Abstract
Infantile hepatic hemangioendothelioma (IHH) is the most common vascular tumor of the liver in infancy. Adult with IHH is extremely rare. We presented a diffuse IHH in an adult patient with computed tomography (CT) and magnetic resonance image (MRI) findings.
A 39-year-old man was admitted to our hospital because of a 2-year history of abnormal liver function tests and a 7-day history of jaundice. Physical examination revealed enlarged liver. Unenhanced abdominal CT showed enlargement of the liver with diffuse hypodensity. Enhanced CT on the arterial phase revealed multiple centrally enhanced lesions diffusely involved the enlarged liver. The enhanced areas of the lesions became larger on the portal phase and all the lesions became homogeneous enhanced on the delayed phase. These lesions showed heterogeneously hyperintense on T2-weighted image, hypointense on T1-weighted image, and early centrally enhanced on dynamic gadolinium-enhanced MRI, with complete tumor enhancement after 180 s. The patient underwent orthotopic liver transplantation. IHH type 2 was confirmed by pathology. The patient died of tumor recurrence in the liver 4 months after transplantation.
Unlike the previously described imaging appearances of IHH, this case showed diffuse nodules with early central enhancement on CT and MRI. Considering the importance of the ability to differentiate IHH from other hepatic tumors, radiologists should be aware of these imaging appearances to establish knowledge of the entire spectrum of IHH.
INTRODUCTION
Infantile hepatic hemangioendothelioma (IHH) is a vascular tumor of the liver composed of anastomosing vascular channels lined by plump endothelial cells.1 It is the most common vascular tumor of the liver in infancy and the third most common hepatic tumor in children. Approximately 85% of affected patients present before 6 months of age, with <5% cases detected beyond 1 year of age.1 To our knowledge, only 2 adult with IHH has been reported in the English literatures.2,3 In this paper, we reported a diffuse IHH in an adult patient, with early central enhancement on CT and MRI.
CASE REPORT
A 39-year-old man was admitted to our hospital because of a 2-year history of abnormal liver function tests and a 7-day history of jaundice. Physical examination revealed that the liver was palpable 2 cm below the right lower costal margin. He had elevated levels of serum total bilirubin (164.9 μmol/L; reference range 2–18 μmol/L), direct bilirubin (83.1 μmol/L; reference range <7 μmol/L), alkaline phosphotase (115 U/L; reference range 32–92 U/L), and gamma glutamyl transpeptidase (173U/L; reference range <47 U/L). The serum hepatitis B virus surface antigen, hepatitis C virus antibody, hepatitis E virus antibody, hepatitis A virus IgM antibody, syphilis antibody, and human immunodeficiency virus antibody were all negative. He had no history of being exposed to hepatotoxins, undergoing any transfusions, regular tobacco or alcohol use, and familial liver disease. Laboratory tests showed normal α-fetoprotein, carcinoembryonic antigen, and cancer antigen 19 to 9 levels.
Unenhanced abdominal CT showed enlargement of the liver with diffuse hypodensity. Enhanced CT on the arterial phase revealed multiple centrally enhanced lesions diffusely involved the enlarged liver. The enhanced areas of the lesions became larger on the portal phase and all the lesions became homogeneous enhanced on the delayed phase (Figure 1). These lesions showed heterogeneously hyperintense on T2-weighted images, hypointense on T1-weighted images, and early centrally enhanced on dynamic gadolinium-enhanced MR imaging, with complete tumor enhancement after 180 s (Figure 2).
FIGURE 1.
