LC) CRC patients were included. MC was found in 9.3% and 9.8% of the patients in SEER and LC, respectively. The clinicopathological characteristics of MC and NMC patients are shown in Table 1. MC was more frequently in right colon compared with NMC in both SEER (57.7% vs 37.2%, P < 0.001) and LC (46.9% vs 27.7%, P < 0.001). In SEER, significant differences existed in MC group compared with NMC group concerning the following clinicopathological characteristics: sex (more females, P < 0.001), age (younger age, P < 0.001), cancer numbers (more cases with multiple cancers, P < 0.001), TNM stage (later stage, P < 0.001), differentiation (poorer differentiation, P < 0.001), surgery (more cases received surgery, P < 0.001), and radiotherapy (less cases received radiotherapy, P < 0.001). In LC, there were more MC with expansive growth than NMC (P = 0.011). There was no significant difference concerning surgical type, chemotherapy, postoperative morbidity, and postoperative 30-day mortality (P > 0.05).
TABLE 1.
Clinicopathological Characteristics of CRC Patients According to Histological Type Groups
Survival in MC and NMC Patients
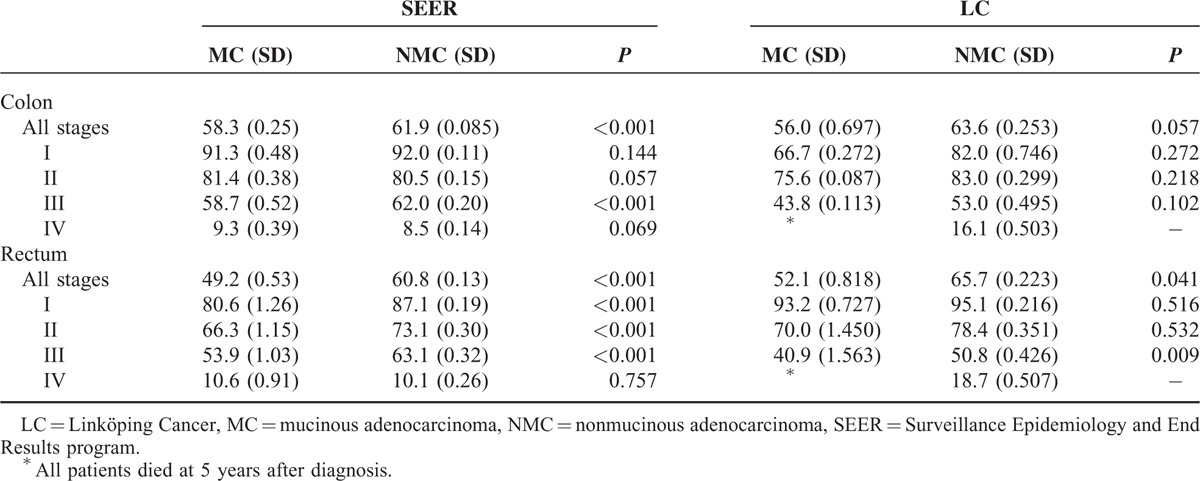
Colorectal MC patients had significantly worse CSS than NMC patients (SEER, P < 0.001; LC, P = 0.026, Fig. 1), prominently in stage III (SEER, P < 0.001, Supplementary Figure 1; LC, P = 0.023, Supplementary Figure 2). The 5-year CSS stratified by tumor location and stage for MC and NMC patients are presented in Table 2. The rectal MC patients had lower 5-year CSS rates compared with NMC patients (SEER, 49.2% vs 60.8%, P < 0.001; LC, 52.1% vs 65.7%, P = 0.041), especially in stage III (SEER, 53.9% vs 63.1%, P < 0.001; LC, 40.9% vs 50.8%, P = 0.009).
FIGURE 1.
