Abstract
Background
Individuals with a family history of alcoholism are at much greater risk for developing an alcohol use disorder (AUD) than youth or adults without such history. A large body of research suggests that there are premorbid differences in brain structure and function in family history positive (FHP) individuals relative to their family history negative (FHN) peers.
Methods
This review summarizes the existing literature on neurobiological phenotypes present in FHP youth and adults by describing findings across neurophysiological and neuroimaging studies.
Results
Neuroimaging studies have shown FHP individuals differ from their FHN peers in amygdalar, hippocampal, basal ganglia, and cerebellar volume. Both increased and decreased white matter integrity has been reported in FHP individuals compared with FHN controls. Functional magnetic resonance imaging studies have found altered inhibitory control and working memory-related brain response in FHP youth and adults, suggesting neural markers of executive functioning may be related to increased vulnerability for developing AUDs in this population. Additionally, brain activity differences in regions involved in bottom-up reward and emotional processing, such as the nucleus accumbens and amygdala, have been shown in FHP individuals relative to their FHN peers.
Conclusions
It is critical to understand premorbid neural characteristics that could be associated with cognitive, reward-related, or emotional risk factors that increase risk for AUDs in FHP individuals. This information may lead to the development of neurobiologically informed prevention and intervention studies focused on reducing the incidence of AUDs in high-risk youth and adults.
Keywords: family history, alcoholism, EEG, MRI, fMRI, DTI
1. FAMILY HISTORY OF ALCOHOLISM
It is well established that family history of alcoholism is a significant risk factor for the development of alcohol use disorders (AUDs; Cloninger et al., 1986; Goodwin, 1985; Schuckit et al., 1972). This evidence comes from the observation that alcoholism is prevalent among relatives (Schuckit et al., 1972), and there is a higher concordance of the disorder in both male and female monozygotic twins (Heath et al., 1997), with an estimated 30-50% of individual risk attributed to genetics (Heath et al., 1997; Kaprio et al., 1987; Knopik et al., 2004). Additionally, adoption studies suggest similar risk in individuals living apart from biological parents, which provides further support for the heritability of the disorder (Bohman, 1978; Cloninger et al., 1981; Goodwin et al., 1974). A quarter of youth in the United States have a family history of alcoholism (Grant, 2000), which increases their likelihood of developing an AUD three-to-five fold (Cotton, 1979). Greater density of alcoholism in one’s family is also associated with higher risk of developing an AUD (Hill and Yuan, 1999). Furthermore, family history of alcoholism increases the risk of alcohol-related problems among adolescents (Lieb et al., 2002). Given the strong evidence that family history of alcoholism significantly increases AUD risk, it is critical to understand the neurobiological underpinnings that contribute towards the heritability of the disorder. Nonetheless, many individuals with a family history of alcoholism do not go on to develop AUDs (Werner, 1986), so it is equally important to identify neurobiological mechanisms that may confer resilience against heavy alcohol use.
Definitions of family history of alcoholism have varied from parental or nonparental presence of AUDs, examination of maternal and/or paternal sides of the family, uni- or multigenerational presence of the disorder, or quantification of multiple relatives with the disorder (Alterman, 1988). Despite these varying definitions, previous neuroimaging research has largely categorized individuals as having a positive family history of alcoholism (FHP) if they had at least one biological parent or two or more second degree relatives diagnosed with AUDs (e.g., Andrews et al., 2011, Cservenka and Nagel, 2012), while family history negative (FHN) individuals had an absence of familial alcoholism in first (e.g., Heitzeg et al., 2010) or first and second degree relatives (e.g., Cservenka and Nagel 2012; Squeglia et al., 2014). While many studies have conducted group-level analyses using these dichotomous definitions (e.g., Herting et al., 2010; Schweinsburg et al., 2004; Sjoerds et al., 2013), others discussed in this review have used continuous measures, such as a quantitative calculation of degree of family history density (FHD; Alterman, 1988) of AUDs (e.g., Cservenka et al., 2015; Silveri et al., 2011; Spadoni et al., 2008) to examine the extent to which the presence of the disorder across multiple relatives may contribute to degree of risk for developing AUDs. Lastly, another common way family history has been defined is by recruiting participants who are considered high-risk due to multi-generational presence of AUDs within families with multiplex alcohol dependence where the first generation in which AUDs were present included two biological brothers with the disorder (e.g., Hill et al., 2001). For simplicity, FHP and FHN will be used in this review to describe group differences between individuals with and without a family history of alcoholism, except in studies of multiplex alcohol dependence where high-risk (HR) and low-risk (LR) offspring are described as those who do and do not come from families with multigenerational alcohol dependence, respectively. Finally, FHD will be used to discuss findings where density of familial AUDs was examined with a quantitative continuous variable.
Using the definitions described above, a multitude of studies have examined neurocognitive, behavioral, and personality characteristics in individuals with familial alcoholism. There is growing research on the neural correlates that may underlie some of the characteristics that could increase risk for the development of AUDs as well as markers that could provide resilience against the development of AUDs, especially in young adult and adult samples with minimal heavy alcohol use. This review will summarize the neurocognitive and neurobiological features present in youth and adults with a family history of alcoholism. Early studies using electroencephalography (EEG) and event-related potentials (ERP) identified electrophysiological differences between FHP and FHN individuals, while more recent studies using structural and functional magnetic resonance imaging (fMRI), as well as diffusion tensor imaging (DTI), have reported a variety of volumetric, functional, and white matter microstructure differences between FHP and FHN youth and adults.
2. NEUROCOGNITION AND AFFECT
Neurocognitive studies consistently report that individuals with familial alcoholism have deficits in verbal and language abilities (Drejer et al., 1985; Knop et al., 1985; Tapert and Brown, 2000), visuomotor, visuospatial, and perception skills (Aronson et al., 1985; Garland et al., 1993; Ozkaragoz et al., 1997; Schaeffer et al., 1984; Tarter et al., 1989), and in various domains of executive functioning (Corral et al., 2003; Gierski et al., 2013; Harden and Pihl, 1995; Hesselbrock et al., 1991). For example, compared with FHN individuals, FHP adults had greater preservative errors on the Wisconsin Card Sorting Task (WCST), and slower reaction time during the Trail Making and Arithmetic Switching Tasks, which reflect weaknesses in set-shifting (Gierski et al., 2013). Similar findings were present in FHP children, who also showed more perseverative errors on the WCST compared with their FHN peers (Corral et al., 2003). The authors suggested that this could be reflective of a developmental delay, as FHP children did not exhibit a reduction in perseverative errors on the WCST when assessments were conducted 3.5 years apart, while control youth did show improvements in performance (Corral et al., 2003). Poor planning and abstract problem solving abilities have also been found in multiple studies of FHP individuals (Drejer et al., 1985; Schaeffer et al., 1984; Tarter et al., 1989), which may also be indicative of executive functioning immaturity, thereby leading FHP youth or adults to make poor choices with regards to alcohol use.
Furthermore, on basic tasks of motor inhibition, FHP individuals were more impulsive and had difficulties in response inhibition compared with their FHN peers (Acheson et al., 2011a; Saunders et al., 2008). Inhibitory control problems have also been found on more cognitively demanding tasks, as FHP adults made more errors than FHN individuals when performing the Stroop (Lovallo et al., 2006), which requires the maintenance of attention, conflict monitoring, and response inhibition. Delay discounting paradigms indicate that FHP adults are also less able to delay reward gratification (Acheson et al., 2011b), perhaps reflecting heightened impulsivity, which may contribute to alcohol-related problems. It is possible that poor decision-making abilities contribute to AUD risk in this population and may vary by sex, since FHP males were reported to be more attentive to financial gains, suggesting a greater propensity for reward-driven behavior compared to FHP females or FHN individuals (Lovallo et al., 2006). These findings also translate to studies of largely alcohol-naïve youth, as poorer response inhibition was present in FHP children and adolescents compared with their FHN peers (Nigg et al., 2004). Thus, a strong body of research points to executive functioning abnormalities, including set-shifting weaknesses and inhibitory control deficits, in FHP individuals in the absence of AUDs. While not all neuropsychological studies have found performance differences between FHP and FHN individuals (Alterman et al., 1989; Bates and Pandina, 1992; Hesselbrock et al., 1985), neuroimaging studies may provide insight into the neurobiological correlates of previously reported abnormalities in cognitive functions in familial alcoholism.
Not only are there top-down cognitive control weaknesses in FHP individuals, but there are also differences in emotional processing and reactivity between FHP adults and their FHN peers, which could be other contributing factors to the emergence of AUDs in this population. Both physiological and subjective affective responses are altered in FHP individuals, as they have shown reduced emotion-modulated startle (Miranda et al., 2002), blunted stress response (Sorocco et al., 2006), and higher rates of internalizing symptoms (West and Prinz, 1987) relative to their FHN peers. Other studies have reported that FHP children experience greater emotional dysregulation and affective problems than their FHN peers (Christensen and Bilenberg, 2000; West and Prinz, 1987). In particular, negative affect in adolescents mediated the relationship between parental history of alcoholism and risk-taking, the latter of which was significantly related to substance use (Ohannessian and Hesselbrock, 2008). These findings suggest that in addition to weaknesses in cognitive control that may lead to maladaptive behaviors in FHP youth and adults, there are also affective pathways that can confer risk for AUDs in this population. Thus, studies examining the neurobiology associated with familial history risk should examine neural markers of risk associated with top-down cognitive control and bottom-up emotion and reward processing, their interaction, and importantly, their ability to predict future escalation of heavy drinking.
3. ELECTROENCEPHALOGRAPHY AND EVENT-RELATED POTENTIALS
Over 30 years ago, the first studies to examine neurobiological correlates of familial risk for alcoholism used EEG and ERP to identify neurophysiological markers of risk for AUDs (Begleiter et al., 1984; Elmasian et al., 1982). Many of these studies have been extensively reviewed by Polich and colleagues (1994), as well as Rangaswamy and Porjesz (2014), but the key findings are described here. The vast majority of these investigations have focused on P3 potentials during the presentation of auditory or visual stimuli. The P3 component is linked with attentional and working memory processes when individuals have to attend to a target stimulus. Initial studies reported lower P3 amplitude in both FHP adult men and FHP boys compared to FHN peers. A meta-analysis by Polich and colleagues (1994) reviewed the mixed evidence regarding the amplitude of the P3 component in familial risk studies, but it appeared that young males show the greatest reduction in amplitude of this component during difficult visual tasks. The authors of this meta-analysis argued that by examining the P3 component, researchers can potentially detect both cognitive dysfunction in those at high risk for alcoholism, as well as find a neural correlate of vulnerability for developing AUDs that can inform prevention efforts aimed at identifying individuals at highest risk for the disorder (Polich et al., 1994). Furthermore, some of the neural markers that may differentiate FHP vs. FHN individuals appear to also be present at rest, as multiple studies have found that beta power is higher in FHP individuals than their FHN peers during resting EEG (Rangaswamy and Porjesz, 2014).
4. BRAIN VOLUME
With the advent of neuroimaging technology, numerous studies have aimed to understand whether a family history of alcoholism is associated with brain volume alterations that could, in part, explain the higher vulnerability of FHP individuals to develop AUDs. These investigations have primarily used region-of-interest analyses to determine whether gray matter volumes may be atypical in familial alcoholism.
Since reward and emotion-serving brain regions may be one pathway of risk towards AUDs, a number of studies have measured amygdalar volume in FHP and HR individuals (Cservenka et al., 2015; Dager et al., 2015; Hill et al., 2001, 2013c), as reductions in amygdalar volume have been shown in alcoholics (Wrase et al., 2008). Hill and colleagues (2001) were the first to report reduced right amygdalar volume in HR adolescents and young adults relative to LR controls. Both this study and subsequent studies by Hill and colleagues (2007a, 2013a, 2007b, 2013b, 2013c, 2011, 2009), compared brain volume between HR and LR individuals, but included some adolescents and adults with alcohol/substance abuse or dependence, so family history and alcohol use effects cannot be completely dissociated. A subsequent study of amygdalar volume with a larger sample showed reduced volume (bilaterally) – a phenotype that was moderated by genetics (Hill et al., 2013c). HR individuals and carriers of the short “S” allele for the serotonin transporter (5-HTTLPR) gene had smaller amygdalar volume compared to those with the long “L” allele. Since the S allele is associated with vulnerability to stress and risk for alcohol dependence, these findings suggest that genotyping those with familial alcoholism is critical to understanding the interplay of gene x family environment interactions that could contribute to AUD risk. A recent study that examined a number of subcortical brain regions implicated in affect and reward, found reduced amygdalar volume in individuals with first degree biological relatives diagnosed with an AUD (Dager et al., 2015), a study in which only 3-4% of individuals had a lifetime diagnosis of substance abuse/dependence across groups, which minimized any alcohol-related effects on brain structure. Thus, there now appears to be compelling evidence that smaller amygdalar volume is present in FHP individuals, even in the absence of personal AUDs. However, when characterizing risk based on FHD of AUDs, no significant relationship was shown between degree of risk and amygdalar volume in a group of adolescents with no heavy alcohol use experience (Cservenka et al., 2015). These discrepancies could be due to participant age, experience with alcohol use even in the absence of abuse or dependence, dichotomous measures of familial risk vs. FHD, or type of amygdalar segmentation used. Nevertheless, it is important to consider the alternative explanation that smaller amygdalar volume in adults largely free of alcohol or substance dependence may be a marker of resilience against the development of AUDs. Thus, it will be important for future studies to examine whether these results can be replicated in alcohol-naïve FHP adolescents, thereby confirming the specific contribution of familial alcoholism to premorbid limbic brain region morphometric alterations.
