Abstract
Objectives
Because in obese youth, pulse wave velocity (PWV), an early CVD marker, is elevated, we tested if obese girls with PCOS have higher PWV and carotid intima-media thickness (cIMT) compared with obese and normal-weight control girls without PCOS and whether PWV and cIMT correlate with inflammatory and circulating endothelial function biomarkers.
Study design
Cross-sectional study of PWV and cIMT in 91 obese girls with PCOS (OB-PCOS), 30 obese controls (OB-non-PCOS), and 19 normal-weight controls (NW-non-PCOS). Body composition, blood pressure, fasting glucose, insulin, lipid concentrations and endothelial function biomarkers were measured. OB-NonPCOS and OB-PCOS girls underwent 2-hour oral glucose tolerance testing.
Results
PWV was higher in OB-PCOS (664±24 cm/s) and OB-NonPCOS (624±37 cm/s) compared with NW-NonPCOS (468±13 cm/s, p<0.001), with no differences in cIMT. Systolic BP (SBP), LDL and non-HDL cholesterol were higher, and HDL cholesterol and indices of insulin sensitivity were lower in OB-PCOS and OB-NonPCOS compared with NW-NonPCOS. Vascular cell adhesion molecule-1 (VCAM-1) and hsCRP were higher in OB-PCOS compared with NW-NonPCOS. PWV correlated with adiposity (rs=.46), insulin sensitivity index (HOMA-IR rs=.31), SBP (rs=.24; p ≤0.003 for all) and free testosterone (rs=.24; p=0.03). In multiple regression analysis with PWV as the dependent variable and age, race, BMI, PCOS and dysglycemia as independent variables, only BMI was an independent contributor to the model (r2=0.068, p=0.003).
Conclusions
In adolescent girls, obesity and not PCOS appears to be associated with heightened CVD risk. Increased PWV, VCAM-1 and hsCRP may be the earliest subclinical atherosclerosis biomarkers in obese girls with PCOS.
Keywords: pulse wave velocity, intima media thickness, adhesion molecules
Polycystic ovary syndrome (PCOS) is believed to be the most common endocrine disorder with a prevalence of approximately 5 – 10% among U.S. women (1). PCOS is frequently associated with obesity, insulin resistance (IR), diabetes, hypertension and dyslipidemia, conditions conducive to increased cardiovascular disease (CVD) risk (1). Indeed the prevalence of the metabolic syndrome is increased in both adult women and adolescents with PCOS (2). Girls with PCOS are not only more overweight but are 4.5 times more likely to have metabolic syndrome than age matched NHANES III girls after adjusting for body mass index (BMI) (2, 3). Moreover, impaired glucose tolerance and type 2 diabetes mellitus are more prevalent in both adult women and adolescents with PCOS (4, 5).
In women with PCOS, evidence of subclinical CVD was shown by increased PWV and cIMT (10). However, controversy continues whether CVD is increased in PCOS. Epidemiological data demonstrate greater CV events and lower survival (11) whereas other studies show no increased prevalence of CVD in women with PCOS compared with non-PCOS women (12, 13).
Data are limited with respect to subclinical CVD using imaging or circulating endothelial function biomarkers in adolescent girls with PCOS. Based on our previous observations of increased PWV in obese adolescent boys and girls compared with their normal weight peers (14), we hypothesized that obese adolescent girls with PCOS will have evidence of subclinical CVD and increased inflammatory and circulating endothelial function biomarkers. Therefore, this study was undertaken to examine PWV and cIMT in obese adolescent girls with PCOS (OB-PCOS), in comparison with obese control peers (OB-NonPCOS) and normal weight controls (NW-NonPCOS), and to assess the relationships between PWV, cIMT, insulin sensitivity, and traditional and non-traditional CVD markers.
Methods
Overweight/obese adolescent girls (n=91) with a diagnosis of PCOS (6, 15) were recruited from the PCOS Center at Children’s Hospital of Pittsburgh, 30 otherwise healthy OB-NonPCOS were recruited from the Weight Management and Wellness Center at Children’s Hospital of Pittsburgh, and 19 healthy NW-NonPCOS (BMI <85th percentile) were recruited through newspaper and hospital advertisements. OB-NonPCOS and NW-NonPCOS had regular menses and no clinical evidence of hyperandrogenism. The diagnosis of PCOS was made based on the presence of clinical signs and symptoms of hyperandrogenism and/or biochemical hyperandrogenemia, consistent with the Endocrine Society Clinical Practice Guidelines and our publications (6, 15–17). Many girls with PCOS were recruited shortly after their diagnosis in our PCOS Center and before pharmacologic therapy was initiated. Inclusion criteria were age 10–20 years, postmenarche, and Tanner stage III-V. Exclusion criteria were: (1) pregnancy; (2) preexisting diabetes; (3) use of medications that impact carbohydrate or lipid metabolism (oral contraceptive pills [OCP], metformin, anti-epileptics, anti-psychotics, statins, fish oil); and (4) smoking history. The investigation was approved by the Institutional Review Board and performed in the Pediatric Clinical and Translational Research Center of Children’s Hospital of Pittsburgh and The Department of Epidemiology Ultrasound Research Laboratory at the University of Pittsburgh. Parental informed consent and child assent were obtained from all participants before participation in accordance with the ethical guidelines of Children’s Hospital of Pittsburgh.
Each participant underwent a physical examination, height, weight, waist and hip circumference measurements. Fasting blood was obtained for lipid profile, glucose, insulin and HbA1c, adipokines (leptin, adiponectin), inflammatory marker (high-sensitivity C-reactive protein or hsCRP) and circulating soluble cell adhesion molecule biomarkers (intercellular adhesion molecule-1 (ICAM-1), vascular adhesion molecule-1 (VCAM-1) and E-selectin). DXA was performed in all participants to assess body composition. Obese participants with and without PCOS had a 2-hour oral glucose tolerance test to assess glucose tolerance, and a free testosterone panel to assess for hyperandrogenemia. Dysglycemia was defined as a fasting plasma glucose ≥ 100 mg/dL, 2-hour glucose ≥ 140 mg/dL, or both. Age-specific BMI z-scores were calculated utilizing Epi Info™ (Version 3.3.2, Centers for Disease).
