Abstract
Background
Weight-based postoperative levothyroxine (LT4) dosing often fails to appropriately dose overweight and underweight patients. We previously created an LT4-dosing algorithm based on Body Mass Index (BMI). We hypothesize that more patients will achieve euthyroidism at their postoperative visit with the use of the protocol.
Methods
A prospective evaluation was performed of our previously published BMI-based LT4 dosing. All adults who underwent thyroidectomy for benign disease between 1/1/2011–12/31/2013 were included; the new protocol was implemented in 10/2012. Serum TSH was measured for all patients 6–8 weeks postoperatively, and adjustments were based on TSH.
Results
330 patients were included, with 54% undergoing thyroidectomy after institution of the protocol. The groups were well matched. Prior to protocol implementation LT4 was dosed solely by weight, and 25% of patients were euthyroid at initial follow-up. After the protocol, 39% of patients were euthyroid (p=0.01). The percentage of patients who were given too high a dose of LT4 remained the same (46% vs. 42%) while there was a significant reduction in the number of patients who were given too little (29% vs. 19%, p = 0.05). The effect was most profound in patients with low and normal BMI, and there were slight gender differences.
Conclusion
Though correct initial dosing of LT4 remains challenging, this dosing protocol that we developed and implemented has improved patient care by increasing the number of patients who achieve euthyroidism at the first postoperative visit. We have made a change to our original protocol to incorporate gender differences into the calculation.
After total thyroidectomy for benign thyroid conditions such as Graves’ disease or multinodular goiter, levothryroxine (LT4) is dosed with the intent of restoring the normal physiologic function of thyroid hormone. This is measured by serum levels of thyroid stimulating hormone (TSH), with the goal of keeping TSH within the normal range. In malignant thyroid cancer, the clinician’s goal becomes TSH suppression, and therefore levothyroxine is often dosed slightly higher1.
Underdosing of LT4 may result in a temporary period of hypothyroidism, associated with unpleasant and disruptive symptoms including fatigue, weight gain, and poor health care quality of life2. Overdosing of LT4 may result in temporary hyperthyroidism symptoms such as arrhythmias, which can be particularly problematic for older adults3. It takes several weeks for TSH levels to adequately reflect any dose changes, and this may lead to prolonged periods of symptoms for patients after surgery. Many authors have recently published dosing algorithms based on a variety of patient factors, such as ideal body weight, age, gender, and body mass index (BMI)4–7 to try and reduce the time between surgery and achievement of a euthyroid state. We designed and published a dosing algorithm for LT4 based on BMI alone from a retrospective analysis of our patient population undergoing thyroidectomy for a variety of benign conditions8. Here we present the results of our prospective evaluation of this dosing algorithm. We hypothesized that application of our novel BMI-based dosing algorithm would result in more patients achieving euthyroid TSH levels at their first laboratory visit 6–8 weeks after total thyroidectomy.
Methods
In October 2012, we instituted a new BMI-based LT4 dosing protocol that was applied to all patients undergoing total or completion thyroidectomy for benign thyroid conditions. We have previously published our protocol, which was derived using regression analysis applied to a cohort of 122 patients. A line of best fit was plotted using euthyroid dose and BMI, and a formula was developed that allowed the surgeon to easily choose a multiplier based on the BMI of a given patient. From that formula, we created a table that grouped patients by BMI for quick calculation of the estimated LT4 dose (Figure 1). We applied this protocol to consecutive patients, and subsequently compared patients who had their surgery prior to the institution of the protocol (but who were not included in the original derivation of the protocol) to those who had their surgery afterward. Prior to the institution of the BMI-based protocol, patients were generally dosed at 1.6 mcg/kg of actual body weight, although variations may have occurred due to available dosing strengths, rounding or calculation errors, or if a patient’s referring endocrinologist preferred to manage LT4.
Figure 1. BMI-based dosing protocol.