While hippocampal morphology appears susceptible to the neurotoxic effects of alcohol (De Bellis et al., 2000; Medina et al., 2007; Nagel et al., 2005; Ozsoy et al., 2013), both a study of FHP adults with very low alcohol-related problems (Sjoerds et al., 2013) and a study of FHP adolescents with minimal to no previous substance use (Hanson et al., 2010) indicated significantly different volume of the parahippocampus and hippocampus, respectively, in FHP compared with FHN individuals. The right parahippocampal gyrus showed significantly smaller grey matter density in FHP relative to FHN adults (Sjoerds et al., 2013), while a group-by-gender interaction was present in alcohol-naïve adolescents, such that FHP males had larger left hippocampal volumes relative to FHN males (Hanson et al., 2010). However, the latter study included a small sample of adolescents, so strong conclusions cannot be made. Given a number of studies that have reported memory impairments (Brown et al., 2000) and altered hippocampal volume in AUDs (De Bellis et al., 2000; Medina et al., 2007; Nagel et al., 2005; Ozsoy et al., 2013), future investigations should examine whether familial AUD risk may be contributing to these impairments, and whether there is a sex-specific pattern.
Since several studies have implicated basal ganglia structure and function in AUDs (Beck et al., 2009; Camchong et al., 2013; Makris et al., 2008; Wrase et al., 2007), a few investigations have also begun to examine volume of this region in at-risk individuals. For example, a positive relationship between FHD of alcoholism and nucleus accumbens volume was present in adolescent girls without personal heavy alcohol use (Cservenka et al., 2015), suggesting that larger volume of an incentive processing region may be associated with reward-related behaviors that confer risk for the development of AUDs, as FHD of alcoholism is believed to represent degree of risk. It is possible that mesolimbic circuitry may be atypical in HR individuals, as orbitofrontal cortex (OFC) volume laterality was reported to be reduced in this population – a phenotype that was related to greater impulsivity (Hill et al., 2009), albeit in a sample in which about 20% of individuals had an alcohol or substance abuse/dependence diagnosis. Future studies should also consider assessing the presence of externalizing disorders in adolescents and adults from multiplex families with alcohol dependence, as these diagnoses accounted for volumetric differences in other basal ganglia structures such as the caudate, which was smaller in volume in those at-risk individuals with externalizing disorders relative to those without these diagnoses (Hill et al., 2013a). Thus, future studies need to carefully consider psychiatric comorbidities when examining the contribution of family history risk to brain morphology.
Altered cerebellar morphometry, which has been associated with AUDs, was also found in two studies of HR individuals with some previous history of alcohol and/or substance abuse/dependence (Hill et al., 2007b, 2011). While cerebellar volume was found to be smaller in alcoholics (Sullivan et al., 2000, 2010), two studies found that HR individuals have larger cerebellar volumes relative to LR controls (Hill et al., 2007b, 2011). Altered postural sway reported in familial alcoholism (Hill et al., 2000) could be related to these morphometric findings. It is still uncertain if altered cerebellar volume may be due to delayed synaptic pruning in FHP individuals, or whether this phenotype could potentially be protective for those with familial alcoholism. Longitudinal investigations of cerebellar volume change are needed in at-risk youth and adults to know if altered cerebellar volume increases risk for or protects against alcoholism.
A study of brain volume and neuropsychological functioning in FHP and FHN early adolescents found that white matter volume as a ratio to intracranial volume (ICV) was significantly related to better performance on reaction time for the Stroop task and correct responses on a Digit symbol task (Silveri et al., 2008). However, this effect was only present in FHN females, suggesting that FH-by-sex interactions may be related to maturation of executive functioning skills, and supports other findings of FH-by-sex effects reported in gray matter volume studies (Cservenka et al., 2015; Hanson et al., 2010).
Further, it should be noted that overall intracranial volume (ICV) may also be affected by familial alcoholism. FHP alcoholics were found to have smaller ICVs than FHN alcoholics or healthy controls (Gilman et al., 2007), suggesting that hereditary or environmental risk factors for AUDs can contribute to overall brain maturation. This finding supports the hypothesis of delayed maturation in alcohol-naïve FHP youth that could put them at risk for maladaptive behaviors. On the other hand, in a different population of adult heavy drinkers and light drinkers, heavy drinkers who had a family history of problem drinking in at least one parent, had smaller cerebrospinal fluid volumes than their FHN peers - effects that were not present in the light drinking group (Cardenas et al., 2005). Thus, it is plausible that the interaction of heavy alcohol use and familial alcoholism as well as the age of onset of use could determine if neurobiological features represent risk for or resilience against AUDs.
Most previous volumetric studies (Table 1) suggest that FHP and HR individuals have altered subcortical brain morphology in reward and affect-related brain regions, including smaller amygdalar volume (Dager et al., 2015; Hill et al., 2001, 2013c), increased NAcc volume in females with higher familial density of the disorder (Cservenka et al., 2015), and display differences in hippocampal volume from their FHN peers (Hanson et al., 2010; Sjoerds et al., 2013). It is possible that decreases in amygdalar volume coupled with increases in NAcc volume could be related to altered emotional processing (Christensen and Bilenberg, 2000; Miranda et al., 2002) and heightened risky drinking (LaBrie et al., 2009) that contributes to vulnerability for developing AUDs in FHP individuals. Furthermore, HR individuals have larger cerebellar volume (Hill et al., 2007b, 2011), a finding opposite to what has been seen in heavy alcohol users (Sullivan et al., 2000, 2010), which could be a risk marker for as opposed to a consequence of alcohol use, or could be indicative of neuroprotective resilience against future cerebellar damage. Further research will need to examine the extent to which cortical areas show alterations in volume in FHP youth or adults as these regions remain understudied.
Table 1.
Brain Volume Findings in Familial Alcoholism
| Structure | Study | Age in Years (M±SD) | Population (N) | Alcohol Use1 | Main Findings2 |
|---|---|---|---|---|---|
| Amygdala | Hill et al., 2001 | HR=17.6±2.9, LR=17.3±2.2 | 17 HR, 17 LR | 18.2% A/D dependence in HR | ↓R Amyg in HR |
| Hill et al., 2013c | HR=18.2±4.2, LR=17.7±5.8 | 71 HR, 58 LR | N=10 with A/D dependence | ↓Amyg in HR | |
| Cservenka et al., 2015 | M=14.1±1.3, F=14.6±1.3 | 75 M/65 F, FHM/FHP | No heavy A/D use4 | ↔FHD on Amyg | |
| Dager et al., 2015 | 1st ± with AUD=52.6±13, Controls=40.2±163 |
137 1st ° with AUD 227 Controls |
3-4% lifetime substance abuse/dependence |
↓Amyg in subjects with 1st ° relative with AUD |
|
| Hippocampus | Hanson et al., 2010 | FHP=13.5±0.9, FHN=13.6±0.9 | 15 FHP, 15 FHN | Minimal/no previous substance use | ↑L Hipp in FHP M vs. FHN M |
| Sjoerds et al., 2013 | FHP=38.2±9.8, FHN=38.1±10.7 | 36 FHP, 107 FHN | AUDIT(mean): FHP=3.3, FHN=3.9 | ↓R PHG in FHP | |
| Nucleus Accumbens | Cservenka et al., 2015 | M=14.1±1.3, F=14.6±1.3 | 75 M/65 F, FHM/FHP | No heavy A/D use4 | ↑FHD, larger NAcc |
| Orbitofrontal Cortex | Hill et al., 2009 | HR=18.3±4.5, LR=16.7±4.9 | 63 HR, 44 LR | N=22 A/D abuse/dependence | ↓R/L OFC ratio in HR |
| Caudate | Hill et al., 2013a | HR=18.1±4.2, LR=17.6±5.8 | 71 HR, 59 LR | N=23 A/D abuse/dependence | ↔risk on caudate5 |
| Cerebellum | Hill et al., 2007b | HR=17.6±2.9, LR=17.5±2.2 | 17 HR, 16 LR | N=5 A/D dependence in HR | ↑cerebellum in HR |
| Hill et al., 2011 | HR=18.3±4.2, LR=17.8±5.8 | 71 HR, 60 LR | N=24 A/D abuse/dependence | ↑cerebellum in HR6 | |
|
Intracranial
Volume |
Gilman et al., 2007 | LO=43.9±8.5, EO=37.0±8.7, Controls=34.6±10.1 |
LO (64 FHP, 38 FHN), EO (82 FHP, 47 FHN), 114 Controls |
N=231 alcoholics (with and without family history of alcoholism) |
↓ICV in FHP alcoholics |
| White Matter | Silveri et al., 2008 | FHP M=12.6±2.7, FHP F=12.4±3.0, FHN M=12.6±2.5, FHP F=12.4±2.1 |
17 FHP, 16 FHN | <3 lifetime episodes of A/D use for all participants |
Information processing speed related to WM/ICV in FHN F |
| Cerebrospinal Fluid | Cardenas et al., 2005 | HD M=40.9±9.7, HD F=43.6±10.4 (ages not specified based on family history) |
26 FHP HD, 22 FHN HD | Heavy drinkers >100 or >80 drinks/month for M, F, respectively for three years7 |
↓frontal, parietal, and occipital CSF in FHP HD |
Amyg = amygdala; A/D = alcohol/drug; AUD = alcohol use disorder; AUDIT = alcohol use disorders identification test; CSF = cerebrospinal fluid; EO = early onset alcoholic; F = female; Hipp = hippocampus; HR = high risk (offspring from multiplex families with alcohol dependence in which two adult brothers with alcohol dependence identified); FHD = family history density (the degree of familial alcoholism in one’s family); FHM = family history mild (individual with at least one 2nd degree relative with AUD); FHN = family history negative (individual with no 1st (or in some cases no 1st or 2nd) degree relatives with AUD); FHP = family history positive (individual with at least one 1st degree relative with AUD or in some cases at least two or more 2nd degree relatives with AUD); HD = heavy drinkers (>100 drinks/month for males and >80 drinks/month for females for three years); ICV = intracranial volume; L = left; LO = late onset alcoholic; LR = low risk (offspring from families in which no Axis I alcohol dependence identified); M = male; NAcc = nucleus accumbens; OFC = orbitofrontal cortex; PHG = parahippocampal gyrus; R = right; WM = white matter;↓ = smaller or decreased; ↑ = larger or increased; ↔ = no difference/no effect;
only alcohol use characteristics are noted, even though some studies also included participants with lifetime diagnoses of other psychiatric disorders not listed in this table (i.e. depression, anxiety, externalizing disorders). In some studies results are reanalyzed without subjects who met criteria for A/D dependence, of which details are not included here.
relative to the LR, control, or FHN group, unless otherwise specified.
study sample also included individuals with lifetime, current, or past AUD diagnoses, and unaffected individuals with 2nd-5th degree relatives with AUDs for which participant characteristics are not listed in table.
no more than 10 lifetime drinks and 2 drinks/occasion, no more than 5 uses of marijuana, 4 cigarettes/day, or any other substance use.
but ↓caudate in those with externalizing disorders in sample.
when 24 subjects with substance use disorder removed from sample (N=107).
light drinkers included in study not described in Table.