Carotid IMT and aortic PWV were measured at the Ultrasound Research Laboratory of the Department of Epidemiology at the University of Pittsburgh, a key laboratory in multiple adult and pediatric trials (7, 14, 18, 19). Using a Toshiba (Toshiba American Medical Systems, Tustin, CA) SSA-270A scanner equipped with a linear 5 MHz transducer, the right and left carotid artery was interrogated. Although this transducer is a lower frequency than recommended in published standards (20), lower frequency probes are used in obese populations where the vessel may be deeper. Detailed B-mode images of the near and far walls of the distal common carotid artery (one cm proximal to the carotid bulb), far wall of the bulb, and first centimeter of the far wall of the internal carotid artery were obtained in end-diastole from each side for a total of 8 images. These images were digitized for later reading using a semi-automated edge detection software (21). The scanning and reading protocols that were used are well established (14, 19). Intima-media thickness measures were obtained by electronically tracing the lumen-intima interface and the media-adventitia interface across a 1-cm segment; one measurement was generated for each pixel over the area, for a total of approximately 140 measures for each segment. For analyses, the mean and maximum values of the average readings at all 8 locations were used. The URL requires certification of sonographers and readers, and monitors quality control with several ongoing quality control systems. Monthly repeat scans were performed by two separate sonographers on the same day and several of the scans were reviewed to monitor the inter-sonographer (scanning) reproducibility. Quality control was also performed between readers on a regular basis. Quarterly, scans were read by two separate readers to monitor inter-reader reproducibility. Reproducibility of cIMT measures was excellent with an intra-class correlation coefficient between sonographers of 0.82–0.97 and within reader of 0.87–0.99 across the study period.
To measure aortic pulse-wave velocity, two unidirectional transcutaneous Doppler flow probes (model 810-a, 10 MHz; Parks Medical Electronics, Aloha, OR) were used; one to detect the pulse wave as it reaches the right carotid artery and one to detect the pulse wave as it reaches the right femoral artery. The time required for the pulse wave to travel from one probe to the other, combined with the distance between the two probes, allowed for the calculation of central pulse wave velocity and was performed several times until waveforms were clear. Heart rate monitoring was used to score the waveforms. Three runs were performed for each participant, and the mean of waveforms from all usable runs was used in analyses, reducing the measurement variability.
Plasma glucose was measured with a glucose analyzer (YSI, Yellow Springs, OH). Insulin, adiponectin, and leptin were measured using a commercially available radioimmunoassay kit (Linco Research, St. Louis, MO). Fasting lipids were analyzed using the standards of the Centers for Disease Control and Prevention. hsCRP was analyzed by nephelometry, total testosterone was measured by HPLC tandem mass spectrometry and free testosterone was measured by equilibrium dialysis at Esoterix Inc (Calabasas Hills, CA). ICAM-1, VCAM-1 and E-selectin were quantified using a commercially available double-sandwich enzyme-linked immunoassay kit (R&D Systems, Minneapolis, MN).
Statistical analyses
HOMA-IR was calculated (www.ihoma.co.uk) using the formula: (fasting glucose (mg/dL) × fasting insulin (µU/mL) × 0.0555)/22.5. Statistical procedures were performed using SPSS version 22 (SPSS Inc., Chicago, IL). Differences in continuous variables among the three groups were tested with either a one-way ANOVA or the nonparametric Kruskall-Wallis test or Mann-Whitney U test for two group comparison, based on the nonviolation of statistical assumptions. Where differences existed among groups, post-hoc multiple comparisons were performed using Bonferroni correction. For categorical variables, three-group comparison was made by Pearson Chi-square. Pearson correlation (r) for parametric and Spearman correlation (rs) for non-parametric measures were performed for PWV and cIMT. Multiple regression analysis was used to quantify the independent contributions to PWV. Statistical significance was set at p ≤ 0.05.
Results
Table I summarizes the characteristics of the participants. The OB-PCOS group was slightly older, but all adiposity measures were comparable between OB-PCOS and OB-NonPCOS and significantly higher than NW-NonPCOS. Total, free and % free testosterone concentrations were higher in OB-PCOS compared with OB-NonPCOS.
Table 1.
Participants’ physical and metabolic characteristics
| OB-PCOS (n = 91) |
OB-NonPCOS (n = 30) |
NW-NonPCOS (n = 19) |
P ANOVA |
|
|---|---|---|---|---|
| Age (years) | 15.8 ± 0.2** | 14.6 ± 0.3 | 14.9 ± 0.5 | 0.006 |
| Race (% of each group) | 0.001 | |||
| AW | 68 | 27 | 58 | |
| AA | 23 | 63 | 42 | |
| Other | 9 | 10 | 0 | |
| Total testosterone (ng/dL) | 41.9 ± 2.7 | 22.5 ± 2.3 | <0.001 | |
| Free testosterone (pg/mL) | 9.2 ± 0.9 | 3.6 ± 0.5 | <0.001 | |
| % free testosterone (%) | 2.4 ± 0.2 | 1.6 ± 0.1 | 0.002 | |
| BMI (kg/m2) | 37.9 ± 0.7 | 35.3 ± 1.1† | 20.7 ± 0.6* | <0.001 |
| BMI z-score | 2.2 ± 0.03 | 2.2 ± 0.1† | 0.2 ± 0.1* | <0.001 |
| Waist circumference (cm) | 107.6 ± 2.0 | 101.4 ± 3.6† | 72.6 ± 1.5* | <0.001 |
| Hip circumference (cm) | 121.3 ± 1.7 | 116.9 ± 3.0† | 86.8 ± 2.0* | <0.001 |
| Waist-to-hip ratio (WHR) | 0.88 ± 0.01 | 0.87 ± 0.01 | 0.84 ± 0.02 | ns |
| % body fat | 46.8 ± 1.0 | 47.6 ± 1.1† | 27.0 ± 2.2* | <0.001 |
| HbA1c (%) | 5.4 ± 0.05 | 5.4 ± 0.1 | 5.3 ± 0.1 | ns |
| Fasting glucose (mg/dL) | 87.2 ± 1.0 | 88.9 ± 1.2 | 87.9 ± 1.6 | ns |
| Glucose tolerance (% of each group) | ||||
| NGT | 76 | 87 | ||
| Dysglycemia | 24 | 13 | ||
| Fasting insulin (µU/mL) | 34.0 ± 1.8 | 32.9 ± 4.2† | 18.3 ± 1.6* | <0.001 |
| HOMA-IR | 8.3 ± 0.5 | 7.2 ± 0.9 | 4.0 ± 0.4* | <0.001 |
| Cholesterol (mg/dL) | 161.4 ± 3.6 | 158.6 ± 5.1 | 144.3 ± 6.6 | ns |
| LDL (mg/dL) | 94.5 ± 3.1 | 99.5 ± 4.9† | 77.0 ± 5.5* | 0.02 |
| TG (mg/dL) | 119.7 ± 6.3** | 83.8 ± 6.7 | 75.3 ± 13.3* | <0.001 |
| HDL (mg/dL) | 42.9 ± 1.2 | 42.9 ± 1.4† | 52.2 ± 2.7* | 0.003 |
| Non-HDL cholesterol (mg/dL) | 118.5 ± 3.4 | 115.7 ± 5.4† | 92.1 ± 5.2* | 0.004 |
| TG/HDL | 3.1 ± 0.2** | 2.1 ± 0.2† | 1.6 ± 0.4* | <0.001 |
| SBP (mmHg) | 123.3 ± 1.4 | 120.0 ± 2.6† | 108.1 ± 2.0* | <0.001 |
| DBP (mmHg) | 64.9 ± 0.8 | 62.3 ± 1.3 | 60.5 ± 1.3 | ns |
| Leptin (ng/mL) | 46.6 ± 2.0 | 44.4 ± 4.2† | 13.8 ± 1.6* | <0.001 |
| Adiponectin (µg/mL) | 6.9 ± 0.3 | 8.1 ± 0.7† | 13.7 ± 1.3* | <0.001 |
| Leptin/Adiponectin | 8.1 ± 0.5 | 7.2 ± 0.9† | 1.2 ± 0.2* | <0.001 |
OB-NonPCOS: obese control, NW-NonPCOS: normal weight control, OB-PCOS: obese PCOS, AW-American White, AA- African American, ns: not significant, NGT: normal glucose tolerance
Post hoc
p<0.05 NW-NonPCOS vs. OB-NonPCOS,
p<0.05 NW-NonPCOS vs. OB-PCOS,
p<0.005 OB-NonPCOS vs. OB-PCOS.