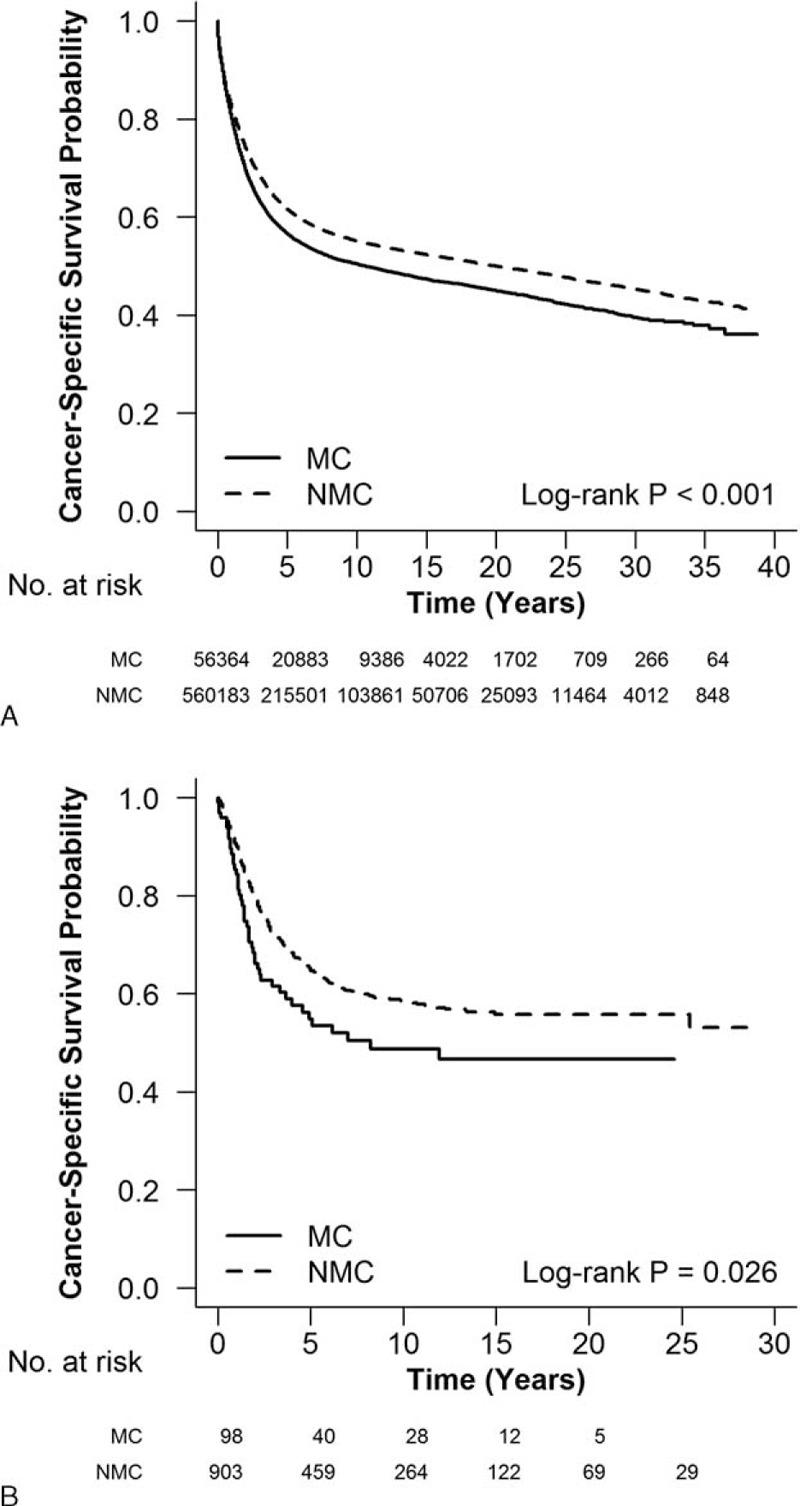
Survival differences between MC and NMC in (A) SEER and (B) LC. Colorectal MC patients had significantly worse cancer-specific survival than NMC patients. LC = Linköping Cancer, MC = mucinous adenocarcinoma, NMC = nonmucinous adenocarcinoma, SEER = Surveillance Epidemiology and End Results program.
TABLE 2.
The 5-Year Cancer-Specific Survival Rates for CRC Patients With AJCC Stages I, II, III, and IV According to Tumor Location and Histological Type
In LC, the DFS (P = 0.294, Supplementary Figure 3), recurrence rate (39.5% vs 33.2%, P = 0.401) including local recurrence rate (11.6% vs 11.2%, P = 0.936) and distant metastasis rate (34.9% vs 28.0%, P = 0.346) had no significant difference between MC and NMC. The similar results had been observed when analyzing on colon and rectum, respectively (data not shown).
