Abstract
Objective
To evaluate the clinical safety of antenatal and postnatal N-acetylcysteine (NAC) as a neuroprotective agent in maternal chorioamnionitis in a randomized, controlled, double-blinded trial.
Study design
Twenty-two mothers >24 weeks gestation presenting within 4 hours of diagnosis of clinical chorioamnionitis were randomized with their 24 infants to NAC or saline treatment. Antenatal NAC (100 mg/kg/dose) or saline was given intravenously every 6 hours until delivery. Postnatally, NAC (12.5–25 mg/kg/dose, n = 12) or saline (n = 12) was given every 12 hours for 5 doses. Doppler studies of fetal umbilical and fetal and infant cerebral blood flow, cranial ultrasounds, echocardiograms, cerebral oxygenation, electroencephalograms, and serum cytokines were evaluated before and after treatment, and 12, 24, and 48 hours after birth. Magnetic resonance spectroscopy and diffusion imaging were performed at term age equivalent. Development was followed for cerebral palsy or autism to 4 years of age.
Results
Cardiovascular measures, cerebral blood flow velocity and vascular resistance, and cerebral oxygenation did not differ between treatment groups. Cerebrovascular coupling was disrupted in infants with chorioamnionitis treated with saline but preserved in infants treated with NAC, suggesting improved vascular regulation in the presence of neuroinflammation. Infants treated with NAC had higher serum anti-inflammatory interleukin-1 receptor antagonist and lower proinflammatory vascular endothelial growth factor over time vs controls. No adverse events related to NAC administration were noted.
Conclusions
In this cohort of newborns exposed to chorioamnionitis, antenatal and postnatal NAC was safe, preserved cerebrovascular regulation, and increased an anti-inflammatory neuroprotective protein.
Trial registration
Intrauterine infection is associated with significant white and gray matter brain injury in newborns and is particularly important in the pathogenesis of periventricular leukomalacia (PVL) and cerebral palsy.1,2 Pathogens initiate toll-like receptor signaling in immune cells at the fetal-maternal interface, amplifying production of inflammatory cytokines in the placental membranes and amniotic fluid.3 Subsequently, fetal inflammatory cells are activated in the cord (funisitis) and in the fetal circulation, and both cytokine storm and leukocyte and endothelial cell activation lead to production of reactive oxygen species, without true infection.4 Endotoxin exposure, toll-like receptor signaling, leukocyte, and vascular endothelial activation all contribute to a fetal inflammatory state (FIRS) induced by chorioamnionitis. Neuroinflammation rapidly follows FIRS, within 1–4 hours in animal models.5
Cytokines and oxidative stress may cause direct central nervous system (CNS) injury.6,7 Oxidative stress before delivery predisposes the fetus to subsequent hypoxic ischemic (HI) injury, even when the interruption of blood flow during delivery is mild.8,9 Not surprisingly, the fetus frequently shows poor tolerance to labor with heart rate decelerations and bradycardia, which may further compromise cerebral perfusion and exacerbate white matter injury.
N-acetylcysteine (NAC) directly scavenges oxygen free radicals through its thiol-reducing group and is a promising neuroprotectant in animal models of chorioamnionitis.10–13 NAC also crosses the blood brain barrier, decreases oxidative stress and cytokine production in the CNS, and replenishes glutathione, a major intracellular antioxidant.14,15 In an animal model of chorioamnionitis, NAC provides optimal neuroprotection when given antenatally.11 However, targeting the fetal brain through the maternal circulation requires consideration of placental metabolism and transfer, and pharmacokinetics (PK) in the fetus and mother.
Although NAC has a favorable safety profile in human infants and pregnant mothers with acetaminophen overdose,16,17 translational studies must carefully consider potential adverse effects in this vulnerable population. In a fetal sheep model of septic shock, antenatal NAC administration increased fetal hypoxemia, raising safety concerns about its application in human chorioamnionitis.18 To address safety and possible pharmacodynamic (PD) side effects of NAC, we conducted a randomized, controlled pilot trial in pregnant women ≥24 weeks gestation within 4 hours of clinical diagnosis of chorioamnionitis, and their infants postnatally. We have previously reported the PK of NAC administered to this cohort of mothers with chorioamnionitis and in their infants.19 Intravenous NAC administered to the mother undergoes rapid placental transport, with slightly more than 1:1 transfer to the fetus, ascertained at delivery. In this report, NAC effects on serial cerebral blood flow (CBF) and oximetry, cardiac function, clotting studies, and blood pressure (BP) in mothers and infants exposed to chorioamnionitis, as well as serum cytokines and magnetic resonance spectroscopy (MRS) are reported with long-term outcomes.
