Abstract
Malfunction of the trabecular meshwork (TM)/schlemm’s canal (SC) conventional outflow pathway is associated with elevated intraocular pressure (IOP) and, therefore, increased risk of developing glaucoma, a potentially blinding disease affecting more than 70 million people worldwide. This TM/SC tissue is subjected to different types of stress, including mechanical, oxidative, and phagocytic stress. Long-term exposure to these stresses is believed to lead to a progressive accumulation of damaged cellular and tissue structures causing permanent alterations in the tissue physiology, and contribute to the pathologic increase in aqueous humor (AH) outflow resistance. Autophagy is emerging as an essential cellular survival mechanism against a variety of stressors. In addition to performing basal functions, autophagy acts as a cellular survival pathway and represents an essential mechanism by which organisms can adapt to acute stress conditions and repair stress-induced damage. A decline in autophagy has been observed in most tissues with aging and has been considered responsible, at least in part, for the accumulation of damaged cellular components in almost all tissues of aging organisms. Dysfunction in the autophagy pathway is associated with several human diseases, from infectious diseases to cancer and neurodegeneration. In this review, we will summarize our current knowledge of the emerging roles of autophagy in outflow tissue physiology and pathophysiology, including novel evidence suggesting compromised autophagy in the glaucomatous outflow pathway.
1. Introduction
Autophagy is a dynamic catabolic process by which cytosolic material, including organelles, proteins and pathogens, are delivered to the lysosome for degradation. Although autophagy was initially thought to be a bulk cytoplasmic degradation mechanism in response to starvation, numerous studies now support a key role of autophagy in maintaining cellular and tissue homeostasis, as well as an adaptive cellular response to stress, providing protective functions during tissue injury. The trabecular meshwork (TM)/schlemm’s canal (SC) outflow pathway is known to be subjected to different types of stress such as mechanical, oxidative, and phagocytic stress. Short-term exposure to these stresses is expected to elicit adaptive responses, however, long-term exposure may lead to permanent alterations in the tissue physiology and contribute to the pathologic increase in aqueous humor (AH) outflow resistance frequently associated with glaucoma. In this review, we will summarize our current knowledge of the emerging roles of autophagy in outflow tissue physiology and pathophysiology, including novel evidence supporting potential alterations of autophagy in the glaucomatous outflow pathway.
2. Autophagy, A Cellular Survival Pathway Against Stress and Adaptation
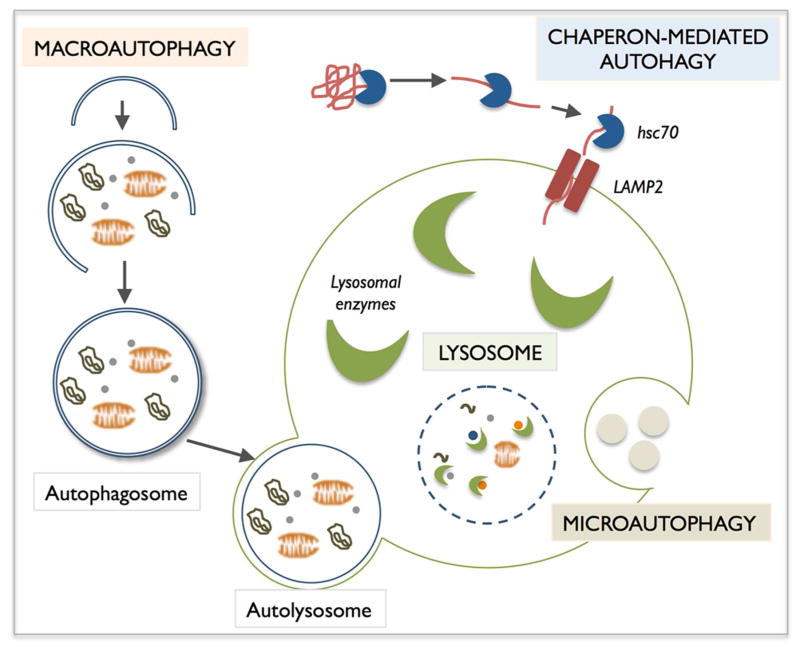
Autophagy, which means self-eating, is an evolutionarily conserved mechanism that allows for the degradation of cytosolic components, such as proteins and organelles, within lysosomes by lysosomal hydrolases. Three main autophagic pathways have been described in mammalian cells based on the delivery route of the cargo material to the lysosomal lumen: macroautophagy, microautophagy and chaperon-mediated-autophagy (CMA) (Figure 1). Macroautophagy, commonly known as simply “autophagy”, involves the formation of a new organelle, the autophagosome, a double-membrane structure that encloses portions of the cytosol, including whole organelles. Autophagosomes then fuse with lysosomes, forming the autolysosomes, in which the luminal material is degraded by resident hydrolases (Mizushima et al., 2008). In microautophagy, small pieces of the cytoplasm are directly engulfed by inward invagination of the lysosomal or late endosomal membrane. In CMA, specific cytosolic soluble proteins, containing a KFERQ-like pentapeptide sequence, are recognized by the chaperone Hsc70 that delivers them to the lysosomal receptor, the lysosome-associated membrane protein type 2A (LAMP-2A), for translocation into the lysosomal lumen (Kon and Cuervo, 2010; Mizushima et al., 2008). After all three types of autophagy, the resultant degradation products are then transported back into the cytosol through the activity of membrane permeases and can be used for different purposes, such as new protein synthesis, energy production, and gluconeogenesis. Among all these types of autophagy, macroautophagy (referred to here as autophagy) is the most extensively studied.
Figure 1.
Summary of the effect of chronic oxidative stress on autophagic lysosomal function in TM cells.
Despite the earliest beliefs, autophagy does not just occur during starvation; autophagy occurs constitutively at basal levels to perform homeostatic functions such as protein and organelle turnover. In fact, the autophagy pathway has emerged as an essential component during development and in maintaining cellular and tissue homeostasis (Mizushima and Komatsu, 2011). A proof of that is that whole-body knockout of essential autophagy genes is incompatible with life; autophagy-incompetent mice die at early embryonic stages because of severe developmental defects, or within the first three days postpartum. In addition to performing basal functions, autophagy acts as a cellular survival pathway. Autophagic activity can be enhanced in response to a wide variety of intracellular and extracellular stimuli and represents an essential mechanism by which organisms can adapt to acute stress conditions and repair stress-induced damage. The importance of autophagy is highlighted by an increasing number of studies linking dysfunction in the autophagy pathway with several human diseases, from infectious diseases to cancer and neurodegeneration (Levine and Kroemer, 2008). Defects in all the steps of the autophagy pathway have been associated to disease; from autophagosome formation, as seen in several cancers, to defects in autophagosome maturation, like in Alzheimer’s disease, or impaired autophagic clearance (i. e. lysosomal storage disorders). Moreover, a decline in autophagy has been observed in most tissues with aging and has been considered responsible, at least in part, for the accumulation of damaged cellular components in almost all tissues of aging organisms (Rubinsztein et al., 2011). This is particularly detriment in patients with proteinopathies, like Huntington’s disease, as autophagy may reach a saturation point in which its capacity to degrade the mutant aggregate-prone proteins is exceeded.
Activation of autophagy is a highly regulated event controlled by a number of evolutionary conserved autophagy related genes (ATG genes) (Mizushima, 2007). The best well-known pathway controlling autophagy is the mTOR pathway, although an mTOR-independent activation of autophagy has been long suspected and is started to be characterized (Sarkar, 2013). In the case of the mTOR-dependent pathway, inactivation of mTOR triggers the recruitment of ATG proteins to the initiation site and activation of two ubiquitin-like conjugation systems (Atg12-Atg5-Atg16 and Atg8/LC3 conjugation systems) that culminate with the formation of the autophagosome. A key event required for autophagosome formation is the lipidation of the autophagosome marker LC3-I to LC3-II (Kimura et al., 2009; Mizushima and Yoshimori, 2007). LC3 is synthesized as a precursor form that is cleaved by the protease ATG4B, resulting in the cytosolic isoform LC3-I. Upon induction of autophagy, LC3-I is conjugated to phosphatidylethanolamine to form LC3-II. LC3-II is incorporated to the nascent and elongating autophagosome membrane and remains on the autophagosome until fusion with the lysosomes. In the autolysosomes LC3-II is then either degraded or delipidated by ATG4 and recycled. A number of different intracellular and extracellular signals and factors have been shown to regulate autophagy, i.e. lack of nutrients, reactive oxygen species or, as we will review here, mechanical strain or ascorbic acid (Kroemer et al., 2010).