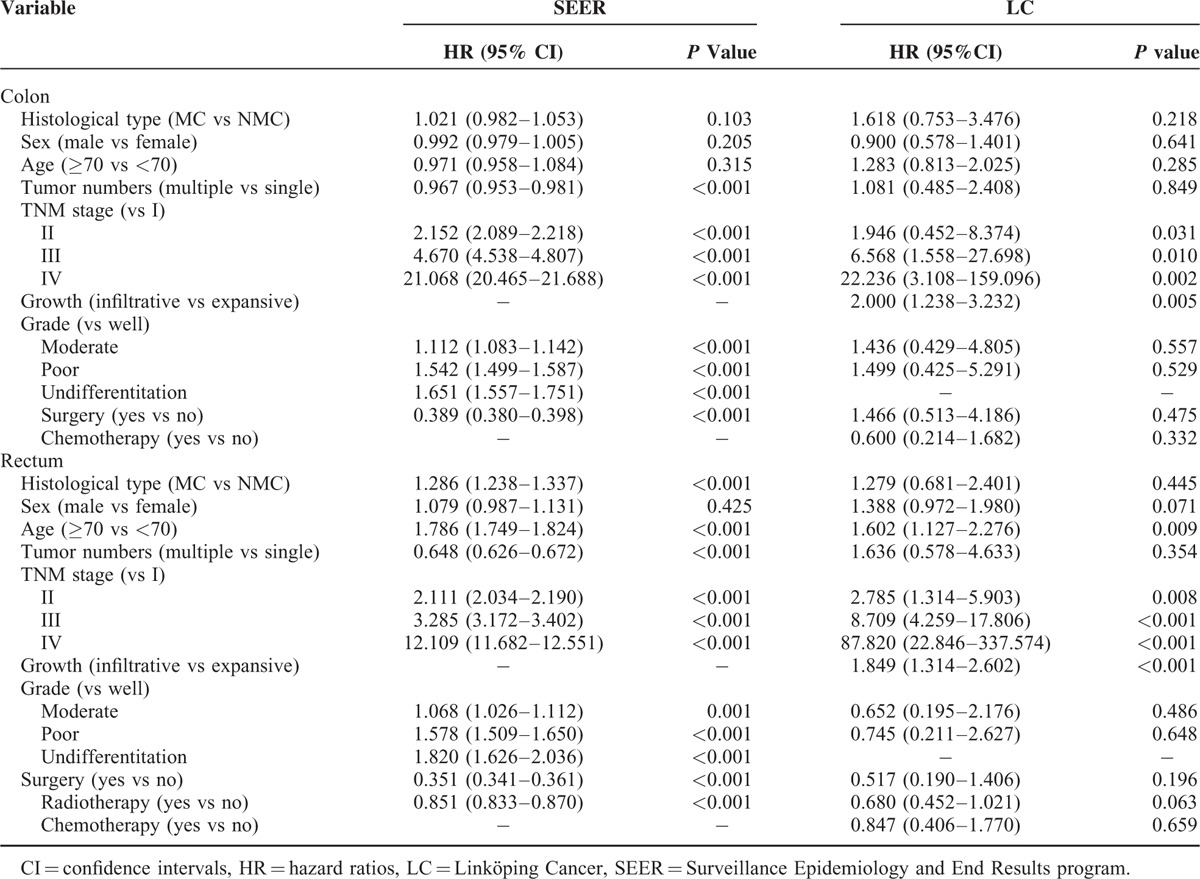
The multivariate analysis showed MC was associated with the poor CSS of CRC patients in SEER (HR, 1.076; 95%, 1.057–1.096; P < 0.001; Supplementary Table 1). Furthermore, MC was an independent predictor of poor CSS for rectal cancer patients in SEER (HR, 1.286; 95%, 1.238–1.337; P < 0.001), but not for colon cancer patients in both SEER and LC (Table 3).
TABLE 3.
Multivariate Survival Analysis of Prognostic Factors in Colon and Rectal Cancer Patients
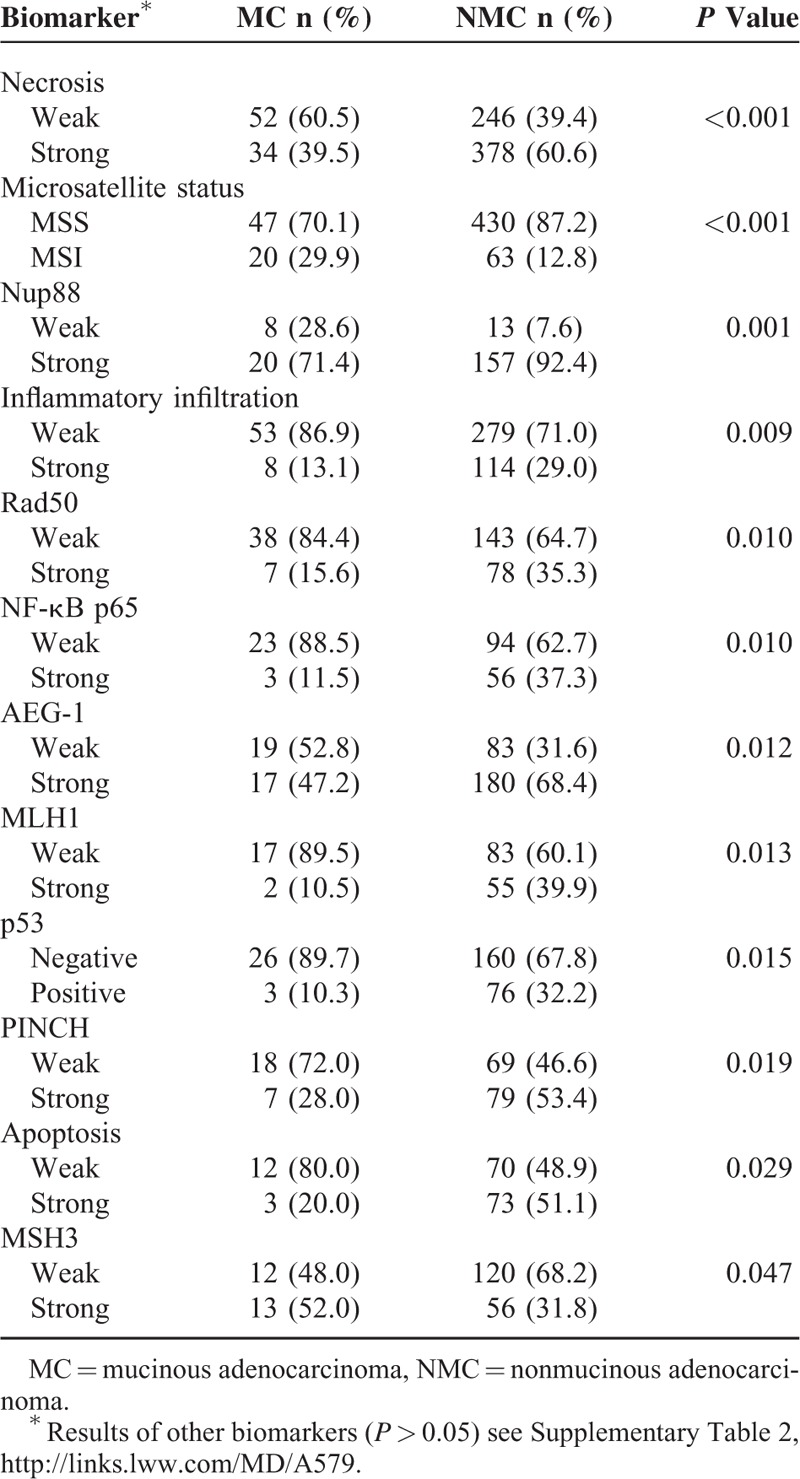
Molecular Features in MC Patients
As is shown in Table 4, significant differences existed in MC compared with NMC concerning the following molecular features and histopathological characteristics: necrosis (P < 0.001), microsatellite status (P < 0.001), Nup88 (P = 0.001), infiltrating infiltration (P = 0.009), RAD50 (P = 0.010), NF-êB p65 (P = 0.010), AEG-1 (P = 0.012), MLH1 (P = 0.013), p53 (P = 0.015), PINCH (P = 0.019), apoptosis (P = 0.029) K-ras mutation (P = 0.041), and MSH3 (P = 0.047) (for the results of other features see Supplementary Tables 2 and 3). No significant difference of DNA methylation of O6-MGMT, p14ARF, p16INK4a, RASSF1A, and APC1A was observed between MC and NMC (Supplementary Table 4).
TABLE 4.
Significant Biomarkers Between MC and NMC Patients
The further survival analysis (Supplementary Table 5) showed that the strong expression of PINCH (HR, 3.954; 95% CI, 1.493–10.47; P = 0.013) and the weak expression of RAD50 (HR 0.348, 95% CI, 0.106–1.192; P = 0.026) were associated with poor CSS of colorectal MC patients (Fig. 2).
FIGURE 2.