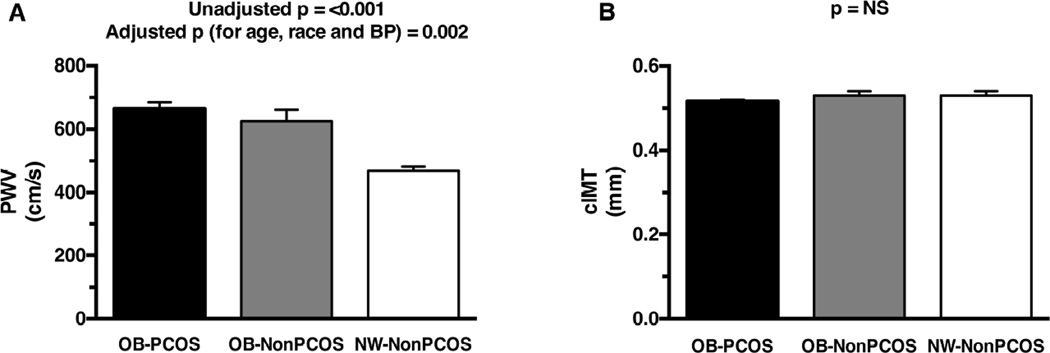
PWV was significantly higher in OB-PCOS and OB-NonPCOS girls compared with NW-NonPCOS before and after adjusting for age, race and blood pressure (Figure 1, A) (adjusted mean: 676 ± 30 cm/s OB-PCOS, 623 ± 64 cm/s OB-NonPCOS, and 457 ± 55 cm/s NW-NonPCOS, p ANOVA=0.002). Mean and maximum cIMT were not different between the three groups (mean: 0.516 ± 0.004 mm OB-PCOS, 0.531 ± 0.01 mm OB-NonPCOS, 0.531 ± 0.01 mm NW-NonPCOS [Figure 1, B]; maximum: 0.634 ± 0.007 mm OB-PCOS, 0.650 ± 0.01 mm OB-NonPCOS, 0.645 ± 0.03 mm NW-NonPCOS, p ANOVA=0.56).
Figure 1.
A, Pulse wave velocity in OB-PCOS girls vs. OB OB-NonPCOS vs. (NW OB-NonPCOS). B, Carotid intima-media thickness in OB-PCOS girls vs. OB-NonPCOS vs. NW-NonPCOS. * Post-hoc OB-PCOS vs. NW-NonPCOS p<0.001; † post-hoc OB-NonPCOS vs. NW-NonPCOS p<0.001; NS, not significant.
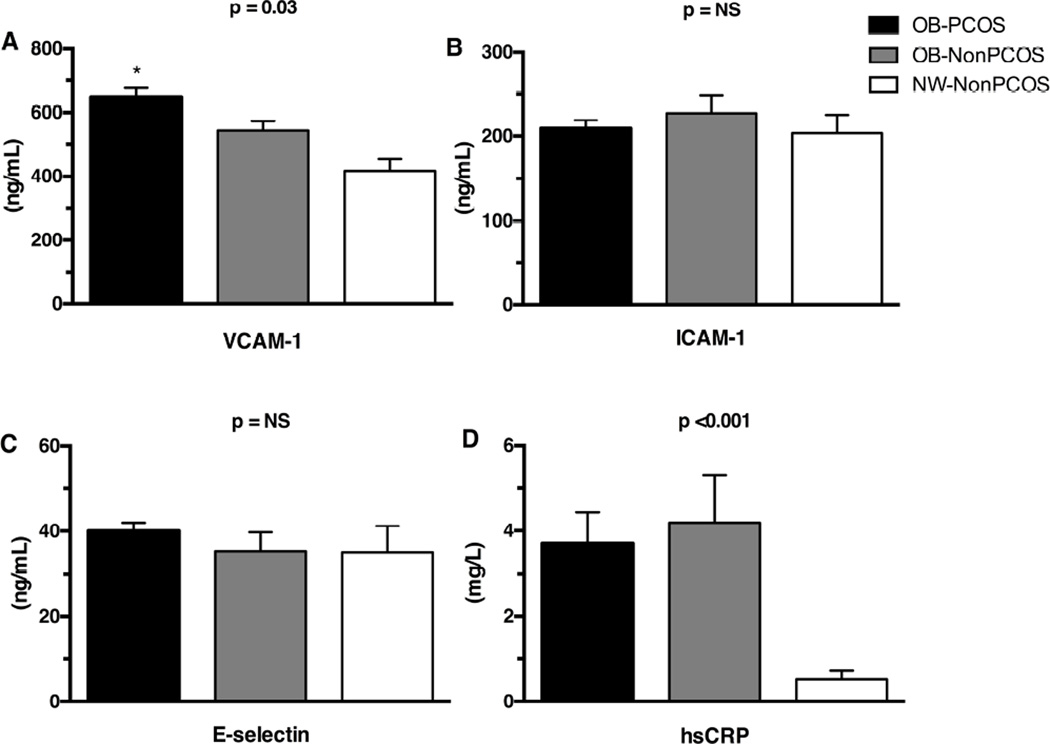
Fasting glucose and HbA1c were similar among the three groups and glucose tolerance did not differ between OB-NonPCOS and OB-PCOS girls (Table I). Surrogate estimates of insulin sensitivity including fasting insulin concentration, HOMA-IR, and leptin/adiponectin were impaired in OB-PCOS and OB-NonPCOS compared with NW-NonPCOS. Although total cholesterol did not differ among the three groups, LDL and non-HDL cholesterols were higher and HDL cholesterol lower in OB-PCOS and OB-NonPCOS vs. NW-NonPCOS girls. TG and TG/HDL ratio were significantly higher in OB-PCOS girls compared with their obese and lean counterparts. SBP was significantly higher in OB-PCOS and OB-NonPCOS compared with NW-NonPCOS, but did not differ between OB-NonPCOS and OB-PCOS even after controlling for age, race and waist circumference. VCAM-1 was significantly different among the three groups, and higher in OB-PCOS compared with NW-NonPCOS (post-hoc p=0.015), and ICAM-1 and E-selectin did not differ among the groups (Figure 2). hsCRP was significantly higher in OB-PCOS and OB-NonPCOS compared with NW-NonPCOS, but did not differ between OB-NonPCOS and OB-PCOS (Figure 2).