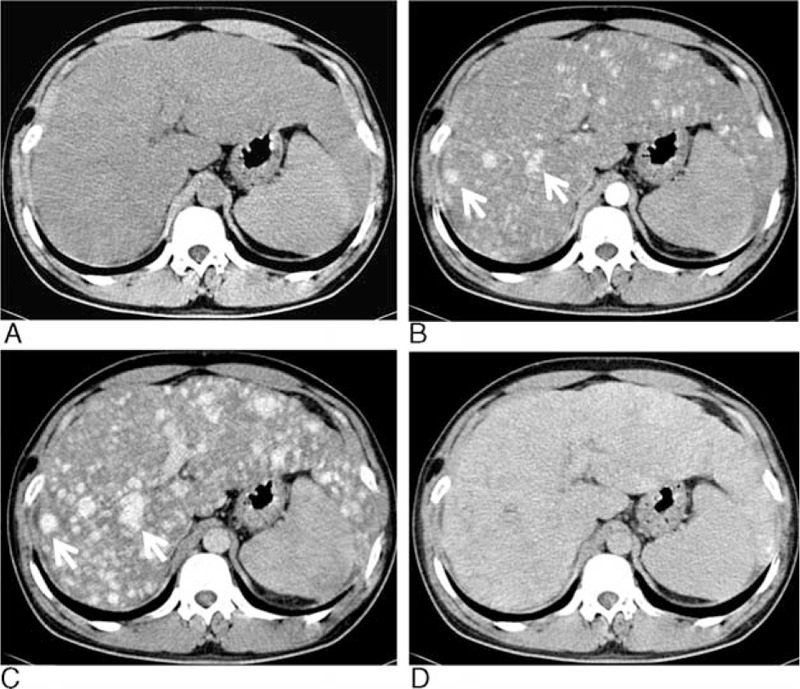
Axial unenhanced CT (A) showed the enlarged liver with homogeneous hypodensity and the lesions were indistinct. Axial enhanced CT (B) in the arterial phase showed numerous enhanced nodules throughout the liver (arrows). In the portal phase (C), the sizes of the enhanced nodules became larger with increased extent of enhancement (arrows). In the delayed phase (D), the nodules became uniform enhancement and nearly indistinct. CT = computed tomography.
FIGURE 2.
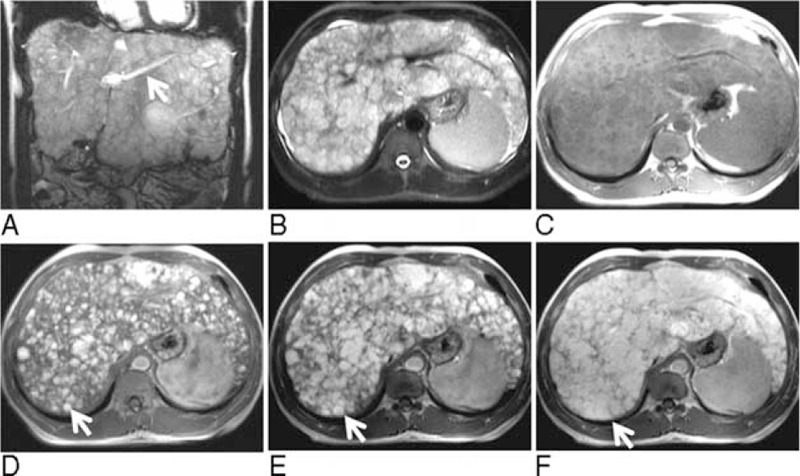
Unenhanced coronary (A) and axial (B) T2-weighted MR images showed diffuse nodules (ranging from a few millimeters to 4.4 cm in diameter) with heterogeneously high signal intensities. The portal branches were compressed (arrow). These nodules were hypointense on the axial unenhanced T1-weighted image (C). The axial enhanced T1-weighted image in the arterial phase (D) showed central enhancement of the nodules (arrow). In the portal phase (E), the hypervascular regions of the nodules became larger (arrow). In the delayed phase (F), the nodules became uniform enhancement (arrow) with reticular regions of hypointensity between the nodules. MR = magnetic resonance.
The patient underwent orthotopic liver transplantation. Gross specimen revealed that the liver was replaced by diffuse numerous grayish brown nodules without evidence of hemorrhage and necrosis. Microscopically (Figure 3), the nodules were not encapsulated and intermixed with the hepatic plates. The vascular cannels were lined by plump endothelial cells with irregular budding and branching. The endothelial cells were hyperchromatic and pleomorphic with nuclear atypia and prominent nucleoli. Mitoses of the endothelial cells nucleus were easily noted. Tumor cells were positive for specific endothelial cell marker CD34. These findings were consistent with IHH type 2. The patient died of tumor recurrence in the liver 4 months after transplantation.
FIGURE 3.