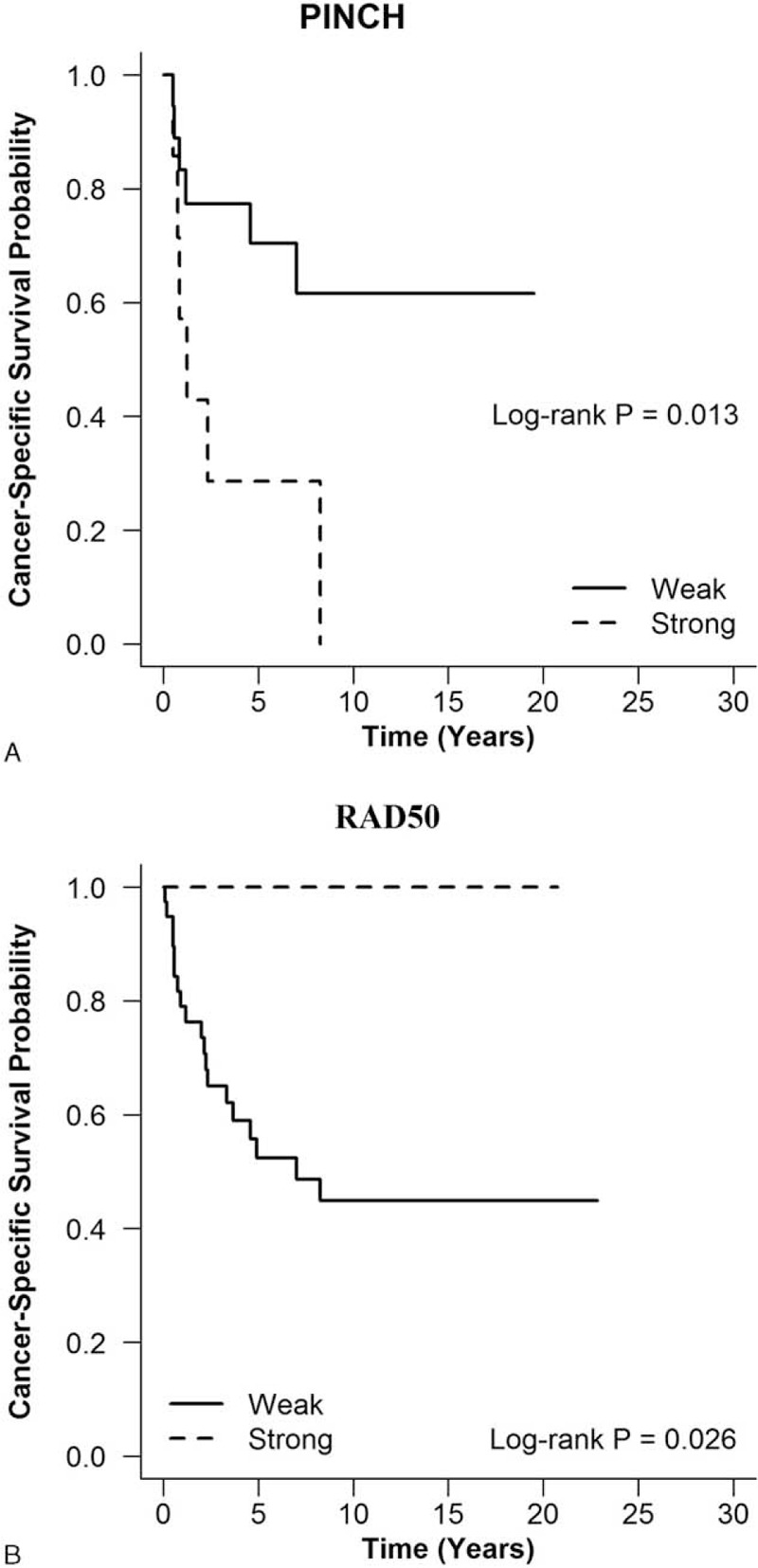
The prognostic value of biomarkers in colorectal MC patients. The strong expression of PINCH (A) and weak expression of Rad 50 (B) were associated with poorer cancer-specific survival of colorectal MC patients. MC = mucinous adenocarcinoma.
DISCUSSION
In the present study, we analyzed 756,683 CRC patients with extensive clinicopathological and survival data from 2 independent databases in the United States and Sweden. MC accounted for 9.3% and 9.8% of all CRCs in SEER and LC, respectively. It is consistent with general accepted finding that MC constitutes 10% to 15% of CRCs in large population-based studies.10 Nevertheless, the regional disparity of histology proportions in CRCs had been observed. The studies from different regions of the world reported the prevalence of MC ranged from approximately 3.9% in Asian countries to 10% to 13.6% in Western countries.3 In addition, a recent research demonstrated that distribution of MC was similar in all patients with regard to race in the United States.4 This regional disparity might be attributed to lifestyle, dietary, and environmental factors, rather than genetic variations between races. Furthermore, the population-based studies showed that notable differences existed between MC and NMC. MC more often affected female and young patients, frequently located on the proximal colon and had relatively later-stage presentations.4,21 Herein, our study corroborated these acknowledged clinicopathological features of MC.