Figure 2.
A, Vascular adhesion molecule-1 (VCAM-1), B, intercellular adhesion molecule-1 (ICAM-1), C, E-selectin, and D, high sensitivity C-reactive protein (hsCRP) in OB-PCOS girls vs. OB-NonPCOS vs. NW-NonPCOS. * Post-hoc OB-PCOS vs. NW-NonPCOS p≤0.015; † post-hoc OB-NonPCOS vs. NW-NonPCOS p<0.001; NS, not significant.
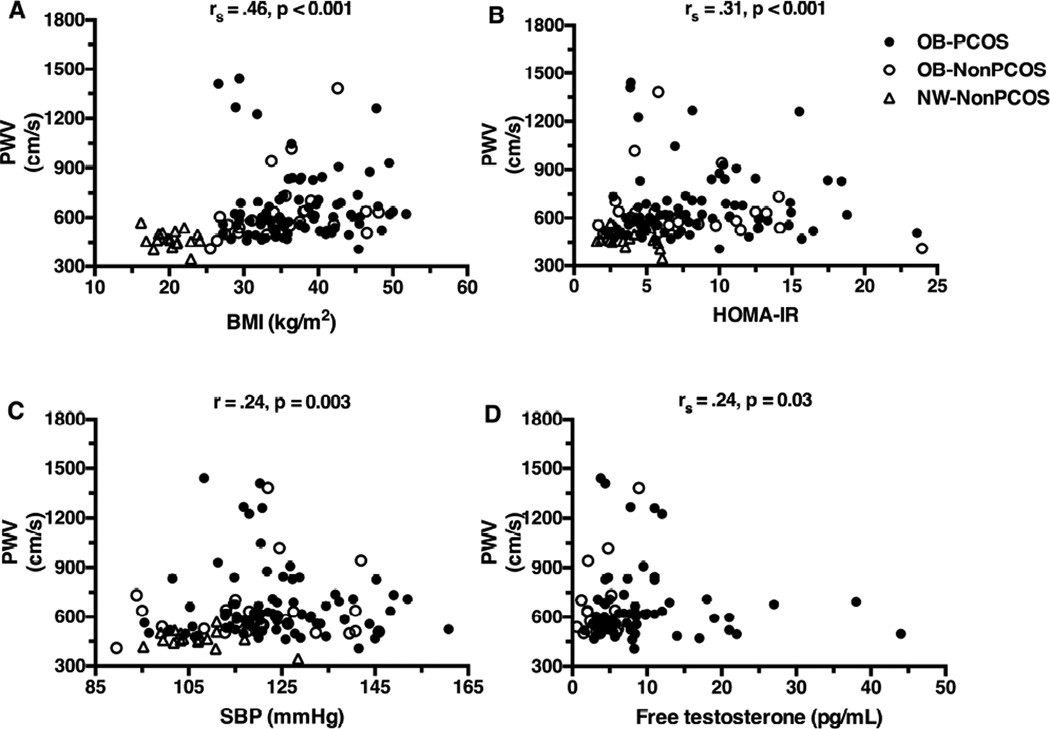
PWV showed the highest correlation with BMI, followed by HOMA-IR, SBP, free testosterone (Figure 3), TG (r=.23, p=0.012), HDL (r=−.25, p=0.005), and hsCRP (rs=.24, p=0.009) but not with total testosterone, LDL, non-HDL cholesterol, VCAM-1, ICAM-1 or E-selectin. cIMT did not correlate with any of the above variables. There were five OB-PCOS girls and one OB-NonPCOS who had PWV >1200 cm/s and three OB-PCOS girls with free testosterone >25 pg/mL (Figure 3). There were no unique physical or metabolic characteristics common among the six with high PWV or the three with high free testosterone that would differentiate them from the others. Excluding these six subjects from the PWV correlations did not change the findings (BMI rs=.52, HOMA-IR rs=.34, SBP rs=.27, free testosterone rs=.27, p <0.05). To examine if among obese girls, hyperandrogenemia or other factors explain an additional component of the variance in PWV besides BMI, stepwise multiple regression analyses were performed with PWV as the dependent variable and age, race, BMI and PCOS status without or with the variables that showed significant univariate correlations to PWV (dysglycemia, SBP, hsCRP, TG, HDL, and HOMA-IR) as independent variables (Table II; available at www.jpeds.com). Seven percent of the variance in PWV was explained by the model which contained age, race, BMI, PCOS and dysglycemia (model p ANOVA=0.003), and BMI was the only significant contributor to the model (Table II).
Figure 3.
Correlation between PWV and A, BMI, B, HOMA-IR, C, systolic blood pressure, and D, free testosterone.
Table 2.
Multivariable Regression Analysis with PWV as the Dependent Variable and Age, Race, BMI, PCOS condition and Cardiometabolic Parameters as Independent Variables
| Model | Independent variables | Standardized β(BMI) | Model R2 | P ANOVA |
|---|---|---|---|---|
| 1 | Age, race, BMI, PCOS condition | .262 | 0.069 | 0.003 |
| 2 | Model 1 + dysglycemia | .262 | 0.068 | 0.003 |
| 3 | Model 2 + SBP, hsCRP | .227 | 0.052 | 0.016 |
| 4 | Model 3 + HOMA-IR, TG, HDL | .227 | 0.052 | 0.016 |
BMI was the only significant contributor within each model. Therefore, standardized β values shown are only for BMI. Standardized β values indicate the number of standard deviations that PWV will change as a result of one standard deviation change in BMI.