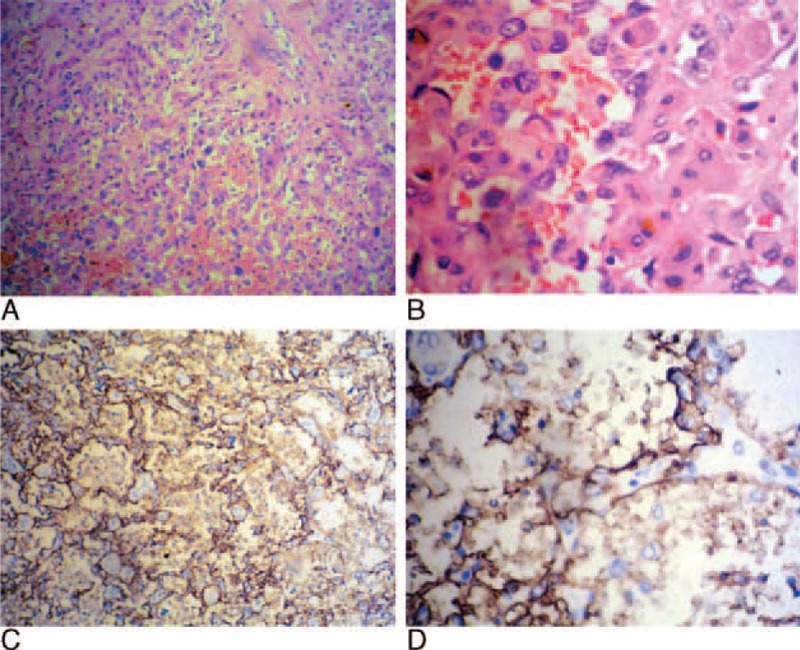
Histopathology (A, hematoxylin and eosin stain, ×200) showed twisting vascular channels lined with proliferating pleomorphic endothelial cells. Histopathology at higher (B, hematoxylin and eosin stain, ×400) magnification showed large hyperchromatic and pleomorphic endothelial cells with abundant mitoses, nuclear atypia, and prominent nucleoli. Immunohistochemically, the endothelial cells showed a strong CD-34 reaction (C, original magnification, ×200; D, original magnification, ×400).
DISCUSSION
Histologically, IHH is subdivided into 2 types by Dehner and Ishak.4 Type 1, tumors are composed of variable sized vascular spaces lined by relatively immature endothelial cells; type 2, tumors are composed of larger, more immature cells with increased cellularity, reflecting more aggressive microscopic appearance. The authors considered type 1 was histologically benign and type 2 is histologically equivalent to angiosarcoma. In addition, a mixture of type 1 and type 2 components within the same liver has been reported.5,6 IHH may be solitary, multifocal, or diffusive. The diffuse lesions are all classified as type 2.7 Larger tumors frequently have central areas of infarction, hemorrhage, and calcification or fibrosis.
On precontrast CT, IHHs are usually hypodense. The incidence of calcification is ∼50%.1 Multifocal lesions are less likely to calcify. On the arterial phase, the most common CT enhancement pattern is peripheral enhancement.8 The nodular, fibrillary, and homogeneous enhancement patterns are less commonly described. Small lesions are usually enhanced homogeneously. On the portal phase, tumors show progressive fill-in of central area. On the delayed phase, small lesions usually enhance completely, whereas some large lesions demonstrate incomplete enhancement, probably due to necrosis or hemorrhage. IHH, generally, is heterogeneous intensity on pre-contrast T1 weighted images because of the presence of hemorrhage, infarction, calcification, or fibrosis. On T2 weighted images, IHH is usually hyperintense due to its vascular nature, similar to the appearance of adult cavernous hemangioma. The dynamic enhanced MRI features are similar to that on enhanced CT.9–11
Unlike the previously described cases, our case showed early central enhancement pattern on both contrast CT and MRI. The early central enhancement pattern was similar to some atypically adult cavernous hemangiomas.12,13 A few reports demonstrate that some hepatic angiosarcomas also exhibit central enhancement on the arterial phase.14–16 Ackermann et al has reported a large tumor of IHH type 2 with early central enhancement.6 To our knowledge, diffuse IHH with early central enhancement has not previously been reported in the English literature. In this case, the reason of central enhancement was unclear. We hypothesized that the central enhancement of the lesions on the arterial phase followed by a centrifugal enhancement in the portal and delayed phases was because the feeding arteries were in the centers of the lesions, and not in the peripheries. This postulation remained to be proven by imaging pathological correlations.
In conclusion, this was a rare case of diffuse IHH in an adult patient. Unlike the previously described imaging appearances of IHH, our case showed diffuse nodules with early central enhancement on CT and MRI. Considering the importance of the ability to differentiate IHH from other hepatic tumors, radiologists should be aware of these imaging appearances to establish knowledge of the entire spectrum of IHH.