5. WHITE MATTER MICROSTRUCTURE
White matter integrity, or more restricted diffusion of water along axons, increases over the course of development (Bava et al., 2010; Lebel et al., 2012) and relates to improvements in executive functioning across adolescence (Treit et al., 2014). Thus, decreased white matter integrity could be related to functional deficits in cognition and thereby increase risk for AUDs. Various studies have reported that relative to FHN adolescents, FHP youth without personal heavy alcohol use have decreased fractional anisotropy (FA) of white matter in pathways that include long-range association tracts, connecting frontal and parietal lobes, such as the superior longitudinal fasciculus (SLF; Acheson et al., 2014c; Herting et al., 2010). Some studies also suggest that degree of risk is negatively related to white matter integrity, such that those with the highest FHD have the lowest FA values (Acheson et al., 2014c). Unfortunately, it is unclear whether this reduced integrity of white matter is a stable characteristic in familial alcoholism or whether lower FA in FHP youth represents developmental delays in white matter maturation. These findings suggest that prevention efforts could focus on strategies to strengthen cognitive functioning prior to the initiation of heavy alcohol use in FHP youth in cognitive domains that are related to reductions in white matter integrity in these adolescents. This is critical, as other studies suggest that interactions between alcohol use and family history of alcoholism may be detrimental to white matter integrity once heavy alcohol use is initiated (Hill et al., 2013b).
Contrary to previous findings, a recent study reported that association, projection, and interhemispheric white matter tracts showed higher FA in alcohol-naïve FHP youth compared with their FHN peers (Squeglia et al., 2014). This could represent compensatory increases in FA in certain pathways, or as the authors hypothesize, could be a marker of more advanced maturation of white matter in FHP adolescents that may increase their susceptibility towards engaging in risky behaviors (Squeglia et al., 2014). Finding associations between white matter integrity and cognitive functioning, as well as assessing risky behaviors, including alcohol use, over the course of adolescence will be needed to answer these questions. Further, many of these studies only included high functioning youth who generally come from affluent families, warranting further research to increase the generalizability of these findings.
Thus, while white matter microstructure has not been extensively explored in studies of familial alcoholism (Table 2), research to date suggests mostly lower FA in FHP relative to FHN youth in white matter tracts connecting fronto-parietal regions, including the SLF, which could explain previous neuropsychological findings of executive functioning deficits in FHP children and adolescents (Corral et al., 2003; Hesselbrock et al., 1991).
Table 2.
White Matter Microstructure Findings in Familial Alcoholism
| Study | Age in Years (M±SD) | Population (N) | Alcohol Use1 | Main Findings2 |
|---|---|---|---|---|
| Herting et al., 2010 | FHP=13.8±1.5, FHN=13.8±1.6 | 15 FHP, 19 FHN | No heavy A/D use3 | ↓FA in prefrontal, subcortical, temporal tracts in FHP |
| Hill et al., 2013b | HR M=25.3±5.0, LR M=21.5±5.2 HR F=24.7±5.1, LR F=24.0±5.7 |
44 HR, 37 LR | N=32 A/D abuse/dependence | Risk x exposure interactions in ILF, SLF, FM, ↓FA in HR |
| Acheson et al., 2014c | FHP Y=12.9±1.2, FHN Y=12.9±1.1 FHP A=24.1±4.2, FHN A=24.5±2.7 |
80 FHP Y, 34 FHN Y, 25 FHP A, 30 FHN A |
No regular substance use in Y4
N=4 past alcohol abuse in A |
↓FA in FOF, SCNin FHP Y; ↓FA with ↑FHD in ACN, genu of CC in FHP A |
| Squeglia et al., 2014 | FHP=13.7±0.7, FHN=13.5±0.6 | 48 FHP, 46 FHN | 0-2 days of lifetime alcohol use | ↑FA in 19 regions in FHP |
A = adult; ACN = anterior corona radiata; A/D = alcohol/drug; CC = corpus callosum; F = female; FA = fractional anisotropy; HR = high risk (offspring from multiplex families with alcohol dependence in which two adult brothers with alcohol dependence identified); FHD = family history density (the degree of familial alcoholism in one’s family); FHN = family history negative (individual with no 1st (or in some cases no 1st or 2nd) degree relatives with AUD); FHP = family history positive (individual with at least one 1st degree relative with AUD or in some cases at least two or more 2nd degree relatives with AUD); FM = forceps major; FOF = fronto-occipital fasciculus; ILF = inferior longitudinal fasciculus; LR = low risk (offspring from families in which no Axis I alcohol dependence identified); M = male; SCN = superior corona radiata; SLF = superior longitudinal fasciculus; Y=youth; ↓ = smaller or decreased; ↑ = larger or increased; ↔ = no difference/no effect;
only alcohol use characteristics are noted, even though some studies also included participants with lifetime diagnoses of other psychiatric disorders not listed in this table (i.e. depression, anxiety, externalizing disorders). In some studies results are reanalyzed without subjects who met criteria for A/D dependence, of which details are not included here.
relative to the LR, control, or FHN group, unless otherwise specified.
no more than 10 lifetime drinks and 2 drinks/occasion, no more than 5 uses of marijuana, 4 cigarettes/day, or any other substance use.
no more than once/month substance use for six consecutive months.
6. BRAIN FUNCTIONING
6.1 Inhibitory Control
Multiple studies indicate that inhibitory control brain activity is altered in FHP youth and adults relative to their FHN peers (Acheson et al., 2014a, 2014b; DeVito et al., 2013; Hardee et al., 2014; Heitzeg et al., 2010; Schweinsburg et al., 2004; Silveri et al., 2011), which could explain impulsive characteristics seen in FHP individuals (Nigg et al., 2004; Saunders et al., 2008). Go NoGo tasks, which assess motor impulsivity and engage fronto-striatal circuitry, have been commonly used to study inhibitory control in FHP individuals. While not all studies have reported behavioral differences between FHP individuals and their FHN peers on laboratory Go NoGo tasks, brain response differences have been observed. Reduced brain activity was demonstrated among fronto-parietal regions in 12-14 year old alcohol-naïve FHP adolescents during response inhibition (NoGo vs. Go activity), despite similar behavioral performance to their FHN peers (Schweinsburg et al., 2004). Additionally, response inhibition may further be derailed in emotionally heated situations, which could exacerbate risk for alcohol abuse, since frontal lobe brain response was reduced during NoGo trials to a greater extent within affective vs. non-affective contexts (Cservenka et al., 2014b). Given alterations in association tracts connecting frontal and parietal areas, such as the SLF, in FHP youth (Herting et al., 2010), functional deficits in these areas could be related to reduced white matter integrity of pathways connecting these regions – tracts that are involved in the maturation of executive functions, such as inhibitory control.
Multiple studies have also found that FHP individuals may exert greater neural effort to perform on par with their FHN peers. Regardless of problem drinking behavior, FHP youth did not deactivate ventral caudate brain response when successfully inhibiting during a Go NoGo task, while FHN youth did deactivate this region (Heitzeg et al., 2010). It is possible that greater neural effort is required in some brain regions for successful performance on this task. In FHP and FHN adults matched on drinking characteristics, family history-by-sex interactions on neural activation during Go NoGo tasks were present. Specifically, in task-positive brain regions such as the anterior insula and inferior frontal gyrus, FHP males had the highest activity during NoGo vs. baseline brain response, which was related to both discounting of rewards and self-reported impulsivity (DeVito et al., 2013). It is possible that increased activity in these brain regions is a function of increased cognitive control effort required for FHP males during successful inhibitions as a result of greater impulsivity in these individuals, or it may be that this neural phenotype provides protection against cognitive control weaknesses.
Furthermore, a longitudinal study of inhibitory control indicated that there are altered trajectories of brain activity during Go NoGo tasks in FHP youth prior to the onset of an AUD. FHP youth showed increased cingulate activity over time, while their FHN peers had reduced fronto-striatal response from baseline to follow-up (Hardee et al., 2014). The authors believed that these findings suggest greater recruitment of inhibitory control regions in FHP youth over time in order to override prepotent responses, which is thought to reflect an altered neurodevelopmental trajectory. Correlating these differing trajectories of brain response during cognitive control to risk-related behaviors that change between childhood and adolescence may help identify patterns of brain activity that predict the onset of heavy alcohol use in FHP youth.
Brain activity differences have also been found between FHP youth and their FHN peers during more complex inhibitory control tasks, such as the counting Stroop or Color-Word Stroop. When contrasting incongruent vs. congruent trials on a counting Stroop task, higher temporo-parietal activity was present in FHP youth (Acheson et al., 2014a). FHD of alcoholism was related both positively and negatively to blood oxygen level-dependent (BOLD) response during a Stroop task in fronto-limbic regions (Silveri et al., 2011).
Overall, these findings indicate both decreased and increased BOLD response during inhibitory control tasks in FHP youth that differs as a function of sex, is related to FHD of alcoholism, changes over the course of development, and is associated with measures of impulsivity (Table 3). Furthermore, in studies of adults with minimal to no alcohol abuse or dependence, increased BOLD activity during successful inhibition in frontal (DeVito et al., 2013) and parietal regions (Acheson et al., 2014a) could be indicative of a protective neural mechanism against the development of AUDs that is reflective of efficient cognitive control functioning in these individuals.
Table 3.