Discussion
Kelly et al (22) first demonstrated increased PWV in obese women with PCOS compared with BMI-matched non-PCOS controls, and further studies confirmed these findings showing that age (23), SBP (23, 24) and presence of PCOS phenotype (23) are significant determinants of PWV (23, 24). In contrast, Moran et al and Cussons et al found no differences in PWV between obese and non-obese PCOS women and their overweight or BMI-matched controls, respectively with age, glucose tolerance, mean arterial pressure and hsCRP, but not adiposity, predicting PWV (25, 26). Our present PWV findings are in agreement with Cussons et al and Ketel et al who demonstrated that obesity and not PCOS was associated with greater arterial stiffness in young adult women with PCOS and obese controls compared with their lean PCOS and normal weight control counterparts (27). On the other hand, early endothelial dysfunction in PCOS is reported by demonstrating lower flow-mediated dilation and nitrate-mediated dilation in the brachial artery compared with matched controls, largely independent of obesity (28). Additionally, numerous adult studies have shown adverse structural changes in cIMT in premenopausal women with PCOS compared with age and/or BMI-matched non-PCOS controls (29). Furthermore, coronary artery and aortic calcification are increased in women with PCOS compared with controls, with the presence of PCOS, age and BMI as significant determinants of coronary artery calcification (30). These contrasting results between our PWV and cIMT findings in adolescent girls with PCOS and adult women are important and could merely be a reflection of chronology, where the initial obesity-driven alteration may over time and with aging evolve into a PCOS-associated abnormality against the backdrop of persistent hyperandrogenism.
Despite the growing evidence of subclinical CVD in adult women with PCOS, the increased risk for CVD in them remains controversial (31). Two large prospective studies each with greater than 20 years of follow-up found that there was no increased prevalence of nonfatal/fatal CVD events in women with PCOS (12, 13). On the other hand, a sub analysis of the NHLBI sponsored Women’s Ischemia Syndrome Evaluation (WISE) study found that women with PCOS had a greater number of CV events (OR 1.71) and lower event free survival compared with non-PCOS women (11).
Insulin resistance is a well-known risk factor for CVD (5, 32). In adult women with PCOS, improvement in insulin sensitivity with troglitazone was associated with reduced carotid IMT progression rates, and worsening of insulin sensitivity with high dose OCP was associated with increased arterial stiffness (33, 34). Elevated TG/HDL, a marker of IR and/or the metabolic syndrome both in adults and in children, has been proposed to be an atherogenic index (35). Based on these, IR and elevated LDL, triglycerides and TG/HDL in the present study suggest a pro-atherogenic state in PCOS and obesity. However, in addition to IR, long-standing hyperandrogenemia may play a role in vascular changes observed in adulthood (10). Even though free testosterone correlated with PWV in our obese adolescents, lack of a significant contribution of the PCOS condition to the variance in PWV in the regression model suggests that PCOS per se in obese adolescents may not play a role over and above obesity in the altered PWV. Queries related to the role of obesity vs. PCOS per se in modulating PWV would best be addressed in a study which includes normal-weight PCOS vs. obese PCOS girls. Until then, we postulate that obesity initially pulls the trigger for changes in PWV in obese PCOS adolescents. However, the persistence of hyperandrogenemia together with dyslipidemia and IR may lead over time and with aging to the reported differences in PWV in adult women with PCOS compared with their BMI-matched controls. Contrary to adult studies cited above, a randomized trial comparing drosperinone/ethinyl estradiol to rosiglitazone in adolescents with PCOS did not show any changes in PWV with either treatment, despite improvement in free testosterone with both treatments and insulin sensitivity with the latter (17). Perhaps the lack of improvement in adiposity in part contributed to the lack of PWV change.
Numerous adult studies from a meta-analysis have also shown adverse structural changes in cIMT in premenopausal and postmenopausal women with PCOS compared with age and/or BMI-matched non-PCOS controls (29). The literature regarding cIMT in pediatric obesity is inconsistent with some studies showing increased cIMT, mediated in part by IR (36), SBP (36, 37), BMI, glucose and hsCRP (37). In contrast, others have shown no differences in cIMT (38), including our current and prior study (14). An additional study showed a correlation between total and calculated free testosterone and cIMT in 160 obese adolescent girls independent of BMI and IR (39), whereas our study found no such associations among our 121 PCOS and OBCN girls combined. These observations in youth and adults suggest that increases in cIMT may evolve over time and with aging against the backdrop of persistence of obesity, other CV risk factors (including but not limited to hsCRP) and hyperandrogenism.
Pediatric literature regarding PWV in obesity is limited and conflicting too, with several studies reporting increased PWV with increasing adiposity in youth (14, 40) with HbA1c and insulin sensitivity being independent predictors of PWV in one study (14) and decreased cardiorespiratory fitness and measures of adiposity as independent predictors in the other (40). Another study showed lower PWV in obese, primarily female subjects compared with lean controls, which the authors suggested may reflect general vasodilatation (41). A study by Urbina et al examined obese and obese insulin resistant adolescents and young adults compared with lean controls and revealed that HOMA, a surrogate index of insulin sensitivity, was not an independent determinant of PWV, but BMI and blood pressure were (42). The latter data are consistent with our findings with respect to BMI and HOMA. These contrasting findings are also likely explained by differences in study populations, including varying sample sizes (40, 42), younger (40) vs. older participants (42), and males (14, 40–42) or prepubertal subjects (40).
Although there are a few cross-sectional adolescent PCOS studies that evaluated CVD risk factors and IR, and incorporated control comparison groups (43, 44), none included ultrasonographic arterial structural or functional imaging modalities. Two interventional studies in adolescent PCOS investigated PWV (17) and cIMT (45), but both were limited in that they did not include control groups. The second study was a 12-month lifestyle intervention that showed that cIMT only improved in those that demonstrated a reduction in BMI SDS (45), further attesting to the important role of obesity in this age group. Our prior work in adolescent girls with PCOS demonstrated that 6 months of insulin sensitization (rosiglitazone) or OCP did not change body weight, cIMT or PWV (17).
Circulating biomarkers of CVD include the soluble cell adhesion molecules ICAM-1, VCAM-1 and E-selectin, elevations in which are believed to reflect early abnormal endothelial status contributing to increased CVD risk. VCAM-1 concentrations in our adolescent girls with OB-PCOS were similar to OB-NonPCOS but greater than NW-NonPCOS, similar to a number of studies in the pediatric obesity literature (46–48) but with no prior data in adolescent PCOS. Several adult studies have shown that VCAM-1 concentrations were higher in PCOS women compared with BMI-matched overweight controls (49, 50). Even though no correlation was demonstrated in our pediatric study, one adult study showed a positive correlation between VCAM-1 and total testosterone concentrations (49). Taken together, it could be hypothesized that in youth the initial alteration in VCAM-1 may be driven by obesity, but over time and with persistence of hyperandrogenemia together with obesity VCAM expression is induced further and becomes pronounced in adulthood in PCOS women. Lastly, with regards to CRP, and a metaanalysis revealed that women with PCOS have elevated circulating CRP independent of obesity compared with controls (51), a study by Talbott et al (52) in adult women with PCOS found that CRP was not a significant independent predictor of cIMT when BMI was taken into account. However, another adult PCOS study found CRP but not adiposity, predicted PWV (25). In our study, hsCRP was greater in OB-PCOS and OB-NonPCOS compared with NW-NonPCOS groups. Although hsCRP correlated with PWV but not cIMT, it was not an independent predictor of PWV. In the pediatric literature, a paucity of data exists on the association of hsCRP and subclinical markers of atherosclerosis (53), and to our knowledge there are no published data in youth with PCOS.