3. The Outflow Pathway, A Tissue Controlling Intraocular Pressure
The TM/SC outflow pathway is a highly specialized tissue located at the angle formed by the cornea and the iris. This tissue is involved in intraocular pressure (IOP) homeostasis by modulating the outflow of AH from the anterior chamber to the venous system. Increased IOP resulting from abnormally high outflow resistance is commonly associated with primary open angle glaucoma (POAG), an age-related disease second leading form of permanent blindness worldwide (Bill and Phillips, 1971; Quigley, 2011; Stamer and Acott, 2012).
In addition to modulating AH outflow resistance, the conventional outflow pathway is believed to be involved in detoxification of the AH, phagocytosis of cellular debris, and the maintenance of immune privilege in the eye. To accomplish all these functions, the conventional outflow pathway is organized, despite its small size (100–150 μg, containing approximately 200,000–300,000 cells), as a complex structure composed of morphologically and functionally different cell types (Lütjen-Drecoll, 1999; Tripathi, 1977). The TM/SC tissue is structured into four differentiated layers through which the AH must pass before leaving the eye: the inner uveal meshwork, the corneoscleral meshwork, the juxtacanalinular tissue (JCT), and the inner wall of the SC. The uveal and the corneoscleral meshworks are composed of sheets of connective tissue beams lined by TM endothelial cells. The beams attach to each other in several layers forming a porous filter-like structure. TM cells covering the beams are involved in phagocytosis and tissue remodeling. The JCT is composed of loosely arranged extracellular matrix (ECM) in which JCT cells are randomly distributed. JCT cells are characterized by the presence of processes or extensions connecting the TM cells from the corneoscleral meshwork with cells from the inner wall of SC. The cells of the inner wall endothelium of SC constitute the only continuous cell layer in the outflow pathway. The JCT/SC region contains the locus of both normal outflow resistance and the abnormal resistance in POAG. The causes for this abnormal resistance are not known, but it is believed to result from failure of the TM/SC cellular function (Stamer and Acott, 2012).
As it will be more extensively explained later within the specific sections, the cells in the outflow pathway are continuously subjected to a variety of physiological stress during their lifetime, as part of their normal metabolism (Liton and Gonzalez, 2008). Three major types of stresses have been identified: oxidative stress, caused by the presence of reactive oxygen species (ROS) in the AH; phagocytic stress, due to the clearing of pigment particles and cell debris; and mechanical stress, in the form of both strain and shear stress, as a result of changes in IOP and fluid flow, respectively. It is therefore imperative that these cells account with protective and repair mechanisms that allows them to cope with these daily challenges. This is particularly important, since cells in the TM are known to be postmitotic differentiated cells characterized by a low renewal rate; injured cells are not readily replaced and damage is not diluted through cell division, as occurs in highly replicative tissues. Although there are still few studies in the literature, the autophagic lysosomal pathway is arising as a critical cellular pathway activated in response to these stressors, contributing to keeping outflow pathway homeostasis. Dysregulation of this pathway, in particularly during aging, might compromise the ability of TM cells to respond to stress and lead to progressing cellular and tissue dysfunction (Liton et al., 2009a; 2009b).
4. The Role of Autophagy in Outflow Pathway Physiology
4. 1. Autophagy, Mechanotransduction and Outflow Pathway Physiology
As Coleman and Trokel showed back in 1969, the TM/SC outflow pathway tissues are constantly exposed to changing levels in IOP (Coleman and Trokel, 1969). These variations in IOP include transient pressure changes of up to 10 mmHg resultant from blinking and eye movement, as well as continuous small cyclic oscillations (2–3 mmHg) associated with the ocular pulse (Johnstone, 2004). Studies performed in human enucleated eyes from postmortem donors fixated at different pressures, as well as in eyes from rhesus monkeys subjected to graded levels of physiologic IOP levels in vivo, have documented dramatic changes in the morphology of the outflow pathway under the influence of changing IOP (Grierson and Lee, 1975a; 1975b; Johnstone and Grant, 1973). Increased IOP results in distention and stretching of the TM and its contained cells, while decreased IOP leads to relaxation of the tissue]. According to measurements conducted by Grierson and Lee, changes in pressure from 8 to 30 mm Hg could result in a level of stretching of the outflow pathway cells that could reach as much as 50% (Grierson and Lee, 1975b; 1975a). Such level of strain causes profound changes to cell morphology, affecting a variety of cellular properties such as motility, stiffness, contraction, orientation and cell alignment. Therefore, TM cells must undoubtedly possess biological mechanisms that allow them to sense and respond to these mechanical forces, so they can adapt and survive. A very recent study performed in our laboratory suggests that autophagy could be one of these cellular adaptative mechanisms in response to strain (Porter et al., 2014). By using an in vitro experimental model mimicking acute sustained elevation of IOP (static biaxial stretch), we found autophagy to be activated quickly after application of mechanical forces. Activation of autophagy was characterized by a dramatic elevation in the levels of LC3-II, which could be prevented by pharmacological blockage of autophagosome formation with 3-MA. Moreover, when autophagic flux was evaluated, our data indicated that not only mechanical stretch does not perturb maturation of autophagosomes into autolysosomes, but also that the number and size of autolysosomes was higher in mechanically stretched cells.
Activation of autophagy was not restricted to in vitro cultured TM cells. We also confirmed the occurrence of autophagy in TM cells in situ in high pressure perfused eyes compared to eyes perfused at normal pressure conditions. Similarly, autophagy was characterized by elevated LC3-II levels and the presence of autophagic figures, preferentially in the cells of the corneoscleral meshwork. These findings nicely corroborated earlier observations by Grierson et al. describing increased size and number of lysosomes and lysosomal complexes in the outflow pathway cells of Rhesus monkeys subjected to high IOP (Grierson and Lee, 1975b).
Two immediate questions arise from these studies, as discussed in our original manuscript: (1) whether autophagy is a primary response to elevated pressure or, in contrast, it is a secondary response to morphological tissue deformations, similar to those described by Battista et al. (Battista et al., 2008); and (2) which is the role of stretch-induced autophagy in cellular and tissue physiology. Regarding the first question, the fact that activation of autophagy was observed as early as 30 min post-stretch reasonably supports a role of the autophagic pathway as one of the initial responses elicited in TM cells to cope with the stress and regain homeostasis. Following on this, we investigated the possibility that stretch-induced autophagy in TM cells could be mediated by chaperone-assisted autophagy (CASA). Chaperon-assisted autophagy is a selective type of autophagy, essential for mechanotransduction, which has been recently described in mammalian cells in response to tension (Arndt et al., 2010; Ulbricht et al., 2013). This type of autophagy integrates tension sensing, autophagosome formation and transcription regulation. The CASA multichaperone complex comprised of Hsc70, HspB8 and BAG3, senses the mechanical unfolding of filamin and initiates an ubiquitin-dependent autophagic sorting of damaged filamin to lysosomes for degradation. At the same time, BAG3 triggers a transcriptional response mediated by the transcription factors YAP-TAZ to compensate disposal of client proteins. Despite the initial enthusiasm, activation of CASA could not be detected in TM cells under static biaxial stretch. However, it cannot be ruled out, and indeed it is supported by unpublished data generated in our laboratory, that CASA is activated in other experimental conditions that might more extensively damage cytoskeleton components.
