Abstract
In contrast to women with relatively asymptomatic endometriosis, women with endometriosis-associated chronic pelvic pain (CPP) exhibit non-pelvic hyperalgesia and decreased gray matter volume in key neural pain processing regions. While these findings suggest central pain amplification in endometriosis-associated CPP, the underlying changes in brain chemistry and function associated with central pain amplification remain unknown. We performed proton spectroscopy and seed-based resting functional connectivity MRI to determine whether women with endometriosis display differences in insula excitatory neurotransmitter concentrations or intrinsic brain connectivity to other pain-related brain regions. Relative to age-matched pain-free controls, women with endometriosis-associated CPP displayed elevated levels of combined glutamine-glutamate (Glx) within the anterior insula, and greater anterior insula connectivity to the medial prefrontal cortex (mPFC). Increased connectivity between these regions was positively correlated with anterior insula Glx concentrations (r=0.87), as well as clinical anxiety (r=0.61,p=0.02), depression (r=0.60,p=0.03), and pain intensity (r=0.55,p=0.05). There were no significant differences in insula metabolite levels or resting-state connectivity in endometriosis without CPP subjects versus controls. We conclude that enhanced anterior insula glutamatergic neurotransmission and connectivity with the mPFC, key regions of the salience and default mode networks, may play a role in the pathophysiology of CPP independent of the presence of endometriosis.
Keywords: endometriosis, chronic pelvic pain, neuroimaging, glutamate, functional connectivity
Introduction
Endometriosis is a common gynecologic condition estimated to affect 10–15% of reproductive-aged women and up to 80% of women with chronic pelvic pain (CPP).21, 43, 45, 62, 68 Endometriosis is defined as the presence of endometrial glands and stroma (referred to as “endometriosis implants”) outside of the uterus. Thus it is a histological diagnosis that requires surgical evaluation and biopsy. Although endometriosis is the most common finding among women undergoing surgery for CPP and likely contributes to pain in at least some of these women, it is unclear whether endometriosis is the underlying cause of pelvic pain in all of these women. Little is known about the mechanisms involved in the development of chronic pain in this population. As with most chronic pain syndromes, the presence and severity of organic pathology do not correlate with symptom severity and many women with endometriosis, even severe, experience little if any pain.7, 38, 54, 67 Furthermore, standard therapies targeting endometriosis implants are not consistently effective and pain frequently recurs, often without evidence of residual pelvic pathology.57, 61, 66 Against this background, endometriosis must be viewed as an important but insufficient factor in the development of CPP.
Central nervous system (CNS) abnormalities in pain processing have been identified in multiple chronic pain syndromes, which often occur in the absence of identifiable pathology in the area of pain. Findings include hyperalgesia within and outside of areas of clinical pain, parallel increases in neural activity, as well as structural alterations in pain-related cortical areas.16, 28, 31, 47, 56 More recently, aberrant brain neurochemistry and functional connectivity have also been identified in patients with other chronic pain conditions. The insula, a brain region involved the integration of interoceptive, affective and cognitive signals, is a primary focus in pain neuroimaging studies as it is one of the most consistently activated regions during acute and chronic pain.3 For example, increased levels of excitatory neurotransmitters within the posterior insula and decreased levels of inhibitory neurotransmitters within the anterior insula have been identified in patients with fibromyalgia.25, 33 Furthermore, resting state brain connectivity of the insula is augmented in neural pain processing regions of fibromyalgia patients relative to pain-free controls.39, 52 These neural signals likely play a role in the underlying pathophysiology of chronic pain and may also have utility in clinical medicine since they are correlated with important patient-centered outcomes, such as clinical pain intensity and response to pharmacotherapy used to treat chronic pain.32, 51
Our group has previously demonstrated that women with CPP, both with and without endometriosis, also exhibit hyperalgesia in a non-pelvic site as well as decreases in regional gray matter volume in brain regions associated with pain processing including the thalamus, cingulate gyrus, putamen and insula, suggesting a similar problem of pain amplification related to CNS changes.5, 6 While various neural mechanisms contributing to pain amplification have been identified in other pain syndromes, the precise nature of these neural changes remain unknown in women with endometriosis-associated pelvic pain. Identifying specific neural mechanisms associated with pain amplification are necessary to develop targeted treatment strategies for women who are not responsive to traditional medical and surgical therapies for endometriosis and other causes of CPP.
The primary objective of this study was to use proton spectroscopy (1H-MRS) and functional connectivity MRI techniques to determine whether women with endometriosis-associated CPP display changes in excitatory neurotransmitters concentrations in the insula and/or changes in intrinsic brain connectivity between the insula and other pain-related brain structures. To investigate whether such neuroimaging changes are related to endometriosis versus the presence of CPP, we also investigated women with similar pelvic pathology (endometriosis) but without CPP. As previously demonstrated in other chronic pain conditions, we hypothesized that endometriosis-associated CPP is associated with elevated levels of excitatory neurotransmitters in the insula and increased intrinsic brain connectivity of the insula to other brain regions associated with pain perception and modulation, and that these findings would not be identified in endometriosis subjects without CPP.
Material and Methods
Study population and case definitions
The following subgroups of patient participants were included in this cross-sectional observational neuroimaging study: 17 women with endometriosis-associated CPP (⊕Endo⊕CPP)and 13 women with endometriosis without chronic pelvic pain (⊕EndoØCPP). For exploratory purposes, an additional group of 6 women with CPP but no evidence of endometriosis (ØEndo⊕CPP) was also evaluated. Participants in this study were included in a previously published study of regional cerebral gray matter differences in women with chronic pelvic pain.6 All participants were premenopausal women aged 18–52 years who had not undergone prior hysterectomy or bilateral oophorectomy. Women with endometriosis were recruited from a tertiary-care endometriosis and pelvic pain referral center, as well as through advertisement to the local community.
A pool of twenty-four pain-free healthy controls was recruited from ongoing studies using the same functional MRI protocol used in this study (mean age ± SD: 29.3 ± 10.1 years). Controls were recruited through local advertisement. Because brain imaging parameters change with age and age varied significantly across patient subgroups, a subset of healthy controls was randomly chosen ratio from pool of 24 healthy control sample to match the age distribution of the patient subgroups (14 controls were available for the ⊕Endo⊕CPP, and 12 controls were available for the ⊕EndoØCPP). Due to the small sample size in the exploratory ØEndo⊕CPP group (n=6), 11 controls were randomly selected to match the age-distribution of this group. This design was used, similar to previously published methods6, because we were unable to directly compare brain imaging findings of ⊕Endo⊕CPP subjects (mean ± SD age, 26.1 ± 6.3 years) to ⊕EndoØCPP subjects (mean ± SD age, 36.6 ± 8.7 years) due to the significant 10 year age difference between these two groups.
Potential participants with a history of endometriosis were screened by a phone interview and were invited to participate only if they met criteria for either (1) endometriosis with chronic pelvic pain (⊕Endo⊕CPP) or (2) endometriosis without chronic pelvic pain (⊕EndoØCPP). All endometriosis participants were required to have a history of surgically confirmed endometriosis within 3 years of study participation. CPP was defined as moderate to severe pelvic pain rated as ≥ 4 on a 0–10 numeric rating scale, pelvic pain was present for greater than 6 months duration, and was non-cyclic occurring for at least 14 days of each month, not just limited to the time of menstrual bleeding. All numeric pain scales in this study defined the value “0” as “no pain” and “10” as “pain as bad as you can imagine”.35 Pelvic pain had to be localized to the anatomic pelvis, and was not exclusively limited to symptoms of dyspareunia (pain with intercourse), dyschezia (pain with bowel movements), or focal low back pain. All pelvic pain patients reported attempting at least one prior medical or surgical therapy and had persistent pelvic pain. Endometriosis without CPP was defined as the absence of any prior history of chronic pelvic pain and the absence of significant dysmenorrhea, defined as pelvic pain during menstruation rated as ≥ 4 on a 0–10 numeric rating scale occurring for 5 or more days of each menstrual cycle (i.e. all study participants falling into the ⊕EndoØCPP group had at maximum 4 days of mild pain associated with menses).
