Abstract
Advantage may be taken of macroautophagy (‘autophagy’) to promote ocular health. Autophagy continually captures aged or damaged cellular material for lysosomal degradation and recyling. When autophagic flux is chronically elevated, or alternatively deficient, health suffers. Chronic elevation of flux and stress are the consequence of inflammatory cytokines or of dry eye tears but not normal tears in vitro. Exogenous tear protein lacritin transiently accelerates flux to restore homeostasis in vitro and corneal health in vivo, and yet the monomeric active form of lacritin appears to be selectively deficient in dry eye. Tissue transglutaminase-dependent cross-linking of monomer decreases monomer quantity and monomer affinity for coreceptor syndecan-1 thereby abrogating activity. Tissue transglutaminase is elevated in dry eye. Mutation of arylsulfatase A, arylsulfatase B, ceroid-lipofuscinosisneuronal 3, mucolipin, or Niemann-Pick disease type C1 respectively underlie several diseases of apparently insufficient autophagic flux that affect the eye, including: metachromatic leukodystrophy, mucopolysaccharidosis type VI, juvenile-onset Batten disease, mucolipidosis IV, and Niemann-Pick type C associated with myelin sheath destruction of corneal sensory and ciliary nerves and of the optic nerve; corneal clouding, ocular hypertension, glaucoma and optic nerve atrophy; accumulation of ‘ceroid-lipofuscin’ in surface conjunctival cells, and in ganglion and neuronal cells; decreased visual acuity and retinal dystrophy; and neurodegeneration. For some, enzyme or gene replacement, or substrate reduction, therapy is proving to be successful. Here we discuss examples of restoring ocular surface homeostasis through alteration of autophagy, with particular attention to lacritin.
Keywords: lacritin, autophagy, lysosomal storage disease, eye, dry eye, cornea
Graphical Abstract
1. Introduction
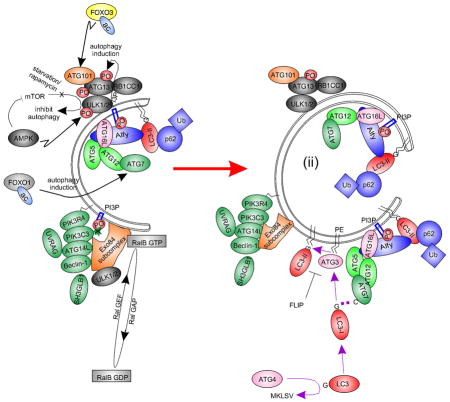
Macroautophagy (‘autophagy’) is a stimulatable self-catabolic process that constitutively clears damaged proteins and organelles to an autolysosomal compartment for degradation (Fig. 1), thus serving as a key regulator of homeostasis (Galluzzi et al., 2014). When insufficient, damaged proteins and organelles accumulate thereby promoting cellular toxicity and inflammation. Insufficient autophagic flux underlies many eye diseases, including stromal corneal dystrophy type 2 (see contribution by Kim in this issue; Choi et al., 2012) and corneal pathogenesis of herpes simplex virus Type 1 via viral sequestration of autophagy protein beclin 1 (Leib et al., 2009). Other examples include: cataract formation in the lens (see contribution by Mizushima and Morishita (Morishita et al., 2013)), glaucoma (see contributions by Liton (Porter et al., 2013) and Dickey (Suntharalingam et al., 2012)), retinal blindness (see contributions by Sinha (Valapala et al., 2014), Swarup (Sirohi et al., 2013), Maeda (Chen et al., 2013) and Yue (Shen et al., 2011)) and axonal degeneration of the optic nerve by Lingor (Knoferle et al., 2010). Accordingly, restoration or transient stimulation of autophagic flux is a potential treatment approach. One example is the tear protein ‘lacritin’ that rapidly stimulates autophagy in stressed human corneal epithelial cells (Wang et al., 2013) and when applied topically largely eliminates corneal lissamine green staining in dry eye mice (Vijmasi et al., 2014).
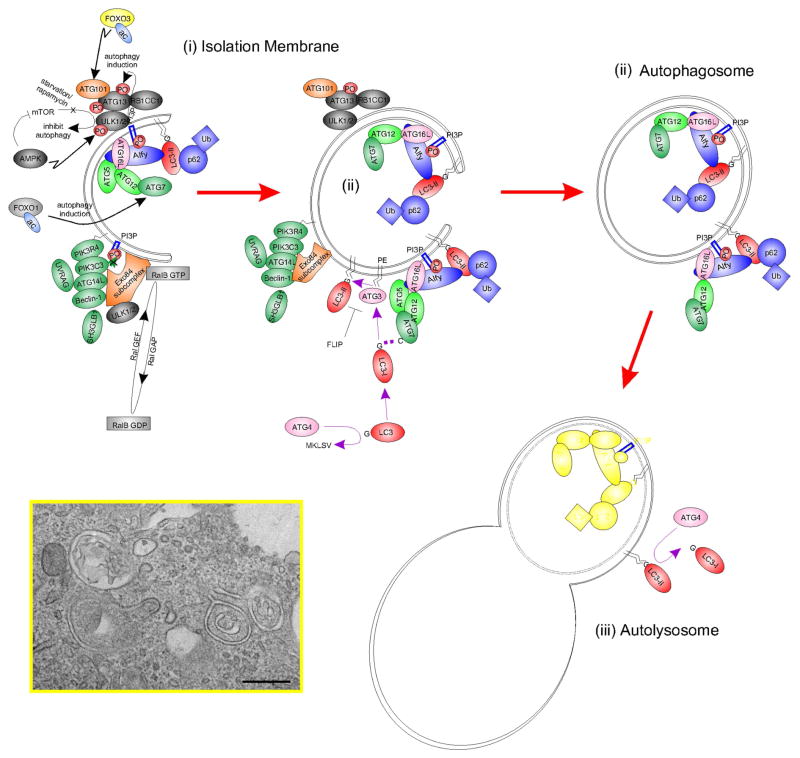
Fig. 1.
Simplified schematic diagram of autophagy with key molecular machinery. Autophagy is a constitutive catabolic mechanism responsible for the turnover of all cellular constituents. With stress (such as starvation), autophagic flux is accelerated. A double membrane ‘isolation membrane’ (ii) forms and fuses as an ‘autophagosome’ (ii) to capture damaged proteins, organelles and cytoplasm. Degradation follows fusion with a lysosome as an ‘autolysosome’ (iii; Figure expanded from Fig. 3B of Wang et al, ‘13). Inset, electron micrography of forming autophagosome-like structures in human corneal epithelial cells stressed with INFG and TNF in the presence of transient autophagic stimulator lacritin, Bar = 0.5 μM.
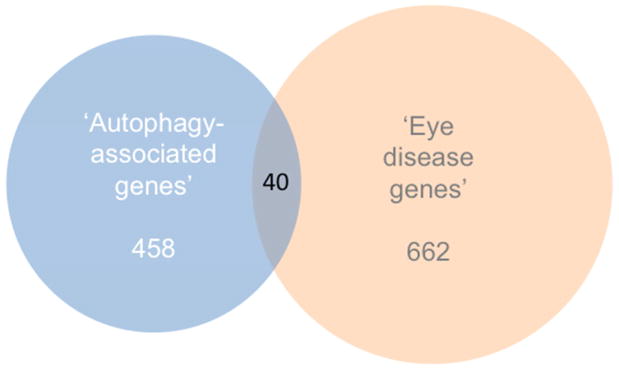
Gene ‘autophagy’ keyword search cross-referenced to expression sequence tag (‘EST’) libraries suggest that at least 460 different autophagy-associated genes are expressed in the eye (Supplemental Table 1). Some are well known autophagy mediators of the AuTophaGy related family ‘ATG’ series (Klionsky et al., 2003), most originally discovered in yeast - including ATG12 (Mizushima et al., 1998) and ATG16L1 (Mizushima et al., 1999) by issue contributor Noboru Mizushima who also discovered ATG101 (Hosokawa et al., 2009) out of HEK293 cells (see each in Fig. 1). Others include members of the upstream AKT serine threonine kinase (AKT1 – 3) family, BCL2 and the BCL2-associated family (BAD, BAG3, BAG5, BAX), BAK1, beclin 1 (BECN1), FOXO1 and FOXO3, the MAP1LC3 family (A, B, B2), MTOR, PIK3C3, RB1CC1, RIPK1 and the ULK1 – 3 family (see several in Fig. 1). Forty are NEIBank ‘eye disease genes’ (Fig. 2; Supplemental Table 1). Here we focus on all known ocular surface disease genes associated with autophagy, beginning with LACRT and its protein product lacritin (Sanghi et al., 2001).
Fig. 2.
Venn diagram illustrating the overlap of ‘autophagy-asociated genes’ (see list in Supplementary Table 1) and NEIBank’s ‘eye disease genes’. The Venn diagram was produced using http://www.stefanjol.nl/venny#ref1 and then redrawn for higher resolution.
2. Lacritin (LACRT)
Lacritin is a multifunctional tear glycoprotein (Fig. 3) (Sanghi et al., 2001) that transiently and rapidly triggers autophagy in cultured corneal epithelial cells under conditions of inflammatory cytokine stress to restore homeostasis (Wang et al., 2013). Lacritin is also a tear secretagogue – although a tear protein itself. It promotes corneal wound healing (Wang et al., 2014), exhibits latent bactericidal activity (McKown et al., 2014) and exists in active monomeric and inactive polymeric forms in human tears (Velez et al., 2013). Several proteomic studies suggest that lacritin monomer is selectively deficient in human dry eye (Aluru et al., 2012; Koo et al., 2005; Nichols and Green-Church, 2009; Srinivasan et al., 2012).