The physiological or pathophysiological implications of mechanically-induced autophagy are not known at present. Induction of autophagy with mechanical stress- either strain, compression or shear stress - is a novel area of research with just a limited number of studies reported in the literature (King, 2012; King et al., 2011; Lien et al., 2013; Tanabe et al., 2011). A very likely role of stretch-induced is adaptation. When subjected to forces or elevated pressure, TM cells must modify and reinforce their cytoskeleton to increase cortical rigidity. Induction of autophagy can help cells to undergo these changes by increasing protein and organelle turnover, which might in turn affect other cellular and metabolic processes to help rebalance cellular and tissue function. Long-term perfusion experiments have suggested the existence of some compensatory pressure-lowering mechanism in the trabecular outflow pathway in response to more physiologic pressure elevations (Borrás et al., 2002; Bradley et al., 2001). Whether the initial activation of autophagy forms part of this compensatory homeostatic mechanism by facilitating secondary adaptation, such as ECM remodeling or mechanical signaling to stretch (Acott et al., 2014), is an exciting possibility that needs to be further explored.
It is also likely that autophagy is activated to protect against long-term stretched-induced injury. Although autophagy is generally regarded as a prosurvival mechanism, a number of studies have indicated a complex relationship between autophagy and apoptosis. Induction of autophagy promotes survival through inhibition of apoptosis via direct interaction between Beclin 1 and Bcl 2 (Levine et al., 2008). However, if autophagy is continuously activated, it can lead to autophagic cell death (Kroemer and Levine, 2008); Lenardo et al., 2009). Thus, mechanical activation of autophagy under normal physiological forces may help remove damaged components and suppress cell death. In this sense, a recent study has shown rapamycin, an autophagy activator, to efficiently protect against the damaging effects of compressive mechanical stress and biochemical stimuli in articular cartilage (Caramés et al., 2012). In contrast, under pathologic conditions where mechanical forces exceed the physiological range, dysregulation of autophagy might trigger cell death and contribute to disease (Tanabe et al., 2011).
Another aspect that needs to be further investigated is the signaling pathway triggering induction of autophagy with mechanical stretch. In agreement with a previous report (Bradley et al., 2003), static stretch activates MTOR pathway in TM cells, suggesting that stretch-induced autophagy occurs in an MTOR-independent manner. A similar finding has been reported in breast cancer cells subjected to mechanical compression (King et al., 2011). This and our study indicate the existence of an alternative mechanosensitive activator of autophagy that override the inhibitory signals triggered from stretch-induced MTOR activation.
4.2 Intralysosomal Degradation of Extracellular Matrix (ECM) Components in TM Cells
Regulation of ECM composition is believed to play a major role in both in outflow pathway physiology (Bradley et al., 1998; Keller et al., 2009; Spiga and Borrás, 2010). Traditionally, it has been thought that proteolysis of ECM components occurs mainly extracellularly meditated by metalloproteinases (MMPs). However, it is now believed that it primarily occurs at the cell membrane and intracellularly, by endocytosis of partially degraded proteins (Broemme and Wilson, 2011). Moreover, several studies have demonstrated the ability of lysosomal cathepsins to contribute to such pericellular and/or intracellular degradation of ECM and basement membrane proteins in some cell types (Broemme and Wilson, 2011; Sloane et al., 2005). In a very recent study, our group has demonstrated the phagocytic uptake of partially degraded ECM components and their delivery to the lysosomal compartment for complete degradation (Porter et al., 2013a). Moreover, phagocytosis of bionic ligands (i.e. E. coli or collagen-coated beads) specifically upregulated and increased the secretion and membrane expression of cathepsin B, a lysosomal cathepsin capable of activating the uPA/plasminogen proteolytic cascade (Aggarwal and Sloane, 2014; Sloane et al., 2005). These findings support a novel role of lysosomal enzymes, in cooperation with phagocytosis and autophagy, in outflow pathway physiology and IOP regulation by modulating ECM remodeling. Failure of this system could explain the abnormal ECM deposition observed in the glaucomatous outflow pathway.
5. Autophagy in Outflow Pathway Pathophysiology: Implications for Glaucoma Pathogenesis
5.1 Autophagy in Aging and Age-Related Diseases
The connection between autophagy and aging comes from studies showing that increase autophagy increases longevity, while autophagy inhibition induces premature aging (Bergamini et al., 2007; Kang et al., 2011; Mariño et al., 2010; Rubinsztein et al., 2011; Vellai et al., 2009; Young and Narita, 2010). During aging, there is a decline in the autophagic activity in all the tissues examined to date. Indeed, age-dependent alterations of the autophagic lysosomal system is considered to be responsible, at least in part, for the accumulation of damaged cellular components in aging organisms. The exact reasons for this reduced autophagic activity with age is not fully elucidated, but it is been proposed to result from a defect in the clearance of autophagic vacuoles (Cuervo, 2008; Terman et al., 2007). Reactive oxygen species (ROS) resulting from normal aerobic metabolism are continuously inflicting macromolecular damage. Most of these oxidatively damaged biomolecules and organelles are successfully removed by the cellular proteolytic systems, namely proteasomes and lysosomes; however, the recycling machinery is inherently imperfect and it is affected itself by the aging process. Thus, there is gradual accumulation of partially degraded or nondegraded intralysosomal material, known as lipofuscin or age pigment, which seems to interfere with the activity of lysosomal hydrolases. This impaired lysosomal proteolytic activity ultimately affects the ability of these secondary lysosomes to fuse with autophagosomes, thus slowing down the overall autophagic clearance. Accordingly, morphological studies showing the expansion of the lysosomal compartments, accumulation of autophagic vacuoles and lipofuscin deposition in the aging lysosomal system. Accumulation of waste material is not limited to within the lysosomes. The recycling of damaged or worn-out organelles (i.e mitochondria, peroxisomes) and cytoplasmic protein aggregates gets also compromised contributing to the cellular dysfunction (Brunk et al., 1992; Cuervo, 2003; Cuervo et al., 2005; Terman et al., 2006). In particular, the inability of the cell to remove damage mitochondria and its consequent cytosolic accumulation is quite detrimental since it initiates a vicious cycle of increased ROS production and further defective autophagic clearance. Failure of the quality control mechanisms is specifically harmful in terminally differentiated post-mitotic cells and in tissues with low renewal rate, since dilution of damaged components by cell division is not possible (Cuervo et al., 2005). Autophagy dysregulation and the presence of biological waste material has been associated with an increasing number of age-related diseases, including age-related macular degeneration, Alzheimer’s disease, Parkinson’s disease, cardiomyopathies, and atherosclerosis (Boya, 2012; Harris and Rubinsztein, 2011; Levine and Kroemer, 2008; Schneider and Cuervo, 2014; Weide and Huber, 2011; Yang et al., 2009)
5.2. Oxidative Stress, Aging and Pathophysiology of the Outflow Pathway
Primary open angle glaucoma (POAG) is a late-onset disease characterized by increased resistance to aqueous humor (AH) outflow through the conventional outflow pathway tissue, leading to elevated intraocular pressure (IOP) and thus increased risk of developing glaucoma (Quigley, 2011). Still nowadays, the exact molecular mechanisms responsible for such resistance are not known; however, aging of the TM tissue or the deleterious effect associated with aging are considered a major risk factor (Liton et al., 2005).
Cells in the outflow pathway are constantly subjected to oxidative insult, both from ROS present in the AH and that generated by normal cellular metabolism (Spector et al., 1998). This continuous exposure to ROS has been suspected to contribute to the morphological and physiological alterations of the aqueous outflow pathway in aging and POAG (Aslan et al., 2008; Kumar and Agarwal, 2007; Saccà et al., 2007; Zanon-Moreno et al., 2008). The particular higher sensitivity of the TM to oxidative radicals compared with other tissues in the anterior chamber of the eye would explain why this tissue is more affected to the oxidative insult (Izzotti et al., 2009). One potential source of intracellular ROS is that generated from H2O2 through iron-catalyzed Fenton reactions, which results in the production of highly reactive hydroxyl radicals (Kruszewski, 2003). These newly generated free radicals can induce the peroxidation of adjacent lipids and proteins and oxidative damage to DNA. Although cells and organisms posses mechanisms to prevent free iron from reacting with H2O2, localized iron dysregulation and consequent iron accumulation has been demonstrated to occur in a variety of tissues and species during normal aging (Killilea et al., 2003; Xu et al., 2008), including senescent and glaucomatous TM cells (Lin et al., 2010). Accumulation of iron primarily occurs within the lysosomal compartment resulting from the autophagic degradation of ferritin (Bridges, 1987). Accordingly, intralysosomal iron chelation effectively suppressed the generation of ROS in TM cells following acute oxidative stress with H2O2, indicating lysosomes as key organelles responsible for ROS generation (Lin et al., 2010). Production of ROS has been also observed by mitochondria in TM cells exposed to chronic oxidative stress, which mediates the upregulation of endothelial leukocyte adhesion molecule-1 (Li et al., 2007), a previously reported glaucoma marker (Liton et al., 2006; Wang et al., 2001). Moreover, increased mitochondrial ROS production has been reported in TM primary cultures from glaucoma donors (He et al., 2008).