A limitation of our study is the absence of normal-weight adolescent girls with PCOS, the numbers of whom diagnosed are few in any PCOS, Endocrinology or Adolescent Medicine clinic, and the relatively small sample size of the healthy normal-weight and obese control groups. However, our NW-NonPCOS and OB-NonPCOS group sample sizes are comparable with other pediatric (43) and adult (9, 10, 27, 50, 54) cross-sectional studies. Although selection bias of NW-NonPCOS and OB-NonPCOS could be contributory, there was no known family history of PCOS in our NW-NonPCOS subjects, and similar to the general US population, most of our OB-NonPCOS subjects had some family history of obesity, diabetes or CVD. Lastly, it remains to be determined whether or not the observed statistically significant increase in PWV in obese girls with PCOS is clinically meaningful. Long-term longitudinal studies are needed to examine the progression of these statistical outcomes and their clinical translation.
Acknowledgments
We thank the nurses of the Pediatric Clinical and Translational Research Center for their assistance and Resa Stauffer for her laboratory expertise at the Children’s Hospital of Pittsburgh of UPMC. We are deeply grateful to the late Kim Sutton-Tyrrell, DrPH (Graduate School of Public Health, University of Pittsburgh), for her conceptual and technical oversight regarding the subclinical cardiovascular imaging studies. Most importantly we are most grateful to the research participants and their parents.
Supported by <> (T32DK07729 [to K.H. and J.W.-U.], K12HD052892 [to K.H.], K24 HD01357 [to S.A.]), Richard L. Day Endowed Chair (to S.A. and H.T.), Children’s Hospital of Pittsburgh Pediatric Clinical and Translational Research Center (UL1TR000005 [Clinical and Translational Science Awards] and RR024153 [<>]), and Endocrine Fellows Foundation (to J.W.-U.).
List of Abbreviations
- PCOS
Polycystic Ovary Syndrome
- PWV
pulse wave velocity
- cIMT
carotid intima-media thickness
- OB-PCOS
obese PCOS
- OB-NonPCOS
obese controls
- NW-NonPCOS
normal-weight controls
- VCAM-1
vascular cell adhesion molecule-1
- IR
insulin resistance
- CVD
cardiovascular disease
- OCP
oral contraceptive pills
- hsCRP
high-sensitivity C-reactive protein
- ICAM-1
intercellular adhesion molecule-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Portions of the study were presented orally at the meeting of the Pediatric Academic Societies, Baltimore, MD, <dates>, as well as a poster at the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Child Health Research Centers annual retreat, Durham, NC, <dates>.
References
- 1.Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;352:1223–1236. doi: 10.1056/NEJMra041536. [DOI] [PubMed] [Google Scholar]
- 2.Coviello AD, Legro RS, Dunaif A. Adolescent girls with polycystic ovary syndrome have an increased risk of the metabolic syndrome associated with increasing androgen levels independent of obesity and insulin resistance. J Clin Endocrinol Metab. 2006;91:492–497. doi: 10.1210/jc.2005-1666. [DOI] [PubMed] [Google Scholar]
- 3.Glueck CJ, Morrison JA, Friedman LA, Goldenberg N, Stroop DM, Wang P. Obesity, free testosterone, and cardiovascular risk factors in adolescents with polycystic ovary syndrome and regularly cycling adolescents. Metabolism. 2006;55:508–514. doi: 10.1016/j.metabol.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Palmert MR, Gordon CM, Kartashov AI, Legro RS, Emans SJ, Dunaif A. Screening for abnormal glucose tolerance in adolescents with polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87:1017–1023. doi: 10.1210/jcem.87.3.8305. [DOI] [PubMed] [Google Scholar]
- 5.McLaughlin T, Allison G, Abbasi F, Lamendola C, Reaven G. Prevalence of insulin resistance and associated cardiovascular disease risk factors among normal weight, overweight, and obese individuals. Metabolism. 2004;53:495–499. doi: 10.1016/j.metabol.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 6.Lewy VD, Danadian K, Witchel SF, Arslanian S. Early metabolic abnormalities in adolescent girls with polycystic ovarian syndrome. J Pediatr. 2001;138:38–44. doi: 10.1067/mpd.2001.109603. [DOI] [PubMed] [Google Scholar]
- 7.Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, et al. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111:3384–3390. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 8.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 9.Lakhani K, Hardiman P, Seifalian AM. Intima-media thickness of elastic and muscular arteries of young women with polycystic ovaries. Atherosclerosis. 2004;175:353–359. doi: 10.1016/j.atherosclerosis.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Meyer C, McGrath BP, Teede HJ. Overweight women with polycystic ovary syndrome have evidence of subclinical cardiovascular disease. J Clin Endocrinol Metab. 2005;90:5711–5716. doi: 10.1210/jc.2005-0011. [DOI] [PubMed] [Google Scholar]
- 11.Shaw LJ, Bairey Merz CN, Azziz R, Stanczyk FZ, Sopko G, Braunstein GD, et al. Postmenopausal women with a history of irregular menses and elevated androgen measurements at high risk for worsening cardiovascular event-free survival: results from the National Institutes of Health--National Heart, Lung, and Blood Institute sponsored Women's Ischemia Syndrome Evaluation. J Clin Endocrinol Metab. 2008;93:1276–1284. doi: 10.1210/jc.2007-0425. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Pierpoint T, McKeigue PM, Isaacs AJ, Wild SH, Jacobs HS. Mortality of women with polycystic ovary syndrome at long-term follow-up. J Clin Epidemiol. 1998;51:581–586. doi: 10.1016/s0895-4356(98)00035-3. [DOI] [PubMed] [Google Scholar]
- 13.Wild S, Pierpoint T, McKeigue P, Jacobs H. Cardiovascular disease in women with polycystic ovary syndrome at long-term follow-up: a retrospective cohort study. Clin Endocrinol (Oxf) 2000;52:595–600. doi: 10.1046/j.1365-2265.2000.01000.x. [DOI] [PubMed] [Google Scholar]