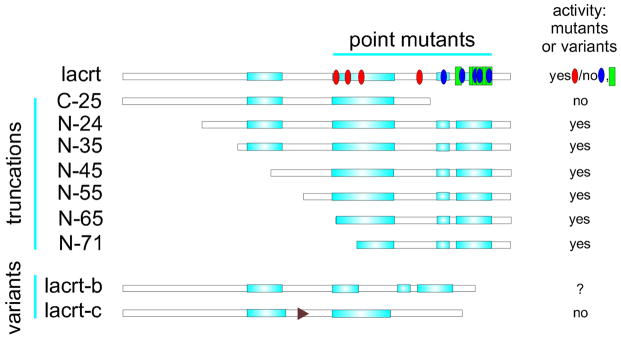
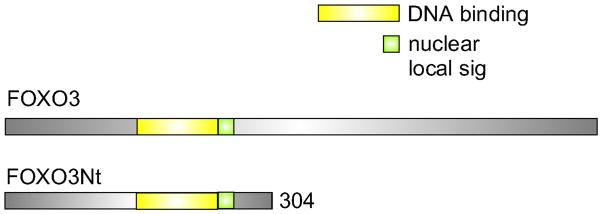
Fig. 3.
Linear diagram of lacritin without signal peptide versus several recombinantly generated truncation and point mutants, and two splice variants. PSIPRED (v3.3) secondar structure prediction. Turquoise rectangles indicate α-helices (C-terminal half α-helices have been confirmed by circular dichroism (Wang et al., 2006; Zhang et al., 2013). Blue and red ovals respectively indicate point mutations that abrogate lacritin prosurvival activity or have no effect. Several are effective only in combination (Wang et al., 2013). Green rectangles indicate point mutations that interefere with syndecan-1 binding. All but one overlaps. C-terminal recombinant lacritin-c (lacrt-c) was inactive (Zhang et al., 2013).
2.1 Discovery as a stimulator of basal tearing
An unbiased screen for factors triggering unstimulated tear protein secretion in rat lacrimal acinar cell culture led indirectly to the discovery of lacritin (Sanghi et al., 2001), an extracellular glycoprotein with SDS-PAGE mobility in tears of ~23 – 25 kDa vs ~18 kDa for lacritin generated recombinantly in E. coli, and 12.3 kDa predicted from primary sequence. Aberrant mobility is in part thought to be a consequence of its C-terminal amphipathic α-helical structure (Fig. 3; (Karnati et al., 2013). Topical recombinant lacritin stimulates tear protein release both in dry eye mice (Vijmasi et al., 2014; Wang et al., 2015) and normal rabbits (Samudre et al., 2011). Similarly, lacritin monomer semi-purified from monkey tears triggers tear lipocalin secretion from monkey lacrimal acinar cells cultured in the presence of dry eye inflammatory cytokines, that under the same conditions are unresponsive to the acetylcholine receptor agonist carbachol (Fujii et al., 2013). Lacritin is itself a tear protein derived largely from the same lacrimal acinar cells that it stimulates (Fujii et al., 2013; Sanghi et al., 2001), and is expressed in human lacrimal gland as the sixth most common mRNA (Ozyildirim et al., 2005). Other human sources include accessory lacrimal glands of Wolfring (Ubels et al., 2012) and meibomian glands (Tsai et al., 2006). RT-PCR of ocular tissues in monkey validate these observations and point also to progressively lesser expression by conjunctiva, corneal, retinal and lens epithelia, as well as by the iris and ciliary body (Nakajima et al., 2007). The lacritin LACRT gene is one of the most eye-specific genes (Karnati et al., 2013).
2.3 Lacritin prosurvival activity
The avascular corneal epithelium is thought to be dependent on tears for nutrition and health. When basal tearing is insufficient and disruptions develop in the tear film, the epithelium becomes stressed and releases inflammatory cytokines such as tumor necrosis factor (Luo et al., 2004) and interferon gamma (Fig. 4) that compromise the plasma membrane. This is the mechanism by which lissamine green is presumed to gain entrance as a sign of dry eye (Chodosh et al., 1994). Topical lacritin largely eliminates lissamine staining in dry eye mice (Vijmasi et al., 2014), and restores health to cultured human corneal epithelial cells subjected to starvation and interferon gamma/tumor necrosis factor stress (Wang et al., 2013). Truncation of lacritin’s C-terminal 25 amino acids 95 – 119 (‘C-25’ mutant; Fig. 3) is sufficient to negate this benefit, in keeping with the inefficacy of lacritin point mutants I98S, F104S, F112S and triple mutant L108/L109/F112S (Wang et al, 2013) that define lacritin’s amphipathic α-helical (Fig. 3) (Wang et al., 2006; Wang et al., 2013) binding surface. Ligation of coreceptor syndecan-1 (Ma et al., 2006; Zhang et al., 2013) is necessary for survival signaling (Wang et al., 2013). Recombinant human lacritin is active over a biphasic dose response curve with an optimum of 10 nM in human cell culture (Wang et al., 2006; Wang et al., 2013) and 4 μM (Samudre et al., 2011; Wang et al., 2015) to 20 μM (Wang et al., 2014) in preclinical studies. 1 μM appears to be the dose optimum for monkey tear lacritin applied to monkey lacrimal acinar cells (Fujii et al., 2013).
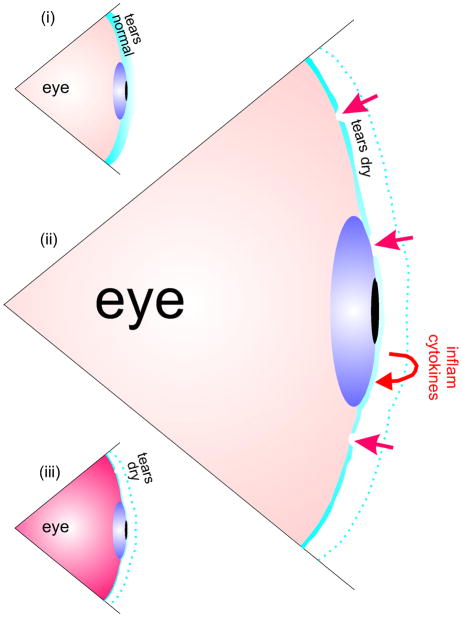
Fig. 4.
Schematic depiction of how some forms of dry eye may be initiated. (i) Normal tears. The avascular corneal epithelium is dependent on tears for health. (ii) When tears are insufficient and holes even develop (straight arrows), the corneal epithelium becomes stressed and secretes inflammatory cytokines (curved arrows). This exacerbates the situation, and (iii) inflammation results.
Human tears comprise a complex fluid of over 1500 different extracellular proteins, including growth factors (Karnati et al., 2013). To assess the relative contribution of lacritin, starved and interferon gamma/tumor necrosis factor stressed human corneal epithelial cells were treated with normal human tears, or with tears immunodepleted of lacritin. Survival was monitored by immunostaining for the transcription factor FOXO3 that exhibits cytoplasmic - nuclear translocation, respectively indicative of health and death or stress (Hu et al., 2004). FOXO3 was cytoplasmic after normal tear treatment. This benefit was lost following immunodepletion of lacritin and when normal tears were replaced with dry eye tears, but was restored by spiking the latter with 10 nM lacritin but not 10 nM C-25 (Wang et al., 2013). Further, 2-D SDS PAGE followed by mass spectrometry suggests that ~23 – 25 kDa lacritin is selectively deficient in tears from aqueous deficient (Aluru et al., 2012) or contact lens-related (Nichols and Green-Church, 2009) dry eye, and in tears from patients with blepharitis (Koo et al., 2005) - a lid inflammatory condition often associated with evaporative dry eye (Mathers, 1993). Deficiency has also been suggested in Sjogren’s syndrome tears, as per 1-D SDS PAGE immunblotting of a small sample group (Vijmasi et al., 2014). That lacritin is solely responsible for survival, at least in this in vitro assay, was unexpected.
2.4 Lacritin transiently stimulates autophagy
What is the prosurvival mechanism, and might FOXO3 be a mediator? Transient elevation of autophagic flux restores homeostasis by shunting stress-damaged proteins and organelles into the lysosomal system for degradation. To ask whether lacritin can stimulate autophagic flux, human corneal epithelial cells were stably transduced with autophagy marker LC3 in a lentiviral construct double tagged with EGFP and mCherry (Wang et al., 2013). Loss of pH sensitive EGFP signal relative to pH insensitive mCherry differentiates LC3 in autolysosomes from LC3 in isolation membranes and autophagosomes (Pankiv et al., 2007). Isolation membranes and autophagosomes respectively capture and envelop stress damaged proteins and organelles. Autophagosomes fuse with endosomes or lysosomes as autolysosomes (Fig. 1). Indeed within 10 min of lacritin addition, the mCherry signal predominated and then declined proportionally with EGFP; whereas the EGFP signal accumulated after blocking autophagosome - lysosome fusion with vinblastine (Wang et al., 2013) – together suggesting that lacritin transiently elevates autophagic flux. In contrast, both signals were equally represented at all times in C-25 treated cells. To ask whether lacritin-accelerated autophagy is capable of capturing damaged proteins, LC3 EGFP/mCherrry cells were transiently transduced with mCFP labeled huntingtin mutants Htt103Q or Htt25Q. Htt103Q, but not Htt25Q, forms toxic aggregates much like stress-damaged proteins. Experiments were performed in the absence of interferon gamma and tumor necrosis factor since Htt103Q is sufficiently stressful. Lacritin enhanced autophagy was observed only in Htt103Q cells, indicating that stress was a prerequisite. Moreover Htt103Q, not Htt25Q, colocalized with mCherry (Wang et al., 2013).
LC3 is a nexus for autophagy machinery, including Alfy and p62 that respectively draw aggregated and polyubiquitinated proteins onto isolation membranes and into autophagosomes (Fig. 1). Lacritin, but not C-25, triggered the association of LC3 with Alfy and p62 within 1 – 5 min that included capture of polyubiquitinated proteins by 5 – 15 min (Wang et al., 2013). Such kinetics are much more rapid than is commonly observed for autophagy. Particularly unusual was the loss within 1 min of blottable LC3-II - often interpreted as inhibition (rather than stimulation) of autophagy. However with leupeptin addition (to block lysosomal degradation) LC3-II accumulated, suggesting that flux had indeed been stimulated. LC3-II is the lipidated and autophagosome associated form of LC3. Lacritin thus appears to stimulate a very rapid form of autophagy in which the kinetics of LC3-II generation may be temporarily and transiently exceeded by degradation to swiftly restore homeostasis (Wang et al., 2013).