Until now, the survival of colorectal MC patients is controversial. In the previous study, there are probably biases associated with single-institution reporting, relatively small sizes, or SRCC as confounding factor. In the present study, we analyzed a large number of CRC patients with long follow-up information from multiple institutions. Excluding SRCC, we found that colorectal MC patients had significantly worse CSS than NMC patients, prominently in stage III. This poor survival impact of MC was demonstrated on multivariate analysis. A previous study of SEER databases from 1991 to 2000 found the worse survival in MC. However, the difference disappeared when analyses were stratified by different stages.10 Consistent with our results, Verhulst et al13 performed meta-analysis of 44 articles and confirmed these significant differences of survival between MC and NMC after correction for stages. In this study, we could not find any difference between MC and NMC concerning recurrence (both local recurrence and distant metastasis) rate or DFS, which is in line with some studies.22–24
In this large population-based study, we analyzed the prognostic impact of MC on colon and rectal cancer patients, respectively. The statistically significant difference in CSS between MC and NMC had been found in stage III rectal cancer patients. The multivariate survival analysis showed MC was associated with a poor prognosis in rectal cancer patients but not in colon cancer patients. The same results had been reported in a study of U.S. National Cancer Data Base (1998–2002).4 MC was associated with increased risk of death in the rectum rather than the colon. Another population-based research from the Netherlands further demonstrated this poorer prognosis only in rectal cancer patients with I and III stages.21 Several studies have reported that MC had poorer response to radiotherapy and higher rate of positive circumferential resection margin resections compared with NMC in the rectum.9,25 These adverse factors might be partly reasons for the poor outcome in rectal MC. Recently, a large European trial cohort analysis showed that the improvement of comprehensive treatment including total mesorectal excision surgery in combination with neoadjuvant chemoradiotherapy could benefit rectal MC patients.26 The prognostic significance of this new comprehensive treatment in rectal MC patients requires a further prospective randomized controlled trial.
For the first time, we performed integrated analysis of genetic and epigenetic features, and their prognostic value in colorectal MC. Overview of the profile of more than 50 biomarkers, we verified some previously reported molecular features associated with MC including increased MSI and decreased p53 expression in accordance with previous studies.14,19,27 There are still conflicting results concerning molecular features such as K-ras mutations and apoptosis in MC. Tanaka et al examined 83 CRC specimens including 26 MCs, and found that K-ras mutations (27% vs 40%) were less frequently in MC. However, this difference was not statistically significant.15 Akino et al28 showed MC had higher apoptotic activity than NMC, but low apoptotic activity was related to poorer prognosis. In the present study, a more frequent of K-ras mutation and weak apoptosis had been found in MC. Furthermore, MC had distinct histopathological characteristics compared with NMC. There were more cases with weak necrosis and inflammatory infiltration in MC. These 2 histopathological characteristics had been reported to be associated with both mucinous subtype and MSI. As the important histopathological characteristics, necrosis and inflammatory infiltration were independent predictors of MSI.29 But we did not find any influence of necrosis and inflammatory infiltration on the prognosis of MC with or without MSI.
We furthermore identified PINCH and RAD50 as prognostic biomarkers of MC. The PINCH expressed more strongly in CRC compared with normal mucosa. Strong of PINCH expression was significantly related to worse survival of poorly differentiated CRC in the multivariate analysis.30 The increased RAD50 expression in MSS CRCs could be a cellular response against tumor from further progression. In addition, weak of RAD50 expression was associated with poor prognosis of MSS CRC.31 Further investigation of these significant biomarkers and their corresponding signaling pathways in a larger population will probably give us a better understanding of the mechanism of underlying cancer differentiation and the prognosis prediction in colorectal MC patients.
To our knowledge, this population-based study included the largest sample sizes of colorectal MC patients, which minimized the biases associated with single-institution experiences. Longer follow-up period has been able to reveal potential differences between histology groups sufficiently. However, there were several limitations of this study deserve comment. First, it is a retrospective nonrandomized exploratory study based on 2 data databases. Despite the national databases with good data quality and completeness, the SEER database only covered approximately 28% of the total U.S. population. LC database had a relatively small number of the patients from Southeast Swedish Care Region (population about 1 million) including the hospitals of Linköping, Norrköping, Motala, Jönköping, Kalmar, Oskarshamn, Västervik, Eksjö, and Värnamo. Second, the SEER database came from multiple registration centers over near 40 years. However, the standardized definition of MC was accepted in 1989.2 The misclassification of MC may exist in SEER database for heterogeneous pathological information and diverse interpretation. We were not able to confirm the histological type of each patient included in this study because of tissue sample being unavailable. To explore the possible heterogeneity among each registration center, we compared the proportion of MC, and there were no significant differences (data not shown). Furthermore, all the specimens were independently reviewed by 2 pathologists in LC database. Third, the specimens are unavailable in SEER, and we only examined the biomarkers in LC patients. The LC selected the patients from the hospitals in the Southeast of Sweden, and may not be reprehensive of the entire Swedish population. Our biomarkers were studied at a small number of the samples, and lack of deeper and systematic analyses such as pathway and interaction investigation. Therefore, the prognostic value of these biomarkers should be considered with caution. The results are needed to be validated in the further study based on a larger and nationwide population.