Using the Complex Medical Symptom Inventory, a standardized diagnostic tool that uses published criteria for the diagnosis of various chronic pain disorders70, all women with endometriosis and/or CPP were formally evaluated and excluded from participation if they had symptoms consistent with one or more of the following chronic pain syndromes: fibromyalgia, chronic fatigue syndrome, interstitial cystitis, vulvodynia, chronic low back pain unrelated to pelvic pain, or temporomandibular disorder. Although comorbid pain conditions are common among women with endometriosis or chronic pelvic pain, we wished to determine whether differences in neuroimaging parameters were identified specifically in women whose primary pain symptom was thought to be secondary to endometriosis, and not secondary to the presence of the above comorbid pain syndromes, which are are known to be associated with CNS changes in pain processing. However, we did not specifically exclude irritable bowel syndrome or migraine headache from our endometriosis or pelvic groups due to the difficulty in recruiting patients without these 2 commonly comorbid pain conditions. However, there was no significant difference in the distribution of these conditions across the endometriosis and CPP samples.
All healthy controls were pain-free women age 18–52 years and had no symptoms of dysmenorrhea, no known history of endometriosis, no history of chronic pain (including pelvic pain), and did not have prior hysterectomy or bilateral oophorectomy. All healthy controls were formally screened in a similar fashion to endometriosis participants, did not meet criteria for any of the chronic pain syndromes previously defined, and did not have a history of chronic, recurrent headaches or irritable bowel syndrome (IBS). Women with current symptoms of major depressive disorder, bipolar disorder, generalized anxiety disorder (according to Diagnostic and Statistical Manual of the American Psychiatric Association IV1 criteria) 1 and those currently on antidepressants for any indication were also not eligible for participation as a healthy control.
Additional exclusion criteria for all participants included severe physical impairment, significant comorbid medical illness that limited physical function, and suicide attempt or substance abuse within 2 years of the study. Women who were pregnant, lactating, or menopausal (defined as no menses for greater than one year unrelated to exogenous hormonal suppression) were also excluded from participation. All participants had to be free of contraindications for an MRI study as determined by a health questionnaire and were right-handed to simplify brain mapping.
This study also included exploratory data on six participants with CPP but no evidence of endometriosis (ØEndo⊕CPP). These women fulfilled all clinical criteria of CPP, had undergone a diagnostic laparoscopy within 3 years of study participation and had no surgical evidence of endometriosis or pelvic adhesions at the time of any prior surgical exploration. No other anatomic sources of pain, such as uterine fibroids or ovarian masses, were identified in these patients. The same additional inclusion and exclusion criteria were applied to this subgroup. Given the smaller sample of ØEndo⊕CPP subgroup [n=6], 2 controls were selected to match each ØEndo⊕CPP participant.
Assessment and analysis of clinical characteristics
All participants completed standardized case report forms to assess their medical history, surgical history, medication use, and any pain symptoms. Participants with endometriosis or CPP completed additional standardized case report forms to assess the severity, pattern, and characteristics of their pelvic pain, as well as validated measures of anxiety and depression. Measurements included numeric ratings (0–10) of pelvic pain during their menses, and the average pain intensity and pain-related disability (“pain interference”) in the last week using the Brief Pain Inventory short-form (BPI-SF).17 The BPI-SF is a self-administered questionnaire that provides 2 summary measures of pain experience: 1) subjective rating of pain intensity and (BPI Severity, range 0–10) and 2) pain interference (BPI Interference, range 0–10) which is defined as the degree to which a subject reports functional and activity-related disability attributed to pain. Higher scores indicate more severe pain intensity and pain interference. Anxiety and depression were measured using the 20-item Trait Anxiety and Trait Depression subscales from the State-Trait Personality Inventory (STPI).60 Scores for each subscale range from 10 to 40, with higher values indicating greater symptoms of anxiety or depression.
Participants with endometriosis or chronic pelvic pain signed medical release forms to obtain operative reports from their most recent pelvic surgery. Each report was reviewed by one of the authors [SA] with significant experience in the surgical evaluation of endometriosis and other gynecologic disease. This author was blinded to the remainder of the study results, and endometriosis was assigned a stage according to the revised American Fertility Society endometriosis scoring system (rAFS).2 Surgical pathology was documented when available but not required for participation since pathologic confirmation was not routinely performed in all participants.
All continuous variables were evaluated for normality. Categorical data were compared using Pearson’s Chi-squared test or Fisher’s exact test. Comparison of continuous variables was conducted using analysis of variance or independent-samples t-test for parametric data or Kruskal-Wallis test for non-parametric data, as appropriate. Proportions, means (standard deviation), medians (range) were presented, as appropriate. The significance threshold was set at p<0.05.
Neuroimaging scanning protocol and analysis
In order to minimize the influence of menstrual cycle variability on study results, all study visits were performed between days 2–10 of the menstrual cycle in women who were not using hormonal contraceptives. Women who were using hormonal contraceptives were scheduled on any day that they were taking an active hormone medication. There was no significant difference in current use of hormonal contraceptive across study groups (p=0.37). Magnetic resonance imaging was performed on a 3.0 Tesla MR scanner (General Electric Medical Systems Signa, Milwaukee, WI). For each subject, a T-1 weighted gradient echo data set (TR/TE=1400/5.5 ms, flip angle=20°, FOV=256×256, yielding 124 sagittal slices with a defined voxel size of 1×1×1.2mm) was acquired. An Eclipse 3.0 T 94 quadrature head coil was used. Inspection of individual T1 MR-images revealed no gross morphological abnormality for any participant.
Acquisition and analysis of proton magnetic resonance spectroscopy
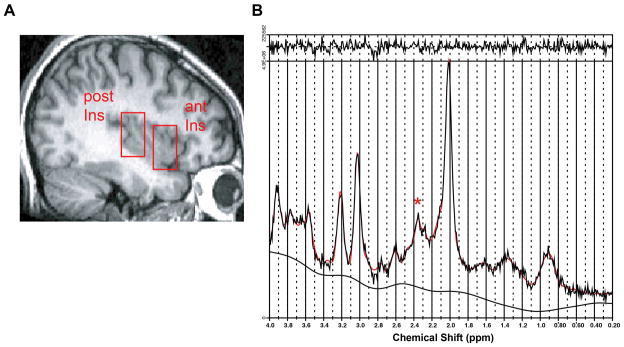
Proton magnetic resonance spectroscopy (1H-MRS), which provides a non-invasive method for the in-vivo study of brain biochemistry and allows measures of chemical concentrations in local brain regions of interest, was used to measure right anterior and posterior insula concentrations of N-acetylaspartate (NAA) and combined glutamate and glutamine (Glx). Since glutamate and glutamine share highly similar structures and have overlapping chemical shifts on spectroscopy, we cannot report individual glutamate or glutamine signals and only report the combined signal that comes from both molecules. Single-voxel proton magnetic resonance spectroscopy (1H-MRS) was performed using the following parameters as previously described by our group33: point resolved spectroscopy, TR/TE=3,000/3 msec, flip angle=90°, number of excitations 8, field of view 16 cm, with a volume of interest (VOIC) of 2 × 2 × 3 cm. Participants were at rest during the 1H-MRS session. During each session, 2 separate single-voxel spectroscopy sequences were performed, once with the VOI placed in the right anterior insula and once in the right posterior insula (Figure 1A). The approximate Montreal Neurological Institute coordinates for the centers of the right anterior and right posterior voxels were 34,19,0 and 38,−17,8, respectively. These coordinates were specifically chosen as the primary area of interest since these include regions shown previously to be activated during acute pain13, and have been shown to have augmented pain activity in patients with fibromyalgia.18, 28
Figure 1.
Anterior and posterior insula regions of interest (ROIs) and resulting spectrum. A. Sagittal T1- weighted images showing single-voxel placement for right anterior insula (ant) and right posterior insula (post). B. Representative proton magnetic resonance spectroscopy spectrum from the posterior insula fit with LCModel (red trace; * = resonance from 2 glutamate proton resonances at 2.35 parts per million). LCModel uses linear combination of individual spectra obtained from pure molecular species to fit the experimental spectra. Absolute concentrations of Glx (glutamine and glutamate) and NAA were calculated in arbitrary units using water as an internal scaling factor.