Brain Function Findings in Familial Alcoholism
| Study | Age in Years (M±SD) | Population (N) | Alcohol Use1 | Main Findings2 | |
|---|---|---|---|---|---|
| Inhibitory Control | Schweinsburg et al., 2004 | FHP=13.8±1.5, FHN=13.8±1.6 | 12 FHP, 14 FHN | None | ↓BOLD in MFG/parietal cortex in FHP for nogo vs. go |
| Heitzeg et al., 2010 | FHP=19.0±1.6, FHP+P=19.3±1.3 FHN=19.2±1.9 |
20 FHP, 21 FHP+P, 20 FHN |
N=8 A/D dependence in FHP+P |
↓BOLD in ventral caudate in FHN; not present in FHP groups for nogo vs. go |
|
| Silveri et al., 2011 | FHP=13.2±3.2, FHN=13.8±2.6 | 18 FHP, 14 FHN | No current or >3 lifetime episodes of A/D use |
↑FHD, ↑BOLD in frontal/insular regions in interference-color naming |
|
| DeVito et al., 2013 | FHP=29.8±11.5, FHN=33.3±13.5 | 28 FHP, 31 FHN | No A/D abuse/dependence | ↑BOLD in frontal/insular regions in FHP during successful inhibition |
|
| Hardee et al., 2014 | Age Range: FHP=7.85-16.74, FHN=7.58-16.83 |
43 FHP, 30 FHN | N=5 A/D use at baseline; N=13 A/D use at follow-up |
↓BOLD in caudate and MFG in FHN over time; absent in FHP |
|
| Acheson et al., 2014a | FHP=23.6±4.0, FHN=24.0±2.6 | 24 FHP, 28 FHN | N=3 past alcohol/abuse | ↑BOLD in parietal, temporal regions in FHP for incongruent vs. congruent |
|
| Acheson et al., 2014b | FHP=12.9±1.0, FHN=12.9±1.1 | 72 FHP, 32 FHN | 3-7% of youth ever used A/D | ↑BOLD in cingulate, temporal, frontal regions in FHP in Go/NoGo blocks |
|
| Cservenka et al., 2014b | FHP=14.9±1.3, FHN=14.7±1.1 | 19 FHP, 17 FHN | No heavy A/D use3 | ↓BOLD in fronto-striatal-parietal regions in FHP during nogo in emotional vs. non-emotional contexts |
|
| Working Memory | Spadoni et al., 2008 | 13.3±0.8 | 72 FHP and FHN | Minimal lifetime use of A/D | ↑FHD, ↓BOLD in vigilance vs. SWM in cingulate, MeFG |
| Cservenka et al., 2012 | FHP=14.0±0.9, FHN=14.2±0.7 | 19 FHP, 16 FHN | No heavy A/D use3 | FHP no difference between VWM and vig BOLD in frontal regions |
|
| Mackiewicz Seghete et al., 2013 | FHP=14.5±0.9, FHN=14.2±0.7 | 18 FHP, 16 FHN | No heavy A/D use3 | FHP no difference between SWM and vig BOLD in frontal regions |
|
|
Reward Processing/
Risky Decision-making |
Bjork et al., 2008 | COA=13.9±0.4, Controls=13.8±0.4 |
13 COA, 13 Controls | Not discussed | No differences in reward anticipation or receipt |
| Acheson et al., 2009 | FHP=24.0±0.8, FHN=23.0±0.7 | 15 FHP, 19 FHN | No A/D abuse/dependence | ↑BOLD in ACC, caudate in FHP during IGT | |
| Andrews et al., 2011 | FHP=33.7±13.6, FHN=33.6±14.4 | 19 FHP, 30 FHN | No past/current A/D abuse | ↓BOLD in NAcc, insula, OFC in FHP during reward anticipation |
|
| Cservenka et al., 2012 | FHP=14.2±0.7, FHN=14.2±0.8 | 18 FHP, 13 FHN | No heavy A/D use3 | ↓BOLD in frontal lobe and cerebellum in FHP during risky vs. safe decision-making |
|
| Yau et al., 2012 | COA=20.2±1.2, Controls=20.1±1.3 |
20 COAs, 20 Controls | A/D use present | ↓BOLD in NAcc in COA during reward anticipation, lower in low-risk COA vs. high-risk COA |
|
| Stice and Yokum, 2014 | FHP=14.7±0.9, FHN=14.9±1.0 | N=26 FHP, N=26 FHN for any substance |
No >1/week use of psychoactive substances |
↑BOLD in midbrain in FHP during food reward receipt; in DLPFC, putamen for anticipated monetary receipt |
|
| Yarosh et al., 2014 | FHP=26.2±5.6, FHN=25.5±6.3 | 40 FHP, 29 FHN | No current/past A/D abuse/dependence |
↑impulsivity, ↑BOLD for reward-punishment across both FHP and FHN |
|
| Muller et al., 2015 | FHP=14.7±0.4, FHN=14.7±0.3 | 77 FHP, 77 FHN | Some lifetime A/D use | No differences between FHP and FHN for NAcc reward anticipation/receipt |
|
| Alcohol/Drug Stimuli | Munro et al., 2006 | FHP=21.7±2.8, FHN=21.9±3.1 | 11 FHP, 30 FHN | <30 alcoholic drinks/month | No differences in BP or DA release between FHP and FHN |
| Kareken et al., 2010 | FHP=23.9±2.3, FHN=23.4±2.9 | 14 FHP heavy drinkers, 12 FHN heavy drinkers |
AUDIT(mean): FHP=10.4, FHN=12.3 |
↑BOLD in MeFG for alcohol vs. appetitive odors in FHP, dampened by acute alcohol |
|
| Dager et al., 2013 | FHP=19.3±0.8, FHN=19.2±0.7 heavy drinkers; FHP=18.9±1.0, FHN=19.4±0.6 light drinkers |
10 FHP heavy drinkers, 25 FHN heavy drinkers, 11 FHP light drinkers, 19 FHN light drinkers |
24-92% A/D abuse/dependence present in heavy drinkers |
↑BOLD in temporo-parietal, hippocampal areas in FHP for repeated alcohol images |
|
| Oberlin et al., 2013 | FHP=24.6±3.8, FHA=24.7±3.9, FHN=24.8±3.2 |
12 FHP, 18 FHA, 19 FHN | N=4 alcohol dependence | Beer flavor stimulates DA release in FHP | |
| Emotional Processing | Glahn et al., 2007 | FHP=23.0±1.1, FHN=24.0±1.2 | 9 FHP, 8 FHN | AUDIT(mean): FHP=3.6, FHN=3.5 |
↓BOLD in Amyg in FHP during face vs. shape matching |
| Hill et al., 2007a | HR=22.6±3.3, LR=23.6±4.2 | 8 HR, 8 LR | N=3 A/D dependence | ↓BOLD in MTG, SFG, IFG, in FHP during emotion vs. gender eyes task |
|
| Heitzeg et al., 2008 | FHP Resilient=18.4±1.0, FHP Vulnerable=17.5±1.3, FHN Controls=17.2±1.6 |
11 FHP Vulnerable, 11 FHP Resilient, 6 FHN Controls |
27.3% A/D dependence in Vulnerable group |
↑BOLD in OFC in resilient vs. controls during negative vs. neutral words | |
| Cservenka et al., 2014b | FHP=14.9±1.3, FHN=14.7±1.1 | 19 FHP, 17 FHN | No heavy A/D use3 | ↓BOLD in STG in FHP to happy vs. calm faces | |
| Peraza et al., 2015 | FHP=13.7±1.5, FHN=13.7±1.6 | 14 FHP, 15 FHN | No heavy A/D use3,4 | ↓BOLD in SPL in FHP to fearful vs. neutral subliminal faces |
|
| Connectivity | Herting et al., 2011 | FHP=13.5±1.3, FHN=13.6±1.6 | 13 FHP, 14 FHN | Alcohol and substance-naïve | ↓fronto-cerebellar connectivity in FHP |
| Wetherill et al., 2012 | FHP=13.6±0.6, FHN=13.5±0.7 | 20 FHP, 20 FHN | Substance-naïve | ↓fronto-parietal connectivity in FHP | |
| Spadoni et al., 2013 | FHP=13.3±0.9, FHN=13.2±0.8, OA=17.7±1.1 |
24 FHP, 26 FHN, 35 OA | Minimal exposure to alcohol, cigarettes, and marijuana |
Removal of SPL-MFG pathway reduced model fit more for FHP than FHN; connectivity of OA more like FHN |
|
| Weiland et al., 2013 | FHP=20.1±1.3, FHN=20.1±1.3 | 49 FHP, 21 FHN | N=8 A/D abuse or dependence |
↑connectivity NAcc-SMA and NAcc-postcentral gyrus connectivity during reward anticipation in FHP |
|
| Cservenka et al., 2014a | FHP=14.6±1.3, FHN=14.3±1.3 | 47 FHP, 50 FHN | No heavy A/D use3 | ↓NAcc-IFG segregation and NAcc-OFC integration in FHP |
|
|
Magnetic Resonance
Spectroscopy |
Meyerhoff et al., 2004 | HD=41.3±9.4 (age not specified based on family history) |
28 FHP HD, 18 FHN HD | Heavy drinkers >100 or >80 drinks/month for M, F, respectively for three years5 |
↑NAA in frontal white matter, ↓mI in brainstem in FHP HD |
| Cohen-Gilbert et al., 2015 | FHP=13.7±1.0, FHN=13.6±0.8 adolescents; FHP=21.0±1.9, FHN=21.7±1.6 young adults |
12 FHP, 19 FHN adolescents, 7 FHP, 22 FHN young adults |
<3 lifetimes uses of alcohol for adolescents; ≤3 drinks/occasion or 5 occasions in 30 days for young adults |
↑Gln/Glu ratio in FHN young adults vs. adolescents in ACC |
ACC = anterior cingulate cortex; Amyg = amygdala; A/D = alcohol/drug; AUDIT = alcohol use disorders identification test; BOLD = blood oxygen level-dependent; BP = binding potential; COA = children of alcoholics; DA = dopamine; DLPFC = dorsolateral prefrontal cortex; FHA = family history ambiguous; FHD = family history density (the degree of familial alcoholism in one’s family); FHM = family history mild (individual with at least one 2nd degree relative with AUD); FHN = family history negative (individual with no 1st (or in some cases no 1st or 2nd) degree relatives with AUD); FHP = family history positive (individual with at least one 1st degree relative with AUD or in some cases at least two or more 2nd degree relatives with AUD); FHP+P = family history positive problem drinker; Gln/Glu = glutamine/glutamate ratio; HR = high risk (offspring from multiplex families with alcohol dependence in which two adult brothers with alcohol dependence identified); IFG = inferior frontal gyrus; IGT = Iowa Gambling Task; LR = low risk (offspring from families in which no Axis I alcohol dependence identified); MFG = middle frontal gyrus; MeFG = medial frontal gyrus; mI = myoinositol; MTG = middle temporal gyrus; NAA = N-acetylaspertate; NAcc = nucleus accumbens; OA = older adolescents; OFC = orbitofrontal cortex; SFG = superior frontal gyrus; SMA = supplementary motor area; SPL = superior parietal lobule; STG = superior temporal gyrus;SWM = spatial working memory; VWM = verbal working memory; ↓ = smaller or decreased; ↑ = larger or increased; ↔ = no difference/no effect
only alcohol use characteristics are noted, even though some studies also included participants with lifetime diagnoses of other psychiatric disorders not listed in this table (i.e. depression, anxiety, externalizing disorders). In some studies results are reanalyzed without subjects who met criteria for A/D dependence, of which details are not included here.
relative to the LR, control, or FHN group, unless otherwise specified.
no more than 10 lifetime drinks and 2 drinks/occasion, no more than 5 uses of marijuana, 4 cigarettes/day, or any other substance use.
one youth had 3 drinks of alcohol on one occasion.
light drinkers included in study not described in Table.
6.2 Working Memory
Poor working memory skills are associated with AUDs (Ambrose et al., 2001), and deficiencies in working memory functioning could lead to poor decision-making skills, thereby increasing vulnerability for alcohol abuse in FHP individuals (Nagel et al., 2012). Specifically, FHN youth showed significantly more frontal lobe engagement during verbal working memory (VWM) relative to a vigilance control condition than FHP youth who showed comparable activity between those conditions (Cservenka et al., 2012). These findings were also present during spatial working memory (SWM; Mackiewicz Seghete et al., 2013). Thus, while FHN youth showed expected disengagement of frontal regions during vigilance, FHP youth activated these areas, indicating that they still utilize neural resources during a relatively simple attentional and motor response condition, which could explain visuospatial and visuomotor deficits reported in this population (Aronson et al., 1985; Garland et al., 1993; Ozkaragoz et al., 1997; Schaeffer et al., 1984; Tarter et al., 1989). Furthermore, working memory relevant brain areas may not be functioning in synchrony in substance-naïve FHP youth, as these adolescents showed weaker fronto-parietal connectivity during visual working memory than their FHN peers (Wetherill et al., 2012), which complements other reports of lower fronto-parietal activity in FHP adults (Rangaswamy et al., 2004). In both of the aforementioned tasks, visual working memory consisted of maintaining and updating information that occurred in the same spatial location on a computer screen, such as remembering if the color array of dots was the same as the previous screen (Wetherill et al., 2012), or silently counting the total number of target stimuli that occurred infrequently during an experiment, and reporting the total number at the end (Rangaswamy et al., 2004). However, the findings above are opposite to those of Spadoni and colleagues (2013), who reported increased connectivity during SWM between the right superior parietal lobe and left middle frontal gyrus in FHP youth relative to their FHN age-matched peers and an older group of adolescents. During the SWM task, participants had to determine if a nonsense design appeared in the same location on a computer screen as previously presented, and there could be one, two, or three distracters between the two stimuli of the same location. Spadoni and colleagues (2013) describe that differences between their study and previous ones may be due to different neural substrates that are relevant to a visual vs. SWM task, although this remains speculative.
Not only is altered task-positive activity present during working memory tasks in FHP youth, but ineffective disengagement of the default mode network (DMN), brain regions including the medial prefrontal cortex and posterior cingulate gyrus, which display functional synchrony at rest (Greicius et al., 2003), has also been related to degree of familial alcoholism (Table 3). During the resting state, the DMN has been associated with introspective, autobiographical thought processes (Gusnard et al., 2001), but active suppression of DMN areas is critical during task engagement to limit intrusion of task-irrelevant thoughts (McKiernan et al., 2003). The increased suppression of DMN activity during SWM relative to vigilance was present to a weaker extent in those with higher FHD of alcoholism (Spadoni et al., 2008). Ineffective DMN modulation during working memory, which is critical for adaptive decision-making, may contribute to risky decisions in FHP youth. Since working memory is important for maintaining and updating information, poor modulation of the DMN during working memory, could result in difficulties with making adaptive decisions, which could subsequently increase risky decision-making in FHP adolescents (Nagel et al., 2012).
6.3 Reward Processing and Decision-making
Alterations in mesolimbic circuitry and reward-related response in AUDs, particularly in the nucleus accumbens (NAcc; Beck et al., 2009; Makris et al., 2008; Wrase et al., 2007), has warranted many investigations of reward-related functioning during fMRI tasks in familial alcoholism. As the NAcc is a major site of dopamine release in the mesolimbic pathway (Oades and Halliday, 1987), a preexisting phenotype that increases risk for reward-driven behaviors could be present in familial alcoholism. Despite numerous studies on this question, there is still mixed evidence for premorbid differences in reward-related functioning between FHP and FHN individuals (Table 3). Several studies have implemented the Monetary Incentive Delay (MID) task to examine neural response to reward anticipation and reward feedback. For example, findings by Bjork and colleagues (2008) and Muller and colleagues (2015) suggested that there are no differences in reward anticipation or reward outcome-related response in the NAcc between FHP and FHN youth for monetary or food rewards, respectively. This is in contrast to studies of adults that used the MID task, where less NAcc activation was present during monetary reward anticipation in FHP adults relative to their FHN peers (Andrews et al., 2011). It is possible this blunted response is related to less incentive motivational processing, as FHP young adults showed this pattern whether or not they were anticipating rewards or losses (Yau et al., 2012). However, it is proposed that this phenotype could be a resilience mechanism against future alcohol abuse as this pattern was only present in FHP young adults with no problematic drinking behavior (Yau et al., 2012), while similarly it was present in adults with no past or current alcohol or drug abuse (Andrews et al., 2011).