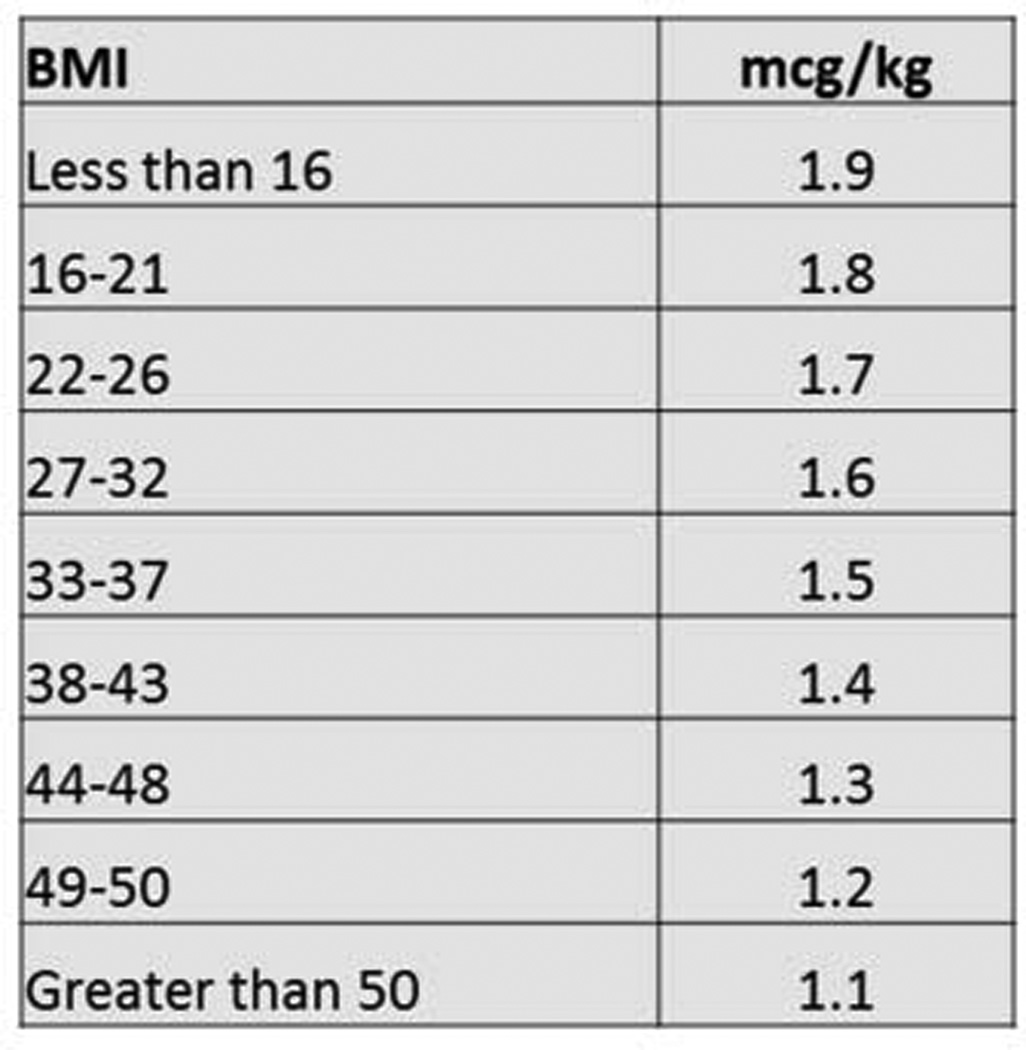
Levothyroxine dose is calculated by choosing the BMI category for the patient and multiplying patient actual weight in kilograms by the multiplier in the second column
Thyroid cancer patients were not included because of the requirement for TSH suppression. We only included patients who underwent total or completion thyroidectomy and were put on LT4 immediately after surgery. A total of 330 patients met criteria and were included in this study, 180 patients were dosed with LT4 after institution of the BMI-based dosing protocol and 150 had their operation prior and were dosed based on weight only. Variables collected included age, gender, weight, pathology, postoperative parathyroid hormone levels, BMI, initial dose of LT4, calculated dose of LT4, postoperative TSH at 6 weeks, and dose adjustments made. Thyroidectomy was performed by 1 of 4 endocrine surgeons at our institution.