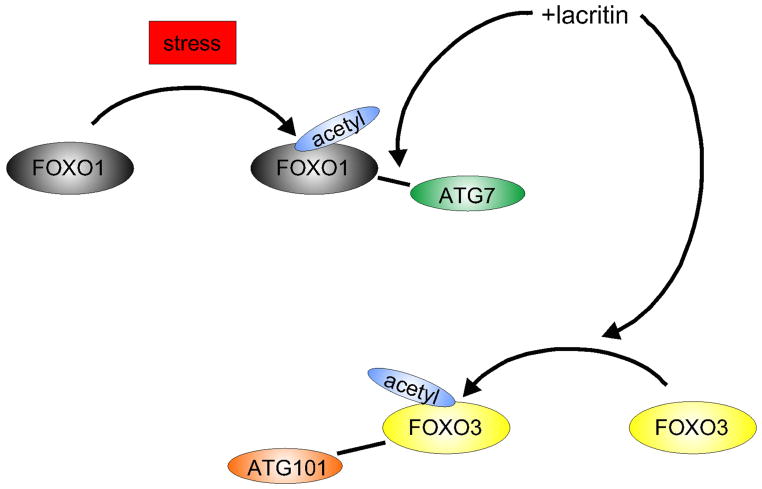
Lacritin dependent survival is not enhanced by mTOR inhibitor rapamycin, nor by class 1 PI3K inhibitor PI103, however the ‘dominant negative’ FOXO3 construct ‘FOXO3Nt’ (Fig. 5) promoted corneal epithelial survival and autophagy in the absence of lacritin (Wang et al., 2013). FOXO3Nt competitively inhibits nuclear DNA binding and cell death-associated transcription of endogenous FOXO3, thereby likely displacing FOXO3 into the cytoplasm. How might cytoplasmic FOXO3 promote autophagy? Zhao et al (2010) proposed that stress-associated acetylation of family member FOXO1 facilitates binding of autophagy mediator ATG7 to trigger autophagy (Fig. 1). In human corneal epithelial cells, stress indeed promoted FOXO1 acetylation, but unexpectedly not binding of ATG7. Only after lacritin, but not C-25, addition did acetylated FOXO1 ligate ATG7 – suggesting that further modification of FOXO1 is required (Fig. 6). Binding was detected as early as 5 min and was maximal at 15 min, thereafter decreasing to background at 60 min (Wang et al., 2013). FOXO3 was also subject to some stress-associated acetylation but most acetylation was entirely lacritin dependent, and did not prompt capture of ATG7. Instead it bound autophagy mediator ATG101 within 1 and 5 min after lacritin, but not C-25, addition (Figs. 1, 6). ATG101 stabilizes ATG13 as part of the ULK1 complex (Fig. 1) necessary for the initiation of autophagy (Hosokawa et al., 2009; Mercer et al., 2009). shRNA depletion of ATG101, ATG7 or FOXO3 abrogated lacritin dependent restoration of homeostasis (Wang et al., 2013).
Fig. 5.
Comparison of FOXO3 and dominant negative FOXO3Nt cDNA constructs. Dominant negative FOXO3Nt preserves the DNA binding domain and nuclear localization signal, but lacks the transactivation domain.
Fig. 6.
Lacritin prosurvival signaling. Lacritin drives coupling of stress acetylated FOXO1 with ATG7. Lacritin also promotes hyperacetylation of FOXO3 necessary for ligation of ATG101, and transient acceleration of autophagy (Wang et al., ’13). Macroautophagy (‘autophagy’) continually captures aged or damaged cellular material for lysosomal degradation and recycling. Advantage may be taken of autophagy to promote ocular health. Here we highlight several examples of dysfunctional ocular surface autophagy and approaches to restoring health.
2.5 Lacritin monomer stimulates mitochondrial fusion and restores oxidative phosphorylation
To examine whether lacritin stimulated autophagy benefits metabolism to restore homeostasis, basal oxygen consumption was monitored. Lacritin restored basal oxygen consumption as early as 8 min after addition. Spare respiratory capacity was also elevated, and was coincident with enhanced mitochondrial fusion, and metabolomic changes in 29 metabolites - some in keeping with acetylation (Wang et al., 2013). Spare respiratory capacity is the difference between basal and maximal oxygen consumption.
2.6 Negative regulation of tear lacritin by tissue transglutaminase (TGM2) and downregulation of active monomer in dry eye
Lacritin is subject to crosslinking by tissue transglutaminase (TGM2) in tears, thereby forming dimers and trimers detectable in immunoblots (Velez et al., 2013). Replication by spiking recombinant lacritin into lacritin depleted tears, or by mixing recombinant lacritin with purchased tissue transglutaminase (TGM2; (Velez et al., 2013) reveals respectively slow and very fast loss of lacritin monomer as it becomes covalently crosslinked between glutamine 106 and lysines 82 or 85 (numbering excludes lacritin’s signal peptide) to substantially interfere with ligation of co-receptor syndecan-1 that requires the glutamine 106 region for affinity (Ma et al., 2006; Zhang et al., 2013). Accordingly, lacritin dimers and trimers (also larger polymers) - unlike monomer - lack cell survival activity (Romano and Laurie, unpublished). Tissue transglutaminase is elevated in cultured human corneal epithelial cells under hyperosmolar stress common to dry eye (Chen et al., 2008), or under UVB stress (Tong et al., 2006), and in conjunctival impression cytologies from dry eye Sjögren’s syndrome patients (Aragona et al., 2015). Thus, lacritin monomer deficiency in dry eye tears may be a consequence of elevated tissue transglutaminase activity. Deficiency has been noted in tears from individuals with Sjögren’s syndrome (Vijmasi et al., 2014), aqueous deficient (Aluru et al., 2012) and contact lens-related (Nichols and Green-Church, 2009) dry eye. Deficiency has also been noted in tears from patients with blepharitis (Koo et al., 2005), a lid inflammatory condition often associated with evaporative dry eye (Mathers, 1993). Yet, total lacritin tear levels (monomer, dimer and trimer) appear to remain unaltered in dry eye. This complicates monitoring and excludes current ELISA’s, and apparently also cationic and reverse phase chromatography followed by mass spectrometry (Boehm et al., 2013; Zhou et al., 2009). On a cautionary note, MyBioSource lists ELISA kits targeting lacritin from camel, cat, cattle, chicken, dog, donkey, duck, fish, goat, horse, human, mouse, monkey, pigeon, pig, rabbit, rat, and sheep, although current genomic alignment by Ensembl (v. 79) supports the existence of only a fraction of these (Karnati et al., 2013). It is suggested that kit antigen and antibody be subjected to independent scrutiny with a collaborative antibody and recombinant lacritin. A library of human lacritin cDNA’s is now available from Addgene.
2.6 Lacritin splice variants and novel activities
Four splice variants (Fig. 3; three depicted) have been detected of which lacritin-a (‘lacritin’, the subject of all studies to date) is by far the most common. cDNA supporting lacritin-a derive largely from human lacrimal gland (currently 77 cDNA ((Ozyildirim et al., 2005); Aceview). Others derive from ‘pooled glandular’ (2), breast (1), breast tumor (1), and from ‘glandular pool – thyroid, parathyroid, adreneal cortex, pineal gland, submandibular gland’ (1; Aceview). One, four and one lacrimal gland cDNAs (Ozyildirim et al., 2005) respectively supports lacritin-b, -c and –d, with lacritin-d likely not translated (Aceview). Lacritin-b lacks S67IVEKSILLTE77, an α-helical region (McKown et al., 2014) without known function but with 82% sequence identity to ‘amino acid adenylation domain-containing protein’ and ‘thioester reductase’, respectively from Bacillus sp.7_6_55CFAA_CT2 and Bacillus cereus. In lacritin-c, S66KSLSLCQINNLEKSLAAGPHHTSTHRDKPGEKQVDSNS104 replaces K66SIVEKSILLTEQALAKAGKGMHGGVPGGKQFIENGSEFAQKLLKKFSLLKPWA119, the latter responsible for all known lacritin activities. Lacritin-c synthetic peptide EKSLAAGPHHTSTHRDKPGEKQV (‘lacrt-I3’) lacks prosurvival activity (Wang et al., 2013). No LACRT nonsense or missense mutations have yet been identified (Supplementary Table 1).
3. Other Autophagy ‘Eye Disease Genes’ of the Ocular Surface
Fourteen other autophagy ‘eye disease gene’ proteins are currently known to be associated with ocular surface pathology, including: arylsulfatase A, arylsulfatase B, ceroid-lipofuscinosis neuronal 3, endoglin, epilepsy progressive myoclonus type 2A, glucosidase beta acid, gap junction protein alpha 1, Kirsten rat sarcoma viral oncogene homolog, mucolipin 1, Niemann-Pick disease type C1, neurotrophic tyrosine kinase receptor type 1, optineurin, prion protein, and transforming growth factor beta-induced protein (Supplementary Table 1). Each is discussed below. Several are associated with lysosomal storage diseases. Optineurin and transforming growth factor beta-induced protein are respectively reviewed in detail by Yue and Kim in this issue.
3.1 Arylsulfatase A (ARSA)
Arylsulfatase A (54 kDa) is a lysosomal and extracellular enzyme responsible for the hydrolysis of glycosphingolipid cerebroside sulfate. Deficiency of arylsulfatase A underlies the lysosomal storage disease metachromatic leukodystrophy manifested by myelin sheath destruction of corneal sensory and ciliary nerves and of the optic nerve (Libert et al., 1979), and lipid accumulation in retinal ganglion cells (Libert et al., 1979) accompanied by retinal pigment epithelial degeneration (Weiter et al., 1980). Rescue has been successful in metachromatic leukodystrophic mice by systemic injection of normal ARSA cloned into an adenovirus vector (Miyake et al., 2014), and appears to be safe as an approach in humans (Zerah et al., 2015). It is thought that dysfunction of glycosphingolipid hydrolysis may disturb autophagosome fusion and autophagic flux (Sun and Grabowski, 2013). Indeed, lysosomal storage diseases are proposed to be ‘autophagy disorders’ in which autophagosome accumulation are associated with inadequate lysosomal fusion (Settembre et al., 2008). 140 nonsense or missense mutations have been documented in ARSA and 13 splice variants (Supplementary Table 1).