While the above studies implemented the MID task as their paradigm, another study used a more socially interactive decision-making task, known as the Domino Game task. In this task participants are told they are playing a competitive game against another human opponent, while they play the game against a computer, in which they have to make risky or safe decisions to dispose of all of their domino chips. This study found that risk-taking on the Balloon Analog Risk Task was positively related to reward-associated NAcc activity during the Domino Game task, but not related to family history status (Yarosh et al., 2014). Thus, future studies should attend to whether personality phenotypes or behavior may account better for reward-related brain activity patterns compared with family history status or whether they may potentially mediate the effects of family history status on reward-associated brain response.
Importantly, more research is needed on whether neural activity to rewards is differentially modulated by monetary rewards or primary rewards, such as food or beverages, in FHP individuals. Research has indicated greater dorsolateral prefrontal cortex and putamen response to monetary reward anticipation in FHP youth compared to their FHN peers, while differences in brain activity to reward receipt were only present during delivery of primary rewards, such as food, and showed that midbrain response was greater in FHP youth than their FHN peers (Stice and Yokum, 2014). However, many of these studies are still confounded by factors that prevent knowing whether family history effects are specific to familial alcoholism or whether lifetime substance use histories of the participants account for some of the findings, as there are frequently subjects with parental histories of multiple substances or an absence of alcohol or substance-naïve participants.
Interestingly, a positron emission tomography (PET) study indicated that tasting beer as opposed to Gatorade induced significantly greater release of dopamine in the striatum in FHP adults than their FHN peers, suggesting inherent differences in striatal dopamine release in response to alcohol in FHP individuals (Oberlin et al., 2013). However, these family history effects were not present when amphetamine was used to stimulate dopamine release in the NAcc (Munro et al., 2006), indicating that inherent risk in FHP individuals may be specific to alcohol-related brain response. Further, the response to alcohol vs. control odors in FHP heavy drinking adults was significantly greater in the medial PFC than in FHN heavy drinking adults – an effect that was absent under intoxication (Kareken et al., 2010). This provides support for the hypothesis that FHP individuals respond differently to rewarding cues than their FHN peers, and that alcohol modulates this response differently in FHP vs. FHN adults. Even visual stimuli themselves, such as the contrast of alcoholic beverages with control images, induced greater BOLD response in visual attention and memory-related brain areas in FHP young adults than their FHN peers, regardless of drinking history (Dager et al., 2013). This could reflect increased sensitivity to rewarding stimuli that may lead to a general predisposition towards risk-related behaviors, including, but not limited to alcohol use.
Moreover, not only is it necessary to understand reward-related brain response in FHP individuals, but it is also critical to know whether risk taking-associated brain activity differs between FHP and FHN youth and adults. Alterations in risky decision-making-related BOLD activity in familial alcoholism would indicate that neural evaluations in the context of risky situations may differ between FHP and FHN individuals, which could explain altered decision-making processes that heighten vulnerability for alcohol abuse in this population. During the Iowa Gambling Task, FHP adults had heightened anterior cingulate cortex (ACC) and caudate activity compared with their FHN peers (Acheson et al., 2009). However, there was no evidence that these differences were related to decision-making components of the task. A study of FHP and FHN youth without a history of personal heavy alcohol or substance use indicated that risky decision-making-related brain response was weaker in FHP youth relative to their FHN peers in key decision-making-related brain regions, such as the DLPFC and cerebellum (Cservenka and Nagel, 2012), which may provide insight into related deficits of planning and problem solving reported on neuropsychological exams in these individuals (Drejer et al., 1985; Schaeffer et al., 1984; Tarter et al., 1989) and help explain maladaptive decisions regarding alcohol use. These findings are relevant as weaker fronto-cerebellar connectivity was also present in FHP youth (Herting et al., 2011), a possible feature or risk that has previously been associated with AUDs (Sullivan et al., 2003). Thus, it is equally important to focus efforts on clarifying whether decision-making or reward-related neural response (or both) may be atypical in FHP individuals, and if these patterns are present prior to the onset of any heavy alcohol use.
Investigating the connectivity of the NAcc with other brain regions is a new avenue of research that could reveal brain network organization of reward-related brain regions and the integration or segregation of NAcc activity with other neural networks in familial alcoholism. For example, during the MID task, increased coupling of the NAcc with sensorimotor regions involved in habit formation mediated the relationship between sensation seeking and drinking in FHP, but not FHN young adults (Weiland et al., 2013). Thus, perhaps the neural risk profile in FHP individuals is more related to the interaction of the NAcc with other brain regions involved in addiction risk, rather than just the response of the NAcc per se. This interpretation is supported by a study that reported differences in resting state connectivity of the NAcc with other brain regions in FHP vs. FHN adolescents. In FHP youth, the NAcc was less integrated with reward evaluation brain regions, such as the OFC, but also less segregated from brain areas involved in top-down cognitive control processing (Cservenka et al., 2014a). Therefore, altered communication within reward-related networks and between the NAcc and networks involved with top-down cognitive control or motor functioning could be preexisting features of brain organization in FHP youth. Additional studies will be needed to assess structural and functional connectivity between pathways connecting the NAcc with the OFC to investigate the coherence of mesolimbic circuitry in FHP individuals.
6.4 Emotional Processing
Studies in alcoholism report that emotional systems, including limbic brain regions, such as the amygdala show altered responses to affective stimuli in those with AUDs (Marinkovic et al., 2009), and alcoholics also have difficulties with socio-affective communication (Thoma et al., 2013). A premorbid phenotype may exist by which atypical emotional processing could lead to coping related reasons for drinking or deficits in emotional processing could lead to the escalation of socio-emotional problems in FHP individuals. Blunted BOLD response was present to positively valenced emotional faces in brain regions associated with socio-emotional processing, such as the temporal lobe, in largely alcohol-naïve FHP youth compared with their FHN peers (Cservenka et al., 2014b). Similarly, HR adolescents/young adults (some of whom met criteria for alcohol dependence or other psychiatric disorders) displayed blunted right middle temporal gyrus activity during a theory of mind task requiring emotional judgments based on pictures of eyes (Hill et al., 2007a). These findings suggest that socio-emotional systems and processing of affective information may be altered in familial alcoholism, and since similar responses have been seen across a variety of emotional facial stimuli, social cues themselves may be processed differently in this population.
Amygdalar activity may also be associated with disinhibited temperament, as response to fearful stimuli in this region is thought to reflect a “breaking” mechanism, by which risk-taking may be curtailed (Ernst et al., 2006). Hyporesponsive amygdalar activity to fearful faces in FHP young adults, which was correlated with impulsive temperament, indicates that reduced limbic response to negatively valenced stimuli could drive engagement with risky behaviors (Glahn et al., 2007). However, blunted amygdalar response to negatively valenced emotional faces was not present in largely alcohol-naïve FHP youth (Cservenka et al., 2014b), which could be due to differences in tasks used, age of participants, or analytical strategies. However, this also begs the question of alcohol-induced alterations that could be driving findings in adult studies, as blunted amygdalar response to emotional words was present in vulnerable (problem drinkers), but not resilient children of alcoholics (Heitzeg et al., 2008).
Importantly, some of the differences in emotional processing between FHP and FHN adolescents (Table 3) are subtle. During a task with presentation of subliminal emotional faces, FHN youth deactivated regions associated with attentional control, such as the superior parietal lobe, in the presence of both fearful and neutral subliminal faces (Peraza et al., 2015). However, FHP youth only deactivated this region during the presentation of fearful subliminal faces. While neutral faces are considered salient during adolescence (Thomas et al., 2001), they may be less salient for FHP youth, which thereby leads them to not deactivate attention-related brain regions in their presence (Peraza et al., 2015).
More studies are necessary to examine the extent to which emotional processing and regulation deficits may be atypical in fMRI studies of FHP individuals. These studies may discover unique neural characteristics of risk towards AUDs in familial alcoholism that are related to stress, coping, and affect regulation, which would not be captured by solely examining brain activity during top-down executive functioning processing tasks.
6.5 Magnetic Resonance Spectroscopy
Only a few studies to date have examined whether brain metabolites differ by family history status (Table 3), with one of these being in a sample of adolescents and young adults with minimal and light alcohol use, respectively (Cohen-Gilbert et al., 2015). Glutamine/glutamate (Gln/Glu) amino acid ratio is believed to represent metabolic turnover that can be used as a marker for neurotransmission (Ongur et al., 2011), and has been shown to be altered as a function of alcohol use (Meyerhoff, 2014). Unexpectedly, Gln/Glu ratios in the anterior cingulate cortex (ACC) were higher in FHN young adults relative to adolescents, but this pattern was not observed in the FHP groups (Cohen-Gilbert et al., 2015). The authors described that these differences were largely due to FHP adolescents already resembling young adults in Gln/Glu ratio. Interestingly, motor impulsivity was negatively related to Gln/Glu ratio in the ACC among FHP adolescents, which was believed to reflect a neuroprotective mechanism (Cohen-Gilbert et al., 2015). Another spectroscopy study, albeit in FHP and FHN adults with alcohol abuse and dependence, found that N-acetylaspertate (NAA), used to infer axonal or neural damage, was not lost to a greater extent in FHP heavy drinkers compared with FHN heavy drinkers, suggesting another potential mechanism of resilience conferred by familial alcoholism, even in adults who have years of alcohol misuse (Meyerhoff et al., 2004). Given the sparsity of research in this area, significantly more work is needed to understand the neurochemical profile related to familial alcoholism.
7. CONCLUSIONS
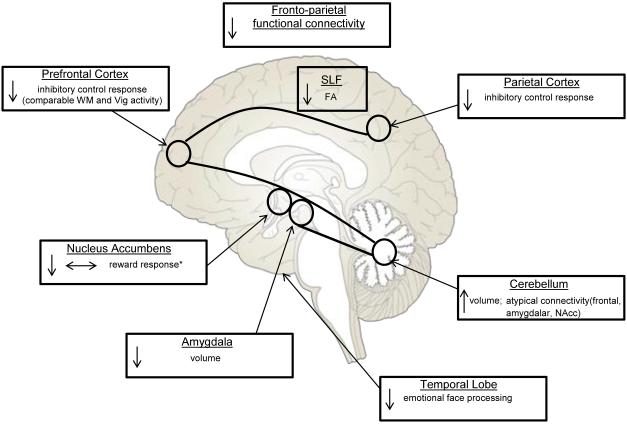
While there is no conclusive evidence for which neural markers of risk in FHP and HR individuals are most related to the higher rates of AUDs seen in this population, many findings have been replicated (Figure 1). Smaller amygdalar volume (Dager et al., 2015; Hill et al., 2001, 2013c) and larger cerebellar volume (Hill et al., 2007b, 2011) have been found in FHP and HR individuals relative to their FHN peers. Future studies that correlate neuropsychological, behavioral, and/or personality variables to these volumetric findings are needed to better understand the functional consequences of altered brain morphometry in familial alcoholism.
Figure 1. Replicated Findings in Youth and Adults with a Family History of Alcoholism.
This figure illustrates volumetric, white matter microstructure, and functional brain imaging findings that have been replicated in neuroimaging studies of family history of alcoholism.
FA = fractional anisotropy, SLF = superior longitudinal fasciculus, Vig = vigilance, WM = working memory, *for monetary rewards, ↓ smaller/decreased, ↑ larger/increased, ↔ no change
Long-range association tracts, such as the SLF, have shown reduced white matter integrity in FHP and HR youth and young adults (Acheson et al., 2014c; Herting et al., 2010; Hill et al., 2013b), while fronto-parietal brain activity has been reduced in largely alcohol-naïve adolescents during inhibitory control in both affective (Cservenka et al., 2014b) and non-affective (Schweinsburg et al., 2004) Go NoGo tasks. FHP adolescents showed comparable activity during both verbal (Cservenka et al., 2012) and spatial working memory (Mackiewicz Seghete et al., 2013) and vigilance in the frontal lobe, while FHN youth showed differences in brain activity between those conditions. Fronto-parietal connectivity (Wetherill et al., 2012) and brain activity (Rangaswamy et al., 2004) was also reduced in FHP individuals during working memory and a visual oddball task compared with their FHN peers. Together, these functional and structural findings suggest executive functioning systems may be compromised in those with familial risk for alcoholism.