ETHICAL REVIEW AND CONSENT
Ethical approval was obtained from the Ethics Committee of Changhai Hospital, Shanghai, China. Written informed consent was obtained from the patient's relative for publication of this case report and any accompanying images.
Footnotes
Abbreviations: CT = computed tomography, IHH = infantile hepatic hemangioendothelioma, MRI = magnetic resonance imaging.
AD and HD contributed equally to the study.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Keslar PJ, Buck JL, Selby DM. From the archives of the AFIP. Infantile hemangioendothelioma of the liver revisited. Radiographics 1993; 13:657–670. [DOI] [PubMed] [Google Scholar]
- 2.Selby DM, Stocker JT, Waclawiw MA, et al. Infantile hemangioendothelioma of the liver. Hepatology 1994; 20:39–45. [DOI] [PubMed] [Google Scholar]
- 3.Diment J, Yurim O, Pappo O. Infantile hemangioendothelioma of the liver in an adult. Arch Pathol Lab Med 2001; 125:931–932. [DOI] [PubMed] [Google Scholar]
- 4.Dehner LP, Ishak KG. Vascular tumors of the liver in infants and children. A study of 30 cases and review of the literature. Arch Pathol 1971; 92:101–111. [PubMed] [Google Scholar]
- 5.Daller JA, Bueno J, Gutierrez J, et al. Hepatic hemangioendothelioma: clinical experience and management strategy. J Pediatr Surg 1999; 34:98–105. [DOI] [PubMed] [Google Scholar]
- 6.Ackermann O, Fabre M, Franchi S, et al. Widening spectrum of liver angiosarcoma in children. J Pediatr Gastroenterol Nutr 2011; 53:615–619. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Z, Chen HJ, Yang WJ, et al. Infantile hepatic hemangioendothelioma: a clinicopathologic study in a Chinese population. World J Gastroenterol 2010; 16:4549–4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng ST, Chan T, Ching AS, et al. CT and MR imaging characteristics of infantile hepatic hemangioendothelioma. Eur J Radiol 2010; 76:e24–e29. [DOI] [PubMed] [Google Scholar]
- 9.Halefoğlu AM. Magnetic resonance imaging of infantile hemangioendothelioma. Turk J Pediatr 2007; 49:77–81. [PubMed] [Google Scholar]
- 10.Mortelé KJ, Vanzieleghem B, Mortelé B, et al. Solitary hepatic infantile hemangioendothelioma: dynamic gadolinium-enhanced MR imaging findings. Eur Radiol 2002; 12:862–865. [DOI] [PubMed] [Google Scholar]
- 11.Mortele KJ, Mergo PJ, Urrutia M, et al. Dynamic gadolinium-enhanced MR findings in infantile hepatic hemangioendothelioma. J Comput Assist Tomogr 1998; 22:714–717. [DOI] [PubMed] [Google Scholar]
- 12.Matsushita M, Takehara Y, Nasu H, et al. Atypically enhanced cavernous hemangiomas of the liver: centrifugal enhancement does not preclude the diagnosis of hepatic hemangioma. J Gastroenterol 2006; 41:1227–1230. [DOI] [PubMed] [Google Scholar]
- 13.Bartolotta TV, Taibbi A, Galia M, et al. Centrifugal (inside-out) enhancement of liver hemangiomas: a possible atypical appearance on contrast-enhanced US. Eur J Radiol 2007; 64:447–455. [DOI] [PubMed] [Google Scholar]
- 14.Koyama T, Fletcher JG, Johnson CD, et al. Primary hepatic angiosarcoma: findings at CT and MR imaging. Radiology 2002; 222:667–673. [DOI] [PubMed] [Google Scholar]
- 15.Kassarjian A, Zurakowski D, Dubois J, et al. Infantile hepatic hemangiomas: clinical and imaging findings and their correlation with therapy. AJR Am J Roentgenol 2004; 182:785–795. [DOI] [PubMed] [Google Scholar]
- 16.Ohmoto K, Hirokawa M, Takesue M, et al. Hepatic angiosarcoma with early central enhancement and arterioportal shunt on dynamic CT. Hepatogastroenterology 2000; 47:1717–1718. [PubMed] [Google Scholar]