Before surgery, all of our patients are given instruction on proper administration of LT4. These instructions did not change when we instituted the new dosing protocol. Patients are instructed to take LT4 on an empty stomach at the same time every day, to wait 30 minutes before eating, and to wait at least 4 hours before taking calcium, multivitamins or iron supplements. LT4 administration was instituted in all patients on postoperative day 1. All patients had TSH levels drawn 6–8 weeks postoperatively and dose adjustments were made by the surgeon or endocrine surgery nurse practitioner. Dose adjustments were rarely made prior to that first postoperative blood draw, and if any provider changed the dose prior to the first TSH measurement, we recorded both the original dose and the adjusted dose. Euthyroidism was defined as a serum TSH level of 0.45 – 4.50 mIU/mL. After that first TSH measurement, some patients continued to be followed by the surgical clinic while others had further adjustments made by an endocrinologist or primary care physician. All dose adjustments and TSH levels were recorded throughout the study period. LT4 dosing can be nuanced and complicated, and sometimes at the high or low ranges of “normal” TSH, a patient may experience symptoms of hypo- or hyperthyroidism. These symptoms are often taken into account for minor dose adjustments, but for the purposes of this study, we defined euthyroidism from a biochemical standpoint only. There were a few patients (<10) who had a TSH that was normal at the first TSH check, but then later developed hypo- or hyperthyroidism. Unless there was some obvious reason to explain this (such as large weight change or pregnancy), we assumed these patients were not on a correct initial dose of LT4, and we used a time point down the road when they achieved euthyroidism again by labs and were on a stable dose of LT4.
Comparisons were made of the entire cohort of patients to identify differences between the groups other than the date of their surgery. Bivariate analysis was done comparing the pre- and post-protocol groups using Pearson chi-square and Student’s t-test for categorical and continuous variables. Patients were then clustered by BMI in the same groupings set by our protocol, and each BMI group was compared for percentage of patients who were found to be euthyroid at the first TSH check in 6–8 weeks. The data that was collected is a part of our IRB-approved endocrine surgery database and informed consent was waived for patients. Statistical analysis was performed using STATA v.11 software (Stata Corp), and a p value < 0.05 was considered significant.
Results
Of the 330 patients who met inclusion criteria, 180 (54%) underwent thyroidectomy after implementation of the BMI-based protocol, leaving 150 patients who were dosed on weight for comparison. There were no differences in gender, age, weight, BMI, or initial dose of levothyroxine for patients operated on prior to versus those operated on after implementation of the protocol, but there were statistically more patients with Graves’ disease in the protocol group (Table 1). This is not from any intentional exclusion or inclusion of patients, it simply reflects an overall trend in our population that more patients are undergoing surgery for Graves’ disease during the time of implementation of this protocol9.
Table 1.
Baseline characteristics of the cohort
| Variable | Pre-BMI protocol (n=150) |
BMI protocol (n=180) |
p |
|---|---|---|---|
| Gender (% female) | 84% | 85% | 0.8 |
| Age, years (mean ± SD) | 50 ± 16 | 48 ± 15 | 0.2 |
| Weight, kg (mean ± SD) | 84 ± 23 | 85 ± 23 | 0.8 |
| Graves' disease (%) | 27% | 44% | 0.001 |
| BMI (mean ± SD) | 29.9 ± 7.8 | 30.4 ± 8.1 | 0.6 |
| BMI Group, % (n) | 0.7 | ||
| 16–21 | 10% (15) | 10% (18) | |
| 22–26 | 28.7% (43) | 27.8% (50) | |
| 27–32 | 35.3% (53) | 28.3% (51) | |
| 33–37 | 9.3% (14) | 15.6% (28) | |
| 38–43 | 10% (15) | 11.7% (21) | |
| 44–48 | 3.3% (5) | 3.9% (7) | |
| 49–50 | 1.3% (2) | 0.6% (1) | |
| ≥51 | 2% (3) | 2.2% (4) | |
| Initial Dose LT4, mcg (mean ± SD) | 138 ± 31 | 132 ± 27 | 0.09 |
SD = standard deviation; LT4 = levothyroxine
Prior to implementation of the protocol, only 25.3% (n=38 of 150) of patients had their first postoperative TSH in the euthyroid range while 38.9% (n=70 of 180) were euthyroid at their initial follow up after the implementation of the BMI-based protocol. This is a statistically significant improvement (p<0.01).