It is uncertain whether reward processing is altered in FHP individuals. Previous studies reported both null effects (Bjork et al., 2008; Muller et al., 2015) and reduced NAcc brain activity during reward anticipation and/or reward receipt (Andrews et al., 2011; Yau et al., 2012), but most research has utilized paradigms with monetary rewards. Studies that use alcohol as a reward either by administering its taste (Oberlin et al., 2013), or odor (Kareken et al., 2010), or presenting alcohol-related cues (Dager et al., 2013), have all indicated increased brain response to alcohol, and this was present across many brain areas, including frontal, reward-related, visual attention, and memory-associated regions. Finally, in response to emotional stimuli, temporal lobe response was reduced in FHP and HR individuals compared with their FHN peers (Cservenka et al., 2014b; Hill et al., 2007a), which is indicative of alterations in socio-affective processing.
Future studies will need to better understand brain network organization in FHP individuals. Is connectivity of brain regions atypical in this population, and which structural and/or functional neural markers are predictive of the development of AUDs? Longitudinal study designs will be critical for answering these questions. Continued efforts towards identifying neural markers that are most predictive of AUD risk will allow for the implementation of neurobiologically informed prevention efforts to reduce the prevalence of AUDs in FHP individuals. Specifically, information gleaned from the studies discussed in this review and future neuroimaging studies of familial alcoholism could be helpful in identifying neural structures, connections, or functions that could be strengthened, modified, or altered with neurobehavioral methods to promote healthy brain functioning and reduce the incidence of AUDs. Similar strategies have recently been examined in neuroimaging studies on the mechanisms of behavior change, which utilize information on brain activity to predict the success of psychosocial interventions (Feldstein Ewing et al., 2011). Developing tasks that promote strong executive functioning skills, such as increased inhibitory control, may be one of many methods that could minimize potential risks associated with reductions in white matter integrity of fronto-parietal pathways (Acheson et al., 2014c; Herting et al., 2010) and altered prefrontal functioning (Cservenka et al., 2012; Cservenka and Nagel, 2012; Schweinsburg et al., 2004) that may be related to elevated risk for AUDs in familial alcoholism.
Highlights.
Family history of alcoholism (FHP) is associated with premorbid subcortical and cerebellar brain volumetric alterations.
FHP individuals have both increased and decreased white matter microstructure integrity relative to their peers (FHN).
Brain activity differences are present between FHP and FHN individuals during executive functioning, reward, and emotion processing tasks.
Understanding premorbid neural characteristics in familial alcoholism may help inform studies focused on reducing the incidence of alcohol abuse in at-risk youth and adults.
Acknowledgements
The author would like to thank Bonnie J. Nagel for guidance and feedback on this review. The author was supported as a trainee on a grant from the National Institute on Alcohol Abuse and Alcoholism: U01 AA021691 (PI: Nagel), and subsequently as a trainee on the Translational Neuroscience of Drug Abuse Training Program T32 DA024635-08 (PI: London) during the preparation of this manuscript.
Role of Funding Source: The grant supporting the trainee played no role in the writing of this review, interpretation of the data, nor the decision to submit the review for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors: A.C. wrote the review.
Conflict of Interest: No conflict declared.
REFERENCES
- Acheson A, Franklin C, Cohoon AJ, Glahn DC, Fox PT, Lovallo WR. Anomalous temporoparietal activity in individuals with a family history of alcoholism: studies from the oklahoma family health patterns project. Alcohol. Clin. Exp. Res. 2014a;38:1639–1645. doi: 10.1111/acer.12420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acheson A, Richard DM, Mathias CW, Dougherty DM. Adults with a family history of alcohol related problems are more impulsive on measures of response initiation and response inhibition. Drug Alcohol Depend. 2011a;117:198–203. doi: 10.1016/j.drugalcdep.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acheson A, Robinson JL, Glahn DC, Lovallo WR, Fox PT. Differential activation of the anterior cingulate cortex and caudate nucleus during a gambling simulation in persons with a family history of alcoholism: studies from the oklahoma family health patterns project. Drug Alcohol Depend. 2009;100:17–23. doi: 10.1016/j.drugalcdep.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acheson A, Tagamets MA, Rowland LM, Mathias CW, Wright SN, Hong LE, Kochunov P, Dougherty DM. Increased forebrain activations in youths with family histories of alcohol and other substance use disorders performing a go/nogo task. Alcohol. Clin. Exp. Res. 2014b;38:2944–2951. doi: 10.1111/acer.12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acheson A, Vincent AS, Sorocco KH, Lovallo WR. Greater discounting of delayed rewards in young adults with family histories of alcohol and drug use disorders: studies from the oklahoma family health patterns project. Alcohol. Clin. Exp. Res. 2011b;35:1607–1613. doi: 10.1111/j.1530-0277.2011.01507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acheson A, Wijtenburg SA, Rowland LM, Winkler AM, Gaston F, Mathias CW, Fox PT, Lovallo WR, Wright SN, Hong LE, Dougherty DM, Kochunov P. Assessment of whole brain white matter integrity in youths and young adults with a family history of substance-use disorders. Hum. Brain Mapp. 2014c;35:5401–5413. doi: 10.1002/hbm.22559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alterman AI. Patterns of familial alcoholism, alcoholism severity, and psychopathology. J. Nerv. Ment. Dis. 1988;176:167–175. doi: 10.1097/00005053-198803000-00005. [DOI] [PubMed] [Google Scholar]
- Alterman AI, Searles JS, Hall JG. Failure to find differences in drinking behavior as a function of familial risk for alcoholism: a replication. J. Abnorm. Psychol. 1989;98:50–53. doi: 10.1037//0021-843x.98.1.50. [DOI] [PubMed] [Google Scholar]
- Ambrose ML, Bowden SC, Whelan G. Working memory impairments in alcohol-dependent participants without clinical amnesia. Alcohol. Clin. Exp. Res. 2001;25:185–191. [PubMed] [Google Scholar]
- Andrews MM, Meda SA, Thomas AD, Potenza MN, Krystal JH, Worhunsky P, Stevens MC, O'Malley S, Book GA, Reynolds B, Pearlson GD. Individuals family history positive for alcoholism show functional magnetic resonance imaging differences in reward sensitivity that are related to impulsivity factors. Biol. Psychiatry. 2011;69:675–683. doi: 10.1016/j.biopsych.2010.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson M, Kyllerman M, Sabel KG, Sandin B, Olegard R. Children of alcoholic mothers. developmental, perceptual and behavioural characteristics as compared to matched controls. Acta Paediatr. Scand. 1985;74:27–35. doi: 10.1111/j.1651-2227.1985.tb10916.x. [DOI] [PubMed] [Google Scholar]
- Bates ME, Pandina RJ. Familial alcoholism and premorbid cognitive deficit: a failure to replicate subtype differences. J. Stud. Alcohol. 1992;53:320–327. doi: 10.15288/jsa.1992.53.320. [DOI] [PubMed] [Google Scholar]
- Bava S, Thayer R, Jacobus J, Ward M, Jernigan TL, Tapert SF. Longitudinal characterization of white matter maturation during adolescence. Brain Res. 2010;1327:38–46. doi: 10.1016/j.brainres.2010.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A, Schlagenhauf F, Wustenberg T, Hein J, Kienast T, Kahnt T, Schmack K, Hagele C, Knutson B, Heinz A, Wrase J. Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biol. Psychiatry. 2009;66:734–742. doi: 10.1016/j.biopsych.2009.04.035. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Bihari B, Kissin B. Event-related brain potentials in boys at risk for alcoholism. Science. 1984;225:1493–1496. doi: 10.1126/science.6474187. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Hommer DW. Incentive-elicited striatal activation in adolescent children of alcoholics. Addiction. 2008;103:1308–1319. doi: 10.1111/j.1360-0443.2008.02250.x. [DOI] [PubMed] [Google Scholar]
- Bohman M. Some genetic aspects of alcoholism and criminality. A population of adoptees. Arch. Gen. Psychiatry. 1978;35:269–276. doi: 10.1001/archpsyc.1978.01770270019001. [DOI] [PubMed] [Google Scholar]
- Brown SA, Tapert SF, Granholm E, Delis DC. Neurocognitive functioning of adolescents: effects of protracted alcohol use. Alcohol. Clin. Exp. Res. 2000;24:164–171. [PubMed] [Google Scholar]
- Camchong J, Stenger A, Fein G. Resting-state synchrony in long-term abstinent alcoholics. Alcohol. Clin. Exp. Res. 2013;37:75–85. doi: 10.1111/j.1530-0277.2012.01859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas VA, Studholme C, Meyerhoff DJ, Song E, Weiner MW. Chronic active heavy drinking and family history of problem drinking modulate regional brain tissue volumes. Psychiatry Res. 2005;138:115–130. doi: 10.1016/j.pscychresns.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Christensen HB, Bilenberg N. Behavioural and emotional problems in children of alcoholic mothers and fathers. Eur. Child. Adolesc. Psychiatry. 2000;9:219–226. doi: 10.1007/s007870070046. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Bohman M, Sigvardsson S. Inheritance of alcohol abuse. Cross-fostering analysis of adopted men. Arch. Gen. Psychiatry. 1981;38:861–868. doi: 10.1001/archpsyc.1981.01780330019001. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Sigvardsson S, Reich T, Bohman M. Inheritance of risk to develop alcoholism. NIDA Res. Monogr. 1986;66:86–96. [PubMed] [Google Scholar]
- Cohen-Gilbert JE, Sneider JT, Crowley DJ, Rosso IM, Jensen JE, Silveri MM. Impact of family history of alcoholism on glutamine/glutamate ratio in anterior cingulate cortex in substance-naive adolescents. Dev. Cogn. Neurosci. 2015 doi: 10.1016/j.dcn.2015.04.005. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corral M, Holguin SR, Cadaveira F. Neuropsychological characteristics of young children from high-density alcoholism families: a three-year follow-up. J. Stud. Alcohol Drugs. 2003;64:195–199. doi: 10.15288/jsa.2003.64.195. [DOI] [PubMed] [Google Scholar]
- Cotton NS. The familial incidence of alcoholism: a review. J. Stud. Alcohol. 1979;40:89–116. doi: 10.15288/jsa.1979.40.89. [DOI] [PubMed] [Google Scholar]
- Cservenka A, Casimo K, Fair DA, Nagel BJ. Resting state functional connectivity of the nucleus accumbens in youth with a family history of alcoholism. Psychiatry Res. 2014a;221:210–219. doi: 10.1016/j.pscychresns.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A, Fair DA, Nagel BJ. Emotional processing and brain activity in youth at high risk for alcoholism. Alcohol. Clin. Exp. Res. 2014b;38:1912–1923. doi: 10.1111/acer.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A, Gillespie AJ, Michael PG, Nagel BJ. Family history density of alcoholism relates to left nucleus accumbens volume in adolescent girls. J. Stud. Alcohol Drugs. 2015;76:47–56. [PMC free article] [PubMed] [Google Scholar]