The raw data from each single-voxel MR spectroscopy sequence underwent manual post-processing using dedicated 1H-MRS software (LCModel; Stephen Provencher, Oakville, Ontario, Canada). LCModel uses a linear combination of individual spectra obtained from pure molecular species to fit the experimental spectra (Figure 1B). Values for combined glutamate and glutamine (Glx) and NAA were calculated as absolute concentrations using the water signal for normalization.55 Resulting metabolite absolute concentrations were reported in arbitrary institutional units. Metabolite levels were corrected for cerebrospinal fluid (CSF) volume in each participant since spectroscopy voxels include CSF, and the volume of CSF dilutes 1H-MRS derived metabolite values. Voxel Based Morphometry, a tool box that operates within the image analysis program Statistical Parametric Mapping (SPM; http://www.fil.ion.ucl.ac.uk/spm/software), was used to correct metabolite levels for CSF volume. High resolution T1-weighted images were segmented into gray matter, white matter, and CSF. Regions of interest within the anterior and posterior insula were used to extract gray matter, whiter matter, and CSF volumes from these images using the SPM2 toolbox Marsbar (http://marsbar.sourceforge.net).12 Metabolite values were corrected by dividing the observed concentration in arbitrary institutional units by the percentage of volume of the entire voxel that was not occupied by CSF (i.e. the percentage of voxel volume occupied by gray matter plus white matter). Corrected metabolite concentrations were entered into SPSS version 16 (SPSS, Chicago, IL) for calculation of difference between patient subgroups and healthy controls groups and for correlational analyses with clinical measures. Independent-samples t-tests and spearman’s correlational analyses were used. Significant correlations were defined as p < 0.05.
Acquisition and analysis of resting-state functional connectivity
Six minutes of resting-state fMRI data were acquired using a whole brain T2*-weighted gradient echo BOLD EPI pulse sequence (TR/TE=2000/30 ms, flip angle=90°, 43 AC-PC aligned axial slices, voxel size=3.125×3.125×4 mm). Subjects were instructed to relax and lie still with their eyes open during the functional MRI scan. A fixation cross was presented on the screen and subjects were told to lie still and fixate on the cross throughout the scan. Minimal cognitive tasks such as staring at a cross typically do not disrupt resting-state networks. Physiological data were collected simultaneously to the fMRI scan to correct cardiorespiratory fluctuations influence on fMRI functional connectivity estimation. Cardiac data were acquired using an infrared pulse oximeter (GE) attached to the right middle finger. Respiratory volume data were acquired using a GE MR- compatible belt placed around the subject’s abdomen. Participants’ motion was minimized using foam pads placed around the head along with a forehead strap.
Functional MRI data were pre-processed using the validated FSL (FMRIB’s Software Library, http://www.fmrib.ox.ac.uk/fsl), AFNI (afni.nimh.nih.gov/afni, and FreeSurfer (http://surfer.nmr.mgh.harvard.edu) software packages. Data were corrected for physiological artifacts (RETROICOR, AFNI27), slice timing, affine head motion (MCFLIRT, FSL40). Non-brain regions were removed from the image (BET, FSL59). Cortical surface reconstruction was completed to perform improved structural-functional co-registration using FreeSurfer’s bbregister tool30. Functional data were then registered to standard Montreal Neurological Institute (MNI) space using FMRIB’s nonlinear co-registration tool (FNIRT, FSL). Data were resampled to 2-mm isotropic voxels and spatially smoothed using a 6-mm FWHM Gaussian kernel. High-pass temporal filtering was applied at 0.008Hz.
Functional connectivity was computed using a seed-based correlation analysis.9, 26, 29, 46 The seed region was defined in the right anterior insula using the same regions evaluated with 1H-MRS. The anterior insula was chosen since this region was found to have elevated excitatory metabolite concentrations relative to healthy controls. The timeseries signals were extracted and averaged from the seed region, and then used as a regressor in a general linear model. Nuisance regressors were included in this model to control for any residual shared variance with non-neuronal signals: the six translation and rotation parameters of the rigid body head motion correction step, fMRI timeseries signals averaged over regions centered in the deep cerebral white matter, cerebral ventricles, and cardiorespiratory artifacts defined by convolving the heart rate and respiratory variation timeseries with appropriate cardiac and respiratory transfer functions, as defined by Chang and colleagues15 and Birn and colleagues8. We did not include the global signal in this analysis. Seed correlation maps, and their variance, were passed up to group level analyses. Such analyses contrast connectivity between different subgroups of endometriosis/CPP patients and age-matched healthy controls, using FLAME (FMRIB’s Local Analysis of Mixed Effects, FSL).
Given the elevated concentrations of excitatory neurotransmitters in the anterior insula of the endometriosis with CPP subgroup, we also performed whole brain voxel-wise linear regression analysis to investigate the link between functional connectivity and levels of excitatory neurotransmitters in the seed regions (anterior and posterior insula), as well as clinical measures of pain and mood. In this analysis, CSF-adjusted levels of excitatory neurotransmitters, BPI pain intensity, STPIDA-A, and SPTIDA-A were used as the independent variables and functional connectivity data (seed correlation and variance maps) were used as the dependent variables. Functional connectivity values (z-statistic) were extracted from the peak voxel, rather than all voxels of the ROI, since peak voxels have previously been demonstrated to have stronger correlation with synaptic activity.4 All brain maps were thresholded using the standard FSL toolbox cluster correction for multiple comparisons. The FSL cluster-forming threshold of Z>2.3 and a cluster-size threshold of p<0.05 to control for familywise error (FWE) is considered widely acceptable and accounts for multiple comparisons.
Ethical approval
Approval for this study was obtained from the University of Michigan Institutional Review Board. All participants provided informed, signed consent prior to participation in the study protocol.
Results
Clinical and demographic characteristics of participant subgroups
The surgical history and clinical characteristics of patients with endometriosis or pelvic pain are presented in Table 1. Participants with CPP (⊕Endo⊕CPP and ØEndo⊕CPP subgroups) reported similar and clinically severe pain intensity scores, with high average pain intensity in the past month and during their menses. There was no significant difference in average duration of pelvic pain symptoms, number of pelvic pain days per month, or pain intensity before or during menses when comparing these two pelvic pain subgroups (p>0.30 for all comparisons). The ⊕EndoØCPP actually had more advanced-stage endometriosis than the ⊕Endo⊕CPP group, but there was no correlation between endometriosis stage and any other clinical pain characteristic. There was no difference in proportion of women who were currently using a method of hormonal contraceptive (Table 1). There was also no difference in proportion of healthy controls currently using hormonal contraceptives as compared to those with endometriosis and/or CPP (p=0.37).
Table 1.
Clinical characteristics of patient subjects with pelvic pain and/or endometriosis patients
| ⊕Endo ⊕CPP (n=17) | ⊕Endo ØCPP (n=13) | ØEndo ⊕CPP (exploratory group, n=6) | ANOVA 3-group P-value |
|
|---|---|---|---|---|
| rAFS Endometriosis score at most recent surgery | ||||
| I | 11 (64.7) | 4 (30.8) | 0 | <0.001 |
| II | 3 (17.7) | 1 (7.7) | 0 | |
| III | 1 (5.9) | 6 (46.2) | 0 | |
| IV | 2 (11.8) | 2 (15.4) | 0 | |
| Currently using hormonal contraceptive | 10 (58.8) | 4 (30.8) | 4 (66.7) | 0.21 |
| Pain duration, median years (95% CI) | 5.5 (3.5, 9.5)* | 0 (0,0)*† | 3.75 (0.90, 9.9)† | <0.001 |
| Months since most recent surgery for endometriosis or pelvic pain | 17.9 ± 13.5 | 18.6 ± 13.7 | 10.7 ± 9.8 | 0.44 |
| Number prior surgeries for endometriosis or pelvic pain | ||||
| 1 | 11 (64.7) | 9 (69.2) | 5 (83.3) | 0.70 |
| ≥ 2 | 6 (35.3) | 4 (30.8) | 1 (16.7) | |
| Number of pelvic pain days per month, median days (95% CI) | 25.0 (14.4, 29.8)* | 0 (0, 1.6)*† | 25.0 (18.4, 29.5)† | <0.001 |
| Measures of clinical pain intensity | ||||
| Average pain intensity in last week (BPI pain intensity score, 0–10) | 4.7 ± 2.2* | 0.5 ± 0.6*† | 5.9 ± 1.0† | <0.001 |
| Average pain-related disability (BPI pain interference score, 0–10) | 3.9 ± 2.7* | 0.6 ± 1.2*† | 3.5 ± 1.2† | <0.001 |
| Average pain intensity during menses (0–10) | 8.8 ± 1.4* | 1.5 ± 2.9*† | 8.8 ± 1.3† | <0.001 |
| Depression score (STPI depression subscore) | 17.9 ± 4.5* | 13.8 ± 4.1* | 14.0 ± 4.1 | 0.02 |
| Anxiety score (STPI anxiety subscore) | 18.3 ± 4.5 | 14.6 ± 4.1 | 16.3 ± 3.9 | 0.08 |
All values are mean ± standard deviation, median (range), n (%)
⊕Endo⊕Pain = endometriosis with chronic pelvic pain; ⊕EndoØPain = endometriosis without chronic pelvic pain, ØEndo⊕Pain = chronic pelvic pain without endometriosis; rAFS = Revised American Fertility Society endometriosis score; BPI = Brief Pain Inventory, STPI=State-Trait Personality Inventory.