In order to ascertain whether this improvement was a result of less overdosing or less underdosing, the patients were further broken down into groups based on whether they were hyperthyroid, euthyroid, or hypothyroid at their first postoperative visit based on serum TSH. We found that the improvement was mostly due to fewer patients being underdosed, as shown on Table 2. There were significantly more patients who were hypothyroid at their first TSH check prior to implementation of the protocol (28.7% vs 19.4%, p=0.05), while the number of hyperthyroid patients did not change significantly (46% vs 41.7%, p=0.4).
Table 2.
Thyroid function of patients at first postoperative visit before and after BMI-based protocol
| Variable | Pre-BMI protocol (n=150) |
BMI protocol (n=180) |
p |
|---|---|---|---|
| Euthyroid at 6–8 weeks | 25.3% | 38.9% | 0.01 |
| Hypothyroid at 6–8 weeks | 28.7% | 19.4% | 0.05 |
| Hyperthyroid at 6–8 weeks | 46% | 41.7% | 0.4 |
Euthyroid TSH reference range = 0.45 – 4.50 uIU/mL; Hypothyroid TSH > 4.5 uIU/mL; Hyperthyroid TSH <0.45 uIU/mL
In order to determine whether the protocol worked equally well for patients across the range of BMI on our protocol, the cohort was divided roughly into tertiles based on BMI. Figure 2 shows the percentage of patients who were hypothyroid, euthyroid, or hyperthyroid before and after the protocol implementation for the lowest (a, BMI 16–26), middle (b, BMI 27–32), and highest (c, BMI >33) BMI categories. The largest and only statistically significant improvement was for patients with BMI of 16–26, who showed improvement in both over- and under-dosing of LT4 (24% of patients were euthyroid prior to the protocol vs. 49% euthyroid after implementation). The patients with BMI of 27–32 showed essentially no change with the protocol (26% euthyroid prior to and after implementation), and patients with BMI greater than 33 had a slight improvement in overdosing of LT4 with the protocol (31% euthyroid prior to the protocol vs 39% after implementation), but this difference was small and not statistically significant.
Figure 2. Thyroid function before and after protocol by BMI tertiles.
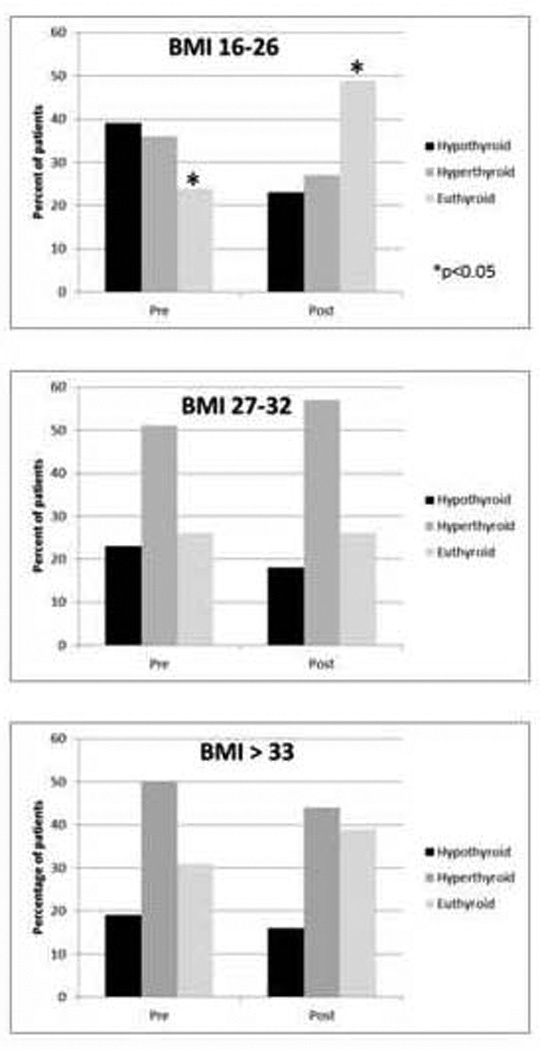
a) BMI 16–26 (n=126); b) BMI 27–32 (n=104); c) BMI > 33 (n=100)
Finally, we obtained the eventual euthyroid dose for 176 of the 180 patients who were dosed according to our protocol. Multiple logistic regression models were created using the variables described on Table 1 in order to develop a more refined protocol to even better predict euthyroid dose of LT4. None of these regression models were able to better dose our patient population, so we manually adjusted our BMI dosing scheme for optimal prediction of the euthyroid dose. Gender was an important contributor in every regression model based on this current patient population, and we observed measurable differences in the higher BMI groups as seen in Figure 2. Pre-operative TSH levels or the presence of Graves’ disease was not a significant contributor. Based on these findings, a revised BMI/Gender dosing protocol was created that seems to better calculate euthyroid dose for our patient cohort and is shown in Figure 3. Based on the known euthyroid dose for this group of patients (as opposed to the first TSH value used in the first portion of this analysis), this revised BMI/Gender protocol predicts eventual euthyroid dose of LT4 to within 20 mcg for 61% of patients, compared to a calculated 53% of patients on the BMI dosing protocol used in the current study. This calculation predicts the eventual euthyroid dose, and whether this directly translates to more patients achieving euthyroid TSH on the first postoperative measurement is not yet known. The revised protocol accomplishes this by reducing both overdosing and underdosing, as well as reducing the magnitude of dosing calculation error for the entire cohort.