3.2 Arylsulfatase B (ARSB)
Arylsulfatase B (60 kDa) is a lysosomal enzyme necessary for the degradation of the glycosaminoglycan dermatan sulfate. Accumulation of dermatan sulfate is the basis of the pediatric lysosomal storage disease mucopolysaccharidosis type VI. In the eye, mucopolysaccharidosis type VI is manifested by corneal clouding, ocular hypertension, glaucoma and optic nerve atrophy (Pitz et al., 2009). Enyzme replacement therapy with idursulfase is approved for human use and is reported to be largely efficacious (Lampe et al., 2014). Hematopoietic cell transplantation (Aldenhoven et al., 2015) is also efficacious, and gene therapy is being developed (Ferla et al., 2015). Unfortunately, ocular benefit limited (Ganesh et al., 2013). 123 nonsense or missense mutations have been documented in ARSB and 4 splice variants (Supplementary Table 1).
3.3 Ceroid-lipofuscinosis, neuronal 3 (CLN3)
Mutations in evolutionarily conserved Ceroid-lipofuscinosis, neuronal 3 (48 kDa) are associated with juvenile-onset Batten disease, a lysosomal storage disease distinguished by an accumulation of ‘ceroid-lipofuscin’ in surface conjunctival (but not corneal) cells, and in ganglion and neuronal cells (Bensaoula et al., 2000) coupled with retinal degeneration (Sarpong et al., 2009). CLN3 codes for a widely expressed 438 amino acid long protein with 6 – 8 predicted transmembrane motifs (Ratajczak et al., 2014) associated with several organelles, including the Golgi apparatus, mitochondria, endosomes and lysosomes. Cells lacking CLN3 display constitutive activation of the Cdc42 pathway, thus altering actin dynamics and indirectly inhibiting fluid phase endocytic uptake (Schultz et al., 2014). In keeping with this mechanism and altered endosomal trafficking, maturation of autophagosomal compartments is not normal (Cao et al., 2006). 31 nonsense or missense mutations have been documented in CLN3 and 47 splice variants (Supplementary Table 1).
3.4 Endoglin (ENG)
Endoglin, a 70.6 kDa protein is a transmembrane constituent of the transforming growth factor beta receptor complex and in the eye is highly expressed throughout, including cornea and conjunctiva (NEIBank), particularly on vascular endothelial cells of trachoma conjunctiva (Abu El-Asrar et al., 2006), and on corneal mesenchymal stromal cells (Bray et al., 2014) that are considered to have stem-like properties (Hashmani et al., 2013). In choroidal neovascularization secondary to age-related macular degeneration, endothelial expression is increased (Grisanti et al., 2004). Endoglin is targeted by transforming growth factor family members and is associated with hereditary haemorrhagic telangiectasia characterized by persistent haemorrhage (McAllister et al., 1994). Endoglin released into sera in patients suffering from preeclampsia suppresses autophagy and promotes endothelial pathology (Nakashima et al., 2013), in keeping with abnormal connections between cerebral arteries and veins as a precursor to hemorrhage in individuals with elevated soluble endoglin (Chen et al., 2009). 141 nonsense or missense mutations have been documented and 14 splice variants (Supplementary Table 1).
3.5 Epilepsy, progressive myoclonus type 2A (EPM2A)
Epilepsy, progressive myoclonus type 2A encodes the 37 kDa glycogen and protein phosphatase ‘laforin’ that binds the E3 ligase malin, and when absent leads to the accumulation of glycogen inclusion bodies termed as lafora bodies characteristic of neurodegenerative myoclonic epilepsy. Lafora bodies have been reported in the retina of a patient with lafora disease (Berard-Badier et al., 1980). In mouse EPM2A knockout fibroblasts, mTOR-dependent autophagy is suppressed, hypothetically as a secondary effect of stress from cellular glycogen accumulation (Garyali et al., 2014). However, overexpression of laforin stimulates autophagy in COS-7 and SK-N-SH cells (Aguado et al., 2010). EPM2A is expressed in several different eye tissues including keratoconus cornea (NEIBank). 36 nonsense or missense mutations have been documented in EPM2A and 19 splice variants (Supplementary Table 1).
3.6 Glucosidase, beta, acid (GBA)
Glucosidase beta acid (60 kDa), also known as glucocerebrosidase, is a lysosomal enzyme responsible for cleavage of the glycosylsphingolipid glycosylceramide. Deficiency of glucosidase beta acid through mutation of GBA is the basis of Gaucher disease, the most common lysosomal storage disease (Osellame and Duchen, 2013). Gaucher disease is characterized by the lysosomal buildup of glycosylceramide (cerebroside) as a consequence of downregulated autophagy. Enzyme replacement replacement therapy with β-glucocerebrosidase is efficacious (Zimran, 2011). GBA mutation increases susceptibility to Parkinson’s disease. Corneal opacification has been noted in a single case study of anindividualhomozygous for GBA D409H (Guemes et al., 1998). GBA is expressed in cornea, retina, iris, and optic nerve (NEIBank), and widely in other tissues (NCBI Unigene). 326 nonsense or missense mutations have been documented in GBA and 28 splice variants (Supplementary Table 1).
3.7 Gap junction protein, alpha 1 (GJA1)
Gap junction protein, alpha 1 (43 kDa; also known as connexin 43) is a cell surface protein with four predicted transmembrane domains, a connexin domain and multiple C-terminal serine, threonine or tyrosine phosphorlyation sites. Gap junction protein alpha 1 plays an important role as a negative modulator of autophagosome biogenesis in cells that is independent of its role in gap junction signaling (Bejarano et al., 2014). Its does so by binding ATG16L1 together with elements of the phosphatidylinositol-3 kinase complex. Upon starvation, ATG14 promotes autophagic degradation of Gap junction protein alpha 1, thus releasing the autophagy block. Single missense mutation of GJA1, that can occur at multiple different sites, underlies oculodentodigital dysplasia (Paznekas et al., 2003). Ocular manifestations include microcornea, optic atrophy, glaucoma, cataracts, abnormalities of the iris and microophthalmia. 88 nonsense or missense mutations have been documented in GJA1 and 2 splice variants (Supplementary Table 1).
3.8 Kirsten rat sarcoma viral oncogene homolog (KRAS)
Kirsten rat sarcoma viral oncogene homolog (KRAS) encodes a 21 kDa GDP/GTP binding protein associated primarily with plasma membrane, or alternatively with mitochondria. Expression in the eye appears to be restricted to keratoconus cornea, optic nerve and retina (NEIBank), although widely expressed in other organs (Unigene). Missense mutations in KRAS are responsible for cardiofaciocutaneous syndrome distinguished by sparse or absence of eyebrows (Stark et al., 2012). KRAS mutation also underlies different forms of cancer, including pancreatic cancer in which cells surviving treatment are dependent on oxidative phosphorylation (Viale et al., 2014). Eight nonsense or missense mutations have been documented in KRAS and 4 splice variants (Supplementary Table 1).
3.9 Mucolipin 1 (MCOLN1)
Mucolipin is a 65 kDa lysosomal calcium channel responsible for lysosomal calcium release associated through calcineurin activation with nuclear translocation of transcription factor EB during starvation (Medina et al., 2015). Transcription factor EB, through transcription, promotes autophagy. Mutation of MCOLN1 underlies the lysosomal storage disease mucolipidosis IV, characterized by an abnormal accumulation of lipids with clinical manifestation in neurodegenerative and ophthalmological disorders, including corneal clouding, decreased visual acuity and retinal dystrophy (Dobrovolny et al., 2007; Goldin et al., 2008). NEIBank detects expression in retina and lens. 15 nonsense or missense mutations have been documented in MCOLN1 and 38 splice variants (Supplementary Table 1).
3.10 Niemann-Pick disease, type C1 (NPC1)
Niemann-Pick disease type C1 is a 142 kDa late endosomal/lysosomal transmembrane protein important in cholesterol trafficking, metabolism and storage, and possibly in the genesis of autophagosomes as a regulator of basal autophagic flux (Sarkar et al., 2014). Niemann-Pick disease type C1 is expressed in normal and keratoconus cornea, retina, and optic nerve (NEIBank), and in other organs widely (NCBI Unigene). When mutated, autophagic flux becomes suboptimal and cholesterol accumulates giving rise to the lysosomal storage disease Niemann-Pick type C associated with neurodegeneration, retinal degeneration (Claudepierre et al., 2010) and slow vertical eye movements (Salsano et al., 2012). Lysosomal substrate reduction therapy is available for Niemann-Pick type C disease that is mild to moderate (Henley et al., 2014). Although corneal involvement is uncommon, cornea of NPC1−/− mice display inclusions (Hovakimyan et al., 2011). 255 nonsense or missense NPC1 mutations have been documented, as well as 14 splice variants (Supplementary Table 1).
3.11 Neurotrophic tyrosine kinase, receptor, type 1 (NTRK1)
Cell surface neurotrophic tyrosine kinase-1 receptor type 1 (88 kDa) is targeted by neurotrophins such as neurotrophin 3 (present in human tears (Karnati et al., 2013)) and nerve growth factor in the promotion of neuronal cell survival, differentiation, axonal growth and innervation (Berard-Badier et al., 1980; Carter and Lewin, 1997). Neurotrophic tyrosine kinase-1 receptor type 1 is expressed in normal not keratoconus cornea, the latter coincident with elevated levels of the repressive short isoform of the Sp3 transcription factor (Lambiase et al., 2005). Expression has also been noted on B lymphocytes and monocytes, in keeping with nerve growth factor dependent stimulation of inflammatory cytokines in inflammation (Prencipe et al., 2014). Loss of neurotrophic tyrosine kinase-1 receptor type 1 expression in brain cortical samples correlates with the development of Alzheimer’s disease (Counts et al., 2004). Despite its link with survival, ectopic expression of neurotrophic tyrosine kinase-1 receptor type 1 in a glioblastoma line that otherwise lacks neurotrophic tyrosine kinase-1 receptor type 1 subjects the cells to nerve growth factor-mediated autophagy that is associated with cell death (Hansen et al., 2007). Similarily, overexpression of neurotrophic tyrosine kinase-1 receptor type 1 in a neuroepithelioma cell line promotes apoptotic cell death, and in an osteosarcoma cell line that then assumes neuronal features, both apoptotic and autophagic cell death are observed (Dadakhujaev et al., 2009). 11 splice variants and 43 non-synonymous NTRK1 point mutations have been reported - most associated with congenital insensitivity to pain with anhidrosis (Supplemental Table 1).