- Cservenka A, Herting MM, Nagel BJ. Atypical frontal lobe activity during verbal working memory in youth with a family history of alcoholism. Drug Alcohol Depend. 2012;123:98–104. doi: 10.1016/j.drugalcdep.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A, Nagel BJ. Risky decision-making: an fmri study of youth at high risk for alcoholism. Alcohol. Clin. Exp. Res. 2012;36:604–615. doi: 10.1111/j.1530-0277.2011.01650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dager AD, Anderson BM, Stevens MC, Pulido C, Rosen R, Jiantonio-Kelly RE, Sisante JF, Raskin SA, Tennen H, Austad CS, Wood RM, Fallahi CR, Pearlson GD. Influence of alcohol use and family history of alcoholism on neural response to alcohol cues in college drinkers. Alcohol. Clin. Exp. Res. 2013;37(Suppl. 1):E161–171. doi: 10.1111/j.1530-0277.2012.01879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dager AD, McKay DR, Kent JW, Jr., Curran JE, Knowles E, Sprooten E, Goring HH, Dyer TD, Pearlson GD, Olvera RL, Fox PT, Lovallo WR, Duggirala R, Almasy L, Blangero J, Glahn DC. Shared genetic factors influence amygdala volumes and risk for alcoholism. Neuropsychopharmacology. 2015;40:412–420. doi: 10.1038/npp.2014.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, Hall J, Kersh A, Keshavan MS. Hippocampal volume in adolescent-onset alcohol use disorders. Am. J. Psychiatry. 2000;157:737–744. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- DeVito EE, Meda SA, Jiantonio R, Potenza MN, Krystal JH, Pearlson GD. Neural correlates of impulsivity in healthy males and females with family histories of alcoholism. Neuropsychopharmacology. 2013;38:1854–1863. doi: 10.1038/npp.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drejer K, Theilgaard A, Teasdale TW, Schulsinger F, Goodwin DW. A prospective study of young men at high risk for alcoholism: neuropsychological assessment. Alcohol. Clin. Exp. Res. 1985;9:498–502. doi: 10.1111/j.1530-0277.1985.tb05590.x. [DOI] [PubMed] [Google Scholar]
- Elmasian R, Neville H, Woods D, Schuckit M, Bloom F. Event-related brain potentials are different in individuals at high and low risk for developing alcoholism. Proc. Natl. Acad. Sci. U. S. A. 1982;79:7900–7903. doi: 10.1073/pnas.79.24.7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychol. Med. 2006;36:299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Filbey FM, Sabbineni A, Chandler LD, Hutchison KE. How psychosocial alcohol interventions work: A preliminary look at what fmri can tell us. Alcohol. Clin. Exp. Res. 2011;35:643–651. doi: 10.1111/j.1530-0277.2010.01382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland MA, Parsons OA, Nixon SJ. Visual-spatial learning in nonalcoholic young adults with and those without a family history of alcoholism. J. Stud. Alcohol. 1993;54:219–224. doi: 10.15288/jsa.1993.54.219. [DOI] [PubMed] [Google Scholar]
- Gierski F, Hubsch B, Stefaniak N, Benzerouk F, Cuervo-Lombard C, Bera-Potelle C, Cohen R, Kahn JP, Limosin F. Executive functions in adult offspring of alcohol-dependent probands: Toward a cognitive endophenotype? Alcohol. Clin. Exp. Res. 2013;37(Suppl. 1):E356–363. doi: 10.1111/j.1530-0277.2012.01903.x. [DOI] [PubMed] [Google Scholar]
- Gilman JM, Bjork JM, Hommer DW. Parental alcohol use and brain volumes in early- and late-onset alcoholics. Biol. Psychiatry. 2007;62:607–615. doi: 10.1016/j.biopsych.2006.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn DC, Lovallo WR, Fox PT. Reduced amygdala activation in young adults at high risk of alcoholism: studies from the oklahoma family health patterns project. Biol. Psychiatry. 2007;61:1306–1309. doi: 10.1016/j.biopsych.2006.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin DW. Alcoholism and genetics. The sins of the fathers. Arch. Gen. Psychiatry. 1985;42:171–174. doi: 10.1001/archpsyc.1985.01790250065008. [DOI] [PubMed] [Google Scholar]
- Goodwin DW, Schulsinger F, Moller N, Hermansen L, Winokur G, Guze SB. Drinking problems in adopted and nonadopted sons of alcoholics. Arch. Gen. Psychiatry. 1974;31:164–169. doi: 10.1001/archpsyc.1974.01760140022003. [DOI] [PubMed] [Google Scholar]
- Grant BF. Estimates of us children exposed to alcohol abuse and dependence in the family. Am. J. Public Health. 2000;90:112–115. doi: 10.2105/ajph.90.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. U. S. A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc. Natl. Acad. Sci. U. S. A. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson KL, Medina KL, Nagel BJ, Spadoni AD, Gorlick A, Tapert SF. Hippocampal volumes in adolescents with and without a family history of alcoholism. Am. J. Drug Alcohol Abuse. 2010;36:161–167. doi: 10.3109/00952991003736397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardee JE, Weiland BJ, Nichols TE, Welsh RC, Soules ME, Steinberg DB, Zubieta JK, Zucker RA, Heitzeg MM. Development of impulse control circuitry in children of alcoholics. Biol. Psychiatry. 2014;76:708–716. doi: 10.1016/j.biopsych.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden PW, Pihl RO. Cognitive function, cardiovascular reactivity, and behavior in boys at high risk for alcoholism. J. Abnorm. Psychol. 1995;104:94–103. doi: 10.1037//0021-843x.104.1.94. [DOI] [PubMed] [Google Scholar]
- Heath AC, Bucholz KK, Madden PA, Dinwiddie SH, Slutske WS, Bierut LJ, Statham DJ, Dunne MP, Whitfield JB, Martin NG. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: consistency of findings in women and men. Psychol. Med. 1997;27:1381–1396. doi: 10.1017/s0033291797005643. [DOI] [PubMed] [Google Scholar]
- Heitzeg MM, Nigg JT, Yau WY, Zubieta JK, Zucker RA. Affective circuitry and risk for alcoholism in late adolescence: differences in frontostriatal responses between vulnerable and resilient children of alcoholic parents. Alcohol. Clin. Exp. Res. 2008;32:414–426. doi: 10.1111/j.1530-0277.2007.00605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzeg MM, Nigg JT, Yau WY, Zucker RA, Zubieta JK. Striatal dysfunction marks preexisting risk and medial prefrontal dysfunction is related to problem drinking in children of alcoholics. Biol. Psychiatry. 2010;68:287–295. doi: 10.1016/j.biopsych.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Fair D, Nagel BJ. Altered fronto-cerebellar connectivity in alcohol-naive youth with a family history of alcoholism. Neuroimage. 2011;54:2582–2589. doi: 10.1016/j.neuroimage.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Schwartz D, Mitchell SH, Nagel BJ. Delay discounting behavior and white matter microstructure abnormalities in youth with a family history of alcoholism. Alcohol. Clin. Exp. Res. 2010;34:1590–1602. doi: 10.1111/j.1530-0277.2010.01244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselbrock V, Bauer LO, Hesselbrock MN, Gillen R. Neuropsychological factors in individuals at high risk for alcoholism. Rec. Dev. Alcohol. 1991;9:21–40. [PubMed] [Google Scholar]
- Hesselbrock VM, Stabenau JR, Hesselbrock MN. Minimal brain dysfunction and neuropsychological test performance in offspring of alcoholics. Rec. Dev. Alcohol. 1985;3:65–82. doi: 10.1007/978-1-4615-7715-7_6. [DOI] [PubMed] [Google Scholar]
- Hill SY, De Bellis MD, Keshavan MS, Lowers L, Shen S, Hall J, Pitts T. Right amygdala volume in adolescent and young adult offspring from families at high risk for developing alcoholism. Biol. Psychiatry. 2001;49:894–905. doi: 10.1016/s0006-3223(01)01088-5. [DOI] [PubMed] [Google Scholar]
- Hill SY, Kostelnik B, Holmes B, Goradia D, McDermott M, Diwadkar V, Keshavan M. Fmri bold response to the eyes task in offspring from multiplex alcohol dependence families. Alcohol. Clin. Exp. Res. 2007a;31:2028–2035. doi: 10.1111/j.1530-0277.2007.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Lichenstein S, Wang S, Carter H, McDermott M. Caudate volume in offspring at ultra high risk for alcohol dependence: comt val158met, drd2, externalizing disorders, and working memory. Adv. J. Mol. Imaging. 2013a;3:43–54. doi: 10.4236/ami.2013.34007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Muddasani S, Prasad K, Nutche J, Steinhauer SR, Scanlon J, McDermott M, Keshavan M. Cerebellar volume in offspring from multiplex alcohol dependence families. Biol. Psychiatry. 2007b;61:41–47. doi: 10.1016/j.biopsych.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Shen S, Locke J, Lowers L, Steinhauer S, Konicky C. Developmental changes in postural sway in children at high and low risk for developing alcohol-related disorders. Biol. Psychiatry. 2000;47:501–511. doi: 10.1016/s0006-3223(99)00175-4. [DOI] [PubMed] [Google Scholar]
- Hill SY, Terwilliger R, McDermott M. White matter microstructure, alcohol exposure, and familial risk for alcohol dependence. Psychiatry Res. 2013b;212:43–53. doi: 10.1016/j.pscychresns.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Wang S, Carter H, McDermott MD, Zezza N, Stiffler S. Amygdala volume in offspring from multiplex for alcohol dependence families: the moderating influence of childhood environment and 5-httlpr. J. Alcohol Drug Depend. 2013c;S1:1–9. doi: 10.4172/2329-6488.S1-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Wang S, Carter H, Tessner K, Holmes B, McDermott M, Zezza N, Stiffler S. Cerebellum volume in high-risk offspring from multiplex alcohol dependence families: association with allelic variation in gabra2 and bdnf. Psychiatry Res. 2011;194:304–313. doi: 10.1016/j.pscychresns.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Wang S, Kostelnik B, Carter H, Holmes B, McDermott M, Zezza N, Stiffler S, Keshavan MS. Disruption of orbitofrontal cortex laterality in offspring from multiplex alcohol dependence families. Biol. Psychiatry. 2009;65:129–136. doi: 10.1016/j.biopsych.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Yuan H. Familial density of alcoholism and onset of adolescent drinking. J. Stud. Alcohol. 1999;60:7–17. doi: 10.15288/jsa.1999.60.7. [DOI] [PubMed] [Google Scholar]
- Kaprio J, Koskenvuo M, Langinvainio H, Romanov K, Sarna S, Rose RJ. Genetic influences on use and abuse of alcohol: a study of 5638 adult finnish twin brothers. Alcohol. Clin. Exp. Res. 1987;11:349–356. doi: 10.1111/j.1530-0277.1987.tb01324.x. [DOI] [PubMed] [Google Scholar]
- Kareken DA, Bragulat V, Dzemidzic M, Cox C, Talavage T, Davidson D, O'Connor SJ. Family history of alcoholism mediates the frontal response to alcoholic drink odors and alcohol in at-risk drinkers. Neuroimage. 2010;50:267–276. doi: 10.1016/j.neuroimage.2009.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knop J, Teasdale TW, Schulsinger F, Goodwin DW. A prospective study of young men at high risk for alcoholism: school behavior and achievement. J. Stud. Alcohol. 1985;46:273–278. doi: 10.15288/jsa.1985.46.273. [DOI] [PubMed] [Google Scholar]
- Knopik VS, Heath AC, Madden PA, Bucholz KK, Slutske WS, Nelson EC, Statham D, Whitfield JB, Martin NG. Genetic effects on alcohol dependence risk: re-evaluating the importance of psychiatric and other heritable risk factors. Psychol. Med. 2004;34:1519–1530. doi: 10.1017/s0033291704002922. [DOI] [PubMed] [Google Scholar]
- LaBrie JW, Kenney SR, Lac A, Migliuri SF. Differential drinking patterns of family history positive and family history negative first semester college females. Addict. Behav. 2009;34:190–196. doi: 10.1016/j.addbeh.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage. 2012;60:340–352. doi: 10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- Lieb R, Merikangas KR, Hofler M, Pfister H, Isensee B, Wittchen HU. Parental alcohol use disorders and alcohol use and disorders in offspring: a community study. Psychol. Med. 2002;32:63–78. doi: 10.1017/s0033291701004883. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Yechiam E, Sorocco KH, Vincent AS, Collins FL. Working memory and decision-making biases in young adults with a family history of alcoholism: studies from the oklahoma family health patterns project. Alcohol. Clin. Exp. Res. 2006;30:763–773. doi: 10.1111/j.1530-0277.2006.00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackiewicz Seghete KL, Cservenka A, Herting MM, Nagel BJ. Atypical spatial working memory and task-general brain activity in adolescents with a family history of alcoholism. Alcohol. Clin. Exp. Res. 2013;37:390–398. doi: 10.1111/j.1530-0277.2012.01948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, Marinkovic K, Breiter HC, Gasic GP, Harris GJ. Decreased volume of the brain reward system in alcoholism. Biol. Psychiatry. 2008;64:192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic K, Oscar-Berman M, Urban T, O'Reilly CE, Howard JA, Sawyer K, Harris GJ. Alcoholism and dampened temporal limbic activation to emotional faces. Alcohol. Clin. Exp. Res. 2009;33:1880–1892. doi: 10.1111/j.1530-0277.2009.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J. Cogn. Neurosci. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicol. Teratol. 2007;29:141–152. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhoff DJ. Brain proton magnetic resonance spectroscopy of alcohol use disorders. Handb. Clin. Neurol. 2014;125:313–337. doi: 10.1016/B978-0-444-62619-6.00019-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhoff DJ, Blumenfeld R, Truran D, Lindgren J, Flenniken D, Cardenas V, Chao LL, Rothlind J, Studholme C, Weiner MW. Effects of heavy drinking, binge drinking, and family history of alcoholism on regional brain metabolites. Alcohol. Clin. Exp. Res. 2004;28:650–661. doi: 10.1097/01.ALC.0000121805.12350.CA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda R, Jr., Meyerson LA, Buchanan TW, Lovallo WR. Altered emotion-modulated startle in young adults with a family history of alcoholism. Alcohol. Clin. Exp. Res. 2002;26:441–448. [PubMed] [Google Scholar]