Figure 3. Revised BMI-based dosing protocol.
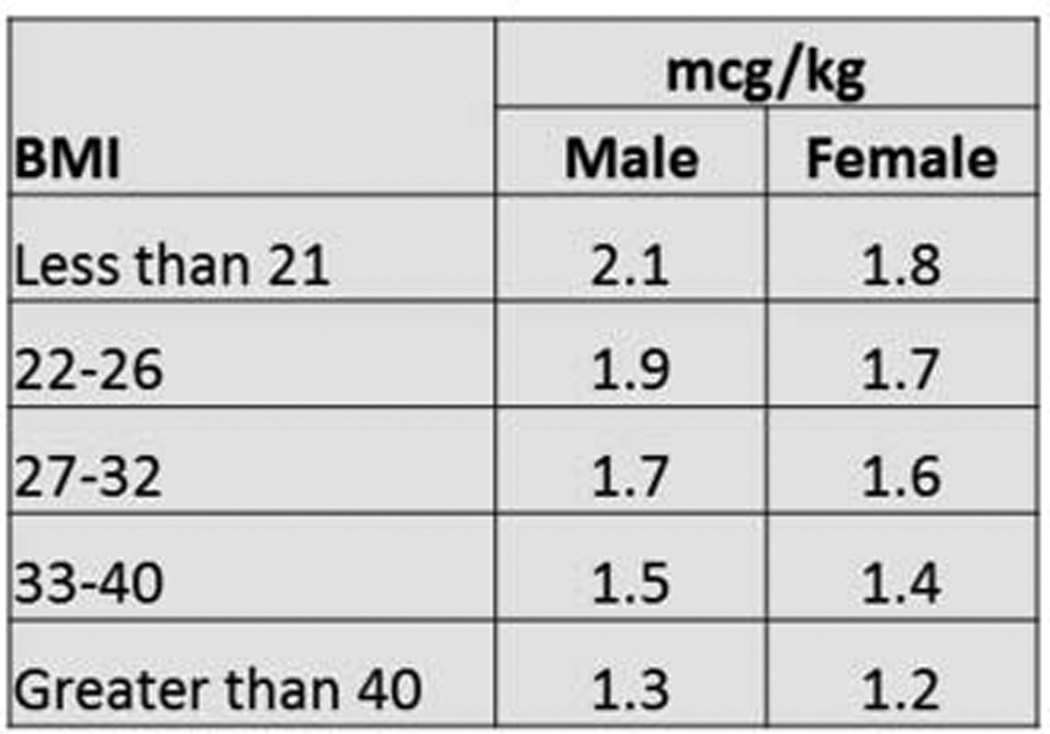
Levothryoxine dose is calculated by choosing the BMI category for the patient and multiplying the patient’s actual weight in kilograms by the multiplier in the column corresponding to the patient’s gender
Discussion
Many authors have discussed the limitations of weight-based dosing of LT4 after thyroidectomy, but it remains fairly controversial in the literature as to the relative importance of BMI, gender, and age on a dosing strategy4–6. Our previous study did not indicate that our population showed a substantial difference in postoperative TSH based on either gender or age, so our protocol was based on BMI alone. The simplicity of having just one variable makes this dosing strategy relatively easy to implement over protocols that take into account multiple variables, and we did find an overall improvement over simple weight based dosing. Di Donna, et al also partially used BMI (in addition to age) to dose LT4, and prospectively validated their dosing scheme on a small (n=31) cohort of patients7. This current study found that, after applying our BMI-based dosing protocol to 180 patients in a prospective manner, important variations were found that led us to make alterations to our original protocol that may not have been apparent in a smaller cohort.