Methods
This prospective, double blinded study was approved by the Medical University of South Carolina’s Institutional Review Board. Written informed consent was obtained prior to enrollment from pregnant women ≥24 completed weeks gestational age (GA), who presented with a clinical diagnosis of chorioamnionitis. Clinical chorioamnionitis was defined as maternal fever ≥38°C in the presence of rupture of membranes or 2 clinical findings: uterine tenderness, maternal white blood cell >15 000 cells/mm, fetal tachycardia >160 bpm, or malodorous amniotic fluid. Maternal exclusion criteria included bronchodilator or steroid-dependent asthma, sepsis, seizure disorder, suspected major abnormalities, fetal weight, or bi-parietal diameter less than the 10th % for GA, or immediate delivery.
Pregnant mothers were recruited in 2 cohorts based on GA at diagnosis of chorioamnionitis: term/late preterm (≥33 completed weeks) and preterm (24–32 completed weeks). GA cut-offs were chosen for fetal renal maturity and estimated NAC elimination.17 After randomized to NAC or saline, NAC (100 mg/kg/dose) or an equivalent volume of saline was given intravenously to mothers over 60 minutes every 6 hours until delivery. NAC/saline (preterm 12.5 mg/kg/dose; term 25 mg/kg/dose) was administered to infants, starting 6 hours after last maternal dose, then every 12 hours for 5 doses.
Primary safety outcomes were the incidence of histaminergic reactions, clinical bleeding, hypotension, seizures, and increase in prothrombin time (PT). Secondary safety outcomes were mean BP (mBP), amount/duration of pressor support, amount of fresh frozen plasma, seizures, and anaphylaxis. Adverse events (AEs) were recorded for 2 days following NAC infusion, and serious AEs (SAEs) for 30 days, verified by an independent clinical research monitor (Table I). Potential AEs expected with NAC treatment are histaminergic reactions or bronchospasm in mothers, longer PT, and lower mBP in mothers and infants. Expected complications from chorioamnionitis are hypotension, cardiac dysfunction, intraventricular hemorrhage (IVH), seizures, disseminated intravascular coagulopathy (DIC), thrombocytopenia, renal failure, pulmonary hypertension, respiratory failure, multisystem organ failure, necrotizing enterocolitis (NEC), PVL, and death in infants.20
Table I.
Demographic and clinical characteristics
| NAC | Control | Total | |
|---|---|---|---|
| Entry strata | |||
| Preterm mothers: n | 6 | 6 | 12 |
| Preterm infants: n | 7 | 7 | 14 |
| GA at birth (wk) | 28.2 ± 1.7 | 29.4 ± 3.3 | NS |
| Birth weight (g) | 1207 ± 235 | 1339 ± 462 | NS |
| Term mothers: n | 5 | 5 | 10 |
| Term infants: n | 5 | 5 | 10 |
| GA at birth (wk) | 38.6 ± 2.4 | 38.4 ± 2.8 | NS |
| Birth weight (g) | 3450 ± 415 | 3150 ± 582 | NS |
| Sex infants | |||
| Male | 7 | 6 | (54%) |
| Female | 5 | 6 | (46%) |
| Race of mothers | |||
| African American | 8 | 3 | (50%) |
| Caucasian | 3 | 8 | (50%) |
| Labor and delivery | |||
| Mean duration rupture of membranes (h, range) | 55 ± 83 (0–229) | 94 ± 127 (0–322) | NS |
| Mode of delivery | |||
| Vaginal (70%) | 8 | 9 | NS |
| Cesarean (29%) | 4 | 3 | NS |
| Maternal dosing | |||
| Mean time first maternal dose to delivery (h, range) (excluding 2 NAC mothers who labored 17 and 32 h) | 2.9 ± 2.2 (0–7) | 1.8 ± 1.8 (0–5) | NS |
| Maternal heart rate change pre- and 1 h postdosing (bpm) | −1.8 ± 17 | −2.5 ± 18 | NS |
| Maternal mBP change pre and postdosing (mm Hg) | 1 ± 12 | 2 ± 10 | NS |
| Maternal hypotension | 1 | 1 | |
| Fetal tachycardia first noted postdosing | 1 | 0 | |
| Maternal mean PT (s) predose | 13.7 ± 0.5 | 14.0 ± 0.9 | NS |
| Maternal mean PT (s) at delivery | 14.2 ± 0.6 | 14.1 ± 1.2 | NS |
| Mean change PT (s) after dose | 0.51 ± 0.5 | 0.45 ± 0.2 | NS |
| Histopathology (missing 3) | |||
| Maternal inflammatory response, any grade | 12/12 (100%) | 8/9 (89%) | |
| Fetal inflammatory response, any grade funisitis | 9/12 (75%) | 5/9 (56%) | |
| Infant | |||
| Apgar at 1 min, median | 6 ± 2.0 | 6.3 ± 2.7 | NS |
| Apgar at 5 min, median | 7.9 ± 1.8 | 7.5 ± 2.5 | NS |
| Cord pH, mean | 7.27 ± 0.08 | 7.22 ± 0.16 | NS |
| WBC (Kcells/mm 3) at birth, mean | 14.4 ± 5.0 | 8.1 ± 6.3 | NS |
| Absolute neutrophil count (cells/mm3) at birth, mean | 8.9 ± 6.2 | 10.8 ± 5.3 | NS |
| Culture proven sepsis/pneumonia at birth | 1 | 1 | |
| PT (s), mean DOL 1 | 19.5 ± 2.5 | 19.0 ± 4.0 | NS |
| SAEs | |||
| Infant resuscitation-chest compressions/CV meds | 0 | 1 | |
| Death | 0 | 1 | |
| PVL/IVH at birth | 1 | 0 | |
| IVH from 1–2 d of age | 1 | 1 | |
| IVH from 5–7 d of age | 2 | 0 | |
| NEC within 30 d | 1 | 1 |
CV, cardiovascular; NS, not significant; WBC, white blood cells.