Post hoc analyses were performed to determine significant differences between subgroups, significant differences (p<0.05, corrected for multiple comparisons) are marked as follows:
⊕Endo⊕Pain vs. ⊕EndoØPain;
⊕EndoØPain vs. ØEndo⊕Pain;
⊕Endo⊕Pain vs. ØEndo⊕Pain
Proton Resonance Spectroscopy of the anterior and posterior insula in subjects with endometriosis with and without chronic pelvic pain compared with healthy controls
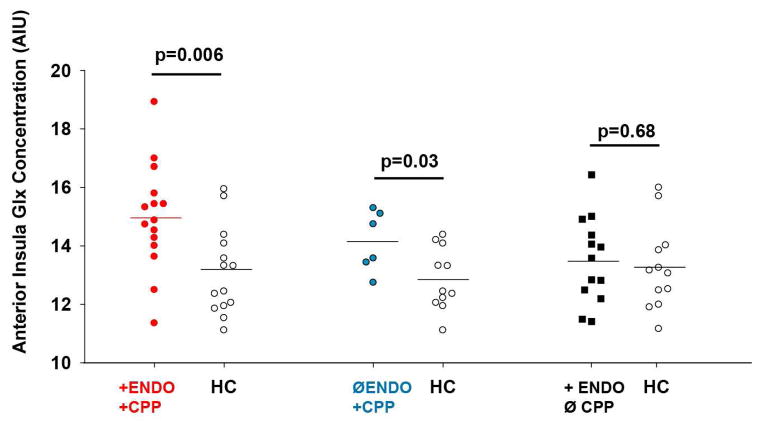
1H-MRS values were not available for two subjects in the ⊕Endo⊕CPP group due to poor quality spectra. As shown in Table 2 and Figure 2, ⊕Endo⊕CPP participants displayed elevated levels of Glx within the anterior insula relative to their age-matched healthy controls. When examining anterior insula metabolite to creatine ratios, participants with ⊕Endo⊕CPP also demonstrated elevated Glx/Cr and reduced NAA/Cr ratios. There were no significant differences in posterior insula metabolite concentrations or metabolite/Cr ratios in the ⊕Endo⊕CPP when compared to age-matched healthy controls. Unlike participants with CPP, endometriosis without CPP participants (⊕EndoØCPP) did not demonstrate any difference in any of the anterior or posterior insula metabolite concentrations or creatine ratios when compared to their age-matched controls.
Table 2.
Anterior and posterior insula corrected metabolite levels and metabolite:creatine ratios.
| ⊕Endo⊕CPP vs controls | ⊕EndoØCPP vs controls | ØEndo⊕CPP vs controls (exploratory analysis) | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| CPP + ENDO (n=15) | Controls (n=14) | p-value | ENDO no CPP (n=13) | Controls (n=12) | p-value | CPP only (n=6) | Controls (n=11) | p-value | |
| Age (years) | 26.7 ± 6.6 | 26.5 ± 6.6 | 0.95 | 36.6 ± 8.7 | 35.0 ± 9.5 | 0.85 | 24.2 ± 4.6 | 24.3 ± 4.0 | 0.96 |
| Anterior insula metabolite concentrations | |||||||||
| Glx | 14.96 ± 1.83 | 13.11 ± 1.50 | 0.006 | 13.50 ± 1.48 | 13.26 ± 1.46 | 0.68 | 14.15 ± 1.04 | 12.86 ± 1.07 | 0.03 |
| NAA | 11.35 ± 0.98 | 11.80 ± 0.89 | 0.21 | 11.77 ± 1.03 | 11.87 ± 1.02 | 0.81 | 11.06 ± 0.88 | 11.46 ± 0.84 | 0.37 |
| Anterior insula metabolite/Creatine ratio | |||||||||
| Glx/Cr | 1.96 ± 0.30 | 1.77 ± 0.17 | 0.05 | 1.72 ± 0.18 | 1.74 ± 0.19 | 0.83 | 1.87 ± 0.22 | 1.80 ± 0.15 | 0.41 |
| NAA/Cr | 1.48 ± 0.08 | 1.60 ± 0.09 | 0.001 | 1.50 ± 0.07 | 1.55 ± 0.08 | 0.07 | 1.46 ± 0.13 | 1.60 ± 0.11 | 0.04 |
| Posterior insula metabolite concentrations | |||||||||
| Glx | 11.74 ± 1.66 | 11.91 ± 1.61 | 0.78 | 11.87 ± 1.22 | 11.46 ± 1.48 | 0.46 | 11.78 ± 1.23 | 12.01 ± 1.55 | 0.76 |
| NAA | 9.66 ± 0.81 | 9.99 ± 0.64 | 0.26 | 10.05 ± 0.86 | 9.98 ± 0.52 | 0.82 | 9.32 ± 0.62 | 10.08 ± 0.47 | 0.01 |
| Posterior insula metabolite/Creatine ratio | |||||||||
| Glx/Cr | 1.80 ± 0.28 | 1.80 ± 0.21 | 1.00 | 1.79 ± 0.15 | 1.75 ± 0.23 | 0.61 | 1.79 ± 0.17 | 1.81 ± 0.22 | 0.88 |
| NAA/Cr | 1.49 ± 0.19 | 1.52 ± 0.13 | 0.57 | 1.52 ± 0.13 | 1.53 ± 0.12 | 0.87 | 1.42 ± 0.12 | 1.52 ± 0.14 | 0.14 |
All values are mean ± standard deviation.
Glx=combined glutamine-glutamate; NAA= N-acetylaspartate.
Figure 2.
Combined glutamine and glutamate (Glx) levels are elevated within the anterior insula in women with chronic pelvic pain (CPP), regardless of endometriosis status.
Linear regression was used to calculate residual values of metabolite concentrations after adjusting for age. Spearman correlational analysis was then performed to evaluate the relationship between age-adjusted metabolite concentrations, clinical pain intensity, depression, and anxiety within each of the endometriosis patient subgroups. Among participants with endometriosis and chronic pelvic pain (⊕Endo⊕CPP), there was no statistically significant correlation (defined as p<0.05) between any anterior or posterior insula metabolite concentrations and measures of anxiety, depression, BPI pain intensity, or BPI pain interference. Similarly, there was no significant correlation between anterior or posterior insula metabolite concentrations and anxiety, depression, or pain measures among participants with endometriosis without CPP (⊕EndoØCPP).
Intrinsic brain connectivity in subjects with endometriosis with and without chronic pelvic pain compared with healthy controls
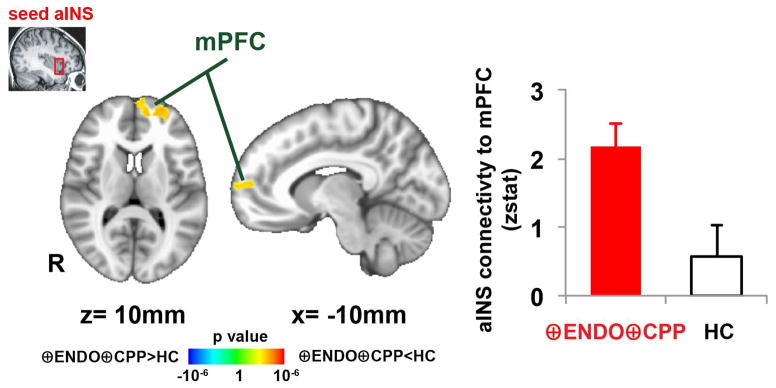
Resting functional connectivity was examined in 16 ⊕Endo⊕CPP participants (data was incomplete in 1 participant) and all 13 ⊕EndoØCPP participants. Since we observed increased Glx within the anterior insula of ⊕Endo⊕CPP subjects, we used this 1H-MRS voxel as a seed in resting connectivity MRI. When comparing ⊕Endo⊕CPP subjects to age-matched controls, greater connectivity was identified between the anterior insula and regions of the medial prefrontal cortex (mPFC), as well as the occipital cortex (Figure 3, Table 3). We did not identify any brain regions with altered connectivity to the posterior insula when comparing ⊕Endo⊕CPP subjects to age-matched controls.