As is evident from figure 2b and 2c, both the weight-based dosing and our novel BMI-based protocol tend to overestimate levothyroxine needs for patients with a BMI greater than 26. For our heaviest patients, there was only a small improvement, and the dosing of these patients remains challenging. On close scrutiny of these BMI groups, we found that the lowest BMI patients are slightly younger and are more likely to be hyperthyroid preoperatively. Being hyperthyroid preoperatively was not independently associated with any differences in the eventual euthyroid dose of LT4. The differences in age and preoperative hyperthyroidism were not statistically significant and were not apparent when we designed the protocol, but it seems that these patients who are normal or even underweight represent a clinically different group than those who are overweight and obese. Our BMI-based dosing works quite well for these patients, and our revised BMI/Gender protocol appears to improve our ability to calculate a dose for these patients even better.
The patients with a BMI greater than 26, however, are slightly older than those in the lowest BMI tertile. Again, this difference is subtle and not statistically significant, but the decreased thyroxine metabolism in older patients 7, as well as the increase in obesity rates in older patients 4 may help explain why our protocol was less effective for these overweight and obese patients. Furthermore, the low numbers of male patients makes it difficult to tease out the role of gender and LT4 dosing, and most studies including ours have not found a statistically significant difference 7. When we looked at the data broken down by tertiles, however, we found that this tendency to overdose patients with a BMI>26 was more pronounced in our female patients. This is in contrast to a small study by Jonklass, et al. that found that women had increased thyroxine needs than men 10. Due to the fact that thyroid disease is rarer in men, our study and others demonstrates the shortcomings of multivariate analysis methods to detect subtle differences. Because of these limitations of regression analysis, we did not find that doing additional regressions improved our estimation of LT4, and we simply manually adjusted our protocol after discovering these subtle relationships between gender, BMI and LT4 needs. Although age may play a small role in BMI dosing, the correlation between age and BMI in our patient population means that age is already accounted for in our protocol, so we decided not to complicate the algorithm by adding a third variable for age. Our revised protocol appears to more closely predict a given patient’s euthyroid dose when we apply it retrospectively to this population. We are currently using this revised dosing protocol in our patient population and plan to evaluate it prospectively once we have accrued significant numbers.
The prospective design and large sample size of our study were major strengths, as well as the ease of use of our single variable dosing protocol, which was subsequently revised with the current study to include gender. The table format proposed here hopefully continues to be convenient to use. However, there are some important limitations to consider. Despite our efforts to train all members of the team about this dosing protocol, often the LT4 dose is determined by the resident who assisted in the operation who rotates temporarily onto our endocrine surgery service for a period of around 4–8 weeks at a time. There are some variation and rounding errors that occur, and because levothyroxine tablets come in 12.5mcg increments at lower doses but only 25 mcg at doses over 150 mcg, these rounding errors occur more at higher doses. Overall, the accuracy of our calculations improved over the study period, but we still have work to do in terms of educating all members of the team about what to do when the calculated dose falls in between the strengths of the tablets (For example, a patient’s calculated daily dose is 130 mcg, which is 910 mcg per week. Rounding down to the 125 mcg strength underdoses the patient at 875 mcg per week, rounding up to 137 mcg overdoses the patient at 959 mcg per week, but giving 150 mcg six days a week gives a weekly total of 900 mcg, which is much closer). We have recently made the table available in read-only form on our electronic order entry system, but have not yet been to electronically integrate the formula and auto-calculate the dose for each patient, which would probably lead to better compliance. Patient compliance is an issue that is difficult to control, but there is no reason to suspect that patients will have different rates of compliance based on BMI, so this limitation is not likely to bias the protocol one way or another. We have a separate protocol for postoperative calcium supplementation based on immediate postoperative serum parathyroid hormone level 11, and patients who require more calcium may experience more interference with LT4 absorption. However, we found no differences in postoperative parathyroid hormone levels based on BMI, so this effect should be equal across all groups.