Maternal clotting studies were measured before NAC infusion and at delivery. Maternal blood samples for NAC serum concentration and cytokines were drawn immediately before and after NAC/saline infusion at 5, 15, and 30 minutes and 1, 2, 3, 4, and 6 hours, or until delivery. NAC PK were previously reported.19 Placentas and umbilical cords were graded for chorioamnionitis and funisitis.21
All infants were evaluated for sepsis at birth, including complete blood count and blood culture, and treated with antibiotics by standardized clinical protocol. Clotting studies were drawn from the cord and at 48 hours. Serial blood samples for NAC and cytokine levels were drawn from the cord, before and at 15 minutes and 8, 12, 36, and 48 hours after the first infant dose of NAC/saline. Lumbar puncture was performed for cell count, culture, and cytokine levels before 12 hours of age, if the infant was stable without coagulopathy. Replicate samples of serum and cerebrospinal fluid (CSF) were prepared for cytokine analysis as previously described.22 Electroencephalograms (EEGs) were recorded during the first dose of NAC/saline. Cranial ultrasounds (CUSs) for IVH or other abnormalities were performed after admission and at 48 hours (term infants) or at 7 days (preterm infants), and magnetic resonance imaging (MRI) with MRS and diffusion tensor imaging, at term age equivalent. Infants were followed in neonatal follow-up clinic at 12–24 months of age and at 3–4 years by the infants’ general and developmental pediatricians.
Doppler blood flow was measured before and after dosing in the umbilical and/or fetal middle cerebral artery (MCA) prior to delivery when possible, and postnatally in the anterior cerebral artery (ACA), MCA, and basilar artery (BA) before and after the first dose (0–6 hours of life [HOLs]), and at 12, 24, 36, and 48 hours. Near-infrared spectroscopy probe placed over the forehead recorded regional cerebral oxygenation (RcSO2) during and after NAC/saline dosing, at 0–6, 12, 24, 36, and 48 HOLs. Echocardiograms were performed before the first dose and at 12, 24, and 48 HOLs. NAC assay was previously reported, with maternal and infant NAC plasma concentrations and PK, determined by high performance liquid chromatography.14,19 Intravenous NAC administration in the mother undergoes rapid placental transfer, and PK of NAC are different in preterm and term infants.19
Additional details for the Methods section are available in the Appendix (available at www.jpeds.com).
Statistical Analyses
Univariate analysis of variables between groups was performed using unpaired Student t test or Kruskal-Wallis tests. Repeated measures were analyzed by ANOVA or generalized linear mixed models as appropriate. Spearman or Pearson correlations were performed for analyses with NAC plasma concentrations. For all safety variables, significance was designated as P < .05 to minimize type II error in measures of drug safety, without corrections for multiple comparisons. For cytokines, logarithmic transformations were performed before statistical analysis. In assessment of beneficial effects of NAC within treatment groups, multiple comparisons adjusted with Bonferroni correction are also reported.
Results
Twenty-two mothers (12 preterm, 10 term) and 24 infants, including 2 sets of twins, were enrolled from August 2008 to January 2010. There were no significant differences in demographic data between the group treated with NAC and controls (Table I). No participant met stop criteria for drug infusion. Two withdrawals occurred: one for maternal preference and one infant treated with NAC for IVH and cystic changes on CUS. The infant was withdrawn from study prior to first NAC dose, and CUS findings were consistent with an event occurring more than a week prior to delivery.
Seventeen mothers had epidural anesthesia, all with rupture of membrane and other signs of chorioamnionitis. Histologic examination of placentas demonstrated 13 had both maternal and fetal inflammatory response; 5 had maternal inflammatory response alone; 2 placentas from the second twin in twin pregnancies had no inflammation; and 4 placentas were missing.