3.12 Optineurin (OPTN)
Optineurin is the subject of a full review in this issue by Yue. Optineurin is a 66 kDa adaptor protein with many binding partners (NCBI Gene). Ocular expression has been noted in keratoconus cornea, the trabecular meshwork, iris, lens and retina (NEIBank), and is widely expressed in other other organs (Unigene). Among different roles, optineurin targeting of damaged, ubiquitinated mitochondria recruits LC3 for autophagosome envelopment and lysosomal degradation (Wong and Holzbaur, 2015). 24 splice varients and 31 non-synonymous mutations have been reported to date (Supplementary Table 1), with association to normal tension and primary open angle glaucoma (Rezaie et al., 2002; Sarfarazi et al., 1998), and to amyotrophic lateral sclerosis (Maruyama et al., 2010).
3.13 Prion protein (PRNP)
Prion protein (28 kDa) is a glycosylphosphatidylinositol anchored cell surface protein in lipid rafts that is well-known for its expression in the central nervous system, although also present widelyin other organs (Unigene). Ocular expression has been detected in keratoconus cornea, as well as normal lens, retina, iris and optic nerve (NEIBank). Prion protein is necessary to bind and draw beclin 1 into lipid rafts where in the context of external stress it can associate with PIK3C3 to initiate autophagy and promote neuronal survival (Nah et al., 2013). Similarly, in herpes simplex virus infected astrocytes from PRNP knockout mice, autophagosome density post starvation is less (Korom et al., 2013). In some cells prion protein appears to promote viability by suppressing autophagy (Barbieri et al., 2011). Protease resistant prion protein is neurotoxic (Aguzzi and Calella, 2009). Whether these roles are played out in ocular surface epithelia is not known. Prion protein binds to many different cellular proteins. To date 8 PRNP splice variants and 59 nonsense or missense mutations have been reported (Supplementary Table 1), including E200K at a prevalency of 0.4% of the general Slovakian population in a corneal donor screen. E200K carriers are at higher risk of eye movement abnormalities (Panegyres et al., 2012) and Creutzfeldt-Jakob disease (Mitrova et al., 2011).
3.14 Transforming growth factor, beta-induced (TGFBI)
Transforming growth factor, beta-induced is the subject of a full review in this issue by Kim. Transforming growth factor, beta-induced is a 68 kDa extracellular matrix protein in cornea (NEIBank), and widely expressed in other organs (Unigene), where it binds collagens I, II and IV, fibronectin, and α-2-macroglobin (Billings et al., 2002) and cells via integrin αvβ5 (Kim et al., 2002a; Kim et al., 2002b). Corneal stromal accumulation of R124H mutated transforming growth factor beta-induced from insufficient autophagic removal underlies progressive loss of vision in granular corneal dystrophy type 2 (Choi et al., 2012). Other TGFBI mutations (R124C, R124L, R124S, A546D, P501T, L509R, F540S, P551Q, R555W, G623D, R666S) are associated with different forms of corneal dystrophy. Fourteen splice variants and 53 non-synonymous mutations have been reported (Supplementary Table 1).
5. Conclusions
Dysfunctional autophagy underlies several ocular diseases – possibly including dry eye. Tear protein lacritin stimulates autophagy, and yet - in its active monomeric form - appears to be selectively deficient in dry eye tears due to tissue transglutaminase cross-linking. Several autophagy diseases are now treatable with systemic enzyme replacement therapy, although ocular benefits are limited. Gene therapy approaches are in development.
Supplementary Material
Autophagy-associated genes and proteins of the normal human eye with the number of ‘eye’ transcripts per 106 from Unigene, splice variants from Aceview and missense or nonsense mutations from the Human Gene Mutation Database. Bold, underline indicates NEIBank ‘eye disease genes’. Bold, italics, underline indicate NEIBank eye disease genes associated with ocular surface pathology – each as further discussed in the text.
Acknowledgments
Grant information: NIH RO1 R01 EY024327 (GWL), SR/FT/LS-157/2012 (RK)
GWL is supported by NIH R01 EY024327. RK is supported by SR/FT/LS-157/2012. The authors acknowledge the multi-institutional Lacritin Consortium for help with much of the lacritin work reviewed.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abu El-Asrar AM, Al-Kharashi SA, Missotten L, Geboes K. Expression of growth factors in the conjunctiva from patients with active trachoma. Eye. 2006;20:362–369. doi: 10.1038/sj.eye.6701884. [DOI] [PubMed] [Google Scholar]
- Aguado C, Sarkar S, Korolchuk VI, Criado O, Vernia S, Boya P, Sanz P, de Cordoba SR, Knecht E, Rubinsztein DC. Laforin, the most common protein mutated in Lafora disease, regulates autophagy. Hum Mol Gen. 2010;19:2867–2876. doi: 10.1093/hmg/ddq190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguzzi A, Calella AM. Prions: protein aggregation and infectious diseases. Physiolog Rev. 2009;89:1105–1152. doi: 10.1152/physrev.00006.2009. [DOI] [PubMed] [Google Scholar]
- Aldenhoven M, Jones SA, Bonney D, Borrill RE, Coussons M, Mercer J, Bierings MB, Versluys B, van Hasselt PM, Wijburg FA, van der Ploeg AT, Wynn RF, Boelens JJ. Hematopoietic Cell Transplantation for Mucopolysaccharidosis Patients Is Safe and Effective: Results after Implementation of International Guidelines. Biology of blood and marrow transplantation. Biol Blood Marrow Transplant 2015. 2015 Feb 10; doi: 10.1016/j.bbmt.2015.02.011. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Aluru SV, Agarwal S, Srinivasan B, Iyer GK, Rajappa SM, Tatu U, Padmanabhan P, Subramanian N, Narayanasamy A. Lacrimal proline rich 4 (LPRR4) protein in the tear fluid is a potential biomarker of dry eye syndrome. PloS One. 2012;7:e51979. doi: 10.1371/journal.pone.0051979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona P, Aguennouz M, Rania L, Postorino E, Sommario MS, Roszkowska AM, De Pasquale MG, Pisani A, Puzzolo D. Matrix metalloproteinase 9 and transglutaminase 2 expression at the ocular surface in patients with different forms of dry eye disease. Ophthalmol. 2015;122:62–71. doi: 10.1016/j.ophtha.2014.07.048. [DOI] [PubMed] [Google Scholar]
- Barbieri G, Palumbo S, Gabrusiewicz K, Azzalin A, Marchesi N, Spedito A, Biggiogera M, Sbalchiero E, Mazzini G, Miracco C, Pirtoli L, Kaminska B, Comincini S. Silencing of cellular prion protein (PrPC) expression by DNA-antisense oligonucleotides induces autophagy-dependent cell death in glioma cells. Autophagy. 2011;7:840–853. doi: 10.4161/auto.7.8.15615. [DOI] [PubMed] [Google Scholar]
- Bejarano E, Yuste A, Patel B, Stout RF, Jr, Spray DC, Cuervo AM. Connexins modulate autophagosome biogenesis. Nat Cell Biol. 2014;16:401–414. doi: 10.1038/ncb2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensaoula T, Shibuya H, Katz ML, Smith JE, Johnson GS, John SK, Milam AH. Histopathologic and immunocytochemical analysis of the retina and ocular tissues in Batten disease. Ophthalmol. 2000;107:1746–1753. doi: 10.1016/s0161-6420(00)00264-5. [DOI] [PubMed] [Google Scholar]
- Berard-Badier M, Pellissier JF, Gambarelli D, de Barsy T, Roger J, Toga M. The retina in Lafora disease: light and electron microscopy. Albrecht von Graefes Archiv Klin Exp Ophthalmol. 1980;212:285–294. doi: 10.1007/BF00410522. [DOI] [PubMed] [Google Scholar]
- Billings PC, Whitbeck JC, Adams CS, Abrams WR, Cohen AJ, Engelsberg BN, Howard PS, Rosenbloom J. The transforming growth factor-beta-inducible matrix protein (beta)ig-h3 interacts with fibronectin. J Biol Chem. 2002;277:28003–28009. doi: 10.1074/jbc.M106837200. [DOI] [PubMed] [Google Scholar]
- Boehm N, Funke S, Wiegand M, Wehrwein N, Pfeiffer N, Grus FH. Alterations in the tear proteome of dry eye patients--a matter of the clinical phenotype. Invest Ophthalmol Vis Sci. 2013;54:2385–2392. doi: 10.1167/iovs.11-8751. [DOI] [PubMed] [Google Scholar]
- Bray LJ, Heazlewood CF, Munster DJ, Hutmacher DW, Atkinson K, Harkin DG. Immunosuppressive properties of mesenchymal stromal cell cultures derived from the limbus of human and rabbit corneas. Cytotherapy. 2014;16:64–73. doi: 10.1016/j.jcyt.2013.07.006. [DOI] [PubMed] [Google Scholar]
- Cao Y, Espinola JA, Fossale E, Massey AC, Cuervo AM, MacDonald ME, Cotman SL. Autophagy is disrupted in a knock-in mouse model of juvenile neuronal ceroid lipofuscinosis. J Biol Chem. 2006;281:20483–20493. doi: 10.1074/jbc.M602180200. [DOI] [PubMed] [Google Scholar]
- Carter BD, Lewin GR. Neurotrophins live or let die: does p75NTR decide? Neuron. 1997;18:187–190. doi: 10.1016/s0896-6273(00)80259-7. [DOI] [PubMed] [Google Scholar]