- 14.Gungor N, Thompson T, Sutton-Tyrrell K, Janosky J, Arslanian S. Early signs of cardiovascular disease in youth with obesity and type 2 diabetes. Diabetes Care. 2005;28:1219–1221. doi: 10.2337/diacare.28.5.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565–4592. doi: 10.1210/jc.2013-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arslanian SA, Lewy VD, Danadian K. Glucose intolerance in obese adolescents with polycystic ovary syndrome: roles of insulin resistance and beta-cell dysfunction and risk of cardiovascular disease. J Clin Endocrinol Metab. 2001;86:66–71. doi: 10.1210/jcem.86.1.7123. [DOI] [PubMed] [Google Scholar]
- 17.Tfayli H, Ulnach JW, Lee S, Sutton-Tyrrell K, Arslanian S. Drospirenone/ethinyl estradiol versus rosiglitazone treatment in overweight adolescents with polycystic ovary syndrome: comparison of metabolic, hormonal, and cardiovascular risk factors. J Clin Endocrinol Metab. 2011;96:1311–1319. doi: 10.1210/jc.2010-2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khan UI, Wang D, Thurston RC, Sowers M, Sutton-Tyrrell K, Matthews KA, et al. Burden of subclinical cardiovascular disease in "metabolically benign" and "at-risk" overweight and obese women: the Study of Women's Health Across the Nation (SWAN) Atherosclerosis. 2011;217:179–186. doi: 10.1016/j.atherosclerosis.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sutton-Tyrrell K, Kuller LH, Matthews KA, Holubkov R, Patel A, Edmundowicz D, et al. Subclinical atherosclerosis in multiple vascular beds: an index of atherosclerotic burden evaluated in postmenopausal women. Atherosclerosis. 2002;160:407–416. doi: 10.1016/s0021-9150(01)00591-3. [DOI] [PubMed] [Google Scholar]
- 20.Urbina EM, Williams RV, Alpert BS, Collins RT, Daniels SR, Hayman L, et al. Noninvasive assessment of subclinical atherosclerosis in children and adolescents: recommendations for standard assessment for clinical research: a scientific statement from the American Heart Association. Hypertension. 2009;54:919–950. doi: 10.1161/HYPERTENSIONAHA.109.192639. [DOI] [PubMed] [Google Scholar]
- 21.Wendelhag I, Gustavsson T, Suurkula M, Berglund G, Wikstrand J. Ultrasound measurement of wall thickness in the carotid artery: fundamental principles and description of a computerized analysing system. Clin Physiol. 1991;11:565–577. doi: 10.1111/j.1475-097x.1991.tb00676.x. [DOI] [PubMed] [Google Scholar]
- 22.Kelly CJ, Speirs A, Gould GW, Petrie JR, Lyall H, Connell JM. Altered vascular function in young women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87:742–746. doi: 10.1210/jcem.87.2.8199. [DOI] [PubMed] [Google Scholar]
- 23.Armeni E, Stamatelopoulos K, Rizos D, Georgiopoulos G, Kazani M, Kazani A, et al. Arterial stiffness is increased in asymptomatic nondiabetic postmenopausal women with a polycystic ovary syndrome phenotype. J Hypertens. 2013;31:1998–2004. doi: 10.1097/HJH.0b013e3283630362. [DOI] [PubMed] [Google Scholar]
- 24.Sasaki A, Emi Y, Matsuda M, Sharula Kamada Y, Chekir C, et al. Increased arterial stiffness in mildly-hypertensive women with polycystic ovary syndrome. J Obstet Gynaecol Res. 2011;37:402–411. doi: 10.1111/j.1447-0756.2010.01375.x. [DOI] [PubMed] [Google Scholar]
- 25.Moran LJ, Cameron JD, Strauss BJ, Teede HJ. Vascular function in the diagnostic categories of polycystic ovary syndrome. Hum Reprod. 2011;26:2192–2199. doi: 10.1093/humrep/der159. [DOI] [PubMed] [Google Scholar]
- 26.Cussons AJ, Watts GF, Stuckey BG. Dissociation of endothelial function and arterial stiffness in nonobese women with polycystic ovary syndrome (PCOS) Clin Endocrinol (Oxf) 2009;71:808–814. doi: 10.1111/j.1365-2265.2009.03598.x. [DOI] [PubMed] [Google Scholar]
- 27.Ketel IJ, Stehouwer CD, Henry RM, Serne EH, Hompes P, Homburg R, et al. Greater arterial stiffness in polycystic ovary syndrome (PCOS) is an obesity--but not a PCOS-associated phenomenon. J Clin Endocrinol Metab. 2010;95:4566–4575. doi: 10.1210/jc.2010-0868. [DOI] [PubMed] [Google Scholar]
- 28.Kravariti M, Naka KK, Kalantaridou SN, Kazakos N, Katsouras CS, Makrigiannakis A, et al. Predictors of endothelial dysfunction in young women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:5088–5095. doi: 10.1210/jc.2005-0151. [DOI] [PubMed] [Google Scholar]
- 29.Meyer ML, Malek AM, Wild RA, Korytkowski MT, Talbott EO. Carotid artery intima-media thickness in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2012;18:112–126. doi: 10.1093/humupd/dmr046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Talbott EO, Zborowski JV, Rager JR, Boudreaux MY, Edmundowicz DA, Guzick DS. Evidence for an association between metabolic cardiovascular syndrome and coronary and aortic calcification among women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89:5454–5461. doi: 10.1210/jc.2003-032237. [DOI] [PubMed] [Google Scholar]
- 31.Legro RS. Polycystic ovary syndrome and cardiovascular disease: a premature association? Endocr Rev. 2003;24:302–312. doi: 10.1210/er.2003-0004. [DOI] [PubMed] [Google Scholar]
- 32.Wild RA. Polycystic ovary syndrome: a risk for coronary artery disease? Am J Obstet Gynecol. 2002;186:35–43. doi: 10.1067/mob.2002.119180. [DOI] [PubMed] [Google Scholar]
- 33.Xiang AH, Peters RK, Kjos SL, Ochoa C, Marroquin A, Goico J, et al. Effect of thiazolidinedione treatment on progression of subclinical atherosclerosis in premenopausal women at high risk for type 2 diabetes. J Clin Endocrinol Metab. 2005;90:1986–1991. doi: 10.1210/jc.2004-1685. [DOI] [PubMed] [Google Scholar]
- 34.Meyer C, McGrath BP, Teede HJ. Effects of medical therapy on insulin resistance and the cardiovascular system in polycystic ovary syndrome. Diabetes Care. 2007;30:471–478. doi: 10.2337/dc06-0618. [DOI] [PubMed] [Google Scholar]
- 35.Hannon TS, Bacha F, Lee SJ, Janosky J, Arslanian SA. Use of markers of dyslipidemia to identify overweight youth with insulin resistance. Pediatr Diabetes. 2006;7:260–266. doi: 10.1111/j.1399-5448.2006.00199.x. [DOI] [PubMed] [Google Scholar]