The TM of patients with POAG has additionally showed increased expression and activity of inducible nitric oxide synthase (iNOS), believed to be responsible for the elevated expression of the powerful oxidant peroxynitrite in the TM of patients with severe POAG (Fernández-Durango et al., 2008). In addition to this pro-oxidant environment, the aging outflow pathway is also characterized by an overall decreased in the antioxidant potential (La Paz and Epstein, 1996) and downregulated expression of the antioxidants paraxonase 3 and ceruloplasmin (Liton et al., 2006). This imbalance between oxidant production and antioxidant defense mechanisms causes oxidative stress to the outflow pathway tissue during aging and in disease. Specific evidence of oxidative stress comes from studies reporting increased presence of peroxidized lipids (Fernández-Durango et al., 2008) and oxidative DNA damage in the glaucomatous outflow pathway; in this latter case, the levels of DNA oxidation significantly correlated with IOP and visual field defects (Saccà et al., 2005). Our own work also reported a 3-fold increase in the levels of carbonylated proteins in TM cells from older donors compared to young ones (Caballero et al., 2004). Interestingly, this increase in carbonylated proteins was associated with a 7.5-fold decrease of proteasome activity, suggesting that a progressive loss of proteolytic proteasomal function in the TM with aging could contribute to the observed increased accumulation of oxidized proteins.
5.3. Effect of Chronic Oxidative Stress on Autophagic Lysosomal Function in TM Cells
Induction of autophagy is one of the potential cellular responses to ROS and oxidative damage, acting at two levels. On the one hand, ROS can initiate autophagosome formation and autophagic degradation functioning as cellular signaling molecules; on the other hand, autophagy serves to reduce oxidative damage as well as ROS levels through the removal of protein aggregates and damage organelles, including mitochondria and lysosomes (Li et al., 2015). Recently, our group investigated the activation of autophagy in TM cells in response to chronic oxidative stress (Porter et al., 2013b). Chronic oxidative stress was applied using our well-established experimental model consisting on culturing confluent monolayers of postmitotic TM cells to hyperoxia (40% O2) for two weeks. Cells grown under these conditions display a phenotype similar to aging cells, characterized by increased intracellular ROS, decreased mitochondrial membrane potential, as well as increased DNA damage, carbonylated protein contents, and the presence of lipofuscin (Liton et al., 2008).
Activation of autophagy, analyzed using three different methodologies, was observed in chronically stressed cultures. Moreover, maturation of newly formed autophagosomes into autolysosomes was also confirmed. Induction of autophagy in TM cells in response to oxidative stress was found to be mTOR-dependent and involved the nuclear translocation of the transcription factor EB (TFEB), a master regulator of autophagy and lysosomal biogenesis (Settembre et al., 2011). Upon nuclear translocation, TFEB drives the expression of autophagy and lysosomal genes, thus linking and coordinating the function of these two cellular organelles, autophagosomes and lysosomes, for an efficient autophagic process. In agreement, induction of autophagy was accompanied by the upregulation of the lysosomal machinery, including lysosomal hydrolases, in TM cells grown at 40% O2 conditions. We observed, however, that despite the transcriptional activation of the autophagic and lysosomal machinery, and the elevated content of lysosomal cathepsins, lysosomal degradation was not enhanced. Furthermore, the oxidatively stressed cultures displayed lower cathepsins activity and improper proteolytic maturation of lysosomal cathepsins (Liton et al., 2008; Porter et al., 2013b). Further analysis revealed that the overall decrease in cathepsin activities and reduced lysosomal degradative potential could be attributed to the basification of the lysosomal lumen in the cells grown under hyperoxia (Porter et al., 2013b). The molecular mechanism leading to lysosomal basification with oxidative stress in TM cells have still not been identified.
Ascorbic acid is a potent antioxidant, which is present at high concentrations in the AH (Delamere, 1996). A reduction of the levels of vitamin C in plasma as well as in the AH of patients with POAG and secondary glaucoma has been documented by several investigators (Ferreira et al., 2009; Koliakos et al., 2002; Leite et al., 2009). The potential relevance of AA in outflow pathway physiology and pathophysiology comes from the observed IOP-lowering effect of AA (LINNER, 1969), and from the recent work by Zanon-Moreno et al. (Zanon-Moreno et al., 2011) reporting an association between a polymorphism in the AA transporter SLC23A2 with lower plasma concentrations of AA and with higher risk of POAG. Very interestingly, supplementation of TM cells with AA has been shown to activate the autophagic lysosomal pathway and increase lysosomal degradation in TM cells in a dose-dependent manner (Xu et al., 2014). This increase in lysosomal degradation was mediated by proteolytic activation of cathepsins, presumably through the stabilization of lysosomal pH, as described in glial cells (Martin et al., 2002). Based on this, it is very tempting to speculate that activation of autophagy might be, at least in part, one of the mechanisms by which AA protects against oxidative stress. The diminished levels of ascorbate or deficient AA cellular uptake can affect lysosomal pH and compromise lysosomal degradation in outflow pathway cells.
The higher number of autophagosomes and autolysosomes - resulting from induction of autophagy - and the increased presence of cargo material within them, while keeping the same cellular degradative capacity, might result in the accumulation of undegraded material within residual bodies. Moreover, as previously explained, renewal of aged or damaged organelles can also be affected. It is therefore possible that decrease in proteasome activity in TM cells from older donors (Caballero et al., 2004) and the increased mitochondrial ROS production in glaucomatous TM primary cultures (He et al., 2008) might result from the poor autophagic turnover of proteasomes and mitochondria, respectively. Also, as it will be discussed elsewhere in this Special Issue, clearance of mutant myocilin can be also compromised since the turnover of myocilin involves ubiquitin-proteasomes and lysosomal pathways (Qiu et al., 2014).
A recent work by Pulliero et al. has reported the occurrence of an age dependent increase in autophagy in the TM tissue (Pulliero et al., 2014). Furthermore, they showed that aging promotes TM senescence due to increased oxidative stress paralleled by autophagy increase. Indeed, both oxidative DNA damage and autophagy were more abundant in subjects older than 60 years. A link between oxidative stress, cellular senescence and autophagy has been also elucidated from our studies in vitro, demonstrating a correlation between increased lysosomal content and detectable senescence-associated-β-galactosidase (SA-β-gal) in TM cells grown under chronic oxidative stress (Liton et al., 2008; Porter et al., 2013b). When application of chronic oxidative stress was conducted in the presence of the autophagy inhibitor 3-MA, the increase in SA-β-gal activity was partially blocked, as well as that in lipofuscin and lysosomal mass suggesting that the occurrence of SA-β-gal activity is mediated by LC3-dependent autophagy and results from reduced autophagic flux within autolysosomes (Porter et al., 2013b). These findings are of special relevance since the glaucomatous outflow pathway contains increased number of cells displaying SA-β-gal activity in the compared with age-matched control tissue (Liton et al., 2005). Impaired autophagic flux induced by oxidative stress might thus represent one of the factors leading to progressive failure of cellular TM function with age and contribute to the pathogenesis of POAG.