- Chen Y, Hao Q, Kim H, Su H, Letarte M, Karumanchi SA, Lawton MT, Barbaro NM, Yang GY, Young WL. Soluble endoglin modulates aberrant cerebral vascular remodeling. Ann Neurol. 2009;66:19–27. doi: 10.1002/ana.21710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Sawada O, Kohno H, Le YZ, Subauste C, Maeda T, Maeda A. Autophagy protects the retina from light-induced degeneration. J Biol Chem. 2013;288:7506–7518. doi: 10.1074/jbc.M112.439935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Tong L, Li Z, Yoon KC, Qi H, Farley W, Li DQ, Pflugfelder SC. Hyperosmolarity-induced cornification of human corneal epithelial cells is regulated by JNK MAPK. Invest 0phthalmol Vis Sci. 2008;49:539–549. doi: 10.1167/iovs.07-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodosh J, Dix RD, Howell RC, Stroop WG, Tseng SC. Staining characteristics and antiviral activity of sulforhodamine B and lissamine green B. Invest 0phthalmol Vis Sci. 1994;35:1046–1058. [PubMed] [Google Scholar]
- Choi SI, Kim BY, Dadakhujaev S, Oh JY, Kim TI, Kim JY, Kim EK. Impaired autophagy and delayed autophagic clearance of transforming growth factor beta-induced protein (TGFBI) in granular corneal dystrophy type 2. Autophagy. 2012;8:1782–1797. doi: 10.4161/auto.22067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claudepierre T, Paques M, Simonutti M, Buard I, Sahel J, Maue RA, Picaud S, Pfrieger FW. Lack of Niemann-Pick type C1 induces age-related degeneration in the mouse retina. Molec Cell Neurosci. 2010;43:164–176. doi: 10.1016/j.mcn.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Counts SE, Nadeem M, Wuu J, Ginsberg SD, Saragovi HU, Mufson EJ. Reduction of cortical TrkA but not p75(NTR) protein in early-stage Alzheimer’s disease. Ann Neurol. 2004;56:520–531. doi: 10.1002/ana.20233. [DOI] [PubMed] [Google Scholar]
- Dadakhujaev S, Jung EJ, Noh HS, Hah YS, Kim CJ, Kim DR. Interplay between autophagy and apoptosis in TrkA-induced cell death. Autophagy. 2009;5:103–105. doi: 10.4161/auto.5.1.7276. [DOI] [PubMed] [Google Scholar]
- Dobrovolny R, Liskova P, Ledvinova J, Poupetova H, Asfaw B, Filipec M, Jirsova K, Kraus J, Elleder M. Mucolipidosis IV: report of a case with ocular restricted phenotype caused by leaky splice mutation. Am J Ophthalmol. 2007;143:663–671. doi: 10.1016/j.ajo.2006.11.049. [DOI] [PubMed] [Google Scholar]
- Ferla R, Claudiani P, Savarese M, Kozarsky K, Parini R, Scarpa M, Donati MA, Sorge G, Hopwood JJ, Parenti G, Fecarotta S, Nigro V, Sivri HS, Van Der Ploeg A, Andria G, Brunetti-Pierri N, Auricchio A. Prevalence of Anti-Adeno-Associated Virus Serotype 8 Neutralizing Antibodies and Arylsulfatase B Cross-Reactive Immunologic Material in Mucopolysaccharidosis VI Patient Candidates for a Gene Therapy Trial. Hum Gene Ther. 2015;26:145–152. doi: 10.1089/hum.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii A, Morimoto-Tochigi A, Walkup RD, Shearer TR, Azuma M. Lacritin-induced secretion of tear proteins from cultured monkey lacrimal acinar cells. Invest Ophthalmol Vis Sci. 2013;54:2533–2540. doi: 10.1167/iovs.12-10394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Pietrocola F, Levine B, Kroemer G. Metabolic control of autophagy. Cell. 2014;159:1263–1276. doi: 10.1016/j.cell.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesh A, Bruwer Z, Al-Thihli K. An update on ocular involvement in mucopolysaccharidoses. Cur Opin Ophthalmol. 2013;24:379–388. doi: 10.1097/ICU.0b013e3283644ea1. [DOI] [PubMed] [Google Scholar]
- Garyali P, Segvich DM, DePaoli-Roach AA, Roach PJ. Protein degradation and quality control in cells from laforin and malin knockout mice. J Biol Chem. 2014;289:20606–20614. doi: 10.1074/jbc.M114.580167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin E, Caruso RC, Benko W, Kaneski CR, Stahl S, Schiffmann R. Isolated ocular disease is associated with decreased mucolipin-1 channel conductance. Invest Ophthalmol Vis Sci. 2008;49:3134–3142. doi: 10.1167/iovs.07-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisanti S, Canbek S, Kaiserling E, Adam A, Lafaut B, Gelisken F, Szurman P, Henke-Fahle S, Oficjalska-Mlynczak J, Bartz-Schmidt KU. Expression of endoglin in choroidal neovascularization. Exp Eye Res. 2004;78:207–213. doi: 10.1016/j.exer.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Guemes A, Kosmorsky GS, Moodie DS, Clark B, Meisler D, Traboulsi EI. Corneal opacities in Gaucher disease. Am J Ophthalmol. 1998;126:833–835. doi: 10.1016/s0002-9394(98)00249-9. [DOI] [PubMed] [Google Scholar]
- Hansen K, Wagner B, Hamel W, Schweizer M, Haag F, Westphal M, Lamszus K. Autophagic cell death induced by TrkA receptor activation in human glioblastoma cells. J Neurochem. 2007;103:259–275. doi: 10.1111/j.1471-4159.2007.04753.x. [DOI] [PubMed] [Google Scholar]
- Hashmani K, Branch MJ, Sidney LE, Dhillon PS, Verma M, McIntosh OD, Hopkinson A, Dua HS. Characterization of corneal stromal stem cells with the potential for epithelial transdifferentiation. Stem Cell Res Ther. 2013;4:75. doi: 10.1186/scrt226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley WE, Anderson LJ, Wyatt KM, Nikolaou V, Anderson R, Logan S. The NCS-LSD cohort study: a description of the methods and analyses used to assess the long-term effectiveness of enzyme replacement therapy and substrate reduction therapy in patients with lysosomal storage disorders. J Inherit Metab Dis. 2014;37:939–944. doi: 10.1007/s10545-014-9679-6. [DOI] [PubMed] [Google Scholar]
- Hosokawa N, Sasaki T, Iemura S, Natsume T, Hara T, Mizushima N. Atg101, a novel mammalian autophagy protein interacting with Atg13. Autophagy. 2009;5:973–979. doi: 10.4161/auto.5.7.9296. [DOI] [PubMed] [Google Scholar]
- Hovakimyan M, Stachs O, Reichard M, Mascher H, Lukas J, Frech MJ, Guthoff R, Witt M, Rolfs A, Wree A. Morphological alterations of the cornea in the mouse model of niemann-pick disease type c1. Cornea. 2011;30:796–803. doi: 10.1097/ICO.0b013e3182012a33. [DOI] [PubMed] [Google Scholar]
- Hu MC, Lee DF, Xia W, Golfman LS, Ou-Yang F, Yang JY, Zou Y, Bao S, Hanada N, Saso H, Kobayashi R, Hung MC. IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell. 2004;117:225–237. doi: 10.1016/s0092-8674(04)00302-2. [DOI] [PubMed] [Google Scholar]
- Karnati R, Laurie DE, Laurie GW. Lacritin and the tear proteome as natural replacement therapy for dry eye. Exp Eye Res. 2013;117:39–52. doi: 10.1016/j.exer.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JE, Jeong HW, Nam JO, Lee BH, Choi JY, Park RW, Park JY, Kim IS. Identification of motifs in the fasciclin domains of the transforming growth factor-beta-induced matrix protein betaig-h3 that interact with the alphavbeta5 integrin. J Biol Chem. 2002a;277:46159–46165. doi: 10.1074/jbc.M207055200. [DOI] [PubMed] [Google Scholar]
- Kim JE, Park RW, Choi JY, Bae YC, Kim KS, Joo CK, Kim IS. Molecular properties of wild-type and mutant betaIG-H3 proteins. Invest Ophthal Vis Sci. 2002b;43:656–661. [PubMed] [Google Scholar]
- Klionsky DJ, Cregg JM, Dunn WA, Jr, Emr SD, Sakai Y, Sandoval IV, Sibirny A, Subramani S, Thumm M, Veenhuis M, Ohsumi Y. A unified nomenclature for yeast autophagy-related genes. Develop Cell. 2003;5:539–545. doi: 10.1016/s1534-5807(03)00296-x. [DOI] [PubMed] [Google Scholar]
- Knoferle J, Koch JC, Ostendorf T, Michel U, Planchamp V, Vutova P, Tonges L, Stadelmann C, Bruck W, Bahr M, Lingor P. Mechanisms of acute axonal degeneration in the optic nerve in vivo. Proc Nat Acad Sci USA. 2010;107:6064–6069. doi: 10.1073/pnas.0909794107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo BS, Lee DY, Ha HS, Kim JC, Kim CW. Comparative analysis of the tear protein expression in blepharitis patients using two-dimensional electrophoresis. J Prot Res. 2005;4:719–724. doi: 10.1021/pr0498133. [DOI] [PubMed] [Google Scholar]
- Korom M, Wylie KM, Wang H, Davis KL, Sangabathula MS, Delassus GS, Morrison LA. A proautophagic antiviral role for the cellular prion protein identified by infection with a herpes simplex virus 1 ICP34.5 mutant. J Virol. 2013;87:5882–5894. doi: 10.1128/JVI.02559-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambiase A, Merlo D, Mollinari C, Bonini P, Rinaldi AM, MDA, Micera A, Coassin M, Rama P, Bonini S, Garaci E. Molecular basis for keratoconus: lack of TrkA expression and its transcriptional repression by Sp3. Proc Nat Acad Sci USA. 2005;102:16795–16800. doi: 10.1073/pnas.0508516102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe C, Atherton A, Burton BK, Descartes M, Giugliani R, Horovitz DD, Kyosen SO, Magalhaes TS, Martins AM, Mendelsohn NJ, Muenzer J, Smith LD. Enzyme Replacement Therapy in Mucopolysaccharidosis II Patients Under 1 Year of Age. JIMD Rep. 2014;14:99–113. doi: 10.1007/8904_2013_289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leib DA, Alexander DE, Cox D, Yin J, Ferguson TA. Interaction of ICP34.5 with Beclin 1 modulates herpes simplex virus type 1 pathogenesis through control of CD4+ T-cell responses. J Virol. 2009;83:12164–12171. doi: 10.1128/JVI.01676-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert J, Van Hoof F, Toussaint D, Roozitalab H, Kenyon KR, Green WR. Ocular findings in metachromatic leukodystrophy. An electron microscopic and enzyme study in different clinical and genetic variants. Arch Ophthal. 1979;97:1495–1504. doi: 10.1001/archopht.1979.01020020157015. [DOI] [PubMed] [Google Scholar]