Conclusion
We previously created a dosing protocol that used a single piece of information accessible in a patient’s electronic medical record – namely BMI – to more accurately dose patients with levothyroxine after thyroidectomy for benign disease. After careful prospective evaluation of this protocol, we found that for patients with a BMI of less than 26, the reduction in the number of hypothyroid patients led to an improvement in our goal of achieving a euthyroid TSH early on after total thyroidectomy. Proper dosing remains a challenge in our obese population, and further fine-tuning of the protocol was done to account for differences between genders and is currently being prospectively verified.
Footnotes
Presented at the American College of Surgeons Clinical Congress, San Francisco, CA 10/27/2014
No disclosures
References
- 1.Wang L, Smith AW, Palmer FL, et al. TSH Suppression increases the risk of osteoporosis without decreasing recurrence in ATA low and intermediate risk patients with differentiated thyroid carcinoma. Thyroid : official journal of the American Thyroid Association. 2014 Nov 11; doi: 10.1089/thy.2014.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vigario Pdos S, Vaisman F, Coeli CM, et al. Inadequate levothyroxine replacement for primary hypothyroidism is associated with poor health-related quality of life-a Brazilian multicentre study. Endocrine. 2013 Oct;44(2):434–440. doi: 10.1007/s12020-013-9886-1. [DOI] [PubMed] [Google Scholar]
- 3.Selmer C, Olesen JB, Hansen ML, et al. The spectrum of thyroid disease and risk of new onset atrial fibrillation: a large population cohort study. BMJ (Clinical research ed.) 2012;345:e7895. doi: 10.1136/bmj.e7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devdhar M, Drooger R, Pehlivanova M, Singh G, Jonklaas J. Levothyroxine replacement doses are affected by gender and weight, but not age. Thyroid : official journal of the American Thyroid Association. 2011 Aug;21(8):821–827. doi: 10.1089/thy.2011.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baehr KM, Lyden E, Treude K, Erickson J, Goldner W. Levothyroxine dose following thyroidectomy is affected by more than just body weight. The Laryngoscope. 2012 Apr;122(4):834–838. doi: 10.1002/lary.23186. [DOI] [PubMed] [Google Scholar]
- 6.Jin J, Allemang MT, McHenry CR. Levothyroxine replacement dosage determination after thyroidectomy. American journal of surgery. 2013 Mar;205(3):360–363. doi: 10.1016/j.amjsurg.2012.10.015. discussion 363–364. [DOI] [PubMed] [Google Scholar]
- 7.Di Donna V, Giannotti Santoro M, de Waure C, et al. A New Strategy to Estimate Levothyroxine Requirement After Total Thyroidectomy for Benign Thyroid Disease. Thyroid : official journal of the American Thyroid Association. 2014 Oct 29; doi: 10.1089/thy.2014.0111. [DOI] [PubMed] [Google Scholar]
- 8.Ojomo KA, Schneider DF, Reiher AE, et al. Using body mass index to predict optimal thyroid dosing after thyroidectomy. Journal of the American College of Surgeons. 2013 Mar;216(3):454–460. doi: 10.1016/j.jamcollsurg.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elfenbein DM, Schneider DF, Havlena J, Chen H, Sippel RS. Clinical and Socioeconomic Factors Influence Treatment Decisions in Graves' Disease. Annals of surgical oncology. 2014 Sep 23; doi: 10.1245/s10434-014-4095-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jonklaas J. Sex and age differences in levothyroxine dosage requirement. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2010 Jan-Feb;16(1):71–79. doi: 10.4158/EP09257.OR. [DOI] [PubMed] [Google Scholar]
- 11.Youngwirth L, Benavidez J, Sippel R, Chen H. Postoperative parathyroid hormone testing decreases symptomatic hypocalcemia and associated emergency room visits after total thyroidectomy. Surgery. 2010 Oct;148(4):841–844. doi: 10.1016/j.surg.2010.07.038. discussion 844–846. [DOI] [PubMed] [Google Scholar]