- 36.Iannuzzi A, Licenziati MR, Acampora C, Salvatore V, Auriemma L, Romano ML, et al. Increased carotid intima-media thickness and stiffness in obese children. Diabetes Care. 2004;27:2506–2508. doi: 10.2337/diacare.27.10.2506. [DOI] [PubMed] [Google Scholar]
- 37.Reinehr T, Kiess W, de Sousa G, Stoffel-Wagner B, Wunsch R. Intima media thickness in childhood obesity: relations to inflammatory marker, glucose metabolism, and blood pressure. Metabolism. 2006;55:113–118. doi: 10.1016/j.metabol.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 38.Tounian P, Aggoun Y, Dubern B, Varille V, Guy-Grand B, Sidi D, et al. Presence of increased stiffness of the common carotid artery and endothelial dysfunction in severely obese children: a prospective study. Lancet. 2001;358:1400–1404. doi: 10.1016/S0140-6736(01)06525-4. [DOI] [PubMed] [Google Scholar]
- 39.de Sousa G, Brodoswki C, Kleber M, Wunsch R, Reinehr T. Association between androgens, intima-media thickness and the metabolic syndrome in obese adolescent girls. Clin Endocrinol (Oxf) 2010;72:770–774. doi: 10.1111/j.1365-2265.2009.03710.x. [DOI] [PubMed] [Google Scholar]
- 40.Sakuragi S, Abhayaratna K, Gravenmaker KJ, O'Reilly C, Srikusalanukul W, Budge MM, et al. Influence of adiposity and physical activity on arterial stiffness in healthy children: the lifestyle of our kids study. Hypertension. 2009;53:611–616. doi: 10.1161/HYPERTENSIONAHA.108.123364. [DOI] [PubMed] [Google Scholar]
- 41.Dangardt F, Osika W, Volkmann R, Gan LM, Friberg P. Obese children show increased intimal wall thickness and decreased pulse wave velocity. Clin Physiol Funct Imaging. 2008;28:287–293. doi: 10.1111/j.1475-097X.2008.00806.x. [DOI] [PubMed] [Google Scholar]
- 42.Urbina EM, Gao Z, Khoury PR, Martin LJ, Dolan LM. Insulin resistance and arterial stiffness in healthy adolescents and young adults. Diabetologia. 2012;55:625–631. doi: 10.1007/s00125-011-2412-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zachurzok-Buczynska A, Szydlowski L, Gawlik A, Wilk K, Malecka-Tendera E. Blood pressure regulation and resting heart rate abnormalities in adolescent girls with polycystic ovary syndrome. Fertil Steril. 2011;96:1519–1525. doi: 10.1016/j.fertnstert.2011.09.043. [DOI] [PubMed] [Google Scholar]
- 44.Rossi B, Sukalich S, Droz J, Griffin A, Cook S, Blumkin A, et al. Prevalence of metabolic syndrome and related characteristics in obese adolescents with and without polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:4780–4786. doi: 10.1210/jc.2008-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lass N, Kleber M, Winkel K, Wunsch R, Reinehr T. Effect of lifestyle intervention on features of polycystic ovarian syndrome, metabolic syndrome, and intima-media thickness in obese adolescent girls. J Clin Endocrinol Metab. 2011;96:3533–3540. doi: 10.1210/jc.2011-1609. [DOI] [PubMed] [Google Scholar]
- 46.Desideri G, De Simone M, Iughetti L, Rosato T, Iezzi ML, Marinucci MC, et al. Early activation of vascular endothelial cells and platelets in obese children. J Clin Endocrinol Metab. 2005;90:3145–3152. doi: 10.1210/jc.2004-1741. [DOI] [PubMed] [Google Scholar]
- 47.Maggio AB, Wacker J, Montecucco F, Galan K, Pelli G, Mach F, et al. Serum resistin and inflammatory and endothelial activation markers in obese adolescents. J Pediatr. 2012;161:1022–1027. doi: 10.1016/j.jpeds.2012.05.063. [DOI] [PubMed] [Google Scholar]
- 48.Kelly AS, Hebbel RP, Solovey AN, Schwarzenberg SJ, Metzig AM, Moran A, et al. Circulating activated endothelial cells in pediatric obesity. J Pediatr. 2010;157:547–551. doi: 10.1016/j.jpeds.2010.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diamanti-Kandarakis E, Alexandraki K, Piperi C, Protogerou A, Katsikis I, Paterakis T, et al. Inflammatory and endothelial markers in women with polycystic ovary syndrome. Eur J Clin Invest. 2006;36:691–697. doi: 10.1111/j.1365-2362.2006.01712.x. [DOI] [PubMed] [Google Scholar]
- 50.Adali E, Yildizhan R, Kurdoglu M, Bugdayci G, Kolusari A, Sahin HG. Increased plasma thrombin-activatable fibrinolysis inhibitor levels in young obese women with polycystic ovary syndrome. Fertil Steril. 2010;94:666–672. doi: 10.1016/j.fertnstert.2009.03.037. [DOI] [PubMed] [Google Scholar]
- 51.Escobar-Morreale HF, Luque-Ramirez M, Gonzalez F. Circulating inflammatory markers in polycystic ovary syndrome: a systematic review and metaanalysis. Fertil Steril. 2011;95:1048–1058. e1–e2. doi: 10.1016/j.fertnstert.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Talbott EO, Zborowski JV, Boudreaux MY, McHugh-Pemu KP, Sutton-Tyrrell K, Guzick DS. The relationship between C-reactive protein and carotid intima-media wall thickness in middle-aged women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89:6061–6067. doi: 10.1210/jc.2003-032110. [DOI] [PubMed] [Google Scholar]
- 53.Juonala M, Viikari JS, Ronnemaa T, Taittonen L, Marniemi J, Raitakari OT. Childhood C-reactive protein in predicting CRP and carotid intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. Arterioscler Thromb Vasc Biol. 2006;26:1883–1888. doi: 10.1161/01.ATV.0000228818.11968.7a. [DOI] [PubMed] [Google Scholar]
- 54.Barcellos CR, Lage SH, Rocha MP, Hayashida SA, Baracat EC, Romano A, et al. Polycystic ovary syndrome and obesity do not affect vascular parameters related to early atherosclerosis in young women without glucose metabolism disturbances, arterial hypertension and severe abnormalities of lipid profile. Gynecol Endocrinol. 2013;29:370–374. doi: 10.3109/09513590.2012.743009. [DOI] [PubMed] [Google Scholar]
- 55.Blankenberg S, Rupprecht HJ, Bickel C, Peetz D, Hafner G, Tiret L, et al. Circulating cell adhesion molecules and death in patients with coronary artery disease. Circulation. 2001;104:1336–1342. doi: 10.1161/hc3701.095949. [DOI] [PubMed] [Google Scholar]
- 56.Mulvihill NT, Foley JB, Murphy RT, Curtin R, Crean PA, Walsh M. Risk stratification in unstable angina and non-Q wave myocardial infarction using soluble cell adhesion molecules. Heart. 2001;85:623–627. doi: 10.1136/heart.85.6.623. [DOI] [PMC free article] [PubMed] [Google Scholar]