5.4. Autophagy in The Glaucomatous Outflow Pathway
Although still not investigated in detail, increasing number of evidence in the literature suggests altered autophagic lysosomal function in the glaucomatous outflow pathway. First evidence come from older studies describing increased hydrolase activities in the TM of glaucomatous eyes (Coupland et al., 1993), as well as those reporting ultrastructural changes, such as the presence of membrane-limited vesicles filled with granular material and accumulation of autophagic vacuoles and pigment granules in the cytoplasm of glaucomatous TM cells (Cracknell et al., 2006; Rohen, 1982; Tektas and Lütjen-Drecoll, 2009). A recent work by Keller et al. also provides a genetic link between autophagy and glaucoma since ASB10, a novel candidate POAG gene, was shown to participate in ubiquitin-mediated degradation pathways (Keller et al., 2013). The importance of autophagy in outflow physiology is additionally supported by the observation that ocular hypertension is an ocular manifestation described in patients with lysosomal storage disorders (Biswas et al., 2008; Nowaczyk et al., 1988; Spellacy et al., 1980).
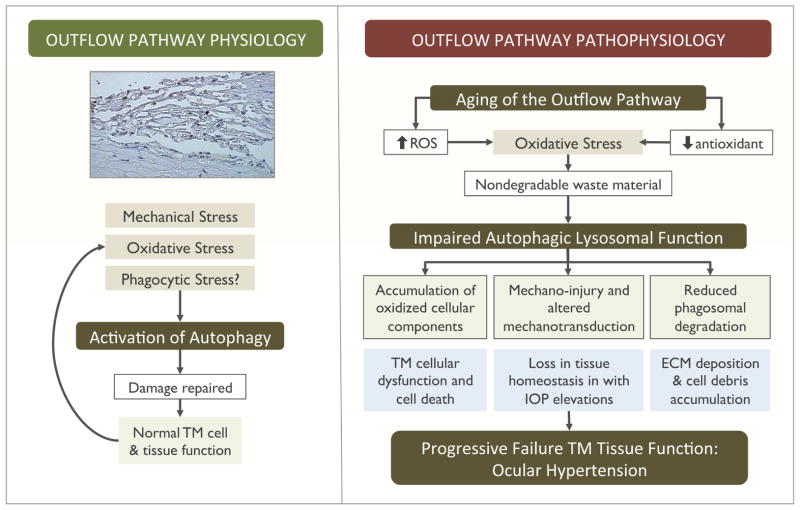
More direct experimental evidence linking autophagy to outflow pathway pathophysiology comes from our latest study, in which we evaluated autophagic lysosomal function in TM cells isolated from glaucomatous donors and compared it to that in age-matched donor eyes (Porter et al., 2015). Glaucomatous TM cells displayed a significant decrease in the steady-state levels of LC3-II, as well as reduced lysosomal proteolysis. Moreover, the levels of RPS6KB phosphorylated at Thr389, a direct mTOR phosphorylation site, were dramatically increased in the glaucomatous cultures, a clear indication of autophagy inhibition. In addition to the constitutive inhibition of autophagic function, the ability to induce autophagy in response to stress, at least to oxidative stress, was similarly compromised in the glaucomatous cultures. A very striking result was that TM cells from glaucoma donors also demonstrated a significant increase in SA-β-gal activity and lipofuscin content, similar to our previous observations in chronically stressed TM cells and in glaucomatous TM cells in situ (Liton et al., 2005). SA-β-gal is an abnormal activity of the lysosomal enzyme β-galactosidase that occurs at pH 6 in senescent cells. Although the exact nature of this abnormal activity is not fully understood, as mentioned earlier, SA-β-gal expression has been co-localized within autolysosomes together with LC3 and has been proposed to results from reduced autophagic flux within autolysosomes during cellular senescence. These findings reinforce a relationship between oxidative stress, autophagic dysfunction, and outflow pathway pathophysiology (Figure 2).
Figure 2.
Summary of the roles of autophagy in outflow pathway physiology and pathophysiology.
6. Concluding Remarks and Future Studies
The study of autophagy is still an emerging, but promising field in outflow pathway tissue physiology and pathophysiology. As summarized in Figure 3, all the experimental evidence available to date supports a key role of autophagy in maintaining outflow pathway tissue homeostasis and response to stress. Morphological and in vitro culture studies also sustain its potential malfunction in glaucoma. However, confirmation of existing observations in vitro in cadaver human tissue and/or transgenic animal models is needed. The study of autophagy in glaucomatous human cadaver tissue can be, conversely, subjected to important limitations, as the supply of human cadaver eyes, access to complete clinical histories and most importantly, to the fact that all glaucoma patients undergo therapy with glaucoma medications, usually a combination of different drugs, and/or might have undergone surgery, either laser or trabeculectomy. So far, there is no information of the effects of these therapeutic treatments on the autophagic lysosomal pathway. Similarly, the use of transgenic animal models is challenging since full body knockouts of relevant autophagic genes are lethal, and no specific promoter has been yet identified for TM cells that can be used to generate tissue specific or conditional knockouts. Also important is the recognition of inducing mechanisms and signaling pathways involved in the autophagic response in TM cells, which could open ways for the modulation of the autophagic lysosomal function in ocular hypertension and possibly define targets for drug design.
Figure 3.
HIGHLIGHTS.
Autophagy is induced in TM cells in response to oxidative and mechanical stress.
The autophagic lysosomal system is emerging as an important regulator of outflow pathway physiology, protecting against stretch-induced injury and modulating ECM composition.
Chronic oxidative stress induces lysosomal basification in TM cells and consequent decrease in autophagic flux.
Glaucomatous TM cells display dysregulated autophagic function.
Acknowledgments
The author would like to thank her funding sources: National Institute of Health Grants R01EY020491 (Liton) and P30EY005722; the Brightfocus Foundation (Liton, G2012022), and the Alcon Foundation (Liton, Young Investigator Grant).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIOGRAPHY
- Acott TS, Kelley MJ, Keller KE, Vranka JA, Abu-Hassan DW, Li X, Aga M, Bradley JM. Intraocular Pressure Homeostasis: Maintaining Balance in a High-Pressure Environment. J Ocul Pharmacol Ther. 2014 doi: 10.1089/jop.2013.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal N, Sloane BF. Cathepsin B: multiple roles in cancer. Proteomics Clin Appl. 2014;8:427–437. doi: 10.1002/prca.201300105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt V, Dick N, Tawo R, Dreiseidler M, Wenzel D, Hesse M, Fürst DO, Saftig P, Saint R, Fleischmann BK, Hoch M, Höhfeld J. Chaperone-assisted selective autophagy is essential for muscle maintenance. Curr Biol. 2010;20:143–148. doi: 10.1016/j.cub.2009.11.022. [DOI] [PubMed] [Google Scholar]
- Aslan M, Cort A, Yucel I. Oxidative and nitrative stress markers in glaucoma. Free Radic Biol Med. 2008;45:367–376. doi: 10.1016/j.freeradbiomed.2008.04.026. [DOI] [PubMed] [Google Scholar]
- Battista SA, Lu Z, Hofmann S, Freddo T, Overby DR, Gong H. Reduction of the available area for aqueous humor outflow and increase in meshwork herniations into collector channels following acute IOP elevation in bovine eyes. Invest Ophthalmol Vis Sci. 2008;49:5346–5352. doi: 10.1167/iovs.08-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergamini E, Cavallini G, Donati A, Gori Z. The role of autophagy in aging: its essential part in the anti-aging mechanism of caloric restriction. Ann N Y Acad Sci. 2007;1114:69–78. doi: 10.1196/annals.1396.020. [DOI] [PubMed] [Google Scholar]
- Bill A, Phillips CI. Uveoscleral drainage of aqueous humour in human eyes. Exp Eye Res. 1971;12:275–281. doi: 10.1016/0014-4835(71)90149-7. [DOI] [PubMed] [Google Scholar]