Figure 3.
Greater connectivity between anterior insula and medial prefrontal cortex (mPFC) in women with endometriosis and chronic pelvic pain (⊕Endo⊕CPP, n=16).
Table 3.
Brain regions showing significant differences in anterior insula connectivity between endometriosis with CPP patients (n=16) and the age-matched healthy controls (n=16).
| size | side (mm3) | MNI coordinates
|
peak z-stat | |||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Dorsomedial prefrontal cortex | L | 4,384 | −26 | 54 | 4 | 3.25 |
| Medial prefrontal cortex | L | 4,384 | −10 | 56 | 10 | 2.96 |
| Occipital cortex | L | 5,168 | −22 | −92 | −14 | 4.12 |
Resting functional connectivity was also compared in ⊕EndoØCPP subjects versus age-matched healthy controls. In contrast to endometriosis subjects with CPP, we did not identify any brain regions with altered connectivity to the anterior or posterior insula in the endometriosis subjects without CPP.
Correlations between spectroscopy and functional connectivity in subjects with endometriosis and chronic pelvic pain (⊕Endo⊕CPP)
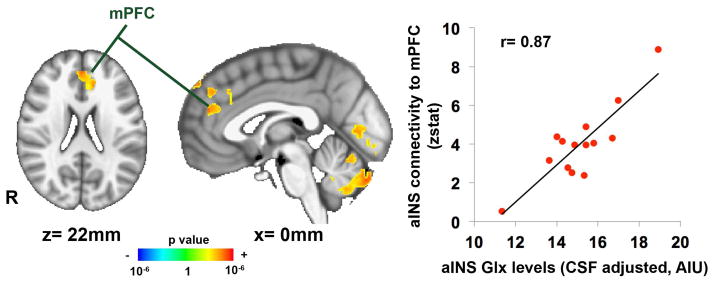
Given the finding of increased connectivity between the anterior insula and medial prefrontal cortex in the ⊕Endo⊕CPP subgroup compared to age-matched controls, we then sought to determine if resting functional connectivity of the anterior insula was correlated with the anterior insula Glx. Whole brain linear regression analysis was performed in 14 ⊕Endo⊕CPP subjects who had both 1H-MRS and intrinsic connectivity data and found positive correlation between Glx metabolite concentrations in the anterior insula and resting anterior insula connectivity to the mPFC (r=0.87) (Table 4, Figure 4).
Table 4.
Brain regions showing significant correlation between Glx levels in the anterior insula and the anterior insula connectivity in endometriosis with CPP patients (n=14).
| side | size (mm3) | MNI coordinates
|
peak z-stat | |||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Dorsomedial prefrontal cortex | - | 4,960 | 0 | 46 | 22 | 3.69 |
| Cerebellum | L | 7,184 | −2 | −82 | −34 | 3.92 |
| Occipital cortex | - | 4,048 | 0 | −76 | 4 | 3.64 |
| Occipital cortex | L | 1,568 | −6 | −90 | −14 | 3.27 |
Figure 4.
Anterior insula connectivity to medial prefrontal cortex (mPFC) is correlated with Glx levels in anterior insula in women with endometriosis and chronic pelvic pain (⊕Endo⊕CPP, n=14), p<0.05.
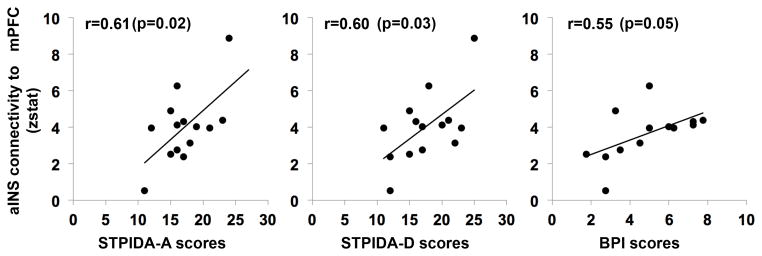
We then performed ROI correlation analysis between resting anterior insula connectivity to mPFC and clinical measures of pain and mood in the ⊕Endo⊕CPP group. Functional connectivity values (z statistics) were extracted from the peak voxel of dorsomedial prefrontal cortex (from the whole brain regression result, Table 4). We found that greater anterior insula connectivity to mPFC was significantly correlated with anxiety scores (STPIDA-A, r(12)=0.61, p=0.02), depression scores (STPIDA-D, r(12)=0.60, p=0.03), as well as clinical pain intensity (BPI, r(11)=0.55, p=0.05) (Figure 5).
Figure 5.
Anterior insula connectivity to medial prefrontal cortex (mPFC) is correlated with anxiety (STPIDA-A), depression (STPIDA-D), and pain intensity (BPI) scores in women with endometriosis and chronic pelvic pain (⊕Endo⊕CPP, n=14).
Exploratory analysis in patients with chronic pelvic pain without endometriosis compared with healthy controls
Participants with ØEndo⊕CPP [n=6] showed a significant increase in Glx concentrations in the anterior insula, as well as reduced NAA/Cr ratios in this region as compared to age-matched healthy controls. There were no significant differences in posterior insula metabolite concentrations or metabolite/Cr ratios in this subgroup, except for decreased NAA concentrations in the posterior insula (Table 2). These findings are thematically similar to those seen in the ⊕Endo⊕CPP versus healthy controls analysis. Given the small sample size of n=6, correlational analysis with age-adjusted metabolite concentrations and clinical variables was not performed. Resting functional connectivity was also examined in these six ØEndo⊕CPP participants. We did not identify any brain regions with altered connectivity to the insula when comparing ⊕Endo⊕CPP subjects and age-matched healthy controls.
Discussion
Consistent with our hypothesis, women with endometriosis-associated CPP demonstrate increased concentrations of excitatory neurotransmitters in the anterior insula and had greater intrinsic connectivity of the same anterior insula region to the mPFC, both known to be regions important in pain processing. Furthermore, increased connectivity between these two regions was positively correlated with anterior insula Glx concentrations, as well as with pain intensity, anxiety and depression symptom severity. These findings are thematically similar to those in other centralized pain states, including fibromyalgia and IBS.32, 37, 39, 51, 52 The CPP patients without endometriosis showed similar findings of elevated excitatory neurotransmitters in the anterior insula, while the endometriosis patients without CPP showed no evidence of changes in insular metabolite levels or resting connectivity relative to pain-free controls, suggesting these alterations in brain physiology may be specific to the pelvic pain state, rather than simply from endometriosis.
Increased excitatory neurotransmitter levels in the anterior insula
The role of the glutamergic system in the neurobiology of chronic pain is an area of active investigation. Glutamate is the brain’s primary excitatory neurotransmitter. Astrocystes transform glutamate to glutamine, which serves as a precursor to both glutamate and γ-aminobutyric acid (GABA), the primary inhibitory neurotransmitter. Using 1H-MRS, we previously reported increased levels of posterior insula Glx in fibromyalgia patients33 and have shown that longitudinal reduction in pain scores were associated with parallel reductions in posterior insula Glx.34 Moreover, pregabalin, with demonstrated efficacy in fibromyalgia and neuropathic pain, is associated with a reduction in posterior insula Glx.32 In line with this work, these results support the notion that increased activity of the insula is an underlying mechanism of endometrisois-associated CPP and future studies are warranted to investigate whether pharmacologic therapy can effectively reduce insular glutamate levels and treat chronic pelvic pain.
Although these findings are thematically similar to findings in fibromyalgia, it is noteworthy that the main 1H-MRS results in CPP are in the anterior, not posterior insula. The reason for the difference is unknown, and several possible explanations underscore the concept that dysfunction in different mechanistic pathways can lead to the same clinical consequence of central pain amplification. The insula has long been recognized to be a multidimensional region that participates in somatic and visceral sensory processing.65 The posterior division plays a prominent role in pain and interoceptive processing, and its activity correlates with linear changes in stimulus intensity.3, 20, 48 In contrast, the anterior insula is involved in the evaluative processing of pain and emotional awareness19, 69, and is active when pain is perceived, even when no innocuous stimulus is present.22 While these topographic differences are often assigned distinctly, evidence suggests that there is significant overlap and integration of neural signals related to both pain and mood. While sensory information is mapped to the posterior insula, it is re-represented in the anterior insula, with significant overlap of pain, emotion, and sensorimotor response.50 Pain signals become consciously accessible in the anterior insula, enabling the affective experience of pain. Furthermore, regions involved in such pathways may shift in disease states, which may explain the significant comorbidity of pain, depression and “emotional allodynia”-the concept that individuals with major depression report pain to stimuli that are not considered painful.