In conclusion, our present study demonstrated that MC more often affected female and young patients, frequently located on the proximal colon with later stage. The colorectal MC patients had significantly worse CSS than NMC patients, prominently in stage III. MC was an independent prognostic factor associated with worse survival in rectal cancer patients. The integrated analysis of genetic and epigenetic features showed that PINCH and RAD50 were prognostic biomarkers for colorectal MC patients. The strong expression of PINCH and the weak expression of RAD50 were associated with poor CSS of colorectal MC patients.
Supplementary Material
Footnotes
Abbreviations: CI = confidence intervals, CRC = colorectal cancer, CSS = cancer-specific survival, HR = hazard ratios, LC = Linköping Cancer, MC = mucinous adenocarcinoma, NMC = nonmucinous adenocarcinoma, PINCH = particularly interesting new cysteine-histidine-rich protein, SEER = Surveillance Epidemiology and End Results program.
M-JW and JP share co-first authorship.
This study was supported by grants from the National Scientific Foundation of China (Grant Nos. 81401949, 81300359), and the Swedish Cancer Foundation, Swedish Research Council and the Health Research Council in the South-East of Sweden.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011; 61:69–90. [DOI] [PubMed] [Google Scholar]
- 2.Jass JR, Sobin LH. Histological Typing of Intestinal Tumours. Berlin: Springer; 1989. [Google Scholar]
- 3.Hugen N, van Beek JJ, de Wilt JH, et al. Insight into mucinous colorectal carcinoma: clues from etiology. Ann Surg Oncol 2014; 21:2963–2970. [DOI] [PubMed] [Google Scholar]
- 4.Hyngstrom JR, Hu CY, Xing Y, et al. Clinicopathology and outcomes for mucinous and signet ring colorectal adenocarcinoma: analysis from the National Cancer Data Base. Ann Surg Oncol 2012; 19:2814–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanemitsu Y, Kato T, Hirai T, et al. Survival after curative resection for mucinous adenocarcinoma of the colorectum. Dis Colon Rectum 2003; 46:160–167. [DOI] [PubMed] [Google Scholar]
- 6.Chiang JM, Yeh CY, Changchien CR, et al. Mucinous adenocarcinoma showing different clinicopathological and molecular characteristics in relation to different colorectal cancer subgroups. Int J Colorectal Dis 2010; 25:941–947. [DOI] [PubMed] [Google Scholar]
- 7.Catalano V, Loupakis F, Graziano F, et al. Prognosis of mucinous histology for patients with radically resected stage II and III colon cancer. Ann Oncol 2012; 23:135–141. [DOI] [PubMed] [Google Scholar]
- 8.Melis M, Hernandez J, Siegel EM, et al. Gene expression profiling of colorectal mucinous adenocarcinomas. Dis Colon Rectum 2010; 53:936–943. [DOI] [PubMed] [Google Scholar]
- 9.Shin US, Yu CS, Kim JH, et al. Mucinous rectal cancer: effectiveness of preoperative chemoradiotherapy and prognosis. Ann Surg Oncol 2011; 18:2232–2239. [DOI] [PubMed] [Google Scholar]
- 10.Kang H, O’Connell JB, Maggard MA, et al. A 10-year outcomes evaluation of mucinous and signet-ring cell carcinoma of the colon and rectum. Dis Colon Rectum 2005; 48:1161–1168. [DOI] [PubMed] [Google Scholar]
- 11.Mekenkamp LJ, Heesterbeek KJ, Koopman M, et al. Mucinous adenocarcinomas: poor prognosis in metastatic colorectal cancer. Eur J Cancer 2012; 48:501–509. [DOI] [PubMed] [Google Scholar]
- 12.Xie L, Villeneuve PJ, Shaw A. Survival of patients diagnosed with either colorectal mucinous or non-mucinous adenocarcinoma: a population-based study in Canada. Int J Oncol 2009; 34:1109–1115. [DOI] [PubMed] [Google Scholar]
- 13.Verhulst J, Ferdinande L, Demetter P, et al. Mucinous subtype as prognostic factor in colorectal cancer: a systematic review and meta-analysis. J Clin Pathol 2012; 65:381–388. [DOI] [PubMed] [Google Scholar]
- 14.Song GA, Deng G, Bell I, et al. Mucinous carcinomas of the colorectum have distinct molecular genetic characteristics. Int J Oncol 2005; 26:745–750. [PubMed] [Google Scholar]