- Biswas J, Nandi K, Sridharan S, Ranjan P. Ocular manifestation of storage diseases. Curr Opin Ophthalmol. 2008;19:507–511. doi: 10.1097/ICU.0b013e32831215c3. [DOI] [PubMed] [Google Scholar]
- Borrás T, Rowlette LLS, Tamm ER, Gottanka J, Epstein DL. Effects of elevated intraocular pressure on outflow facility and TIGR/MYOC expression in perfused human anterior segments. Invest Ophthalmol Vis Sci. 2002;43:33–40. [PubMed] [Google Scholar]
- Boya P. Lysosomal function and dysfunction: mechanism and disease. Antioxid Redox Signal. 2012;17:766–774. doi: 10.1089/ars.2011.4405. [DOI] [PubMed] [Google Scholar]
- Bradley JM, Kelley MJ, Zhu X, Anderssohn AM, Alexander JP, Acott TS. Effects of mechanical stretching on trabecular matrix metalloproteinases. Invest Ophthalmol Vis Sci. 2001;42:1505–1513. [PubMed] [Google Scholar]
- Bradley JM, Vranka J, Colvis CM, Conger DM, Alexander JP, Fisk AS, Samples JR, Acott TS. Effect of matrix metalloproteinases activity on outflow in perfused human organ culture. Invest Ophthalmol Vis Sci. 1998;39:2649–2658. [PubMed] [Google Scholar]
- Bradley JMB, Kelley MJ, Rose A, Acott TS. Signaling pathways used in trabecular matrix metalloproteinase response to mechanical stretch. Invest Ophthalmol Vis Sci. 2003;44:5174–5181. doi: 10.1167/iovs.03-0213. [DOI] [PubMed] [Google Scholar]
- Bridges KR. Ascorbic acid inhibits lysosomal autophagy of ferritin. J Biol Chem. 1987;262:14773–14778. [PubMed] [Google Scholar]
- Broemme D, Wilson S. Role of Cysteine Cathepsins in Extracellular Proteolysis. In: parks WC, PMR, editors. The Extracellular Matrix: an Overview. Springer-Verlag; Heidelberg: n.d. pp. 23–51. [Google Scholar]
- Brunk UT, Jones CB, Sohal RS. A novel hypothesis of lipofuscinogenesis and cellular aging based on interactions between oxidative stress and autophagocytosis. Mutat Res. 1992;275:395–403. doi: 10.1016/0921-8734(92)90042-n. [DOI] [PubMed] [Google Scholar]
- Caballero M, Liton PB, Challa P, Epstein DL, Gonzalez P. Effects of donor age on proteasome activity and senescence in trabecular meshwork cells. Biochem Biophys Res Commun. 2004;323:1048–1054. doi: 10.1016/j.bbrc.2004.08.195. [DOI] [PubMed] [Google Scholar]
- Caramés B, Taniguchi N, Seino D, Blanco FJ, D’Lima D, Lotz M. Mechanical injury suppresses autophagy regulators and pharmacologic activation of autophagy results in chondroprotection. Arthritis Rheum. 2012;64:1182–1192. doi: 10.1002/art.33444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman DJ, Trokel S. Direct-recorded intraocular pressure variations in a human subject. Arch Ophthalmol. 1969;82:637–640. doi: 10.1001/archopht.1969.00990020633011. [DOI] [PubMed] [Google Scholar]
- Coupland SE, Heimann H, Hoffmann F, Penfold PL, Billson FA. Increased hydrolase activities in the human trabecular meshwork of glaucomatous eyes. Ger J Ophthalmol. 1993;2:107–112. [PubMed] [Google Scholar]
- Cracknell KPB, Grierson I, Hogg P, Majekodunmi AA, Watson P, Marmion V. Melanin in the trabecular meshwork is associated with age, POAG but not Latanoprost treatment. A masked morphometric study. Exp Eye Res. 2006;82:986–993. doi: 10.1016/j.exer.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Cuervo AM. Autophagy and aging--when “all you can eat” is yourself. Sci Aging Knowledge Environ. 2003:pe25. doi: 10.1126/sageke.2003.36.pe25. [DOI] [PubMed] [Google Scholar]
- Cuervo AM. Autophagy and aging: keeping that old broom working. Trends Genet. 2008;24:604–612. doi: 10.1016/j.tig.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo AM, Bergamini E, Brunk UT, Dröge W, Ffrench M, Terman A. Autophagy and aging: the importance of maintaining “clean” cells. Autophagy. 2005;1:131–140. doi: 10.4161/auto.1.3.2017. [DOI] [PubMed] [Google Scholar]
- Delamere NA. Ascorbic acid and the eye. Subcell Biochem. 1996;25:313–329. doi: 10.1007/978-1-4613-0325-1_16. [DOI] [PubMed] [Google Scholar]
- Fernández-Durango R, Fernández-Martínez A, García-Feijoo J, Castillo A, de la Casa JM, García-Bueno B, Pérez-Nievas BG, Fernández-Cruz A, Leza JC. Expression of nitrotyrosine and oxidative consequences in the trabecular meshwork of patients with primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2008;49:2506–2511. doi: 10.1167/iovs.07-1363. [DOI] [PubMed] [Google Scholar]
- Ferreira SM, Lerner SF, Brunzini R, Evelson PA, Llesuy SF. Antioxidant status in the aqueous humour of patients with glaucoma associated with exfoliation syndrome. Eye (Lond) 2009;23:1691–1697. doi: 10.1038/eye.2008.352. [DOI] [PubMed] [Google Scholar]
- Grierson I, Lee WR. The fine structure of the trabecular meshwork at graded levels of intraocular pressure. (2) Pressures outside the physiological range (0 and 50 mmHg) Exp Eye Res. 1975a;20:523–530. doi: 10.1016/0014-4835(75)90219-5. [DOI] [PubMed] [Google Scholar]
- Grierson I, Lee WR. The fine structure of the trabecular meshwork at graded levels of intraocular pressure. (1) Pressure effects within the near-physiological range (8–30 mmHg) Exp Eye Res. 1975b;20:505–521. doi: 10.1016/0014-4835(75)90218-3. [DOI] [PubMed] [Google Scholar]
- Harris H, Rubinsztein DC. Control of autophagy as a therapy for neurodegenerative disease. Nat Rev Neurol. 2011;8:108–117. doi: 10.1038/nrneurol.2011.200. [DOI] [PubMed] [Google Scholar]
- He Y, Leung KW, Zhang YH, Duan S, Zhong XF, Jiang RZ, Peng Z, Tombran-Tink J, Ge J. Mitochondrial complex I defect induces ROS release and degeneration in trabecular meshwork cells of POAG patients: protection by antioxidants. Invest Ophthalmol Vis Sci. 2008;49:1447–1458. doi: 10.1167/iovs.07-1361. [DOI] [PubMed] [Google Scholar]
- Izzotti A, Saccà SC, Longobardi M, Cartiglia C. Sensitivity of ocular anterior chamber tissues to oxidative damage and its relevance to the pathogenesis of glaucoma. Invest Ophthalmol Vis Sci. 2009;50:5251–5258. doi: 10.1167/iovs.09-3871. [DOI] [PubMed] [Google Scholar]
- Johnstone MA. The aqueous outflow system as a mechanical pump: evidence from examination of tissue and aqueous movement in human and non-human primates. J Glaucoma. 2004;13:421–438. doi: 10.1097/01.ijg.0000131757.63542.24. [DOI] [PubMed] [Google Scholar]
- Johnstone MA, Grant WG. Pressure-dependent changes in structures of the aqueous outflow system of human and monkey eyes. Am J Ophthalmol. 1973;75:365–383. doi: 10.1016/0002-9394(73)91145-8. [DOI] [PubMed] [Google Scholar]
- Kang HT, Lee KB, Kim SY, Choi HR, Park SC. Autophagy impairment induces premature senescence in primary human fibroblasts. PLoS ONE. 2011;6:e23367. doi: 10.1371/journal.pone.0023367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller KE, Aga M, Bradley JM, Kelley MJ, Acott TS. Extracellular matrix turnover and outflow resistance. Exp Eye Res. 2009;88:676–682. doi: 10.1016/j.exer.2008.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller KE, Yang YF, Sun YY, Sykes R, Acott TS, Wirtz MK. Ankyrin repeat and suppressor of cytokine signaling box containing protein-10 is associated with ubiquitin-mediated degradation pathways in trabecular meshwork cells. Mol Vis. 2013;19:1639–1655. [PMC free article] [PubMed] [Google Scholar]