- Luo L, Li DQ, Doshi A, Farley W, Corrales RM, Pflugfelder SC. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthal Vis Sci. 2004;45:4293–4301. doi: 10.1167/iovs.03-1145. [DOI] [PubMed] [Google Scholar]
- Ma P, Beck SL, Raab RW, McKown RL, Coffman GL, Utani A, Chirico WJ, Rapraeger AC, Laurie GW. Heparanase deglycanation of syndecan-1 is required for binding of the epithelial-restricted prosecretory mitogen lacritin. The J Cell Biol. 2006;174:1097–1106. doi: 10.1083/jcb.200511134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama H, Morino H, Ito H, Izumi Y, Kato H, Watanabe Y, Kinoshita Y, Kamada M, Nodera H, Suzuki H, Komure O, Matsuura S, Kobatake K, Morimoto N, Abe K, Suzuki N, Aoki M, Kawata A, Hirai T, Kato T, Ogasawara K, Hirano A, Takumi T, Kusaka H, Hagiwara K, Kaji R, Kawakami H. Mutations of optineurin in amyotrophic lateral sclerosis. Nature. 2010;465:223–226. doi: 10.1038/nature08971. [DOI] [PubMed] [Google Scholar]
- Mathers WD. Ocular evaporation in meibomian gland dysfunction and dry eye. Ophthal. 1993;100:347–351. doi: 10.1016/s0161-6420(93)31643-x. [DOI] [PubMed] [Google Scholar]
- McAllister KA, Grogg KM, Johnson DW, Gallione CJ, Baldwin MA, Jackson CE, Helmbold EA, Markel DS, McKinnon WC, Murrell J, et al. Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat Gen. 1994;8:345–351. doi: 10.1038/ng1294-345. [DOI] [PubMed] [Google Scholar]
- McKown RL, Coleman Frazier EV, Zadrozny KK, Deleault AM, Raab RW, Ryan DS, Sia RK, Lee JK, Laurie GW. A cleavage-potentiated fragment of tear lacritin is bactericidal. J Biol Chem. 2014;289:22172–22182. doi: 10.1074/jbc.M114.570143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina DL, Di Paola S, Peluso I, Armani A, De Stefani D, Venditti R, Montefusco S, Scotto-Rosato A, Prezioso C, Forrester A, Settembre C, Wang W, Gao Q, Xu H, Sandri M, Rizzuto R, De Matteis MA, Ballabio A. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat Cell Biol. 2015;17:288–299. doi: 10.1038/ncb3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer CA, Kaliappan A, Dennis PB. A novel, human Atg13 binding protein, Atg101, interacts with ULK1 and is essential for macroautophagy. Autophagy. 2009;5:649–662. doi: 10.4161/auto.5.5.8249. [DOI] [PubMed] [Google Scholar]
- Mitrova E, Cernak A, Slivarichova D, Koscova S, Bernovska V, Cernak M. Experience with preventive genetic testing of corneal donors in Slovakia. Cornea. 2011;30:987–990. doi: 10.1097/ICO.0b013e3182035ac1. [DOI] [PubMed] [Google Scholar]
- Miyake N, Miyake K, Asakawa N, Yamamoto M, Shimada T. Long-term correction of biochemical and neurological abnormalities in MLD mice model by neonatal systemic injection of an AAV serotype 9 vector. Gene Ther. 2014;21:427–433. doi: 10.1038/gt.2014.17. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Noda T, Ohsumi Y. Apg16p is required for the function of the Apg12p-Apg5p conjugate in the yeast autophagy pathway. EMBO J. 1999;18:3888–3896. doi: 10.1093/emboj/18.14.3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Noda T, Yoshimori T, Tanaka Y, Ishii T, George MD, Klionsky DJ, Ohsumi M, Ohsumi Y. A protein conjugation system essential for autophagy. Nature. 1998;395:395–398. doi: 10.1038/26506. [DOI] [PubMed] [Google Scholar]
- Morishita H, Eguchi S, Kimura H, Sasaki J, Sakamaki Y, Robinson ML, Sasaki T, Mizushima N. Deletion of autophagy-related 5 (Atg5) and Pik3c3 genes in the lens causes cataract independent of programmed organelle degradation. J Biol Chem. 2013;288:11436–11447. doi: 10.1074/jbc.M112.437103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nah J, Pyo JO, Jung S, Yoo SM, Kam TI, Chang J, Han J, Soo AAS, Onodera T, Jung YK. BECN1/Beclin 1 is recruited into lipid rafts by prion to activate autophagy in response to amyloid beta 42. Autophagy. 2013;9:2009–2021. doi: 10.4161/auto.26118. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Walkup RD, Tochigi A, Shearer TR, Azuma M. Establishment of an appropriate animal model for lacritin studies: cloning and characterization of lacritin in monkey eyes. Exp Eye Res. 2007;85:651–658. doi: 10.1016/j.exer.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Nakashima A, Yamanaka-Tatematsu M, Fujita N, Koizumi K, Shima T, Yoshida T, Nikaido T, Okamoto A, Yoshimori T, Saito S. Impaired autophagy by soluble endoglin, under physiological hypoxia in early pregnant period, is involved in poor placentation in preeclampsia. Autophagy. 2013;9:303–316. doi: 10.4161/auto.22927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols JJ, Green-Church KB. Mass spectrometry-based proteomic analyses in contact lens-related dry eye. Cornea. 2009;28:1109–1117. doi: 10.1097/ICO.0b013e3181a2ad81. [DOI] [PubMed] [Google Scholar]
- Osellame LD, Duchen MR. Defective quality control mechanisms and accumulation of damaged mitochondria link Gaucher and Parkinson diseases. Autophagy. 2013;9:1633–1635. doi: 10.4161/auto.25878. [DOI] [PubMed] [Google Scholar]
- Ozyildirim AM, Wistow GJ, Gao J, Wang J, Dickinson DP, Frierson HF, Jr, Laurie GW. The lacrimal gland transcriptome is an unusually rich source of rare and poorly characterized gene transcripts. Invest Ophthal Vis Sci. 2005;46:1572–1580. doi: 10.1167/iovs.04-1380. [DOI] [PubMed] [Google Scholar]
- Panegyres PK, Goh JG, Goldblatt J. Codon 200 mutation of the prion gene: genotype-phenotype correlations. J Neurol. 2012;259:2579–2584. doi: 10.1007/s00415-012-6539-x. [DOI] [PubMed] [Google Scholar]
- Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, Overvatn A, Bjorkoy G, Johansen T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282:24131–24145. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- Paznekas WA, Boyadjiev SA, Shapiro RE, Daniels O, Wollnik B, Keegan CE, Innis JW, Dinulos MB, Christian C, Hannibal MC, Jabs EW. Connexin 43 (GJA1) mutations cause the pleiotropic phenotype of oculodentodigital dysplasia. Am J Hum Gen. 2003;72:408–418. doi: 10.1086/346090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitz S, Ogun O, Arash L, Miebach E, Beck M. Does enzyme replacement therapy influence the ocular changes in type VI mucopolysaccharidosis? Albrecht von Graefes Arch Kin Exp Ophthal. 2009;247:975–980. doi: 10.1007/s00417-008-1030-1. [DOI] [PubMed] [Google Scholar]
- Porter K, Nallathambi J, Lin Y, Liton PB. Lysosomal basification and decreased autophagic flux in oxidatively stressed trabecular meshwork cells: implications for glaucoma pathogenesis. Autophagy. 2013;9:581–594. doi: 10.4161/auto.23568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prencipe G, Minnone G, Strippoli R, De Pasquale L, Petrini S, Caiello I, Manni L, De Benedetti F, Bracci-Laudiero L. Nerve growth factor downregulates inflammatory response in human monocytes through TrkA. J Immunol. 2014;192:3345–3354. doi: 10.4049/jimmunol.1300825. [DOI] [PubMed] [Google Scholar]
- Ratajczak E, Petcherski A, Ramos-Moreno J, Ruonala MO. FRET- assisted determination of CLN3 membrane topology. PloS One. 2014;9:e102593. doi: 10.1371/journal.pone.0102593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaie T, Child A, Hitchings R, Brice G, Miller L, Coca-Prados M, Heon E, Krupin T, Ritch R, Kreutzer D, Crick RP, Sarfarazi M. Adult-onset primary open-angle glaucoma caused by mutations in optineurin. Science. 2002;295:1077–1079. doi: 10.1126/science.1066901. [DOI] [PubMed] [Google Scholar]
- Salsano E, Umeh C, Rufa A, Pareyson D, Zee DS. Vertical supranuclear gaze palsy in Niemann-Pick type C disease. Neurol Scien. 2012;33:1225–1232. doi: 10.1007/s10072-012-1155-1. [DOI] [PubMed] [Google Scholar]
- Samudre S, Lattanzio FA, Jr, Lossen V, Hosseini A, Sheppard JD, Jr, McKown RL, Laurie GW, Williams PB. Lacritin, a novel human tear glycoprotein, promotes sustained basal tearing and is well tolerated. Invest Ophthal Vis Scien. 2011;52:6265–6270. doi: 10.1167/iovs.10-6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanghi S, Kumar R, Lumsden A, Dickinson D, Klepeis V, Trinkaus-Randall V, Frierson HF, Jr, Laurie GW. cDNA and genomic cloning of lacritin, a novel secretion enhancing factor from the human lacrimal gland. J Mol Biol. 2001;310:127–139. doi: 10.1006/jmbi.2001.4748. [DOI] [PubMed] [Google Scholar]