- Muller KU, Gan G, Banaschewski T, Barker GJ, Bokde AL, Buchel C, Conrod P, Fauth-Buhler M, Flor H, Gallinat J, Garavan H, Gowland P, Heinz A, Ittermann B, Lawrence C, Loth E, Mann K, Martinot JL, Nees F, Paus T, Pausova Z, Rietschel M, Strohle A, Struve M, Schumann G, Smolka MN. No differences in ventral striatum responsivity between adolescents with a positive family history of alcoholism and controls. Addict. Biol. 2015;20:534–545. doi: 10.1111/adb.12136. [DOI] [PubMed] [Google Scholar]
- Munro CA, McCaul ME, Oswald LM, Wong DF, Zhou Y, Brasic J, Kuwabara H, Kumar A, Alexander M, Ye W, Wand GS. Striatal dopamine release and family history of alcoholism. Alcohol. Clin. Exp. Res. 2006;30:1143–1151. doi: 10.1111/j.1530-0277.2006.00130.x. [DOI] [PubMed] [Google Scholar]
- Nagel BJ, Herting MM, Cservenka A. Working memory and addictive behavior. In: Alloway TP, Alloway RG, editors. Working Memory: The Connected Intelligence. East Sussex, UK; Psychology Press: 2012. [Google Scholar]
- Nagel BJ, Schweinsburg AD, Phan V, Tapert SF. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Res. 2005;139:181–190. doi: 10.1016/j.pscychresns.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT, Glass JM, Wong MM, Poon E, Jester JM, Fitzgerald HE, Puttler LI, Adams KM, Zucker RA. Neuropsychological executive functioning in children at elevated risk for alcoholism: findings in early adolescence. J. Abnorm. Psychol. 2004;113:302–314. doi: 10.1037/0021-843X.113.2.302. [DOI] [PubMed] [Google Scholar]
- Oades RD, Halliday GM. Ventral tegmental (a10) system: neurobiology. 1. Anatomy and connectivity. Brain Res. 1987;434:117–165. doi: 10.1016/0165-0173(87)90011-7. [DOI] [PubMed] [Google Scholar]
- Oberlin BG, Dzemidzic M, Tran SM, Soeurt CM, Albrecht DS, Yoder KK, Kareken DA. Beer flavor provokes striatal dopamine release in male drinkers: Mediation by family history of alcoholism. Neuropsychopharmacology. 2013;38:1617–1624. doi: 10.1038/npp.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohannessian CM, Hesselbrock VM. Paternal alcoholism and youth substance abuse: the indirect effects of negative affect, conduct problems, and risk taking. J. Adolesc. Health. 2008;42:198–200. doi: 10.1016/j.jadohealth.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, Haddad S, Prescot AP, Jensen JE, Siburian R, Cohen BM, Renshaw PF, Smoller JW. Relationship between genetic variation in the glutaminase gene gls1 and brain glutamine/glutamate ratio measured in vivo. Biol. Psychiatry. 2011;70:169–174. doi: 10.1016/j.biopsych.2011.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkaragoz T, Satz P, Noble EP. Neuropsychological functioning in sons of active alcoholic, recovering alcoholic, and social drinking fathers. Alcohol. 1997;14:31–37. doi: 10.1016/s0741-8329(96)00084-5. [DOI] [PubMed] [Google Scholar]
- Ozsoy S, Durak AC, Esel E. Hippocampal volumes and cognitive functions in adult alcoholic patients with adolescent-onset. Alcohol. 2013;47:9–14. doi: 10.1016/j.alcohol.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Peraza J, Cservenka A, Herting MM, Nagel BJ. Atypical parietal lobe activity to subliminal faces in youth with a family history of alcoholism. Am. J. Drug Alcohol Abuse. 2015;41:139–145. doi: 10.3109/00952990.2014.953251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, Pollock VE, Bloom FE. Meta-analysis of p300 amplitude from males at risk for alcoholism. Psychol. Bull. 1994;115:55–73. doi: 10.1037/0033-2909.115.1.55. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B. Understanding alcohol use disorders with neuroelectrophysiology. Handb. Clin. Neurol. 2014;125:383–414. doi: 10.1016/B978-0-444-62619-6.00023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B, Ardekani BA, Choi SJ, Tanabe JL, Lim KO, Begleiter H. A functional mri study of visual oddball: Evidence for frontoparietal dysfunction in subjects at risk for alcoholism. Neuroimage. 2004;21:329–339. doi: 10.1016/j.neuroimage.2003.09.018. [DOI] [PubMed] [Google Scholar]
- Saunders B, Farag N, Vincent AS, Collins FL, Jr., Sorocco KH, Lovallo WR. Impulsive errors on a go-nogo reaction time task: Disinhibitory traits in relation to a family history of alcoholism. Alcohol. Clin. Exp. Res. 2008;32:888–894. doi: 10.1111/j.1530-0277.2008.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer KW, Parsons OA, Yohman JR. Neuropsychological differences between male familial and nonfamilial alcoholics and nonalcoholics. Alcohol. Clin. Exp. Res. 1984;8:347–351. doi: 10.1111/j.1530-0277.1984.tb05678.x. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Goodwin DA, Winokur G. A study of alcoholism in half siblings. Am. J. Psychiatry. 1972;128:1132–1136. doi: 10.1176/ajp.128.9.1132. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Paulus MP, Barlett VC, Killeen LA, Caldwell LC, Pulido C, Brown SA, Tapert SF. An fmri study of response inhibition in youths with a family history of alcoholism. Ann. N. Y. Acad. Sci. 2004;1021:391–394. doi: 10.1196/annals.1308.050. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Rogowska J, McCaffrey A, Yurgelun-Todd DA. Adolescents at risk for alcohol abuse demonstrate altered frontal lobe activation during stroop performance. Alcohol. Clin. Exp. Res. 2011;35:218–228. doi: 10.1111/j.1530-0277.2010.01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri MM, Tzilos GK, Yurgelun-Todd DA. Relationship between white matter volume and cognitive performance during adolescence: effects of age, sex and risk for drug use. Addiction. 2008;103:1509–1520. doi: 10.1111/j.1360-0443.2008.02272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoerds Z, Van Tol MJ, Van den Brink W, Van der Wee NJ, Van Buchem MA, Aleman A, Penninx BW, Veltman DJ. Family history of alcohol dependence and gray matter abnormalities in non-alcoholic adults. World J. Biol. Psychiatry. 2013;14:565–573. doi: 10.3109/15622975.2011.640942. [DOI] [PubMed] [Google Scholar]
- Sorocco KH, Lovallo WR, Vincent AS, Collins FL. Blunted hypothalamic-pituitary-adrenocortical axis responsivity to stress in persons with a family history of alcoholism. Int. J. Psychophysiol. 2006;59:210–217. doi: 10.1016/j.ijpsycho.2005.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadoni AD, Norman AL, Schweinsburg AD, Tapert SF. Effects of family history of alcohol use disorders on spatial working memory bold response in adolescents. Alcohol. Clin. Exp. Res. 2008;32:1135–1145. doi: 10.1111/j.1530-0277.2008.00694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadoni AD, Simmons AN, Yang TT, Tapert SF. Family history of alcohol use disorders and neuromaturation: a functional connectivity study with adolescents. Am. J. Drug Alcohol Abuse. 2013;39:356–364. doi: 10.3109/00952990.2013.818680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Jacobus J, Brumback T, Meloy MJ, Tapert SF. White matter integrity in alcohol-naive youth with a family history of alcohol use disorders. Psychol. Med. 2014;44:2775–2786. doi: 10.1017/S0033291714000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S. Brain reward region responsivity of adolescents with and without parental substance use disorders. Psychol. Addict. Behav. 2014;28:805–815. doi: 10.1037/a0034460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Deshmukh A, Desmond JE, Lim KO, Pfefferbaum A. Cerebellar volume decline in normal aging, alcoholism, and korsakoff's syndrome: relation to ataxia. Neuropsychology. 2000;14:341–352. doi: 10.1037//0894-4105.14.3.341. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Harding AJ, Pentney R, Dlugos C, Martin PR, Parks MH, Desmond JE, Chen SH, Pryor MR, De Rosa E, Pfefferbaum A. Disruption of frontocerebellar circuitry and function in alcoholism. Alcohol. Clin. Exp. Res. 2003;27:301–309. doi: 10.1097/01.ALC.0000052584.05305.98. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rohlfing T, Pfefferbaum A. Pontocerebellar volume deficits and ataxia in alcoholic men and women: no evidence for "telescoping". Psychopharmacology (Berl.) 2010;208:279–290. doi: 10.1007/s00213-009-1729-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapert SF, Brown SA. Substance dependence, family history of alcohol dependence and neuropsychological functioning in adolescence. Addiction. 2000;95:1043–1053. doi: 10.1046/j.1360-0443.2000.95710436.x. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Jacob T, Bremer DA. Cognitive status of sons of alcoholic men. Alcohol. Clin. Exp. Res. 1989;13:232–235. doi: 10.1111/j.1530-0277.1989.tb00318.x. [DOI] [PubMed] [Google Scholar]
- Thoma P, Friedmann C, Suchan B. Empathy and social problem solving in alcohol dependence, mood disorders and selected personality disorders. Neurosci. Biobehav. Rev. 2013;37:448–470. doi: 10.1016/j.neubiorev.2013.01.024. [DOI] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Whalen PJ, Eccard CH, Dahl RE, Ryan ND, Casey BJ. Amygdala response to facial expressions in children and adults. Biol. Psychiatry. 2001;49:309–316. doi: 10.1016/s0006-3223(00)01066-0. [DOI] [PubMed] [Google Scholar]
- Treit S, Chen Z, Rasmussen C, Beaulieu C. White matter correlates of cognitive inhibition during development: a diffusion tensor imaging study. Neuroscience. 2014;276:87–97. doi: 10.1016/j.neuroscience.2013.12.019. [DOI] [PubMed] [Google Scholar]
- Weiland BJ, Welsh RC, Yau WY, Zucker RA, Zubieta JK, Heitzeg MM. Accumbens functional connectivity during reward mediates sensation-seeking and alcohol use in high-risk youth. Drug Alcohol Depend. 2013;128:130–139. doi: 10.1016/j.drugalcdep.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner EE. Resilient offspring of alcoholics: a longitudinal study from birth to age 18. J. Stud. Alcohol. 1986;47:34–40. doi: 10.15288/jsa.1986.47.34. [DOI] [PubMed] [Google Scholar]
- West MO, Prinz RJ. Parental alcoholism and childhood psychopathology. Psychol. Bull. 1987;102:204–218. [PubMed] [Google Scholar]
- Wetherill RR, Bava S, Thompson WK, Boucquey V, Pulido C, Yang TT, Tapert SF. Frontoparietal connectivity in substance-naive youth with and without a family history of alcoholism. Brain Res. 2012;1432:66–73. doi: 10.1016/j.brainres.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrase J, Makris N, Braus DF, Mann K, Smolka MN, Kennedy DN, Caviness VS, Hodge SM, Tang L, Albaugh M, Ziegler DA, Davis OC, Kissling C, Schumann G, Breiter HC, Heinz A. Amygdala volume associated with alcohol abuse relapse and craving. Am. J. Psychiatry. 2008;165:1179–1184. doi: 10.1176/appi.ajp.2008.07121877. [DOI] [PubMed] [Google Scholar]
- Wrase J, Schlagenhauf F, Kienast T, Wustenberg T, Bermpohl F, Kahnt T, Beck A, Strohle A, Juckel G, Knutson B, Heinz A. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage. 2007;35:787–794. doi: 10.1016/j.neuroimage.2006.11.043. [DOI] [PubMed] [Google Scholar]
- Yarosh HL, Hyatt CJ, Meda SA, Jiantonio-Kelly R, Potenza MN, Assaf M. Relationships between reward sensitivity, risk-taking and family history of alcoholism during an interactive competitive fmri task. PLoS One. 2014;9:e88188. doi: 10.1371/journal.pone.0088188. G, D.P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau WY, Zubieta JK, Weiland BJ, Samudra PG, Zucker RA, Heitzeg MM. Nucleus accumbens response to incentive stimuli anticipation in children of alcoholics: relationships with precursive behavioral risk and lifetime alcohol use. J. Neurosci. 2012;32:2544–2551. doi: 10.1523/JNEUROSCI.1390-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]