- 15.Tanaka H, Deng G, Matsuzaki K, et al. BRAF mutation, CpG island methylator phenotype and microsatellite instability occur more frequently and concordantly in mucinous than non-mucinous colorectal cancer. Int J Cancer 2006; 118:2765–2771. [DOI] [PubMed] [Google Scholar]
- 16.Hawkins N, Norrie M, Cheong K, et al. CpG island methylation in sporadic colorectal cancers and its relationship to microsatellite instability. Gastroenterology 2002; 122:1376–1387. [DOI] [PubMed] [Google Scholar]
- 17.Kim DH, Kim JW, Cho JH, et al. Expression of mucin core proteins, trefoil factors, APC and p21 in subsets of colorectal polyps and cancers suggests a distinct pathway of pathogenesis of mucinous carcinoma of the colorectum. Int J Oncol 2005; 27:957–964. [PubMed] [Google Scholar]
- 18.Enriquez JM, Diez M, Tobaruela E, et al. Clinical, histopathological, cytogenetic and prognostic differences between mucinous and nonmucinous colorectal adenocarcinomas. Rev Esp Enferm Dig 1998; 90:563–572. [PubMed] [Google Scholar]
- 19.Zhang H, Evertsson S, Sun X. Clinicopathological and genetic characteristics of mucinous carcinomas in the colorectum. Int J Oncol 1999; 14:1057–1061. [DOI] [PubMed] [Google Scholar]
- 20.Bosman FT, Carneiro F, Hruban RH, et al. WHO Classification of Tumours of the Digestive System. 4th edGeneva, Switzerland: World Health Organization; 2010. [Google Scholar]
- 21.Hugen N, Verhoeven RH, Radema SA, et al. Prognosis and value of adjuvant chemotherapy in stage III mucinous colorectal carcinoma. Ann Oncol 2013; 24:2819–2824. [DOI] [PubMed] [Google Scholar]
- 22.Consorti F, Lorenzotti A, Midiri G, et al. Prognostic significance of mucinous carcinoma of colon and rectum: a prospective case-control study. J Surg Oncol 2000; 73:70–74. [DOI] [PubMed] [Google Scholar]
- 23.Green JB, Timmcke AE, Mitchell WT, et al. Mucinous carcinoma: just another colon cancer? Dis Colon Rectum 1993; 36:49–54. [DOI] [PubMed] [Google Scholar]
- 24.Papadopoulos VN, Michalopoulos A, Netta S, et al. Prognostic significance of mucinous component in colorectal carcinoma. Tech Coloproctol 2004; 8 Suppl 1:s123–125. [DOI] [PubMed] [Google Scholar]
- 25.Grillo-Ruggieri F, Mantello G, Berardi R, et al. Mucinous rectal adenocarcinoma can be associated to tumor downstaging after preoperative chemoradiotherapy. Dis Colon Rectum 2007; 50:1594–1603. [DOI] [PubMed] [Google Scholar]
- 26.Hugen N, van de Velde CJ, Bosch SL, et al. Modern treatment of rectal cancer closes the gap between common adenocarcinoma and mucinous carcinoma. Ann Surg Oncol 2015; 22:2669–2676. [DOI] [PubMed] [Google Scholar]
- 27.Messerini L, Vitelli F, De Vitis LR, et al. Microsatellite instability in sporadic mucinous colorectal carcinomas: relationship to clinico-pathological variables. J Pathol 1997; 182:380–384. [DOI] [PubMed] [Google Scholar]
- 28.Akino F, Mitomi H, Nakamura T, et al. High apoptotic activity and low epithelial cell proliferation with underexpression of p21(WAF1/CIP1) and p27Kip1 of mucinous carcinomas of the colorectum: comparison with well-differentiated type. Am J Clin Pathol 2002; 117:908–915. [DOI] [PubMed] [Google Scholar]
- 29.Greenson JK, Bonner JD, Ben-Yzhak O, et al. Phenotype of microsatellite unstable colorectal carcinomas: well-differentiated and focally mucinous tumors and the absence of dirty necrosis correlate with microsatellite instability. Am J Surg Pathol 2003; 27:563–570. [DOI] [PubMed] [Google Scholar]
- 30.Loof J, Rosell J, Bratthall C, et al. Impact of PINCH expression on survival in colorectal cancer patients. BMC Cancer 2011; 11:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao J, Zhang H, Arbman G, et al. The different roles of hRAD50 in microsatellite stable and unstable colorectal cancers. Dis Markers 2008; 24:127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