- Killilea DW, Atamna H, Liao C, Ames BN. Iron accumulation during cellular senescence in human fibroblasts in vitro. Antioxid Redox Signal. 2003;5:507–516. doi: 10.1089/152308603770310158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S, Fujita N, Noda T, Yoshimori T. Monitoring autophagy in mammalian cultured cells through the dynamics of LC3. Meth Enzymol. 2009;452:1–12. doi: 10.1016/S0076-6879(08)03601-X. [DOI] [PubMed] [Google Scholar]
- King JS. Mechanical stress meets autophagy: potential implications for physiology and pathology. Trends in molecular medicine. 2012;18:583–588. doi: 10.1016/j.molmed.2012.08.002. [DOI] [PubMed] [Google Scholar]
- King JS, Veltman DM, Insall RH. The induction of autophagy by mechanical stress. Autophagy. 2011;7:1490–1499. doi: 10.4161/auto.7.12.17924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koliakos GG, Konstas AGP, Schlötzer-Schrehardt U, Bufidis T, Georgiadis N, Ringvold A. Ascorbic acid concentration is reduced in the aqueous humor of patients with exfoliation syndrome. Am J Ophthalmol. 2002;134:879–883. doi: 10.1016/s0002-9394(02)01797-x. [DOI] [PubMed] [Google Scholar]
- Kon M, Cuervo AM. Chaperone-mediated autophagy in health and disease. FEBS Lett. 2010;584:1399–1404. doi: 10.1016/j.febslet.2009.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol. 2008;9:1004–1010. doi: 10.1038/nrm2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G, Mariño G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40:280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruszewski M. Labile iron pool: the main determinant of cellular response to oxidative stress. Mutat Res. 2003;531:81–92. doi: 10.1016/j.mrfmmm.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Kumar DM, Agarwal N. Oxidative stress in glaucoma: a burden of evidence. J Glaucoma. 2007;16:334–343. doi: 10.1097/01.ijg.0000243480.67532.1b. [DOI] [PubMed] [Google Scholar]
- De La Paz MA, Epstein DL. Effect of age on superoxide dismutase activity of human trabecular meshwork. Invest Ophthalmol Vis Sci. 1996;37:1849–1853. [PubMed] [Google Scholar]
- Leite MT, Prata TS, Kera CZ, Miranda DV, de Moraes Barros SB, Melo LA. Ascorbic acid concentration is reduced in the secondary aqueous humour of glaucomatous patients. Clin Experiment Ophthalmol. 2009;37:402–406. doi: 10.1111/j.1442-9071.2009.02046.x. [DOI] [PubMed] [Google Scholar]
- Lenardo MJ, McPhee CK, Yu L. Methods in Enzymology, Methods in Enzymology. Elsevier; 2009. Chapter 2 Autophagic Cell Death; pp. 17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Sinha S, Kroemer G. Bcl-2 family members: dual regulators of apoptosis and autophagy. Autophagy. 2008;4:600–606. doi: 10.4161/auto.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Luna C, Liton PB, Navarro I, Epstein DL, Gonzalez P. Sustained stress response after oxidative stress in trabecular meshwork cells. Mol Vis. 2007;13:2282–2288. [PMC free article] [PubMed] [Google Scholar]
- Li L, Tan J, Miao Y, Lei P, Zhang Q. ROS and Autophagy: Interactions and Molecular Regulatory Mechanisms. Cell Mol Neurobiol. 2015 doi: 10.1007/s10571-015-0166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien SC, Chang SF, Lee PL, Wei SY, Chang MDT, Chang JY, Chiu JJ. Mechanical regulation of cancer cell apoptosis and autophagy: roles of bone morphogenetic protein receptor, Smad1/5, and p38 MAPK. Biochim Biophys Acta. 2013;1833:3124–3133. doi: 10.1016/j.bbamcr.2013.08.023. [DOI] [PubMed] [Google Scholar]
- Lin Y, Epstein DL, Liton PB. Intralysosomal iron induces lysosomal membrane permeabilization and cathepsin D-mediated cell death in trabecular meshwork cells exposed to oxidative stress. Invest Ophthalmol Vis Sci. 2010;51:6483–6495. doi: 10.1167/iovs.10-5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINNER E. The pressure lowering effect of ascorbic acid in ocular hypertension. Acta Ophthalmol. 1969;47:685–689. doi: 10.1111/j.1755-3768.1969.tb08156.x. [DOI] [PubMed] [Google Scholar]
- Liton PB, Challa P, Stinnett S, Luna C, Epstein DL, Gonzalez P. Cellular senescence in the glaucomatous outflow pathway. Exp Gerontol. 2005;40:745–748. doi: 10.1016/j.exger.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liton PB, Gonzalez P. Stress response of the trabecular meshwork. J Glaucoma. 2008;17:378–385. doi: 10.1097/IJG.0b013e31815f52a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liton PB, Gonzalez P, Epstein DL. The role of proteolytic cellular systems in trabecular meshwork homeostasis. Exp Eye Res. 2009a;88:724–728. doi: 10.1016/j.exer.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liton PB, Lin Y, Gonzalez P, Epstein DL. Potential role of lysosomal dysfunction in the pathogenesis of primary open angle glaucoma. Autophagy. 2009b;5:122–124. doi: 10.4161/auto.5.1.7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liton PB, Lin Y, Luna C, Li G, Gonzalez P, Epstein DL. Cultured porcine trabecular meshwork cells display altered lysosomal function when subjected to chronic oxidative stress. Invest Ophthalmol Vis Sci. 2008;49:3961–3969. doi: 10.1167/iovs.08-1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liton PB, Luna C, Challa P, Epstein DL, Gonzalez P. Genome-wide expression profile of human trabecular meshwork cultured cells, nonglaucomatous and primary open angle glaucoma tissue. Mol Vis. 2006;12:774–790. [PMC free article] [PubMed] [Google Scholar]
- Lütjen-Drecoll E. Functional morphology of the trabecular meshwork in primate eyes. Prog Retin Eye Res. 1999;18:91–119. doi: 10.1016/s1350-9462(98)00011-1. [DOI] [PubMed] [Google Scholar]
- Mariño G, Fernández AF, López-Otín C. Autophagy and aging: lessons from progeria models. Adv Exp Med Biol. 2010;694:61–68. doi: 10.1007/978-1-4419-7002-2_6. [DOI] [PubMed] [Google Scholar]
- Martin A, Joseph JA, Cuervo AM. Stimulatory effect of vitamin C on autophagy in glial cells. J Neurochem. 2002;82:538–549. doi: 10.1046/j.1471-4159.2002.00978.x. [DOI] [PubMed] [Google Scholar]
- Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting. Autophagy. 2007;3:542–545. doi: 10.4161/auto.4600. [DOI] [PubMed] [Google Scholar]
- Nowaczyk MJ, Clarke JT, Morin JD. Glaucoma as an early complication of Hurler’s disease. Arch Dis Child. 1988;63:1091–1093. doi: 10.1136/adc.63.9.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter K, Hirt J, Stamer WD, Liton PB. Autophagic dysregulation in glaucomatous trabecular meshwork cells. Biochim Biophys Acta. 2015;1852:379–385. doi: 10.1016/j.bbadis.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter K, Lin Y, Liton PB. Cathepsin B is up-regulated and mediates extracellular matrix degradation in trabecular meshwork cells following phagocytic challenge. PLoS ONE. 2013a;8:e68668. doi: 10.1371/journal.pone.0068668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter K, Nallathambi J, Lin Y, Liton PB. Lysosomal basification and decreased autophagic flux in oxidatively stressed trabecular meshwork cells: implications for glaucoma pathogenesis. Autophagy. 2013b;9:581–594. doi: 10.4161/auto.23568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter KM, Jeyabalan N, Liton PB. MTOR-independent induction of autophagy in trabecular meshwork cells subjected to biaxial stretch. Biochim Biophys Acta. 2014;1843:1054–1062. doi: 10.1016/j.bbamcr.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulliero A, Seydel A, Camoirano A, Saccà SC, Sandri M, Izzotti A. Oxidative damage and autophagy in the human trabecular meshwork as related with ageing. PLoS ONE. 2014;9:e98106. doi: 10.1371/journal.pone.0098106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Shen X, Shyam R, Yue BYJT, Ying H. Cellular processing of myocilin. PLoS ONE. 2014;9:e92845. doi: 10.1371/journal.pone.0092845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley HA. Glaucoma. The Lancet. 2011;377:1367–1377. doi: 10.1016/S0140-6736(10)61423-7. [DOI] [PubMed] [Google Scholar]