Although prior work from our group did not identify differences in excitatory neurotransmitter metabolites in the anterior insula of patients with fibromyalgia33, we have demonstrated lower levels of GABA, the primary inhibitory neurotransmitter, in the right anterior insula.25 Thus, while the molecular underpinnings may not be the same in CPP and fibromyalgia patients, both chronic pain populations demonstrate “over-activity” of the anterior insula, the former group due to elevated excitatory neurotransmitters and the latter due to decreased inhibitory neurotransmitters.
Increased connectivity of the anterior insula and the default mode network
In addition to differences in neurotransmitter activity, accumulating evidence also suggests that altered intrinsic brain connectivity is a pathologic factor in the mechanism of chronic pain. One particular area of focus is altered connectivity between the anterior insula, a key region of the salience network, and the default mode network (DMN).10 Recently, a three dimensional model has been proposed in which the salience network, involved in the integration of internal and external signals to identify salient stimuli, mediates the switching between activation of the DMN and the central executive network (CEN).49, 65 The DMN is thought to support self-referential and internally related cognition, while the CEN supports goal-oriented or externally-directed cognition. Resting insula connectivity to areas of the DMN is augmented in patients with fibromyalgia51, 52, IBS37, and chronic low back pain44. Moreover, reductions in clinical pain after treatment with pregabalin32 and acupuncture51 were both associated with reduced insular Glx and reduced resting connectivity between the insula and regions of the DMN. Our findings in endometriosis-associated CPP patients suggest a similar underlying mechanism of pain, since we also demonstrate similar increases in connectivity between the insula and mPFC, a key component of the DMN. One may speculate that patients with chronic pain may have impaired ability to disengage from internal stimuli due to increased connectivity between regions of the salience network and DMN.
Endometriosis and CPP – growing evidence supports a central pain disorder
Our findings add to the growing body of literature, recently summarized by Brawn11, that dysfunction in the CNS pain regulatory system may play a role in the underlying mechanism of endometriosis-associated CPP. For example, our group found that women with CPP exhibit hyperalgesia at a non-pelvic site, which was independent of the presence and severity of endometriosis or the presence or number of comorbid pain disorders.5 We have also demonstrated decreased gray matter volume (GMV) in key pain regulatory regions such as the thalamus, cingulate gyrus, putamen and insula in women with CPP, with and without endometriosis.6 Among those with endometriosis-associated CPP, decreases in GMV in the thalamus, mid-cingulate cortex, and posterior insula negatively correlated with pain unpleasantness. Furthermore, endometriosis without CPP did not exhibit hyperalgesia or changes in regional GMV, but did demonstrate increased GMV in the periaqueductal gray (PAG), a key structure in the endogenous, antinociceptive pain regulatory system. Thus, it is possible that endometriosis without CPP patients experience little if any pelvic pain due to adaptive, antinociceptive activity. Our findings and others suggest that each person’s clinical pain experience is likely determined by a complex interaction between peripheral factors (e.g. endometriosis), anti-nociceptive capacity (e.g. activity of the PAG), and maladaptive changes in the CNS pain regulatory system. A growing body of literature also suggests that even among visceral CPP conditions such as endometriosis and painful bladder syndrome, pelvic pain is often associated with alterations in the mechanical sensitivity of the pelvic floor musculature36, 63, 64 and altered muscle activity in chronic pain may be related to changes in motor cortical structure and function.42 Genetic factors, environmental stressors, and cognitive/behavioral factors likely modulate chronic pain14, 23, and all these factors deserve further investigation in women with endometriosis-associated CPP.
Strengths and limitations
Strengths of this study include the use of patient subgroups with various combinations of pain severity and pelvic pathology, the prospective collection of data regarding clinical pain and mood, and the systematic exclusion of patients with several comorbid pain syndromes using standardized criteria. While positive correlation between intrinsic connectivity and glutamate concentrations has been demonstrated in previous studies using healthy controls24, 41, this is the first study to our knowledge to use multimodal imaging to demonstrate a correlation between Glu and increased connectivity of the insula to areas of the DMN in a chronic pain condition. These findings and others point to the important interplay of neurotransmitter balances and inter-regional brain networks on health and disease.
Although our small sample size limited our ability to disentangle the complex and commonly co-occurring relationship between pain and mood, our findings support prior work that suggests the anterior insula plays a role in both pain and mood.50, 58 Larger samples that include chronic pain patients with and without mood disorders are needed to better differentiate the independent effects of mood and pain. Our sample of endometriosis and CPP patients were recruited through a tertiary care clinic and may not be generalizable to community samples. The cross-sectional design of this study also precludes our ability to determine whether the neuroimaging changes are a cause or consequence of chronic pain. Longitudinal studies are needed to address this important question. We also did not include CPP or endometriosis patients who experienced improvement in pain following therapy and therefore cannot comment on how this group may be similar or different than those who have asymptomatic endometriosis or those whose pain is unresponsive to usual therapy. Furthermore, the endometriosis and pelvic pain subgroups (⊕Endo⊕CPP, ØEndo⊕CPP, and ⊕EndoØCPP) were not directly compared to each other because of the significant age difference between the ⊕Endo⊕CPP and ⊕EndoØCPP groups and the small sample in the ØEndo⊕CPP group. While we have postulated that the ⊕Endo⊕CPP subjects are likely different than the ⊕EndoØCPP group (since the findings in the ⊕Endo⊕CPP group were robust and no findings were identified in the ⊕EndoØCPP), future studies should recruit adequate age-matched subjects that can directly compare these patient subgroups. Also, not all controls underwent prior surgery to exclude the diagnosis of asymptomatic endometriosis. However, given a 0.07–11% prevalence of endometriosis in asymptomatic women53, we would not expect this to significantly influence our results. Finally, the ØEndo⊕CPP subgroup was very small due to challenges recruiting eligible participants in our referral setting that primarily sees endometriosis patients, and this analysis was considered exploratory. Although we did identify similar differences in anterior insula metabolite levels in this subgroup, we did not identify any differences intrinsic brain connectivity. While this sample is likely underpowered to detect this difference if it exists, larger samples that directly compare all subgroups to each other are needed to determine if these central changes are specific to the pelvic pain state, or unique to endometriosis-associated CPP.
Conclusion
In summary, these findings add to the growing body of literature that suggests that, in at least some with women with CPP, pelvic pain is not sufficiently explained by the presence of endometriosis and may be due to dysfunction in the CNS pain regulatory system. These findings have important implications for the evaluation and management of women with CPP and may explain why some of these women do not respond to therapies aimed at eliminating endometriosis. Almost certainly, CPP patients represent highly heterogeneous subgroups that have variable degrees of peripheral and central contributions to pain symptoms. For example, it may be that some women will benefit more from pharmacologic and cognitive therapies aimed at central pain amplification and could avoid repetitive surgeries directed at pelvic pathology. Identification of these subgroups likely represents a critical step in the development of a personalized, mechanism-based approach for individuals with CPP.
Perspective.
Similar to other chronic pain conditions, endometriosis-associated pelvic pain is associated with altered brain chemistry and function in the pain matrix. These findings support central pain amplification as a mechanism of CPP, and clinicians should consider the use of adjunctive therapies that target central pain dysfunction in these women.
Highlights.
Chronic pelvic pain is a symptom in some, but not all women with endometriosis
The mechanism by which only some women with endometriosis experience pain is not understood
Endometriosis-associated pelvic pain is associated with altered brain chemistry and function
These differences were not identified in a pain-free endometriosis subgroup
Central nervous system changes likely contribute to the mechanism of endometriosis pain
Acknowledgments
We would like to thank Keith Newnham for MRI technical assistance.
Footnotes
Presented in part at the International Association for the Study of Pain Annual Scientific Meeting, August 2012, Milan, Italy and the Biennial Conference on Resting State/Brain Connectivity, September 2014, Boston, Massachusetts.