- Sarfarazi M, Child A, Stoilova D, Brice G, Desai T, Trifan OC, Poinoosawmy D, Crick RP. Localization of the fourth locus (GLC1E) for adult-onset primary open-angle glaucoma to the 10p15-p14 region. Am J Hum Gen. 1998;62:641–652. doi: 10.1086/301767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Maetzel D, Korolchuk VI, Jaenisch R. Restarting stalled autophagy a potential therapeutic approach for the lipid storage disorder, Niemann-Pick type C1 disease. Autophagy. 2014;10:1137–1140. doi: 10.4161/auto.28623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarpong A, Schottmann G, Ruther K, Stoltenburg G, Kohlschutter A, Hubner C, Schuelke M. Protracted course of juvenile ceroid lipofuscinosis associated with a novel CLN3 mutation (p.Y199X) Clin Gen. 2009;76:38–45. doi: 10.1111/j.1399-0004.2009.01179.x. [DOI] [PubMed] [Google Scholar]
- Schultz ML, Tecedor L, Stein CS, Stamnes MA, Davidson BL. CLN3 deficient cells display defects in the ARF1-Cdc42 pathway and actin-dependent events. PloS One. 2014;9:e96647. doi: 10.1371/journal.pone.0096647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre C, Fraldi A, Jahreiss L, Spampanato C, Venturi C, Medina D, de Pablo R, Tacchetti C, Rubinsztein DC, Ballabio A. A block of autophagy in lysosomal storage disorders. Hum Mol Gen. 2008;17:119–129. doi: 10.1093/hmg/ddm289. [DOI] [PubMed] [Google Scholar]
- Shen X, Ying H, Qiu Y, Park JS, Shyam R, Chi ZL, Iwata T, Yue BY. Processing of optineurin in neuronal cells. J Biol Chem. 2011;286:3618–3629. doi: 10.1074/jbc.M110.175810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirohi K, Chalasani ML, Sudhakar C, Kumari A, Radha V, Swarup G. M98K-OPTN induces transferrin receptor degradation and RAB12-mediated autophagic death in retinal ganglion cells. Autophagy. 2013;9:510–527. doi: 10.4161/auto.23458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S, Thangavelu M, Zhang L, Green KB, Nichols KK. iTRAQ quantitative proteomics in the analysis of tears in dry eye patients. Invest Ophthal Vis Sci. 2012;53:5052–5059. doi: 10.1167/iovs.11-9022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark Z, Gillessen-Kaesbach G, Ryan MM, Cirstea IC, Gremer L, Ahmadian MR, Savarirayan R, Zenker M. Two novel germline KRAS mutations: expanding the molecular and clinical phenotype. Clin Gen. 2012;81:590–594. doi: 10.1111/j.1399-0004.2011.01754.x. [DOI] [PubMed] [Google Scholar]
- Sun Y, Grabowski GA. Altered autophagy in the mice with a deficiency of saposin A and saposin B. Autophagy. 2013;9:1115–1116. doi: 10.4161/auto.24919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suntharalingam A, Abisambra JF, O’Leary JC, 3rd, Koren J, 3rd, Zhang B, Joe MK, Blair LJ, Hill SE, Jinwal UK, Cockman M, Duerfeldt AS, Tomarev S, Blagg BS, Lieberman RL, Dickey CA. Glucose-regulated protein 94 triage of mutant myocilin through endoplasmic reticulum-associated degradation subverts a more efficient autophagic clearance mechanism. J Biol Chem. 2012;287:40661–40669. doi: 10.1074/jbc.M112.384800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong L, Chen Z, De Paiva CS, Beuerman R, Li DQ, Pflugfelder SC. Transglutaminase participates in UVB-induced cell death pathways in human corneal epithelial cells. Invest Ophthal Vis Sci. 2006;47:4295–4301. doi: 10.1167/iovs.06-0412. [DOI] [PubMed] [Google Scholar]
- Tsai PS, Evans JE, Green KM, Sullivan RM, Schaumberg DA, Richards SM, Dana MR, Sullivan DA. Proteomic analysis of human meibomian gland secretions. Brit J Ophthal. 2006;90:372–377. doi: 10.1136/bjo.2005.080846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubels JL, Gipson IK, Spurr-Michaud SJ, Tisdale AS, Van Dyken RE, Hatton MP. Gene expression in human accessory lacrimal glands of Wolfring. Invest Ophthal Vis Sci. 2012;53:6738–6747. doi: 10.1167/iovs.12-10750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valapala M, Wilson C, Hose S, Bhutto IA, Grebe R, Dong A, Greenbaum S, Gu L, Sengupta S, Cano M, Hackett S, Xu G, Lutty GA, Dong L, Sergeev Y, Handa JT, Campochiaro P, Wawrousek E, Zigler JS, Jr, Sinha D. Lysosomal-mediated waste clearance in retinal pigment epithelial cells is regulated by CRYBA1/betaA3/A1-crystallin via V-ATPase-MTORC1 signaling. Autophagy. 2014;10:480–496. doi: 10.4161/auto.27292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velez VF, Romano JA, McKown RL, Green K, Zhang L, Raab RW, Ryan DS, Hutnik CM, Frierson HF, Jr, Laurie GW. Tissue transglutaminase is a negative regulator of monomeric lacritin bioactivity. Invest Ophthal Vis Sci. 2013;54:2123–2132. doi: 10.1167/iovs.12-11488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viale A, Pettazzoni P, Lyssiotis CA, Ying H, Sanchez N, Marchesini M, Carugo A, Green T, Seth S, Giuliani V, Kost-Alimova M, Muller F, Colla S, Nezi L, Genovese G, Deem AK, Kapoor A, Yao W, Brunetto E, Kang Y, Yuan M, Asara JM, Wang YA, Heffernan TP, Kimmelman AC, Wang H, Fleming JB, Cantley LC, DePinho RA, Draetta GF. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature. 2014;514:628–632. doi: 10.1038/nature13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijmasi T, Chen FY, Balasubbu S, Gallup M, McKown RL, Laurie GW, McNamara NA. Topical administration of lacritin is a novel therapy for aqueous-deficient dry eye disease. Invest Ophthal Vis Sci. 2014;55:5401–5409. doi: 10.1167/iovs.14-13924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wang N, Xie J, Walton SC, McKown RL, Raab RW, Ma P, Beck SL, Coffman GL, Hussaini IM, Laurie GW. Restricted epithelial proliferation by lacritin via PKCalpha-dependent NFAT and mTOR pathways. J Cell Biol. 2006;174:689–700. doi: 10.1083/jcb.200605140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Zimmerman K, Raab RW, McKown RL, Hutnik CM, Talla V, Tyler MF, Lee JK, Laurie GW. Lacritin rescues stressed epithelia via rapid forkhead box O3 (FOXO3)-associated autophagy that restores metabolism. J Biol Chem. 2013;288:18146–18161. doi: 10.1074/jbc.M112.436584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Despanie J, Shi P, Edman-Woolcott MC, Lin YA, Cui H, Heur JM, Fini ME, Hamm-Alvarez SF, MacKay JA. Lacritin-mediated regeneration of the corneal epithelia by protein polymer nanoparticles. J Mat Chem B Mat Biol Med. 2014;2:8131–8141. doi: 10.1039/C4TB00979G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Jashnani A, Aluri SR, Gustafson JA, Hsueh PY, Yarber F, McKown RL, Laurie GW, Hamm-Alvarez SF, MacKay JA. A thermo-responsive protein treatment for dry eyes. J Control Release. 2015;199:156–167. doi: 10.1016/j.jconrel.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiter JJ, Feingold M, Kolodny EH, Raghaven SS. Retinal pigment epithelial degeneration associated with leukocytic arylsulfatase A deficiency. Am J Ophthalmol. 1980;90:768–772. doi: 10.1016/s0002-9394(14)75191-8. [DOI] [PubMed] [Google Scholar]
- Wong YC, Holzbaur EL. Temporal dynamics of PARK2/parkin and OPTN/optineurin recruitment during the mitophagy of damaged mitochondria. Autophagy. 2015;11:422–424. doi: 10.1080/15548627.2015.1009792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerah M, Piguet F, Colle MA, Raoul S, Deschamps JY, Deniaud J, Gautier B, Toulgoat F, Bieche I, Laurendeau I, Sondhi D, Souweidane MM, Cartier-Lacave N, Moullier P, Crystal RG, Roujeau T, Sevin C, Aubourg P. Intracerebral gene therapy using AAVrh.10-hARSA recombinant vector to treat patients with early-onset forms of metachromatic leukodystrophy: preclinical feasibility and safety assessments in non-human primates. Hum Gene Ther Clin Dev 2015. 2015 Mar 10; doi: 10.1089/humc.2014.139. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang N, Raab RW, McKown RL, Irwin JA, Kwon I, van Kuppevelt TH, Laurie GW. Targeting of heparanase-modified syndecan-1 by prosecretory mitogen lacritin requires conserved core GAGAL plus heparan and chondroitin sulfate as a novel hybrid binding site that enhances selectivity. J Biol Chem. 2013;288:12090–12101. doi: 10.1074/jbc.M112.422717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Yang J, Liao W, Liu X, Zhang H, Wang S, Wang D, Feng J, Yu L, Zhu WG. Cytosolic FoxO1 is essential for the induction of autophagy and tumour suppressor activity. Nat Cell Biol. 2010;12:665–675. doi: 10.1038/ncb2069. [DOI] [PubMed] [Google Scholar]
- Zhou L, Beuerman RW, Chan CM, Zhao SZ, Li XR, Yang H, Tong L, Liu S, Stern ME, Tan D. Identification of tear fluid biomarkers in dry eye syndrome using iTRAQ quantitative proteomics. J Prot Res. 2009;8:4889–4905. doi: 10.1021/pr900686s. [DOI] [PubMed] [Google Scholar]
- Zimran A. How I treat Gaucher disease. Blood. 2011;118:1463–1471. doi: 10.1182/blood-2011-04-308890. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Autophagy-associated genes and proteins of the normal human eye with the number of ‘eye’ transcripts per 106 from Unigene, splice variants from Aceview and missense or nonsense mutations from the Human Gene Mutation Database. Bold, underline indicates NEIBank ‘eye disease genes’. Bold, italics, underline indicate NEIBank eye disease genes associated with ocular surface pathology – each as further discussed in the text.