- Rohen JW. Presence of matrix vesicles in the trabecular meshwork of glaucomatous eyes. Graefes Arch Clin Exp Ophthalmol. 1982;218:171–176. doi: 10.1007/BF02150090. [DOI] [PubMed] [Google Scholar]
- Rubinsztein DC, Mariño G, Kroemer G. Autophagy and aging. Cell. 2011;146:682–695. doi: 10.1016/j.cell.2011.07.030. [DOI] [PubMed] [Google Scholar]
- Saccà SC, Izzotti A, Rossi P, Traverso C. Glaucomatous outflow pathway and oxidative stress. Exp Eye Res. 2007;84:389–399. doi: 10.1016/j.exer.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Saccà SC, Pascotto A, Camicione P, Capris P, Izzotti A. Oxidative DNA damage in the human trabecular meshwork: clinical correlation in patients with primary open-angle glaucoma. Arch Ophthalmol. 2005;123:458–463. doi: 10.1001/archopht.123.4.458. [DOI] [PubMed] [Google Scholar]
- Sarkar S. Regulation of autophagy by mTOR-dependent and mTOR-independent pathways: autophagy dysfunction in neurodegenerative diseases and therapeutic application of autophagy enhancers. Biochem Soc Trans. 2013;41:1103–1130. doi: 10.1042/BST20130134. [DOI] [PubMed] [Google Scholar]
- Schneider JL, Cuervo AM. Autophagy and human disease: emerging themes. Curr Opin Genet Dev. 2014;26C:16–23. doi: 10.1016/j.gde.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre C, Di Malta C, Polito VA, Garcia Arencibia M, Vetrini F, Erdin S, Erdin SU, Huynh T, Medina D, Colella P, Sardiello M, Rubinsztein DC, Ballabio A. TFEB links autophagy to lysosomal biogenesis. Science. 2011;332:1429–1433. doi: 10.1126/science.1204592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloane BF, Yan S, Podgorski I, Linebaugh BE, Cher ML, Mai J, Cavallo-Medved D, Sameni M, Dosescu J, Moin K. Cathepsin B and tumor proteolysis: contribution of the tumor microenvironment. Semin Cancer Biol. 2005;15:149–157. doi: 10.1016/j.semcancer.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Spector A, Ma W, Wang RR. The aqueous humor is capable of generating and degrading H2O2. 1998;39:1188–1197. [PubMed] [Google Scholar]
- Spellacy E, Bankes JL, Crow J, Dourmashkin R, Shah D, Watts RW. Glaucoma in a case of Hurler disease. Br J Ophthalmol. 1980;64:773–778. doi: 10.1136/bjo.64.10.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiga MG, Borrás T. Development of a gene therapy virus with a glucocorticoid-inducible MMP1 for the treatment of steroid glaucoma. Invest Ophthalmol Vis Sci. 2010;51:3029–3041. doi: 10.1167/iovs.09-4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamer WD, Acott TS. Current understanding of conventional outflow dysfunction in glaucoma. Curr Opin Ophthalmol. 2012;23:135–143. doi: 10.1097/ICU.0b013e32834ff23e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe F, Yone K, Kawabata N, Sakakima H, Matsuda F, Ishidou Y, Maeda S, Abematsu M, Komiya S, Setoguchi T. Accumulation of p62 in degenerated spinal cord under chronic mechanical compression: functional analysis of p62 and autophagy in hypoxic neuronal cells. Autophagy. 2011;7:1462–1471. doi: 10.4161/auto.7.12.17892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tektas OY, Lütjen-Drecoll E. Structural changes of the trabecular meshwork in different kinds of glaucoma. Exp Eye Res. 2009;88:769–775. doi: 10.1016/j.exer.2008.11.025. [DOI] [PubMed] [Google Scholar]
- Terman A, Gustafsson B, Brunk UT. Mitochondrial damage and intralysosomal degradation in cellular aging. Mol Aspects Med. 2006;27:471–482. doi: 10.1016/j.mam.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Terman A, Gustafsson B, Brunk UT. Autophagy, organelles and ageing. J Pathol. 2007;211:134–143. doi: 10.1002/path.2094. [DOI] [PubMed] [Google Scholar]
- Tripathi RC. The functional morphology of the outflow systems of ocular and cerebrospinal fluids. Exp Eye Res. 1977;25(Suppl):65–116. doi: 10.1016/s0014-4835(77)80010-9. [DOI] [PubMed] [Google Scholar]
- Ulbricht A, Eppler FJ, Tapia VE, van der Ven PFM, Hampe N, Hersch N, Vakeel P, Stadel D, Haas A, Saftig P, Behrends C, Fürst DO, Volkmer R, Hoffmann B, Kolanus W, Höhfeld J. Cellular Mechanotransduction Relies on Tension-Induced and Chaperone-Assisted Autophagy. Curr Biol. 2013 doi: 10.1016/j.cub.2013.01.064. [DOI] [PubMed] [Google Scholar]
- Vellai T, Takács-Vellai K, Sass M, Klionsky DJ. The regulation of aging: does autophagy underlie longevity? Trends Cell Biol. 2009;19:487–494. doi: 10.1016/j.tcb.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Chintala SK, Fini ME, Schuman JS. Activation of a tissue-specific stress response in the aqueous outflow pathway of the eye defines the glaucoma disease phenotype. Nat Med. 2001;7:304–309. doi: 10.1038/85446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weide T, Huber TB. Implications of autophagy for glomerular aging and disease. Cell Tissue Res. 2011;343:467–473. doi: 10.1007/s00441-010-1115-0. [DOI] [PubMed] [Google Scholar]
- Xu J, Knutson MD, Carter CS, Leeuwenburgh C. Iron accumulation with age, oxidative stress and functional decline. PLoS ONE. 2008;3:e2865. doi: 10.1371/journal.pone.0002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Lin Y, Porter K, Liton PB. Ascorbic Acid Modulation of Iron Homeostasis and Lysosomal Function in Trabecular Meshwork Cells. J Ocul Pharmacol Ther. 2014 doi: 10.1089/jop.2013.0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang DS, Lee JH, Nixon RA. Monitoring autophagy in Alzheimer’s disease and related neurodegenerative diseases. Meth Enzymol. 2009;453:111–144. doi: 10.1016/S0076-6879(08)04006-8. [DOI] [PubMed] [Google Scholar]
- Young AR, Narita M. Connecting autophagy to senescence in pathophysiology. Curr Opin Cell Biol. 2010;22:234–240. doi: 10.1016/j.ceb.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Zanon-Moreno V, Ciancotti-Olivares L, Asencio J, Sanz P, Ortega-Azorin C, Pinazo-Duran MD, Corella D. Association between a SLC23A2 gene variation, plasma vitamin C levels, and risk of glaucoma in a Mediterranean population. Mol Vis. 2011;17:2997–3004. [PMC free article] [PubMed] [Google Scholar]
- Zanon-Moreno V, Marco-Ventura P, Lleo-Perez A, Pons-Vazquez S, Garcia-Medina JJ, Vinuesa-Silva I, Moreno-Nadal MA, Pinazo-Duran MD. Oxidative stress in primary open-angle glaucoma. J Glaucoma. 2008;17:263–268. doi: 10.1097/IJG.0b013e31815c3a7f. [DOI] [PubMed] [Google Scholar]