Disclosures: This study was funded by NIH K12HD001438, NIH UL1RR024986, NIH 1S10OD012240-01A1, and the Bayer Droegemueller Award in Clinical Research. TS-W was supported by a grant of the DFG (Deutsche Forschungsgemeinschaft, GZ: SchM 2665/1). VN was supported by NCCAM, NIH: P01-AT006663, R01-AT007550; NIAMS, NIH: R01-AR064367. REH was supported by the Dana Foundation, the Department of Defense (Army grant: DAMD-W81XWH- 07-2-0050), GCRC M01-RR-01066, and NIH: 1R01 AT007550. JK was supported by a grant of the Korea Institute of Oriental Medicine (K15050). REH has previously consulted for Pfizer Inc. DJC has received grants/research support from Pfizer Inc. and Forest Laboratories. He has been a consultant for and served on advisory boards for Pfizer Inc., Eli Lilly and Company, Forest Laboratories, Inc., Cypress Bioscience, Inc, Pierre Fabre Pharmaceuticals, UCB, and AstraZeneca. All other authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (Revised 4th ed.) Washington, DC: Author; 2000. [Google Scholar]
- 2.Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67:817–821. doi: 10.1016/s0015-0282(97)81391-x. [DOI] [PubMed] [Google Scholar]
- 3.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Arthurs OJ, Boniface SJ. What aspect of the fMRI BOLD signal best reflects the underlying electrophysiology in human somatosensory cortex? Clin Neurophysiol. 2003;114:1203–1209. doi: 10.1016/s1388-2457(03)00080-4. [DOI] [PubMed] [Google Scholar]
- 5.As-Sanie S, Harris RE, Harte SE, Tu FF, Neshewat G, Clauw DJ. Increased pressure pain sensitivity in women with chronic pelvic pain. Obstet Gynecol. 2013;122:1047–1055. doi: 10.1097/AOG.0b013e3182a7e1f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.As-Sanie S, Harris RE, Napadow V, Kim J, Neshewat G, Kairys A, Williams D, Clauw DJ, Schmidt-Wilcke T. Changes in regional gray matter volume in women with chronic pelvic pain: a voxel-based morphometry study. Pain. 2012;153:1006–1014. doi: 10.1016/j.pain.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balasch J, Creus M, Fabregues F, Carmona F, Ordi J, Martinez-Roman S, Vanrell JA. Visible and non-visible endometriosis at laparoscopy in fertile and infertile women and in patients with chronic pelvic pain: a prospective study. Hum Reprod. 1996;11:387–391. doi: 10.1093/humrep/11.2.387. [DOI] [PubMed] [Google Scholar]
- 8.Birn RM, Smith MA, Jones TB, Bandettini PA. The respiration response function: the temporal dynamics of fMRI signal fluctuations related to changes in respiration. Neuroimage. 2008;40:644–654. doi: 10.1016/j.neuroimage.2007.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 10.Borsook D, Edwards R, Elman I, Becerra L, Levine J. Pain and analgesia: the value of salience circuits. Prog Neurobiol. 2013;104:93–105. doi: 10.1016/j.pneurobio.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brawn J, Morotti M, Zondervan KT, Becker CM, Vincent K. Central changes associated with chronic pelvic pain and endometriosis. Hum Reprod Update. 2014;20:737–747. doi: 10.1093/humupd/dmu025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox [abstract]. Presented at the 8th International Conference on Functional Mapping of the Human Brain; June 2–6, 2002; Sendai, Japan. Available on CD-ROM in NeuroImage. [Google Scholar]
- 13.Brooks JC, Nurmikko TJ, Bimson WE, Singh KD, Roberts N. fMRI of thermal pain: effects of stimulus laterality and attention. Neuroimage. 2002;15:293–301. doi: 10.1006/nimg.2001.0974. [DOI] [PubMed] [Google Scholar]
- 14.Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci. 2013;14:502–511. doi: 10.1038/nrn3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang C, Cunningham JP, Glover GH. Influence of heart rate on the BOLD signal: the cardiac response function. Neuroimage. 2009;44:857–869. doi: 10.1016/j.neuroimage.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clauw DJ, Schmidt M, Radulovic D, Singer A, Katz P, Bresette J. The relationship between fibromyalgia and interstitial cystitis. J Psychiatr Res. 1997;31:125–131. doi: 10.1016/s0022-3956(96)00051-9. [DOI] [PubMed] [Google Scholar]
- 17.Cleeland C. Measurement of pain by subjective report. In: Chapman C, Loeser J, editors. Issues in pain measurement: advances in pain research and therapy. Raven Press; New York, NY: 1989. pp. 391–403. [Google Scholar]
- 18.Cook DB, Lange G, Ciccone DS, Liu WC, Steffener J, Natelson BH. Functional imaging of pain in patients with primary fibromyalgia. J Rheumatol. 2004;31:364–378. [PubMed] [Google Scholar]
- 19.Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 20.Craig AD, Chen K, Bandy D, Reiman EM. Thermosensory activation of insular cortex. Nat Neurosci. 2000;3:184–190. doi: 10.1038/72131. [DOI] [PubMed] [Google Scholar]
- 21.Crosignani P, Olive D, Bergqvist A, Luciano A. Advances in the management of endometriosis: an update for clinicians. Hum Reprod Update. 2006;12:179–189. doi: 10.1093/humupd/dmi049. [DOI] [PubMed] [Google Scholar]
- 22.Davis KD, Pope GE, Crawley AP, Mikulis DJ. Perceptual illusion of “paradoxical heat” engages the insular cortex. J Neurophysiol. 2004;92:1248–1251. doi: 10.1152/jn.00084.2004. [DOI] [PubMed] [Google Scholar]
- 23.Denk F, McMahon SB, Tracey I. Pain vulnerability: a neurobiological perspective. Nat Neurosci. 2014;17:192–200. doi: 10.1038/nn.3628. [DOI] [PubMed] [Google Scholar]
- 24.Duncan NW, Wiebking C, Tiret B, Marjanska M, Hayes DJ, Lyttleton O, Doyon J, Northoff G. Glutamate concentration in the medial prefrontal cortex predicts resting-state corticalsubcortical functional connectivity in humans. PLoS One. 2013;8:e60312. doi: 10.1371/journal.pone.0060312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foerster BR, Petrou M, Edden RA, Sundgren PC, Schmidt-Wilcke T, Lowe SE, Harte SE, Clauw DJ, Harris RE. Reduced insular gamma-aminobutyric acid in fibromyalgia. Arthritis Rheum. 2012;64:579–583. doi: 10.1002/art.33339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med. 2000;44:162–167. doi: 10.1002/1522-2594(200007)44:1<162::aid-mrm23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 28.Gracely RH, Petzke F, Wolf JM, Clauw DJ. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002;46:1333–1343. doi: 10.1002/art.10225. [DOI] [PubMed] [Google Scholar]
- 29.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. NeuroImage. 2009;48:63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hampson JP, Reed BD, Clauw DJ, Bhavsar R, Gracely RH, Haefner HK, Harris RE. Augmented Central Pain Processing in Vulvodynia. J Pain. 2013 doi: 10.1016/j.jpain.2013.01.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris RE, Napadow V, Huggins JP, Pauer L, Kim J, Hampson J, Sundgren PC, Foerster B, Petrou M, Schmidt-Wilcke T, Clauw DJ. Pregabalin rectifies aberrant brain chemistry, connectivity, and functional response in chronic pain patients. Anesthesiology. 2013;119:1453–1464. doi: 10.1097/ALN.0000000000000017. [DOI] [PubMed] [Google Scholar]
- 33.Harris RE, Sundgren PC, Craig AD, Kirshenbaum E, Sen A, Napadow V, Clauw DJ. Elevated insular glutamate in fibromyalgia is associated with experimental pain. Arthritis Rheum. 2009;60:3146–3152. doi: 10.1002/art.24849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harris RE, Sundgren PC, Pang Y, Hsu M, Petrou M, Kim SH, McLean SA, Gracely RH, Clauw DJ. Dynamic levels of glutamate within the insula are associated with improvements in multiple pain domains in fibromyalgia. Arthritis Rheum. 2008;58:903–907. doi: 10.1002/art.23223. [DOI] [PubMed] [Google Scholar]
- 35.Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP) Arthritis Care Res (Hoboken) 2011;63 (Suppl 11):S240–252. doi: 10.1002/acr.20543. [DOI] [PubMed] [Google Scholar]
- 36.Hellman KM, Patanwala IY, Pozolo KE, Tu FF. Multimodal nociceptive mechanisms underlying chronic pelvic pain. Am J Obstet Gynecol. 2015 doi: 10.1016/j.ajog.2015.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hong JY, Kilpatrick LA, Labus JS, Gupta A, Katibian D, Ashe-McNalley C, Stains J, Heendeniya N, Smith SR, Tillisch K, Naliboff B, Mayer EA. Sex and disease-related alterations of anterior insula functional connectivity in chronic abdominal pain. J Neurosci. 2014;34:14252–14259. doi: 10.1523/JNEUROSCI.1683-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsu AL, Sinaii N, Segars J, Nieman LK, Stratton P. Relating pelvic pain location to surgical findings of endometriosis. Obstet Gynecol. 2011;118:223–230. doi: 10.1097/AOG.0b013e318223fed0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ichesco E, Schmidt-Wilcke T, Bhavsar R, Clauw DJ, Peltier SJ, Kim J, Napadow V, Hampson JP, Kairys AE, Williams DA, Harris RE. Altered resting state connectivity of the insular cortex in individuals with fibromyalgia. J Pain. 2014;15:815–826. e811. doi: 10.1016/j.jpain.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 41.Kapogiannis D, Reiter DA, Willette AA, Mattson MP. Posteromedial cortex glutamate and GABA predict intrinsic functional connectivity of the default mode network. Neuroimage. 2013;64:112–119. doi: 10.1016/j.neuroimage.2012.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kutch JJ, Yani MS, Asavasopon S, Kirages DJ, Rana M, Cosand L, Labus JS, Kilpatrick LA, Ashe-McNalley C, Farmer MA, Johnson KA, Ness TJ, Deutsch G, Harris RE, Apkarian AV, Clauw DJ, Mackey SC, Mullins C, Mayer EA. Altered resting state neuromotor connectivity in men with chronic prostatitis/chronic pelvic pain syndrome: A MAPP: Research Network Neuroimaging Study. Neuroimage Clin. 2015;8:493–502. doi: 10.1016/j.nicl.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ling FW. Randomized controlled trial of depot leuprolide in patients with chronic pelvic pain and clinically suspected endometriosis. Pelvic Pain Study Group. Obstet Gynecol. 1999;93:51–58. doi: 10.1016/s0029-7844(98)00341-x. [DOI] [PubMed] [Google Scholar]
- 44.Loggia ML, Kim J, Gollub RL, Vangel MG, Kirsch I, Kong J, Wasan AD, Napadow V. Default mode network connectivity encodes clinical pain: an arterial spin labeling study. Pain. 2013;154:24–33. doi: 10.1016/j.pain.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mao AJ, Anastasi JK. Diagnosis and management of endometriosis: the role of the advanced practice nurse in primary care. J Am Acad Nurse Pract. 2010;22:109–116. doi: 10.1111/j.1745-7599.2009.00475.x. [DOI] [PubMed] [Google Scholar]
- 46.Margulies DS, Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Mapping the functional connectivity of anterior cingulate cortex. NeuroImage. 2007;37:579–588. doi: 10.1016/j.neuroimage.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 47.Mayer EA, Aziz Q, Coen S, Kern M, Labus JS, Lane R, Kuo B, Naliboff B, Tracey I. Brain imaging approaches to the study of functional GI disorders: a Rome working team report. Neurogastroenterol Motil. 2009;21:579–596. doi: 10.1111/j.1365-2982.2009.01304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mazzola L, Faillenot I, Barral FG, Mauguiere F, Peyron R. Spatial segregation of somato-sensory and pain activations in the human operculo-insular cortex. Neuroimage. 2012;60:409–418. doi: 10.1016/j.neuroimage.2011.12.072. [DOI] [PubMed] [Google Scholar]
- 49.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mutschler I, Ball T, Wankerl J, Strigo IA. Pain and emotion in the insular cortex: evidence for functional reorganization in major depression. Neurosci Lett. 2012;520:204–209. doi: 10.1016/j.neulet.2012.03.095. [DOI] [PubMed] [Google Scholar]
- 51.Napadow V, Kim J, Clauw DJ, Harris RE. Decreased intrinsic brain connectivity is associated with reduced clinical pain in fibromyalgia. Arthritis Rheum. 2012;64:2398–2403. doi: 10.1002/art.34412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Napadow V, LaCount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 2010;62:2545–2555. doi: 10.1002/art.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peterson CM, Johnstone EB, Hammoud AO, Stanford JB, Varner MW, Kennedy A, Chen Z, Sun L, Fujimoto VY, Hediger ML, Buck Louis GM. Risk factors associated with endometriosis: importance of study population for characterizing disease in the ENDO Study. Am J Obstet Gynecol. 2013;208:451 e451–411. doi: 10.1016/j.ajog.2013.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Porpora MG, Koninckx PR, Piazze J, Natili M, Colagrande S, Cosmi EV. Correlation between endometriosis and pelvic pain. J Am Assoc Gynecol Laparosc. 1999;6:429–434. doi: 10.1016/s1074-3804(99)80006-1. [DOI] [PubMed] [Google Scholar]
- 55.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 56.Schmidt-Wilcke T. Variations in brain volume and regional morphology associated with chronic pain. Current rheumatology reports. 2008;10:467–474. doi: 10.1007/s11926-008-0077-7. [DOI] [PubMed] [Google Scholar]
- 57.Shakiba K, Bena JF, McGill KM, Minger J, Falcone T. Surgical treatment of endometriosis: a 7-year follow-up on the requirement for further surgery. Obstet Gynecol. 2008;111:1285–1292. doi: 10.1097/AOG.0b013e3181758ec6. [DOI] [PubMed] [Google Scholar]
- 58.Sliz D, Hayley S. Major depressive disorder and alterations in insular cortical activity: a review of current functional magnetic imaging research. Front Hum Neurosci. 2012;6:323. doi: 10.3389/fnhum.2012.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith SM. Fast robust automated brain extraction. Human brain mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spielberger CD, Gorsuch RL. Manual for the State-Trait Anxiety Inventory: (STAI) “(Self-Evaluation Questionnaire)”. Palo Alto: Consulting Psychologists; 1979. [Google Scholar]
- 61.Sutton CJ, Ewen SP, Whitelaw N, Haines P. Prospective, randomized, double-blind, controlled trial of laser laparoscopy in the treatment of pelvic pain associated with minimal, mild, and moderate endometriosis. Fertil Steril. 1994;62:696–700. doi: 10.1016/s0015-0282(16)56990-8. [DOI] [PubMed] [Google Scholar]
- 62.Treloar SA, Bell TA, Nagle CM, Purdie DM, Green AC. Early menstrual characteristics associated with subsequent diagnosis of endometriosis. Am J Obstet Gynecol. 2010;202:534, e531–536. doi: 10.1016/j.ajog.2009.10.857. [DOI] [PubMed] [Google Scholar]
- 63.Tu FF, As-Sanie S, Steege JF. Musculoskeletal causes of chronic pelvic pain: a systematic review of existing therapies: part II. Obstet Gynecol Surv. 2005;60:474–483. doi: 10.1097/01.ogx.0000162246.06900.9f. [DOI] [PubMed] [Google Scholar]
- 64.Tu FF, As-Sanie S, Steege JF. Prevalence of pelvic musculoskeletal disorders in a female chronic pelvic pain clinic. J Reprod Med. 2006;51:185–189. [PubMed] [Google Scholar]
- 65.Uddin LQ. Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci. 2015;16:55–61. doi: 10.1038/nrn3857. [DOI] [PubMed] [Google Scholar]
- 66.Vercellini P, Barbara G, Abbiati A, Somigliana E, Vigano P, Fedele L. Repetitive surgery for recurrent symptomatic endometriosis: what to do? Eur J Obstet Gynecol Reprod Biol. 2009;146:15–21. doi: 10.1016/j.ejogrb.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 67.Vercellini P, Trespidi L, De Giorgi O, Cortesi I, Parazzini F, Crosignani PG. Endometriosis and pelvic pain: relation to disease stage and localization. Fertil Steril. 1996;65:299–304. [PubMed] [Google Scholar]
- 68.Vigano P, Parazzini F, Somigliana E, Vercellini P. Endometriosis: epidemiology and aetiological factors. Best Pract Res Clin Obstet Gynaecol. 2004;18:177–200. doi: 10.1016/j.bpobgyn.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 69.Wiech K, Lin CS, Brodersen KH, Bingel U, Ploner M, Tracey I. Anterior insula integrates information about salience into perceptual decisions about pain. J Neurosci. 2010;30:16324–16331. doi: 10.1523/JNEUROSCI.2087-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Williams DA, Schilling S. Advances in the assessment of fibromyalgia. Rheum Dis Clin North Am. 2009;35:339–357. doi: 10.1016/j.rdc.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]