Additional details for the Results section are available in the Appendix (available at www.jpeds.com).
Maternal/Fetal AEs
There were no differences in maternal AEs between the group treated with NAC or saline. Hypotension during delivery (1 mother treated with NAC and 1 control), resolved with phenylephrine and volume expansion. One mother treated with NAC had an urticarial rash, but no anaphylactoid reactions were noted. Maternal heart rate, BP, and PTs did not show significant changes pre- and post-NAC dosing or over time between mothers treated with NAC and controls (Table I), and did not correlate with NAC concentrations. A single maternal SAE was reported in a control mother at 24 weeks GA with hypotension, fetal heart rate abnormalities, purulent amniotic fluid, severe necrotizing chorioamnionitis, and trivascular funisitis. Her infant died in the delivery room with severe mixed acidosis (cord pH <6.8, base deficit 24).
Fetal Hemodynamics
Two mothers (1 mother treated with NAC and 1 control) had nonreassuring fetal heart rate tracings, with late decelerations and fetal bradycardia. One mother treated with NAC with 39.4°C had new-onset fetal tachycardia. Fetal metabolic acidosis was not different between groups (Table I).
Umbilical cord blood flow was measured predosing (n = 13), and postdosing (n = 10; NAC 8, saline 2), and remained stable without reversal of flow in diastole. Mean cord pulsatility index was similar pre-NAC (1.16 ± 0.28, 95% CI) and post-NAC (1.12 ± 0.15, 95% CI, n = 8). Fetal MCA blood flow was measured pre- (n = 8) and postdosing (n = 6; NAC 4, saline 2) (Figure 1; available atwww.jpeds.com). Preterm and term fetuses treated with NAC had no evidence of systemic or cardiac compromise in utero. An increase in fetal MCA time average maximum velocity (TAMX) after NAC dosing was observed in the 4 preterm fetuses.
Figure 1.
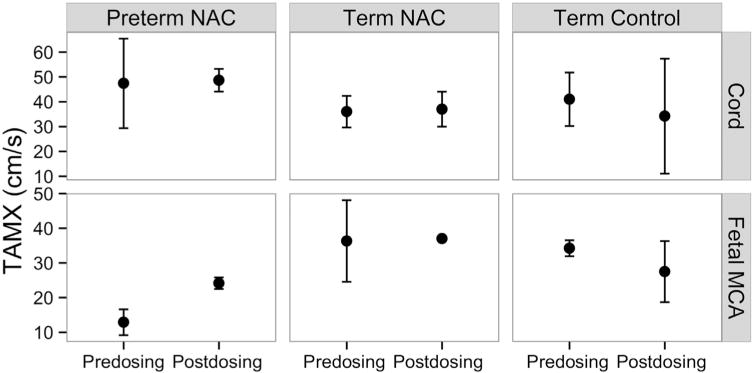
Mean fetal umbilical cord and MCA blood flow velocity (TAMX) before and after NAC dosing by treatment and GA (n = 4 preterm, n = 2−3 term) with 95% CIs. Doppler measures could not be obtained in preterm control fetuses.
Infant AEs
Eight infants had SAEs (Table I). Sepsis-related events were death (1 control), listeria sepsis (1 control), and pneumonia at birth (1 NAC). All CSF cultures were negative. NEC before 30 days of life (DOLs) occurred in 1 NAC and 1 control infant. Late NEC developed in 1 infant treated with NAC with Escherichia coli sepsis and grade IV IVH (DOL 35), PVL (DOL 45), cerebral palsy, and cognitive deficits at 4 years.
PT was not significantly different in infants treated with NAC or saline (Table I). At birth, PT was >18 seconds in 50% preterm infants (2 infants treated with NAC and 2 controls out of 8 preterm infants with clotting studies at birth), and one-quarter of term infants (1 infant treated with NAC with HI encephalopathy [HIE] out of 4 term infants with clotting studies at birth). Overall, 57% infants with clotting studies within 48 hours of birth had PT >18 seconds (3/6 control; 5/8 NAC). Two infants treated with NAC and 1 control received fresh frozen plasma.
In total 4 preterm infants had IVH grades II-III during first week of life, 3 had DIC at birth (2 infants treated with NAC and 1 control). One infant treated with NAC had grade I IVH bilaterally with cystic changes at 4.5 HOL, consistent with an intrauterine event prior to study drug infusion. NAC was undetectable in this infant’s cord blood, and coagulation studies clotted.
Hemodynamic Variables in Infants
Cardiovascular measures (heart rate, systolic, diastolic, mBP) were not significant between treatment groups or before and after study drug dosing (Figure 2). No infant required vasopressors during the period of NAC infusion. Cord, pre-, or postinfusion NAC plasma concentrations showed no PD correlation with BP measurements.
Figure 2.
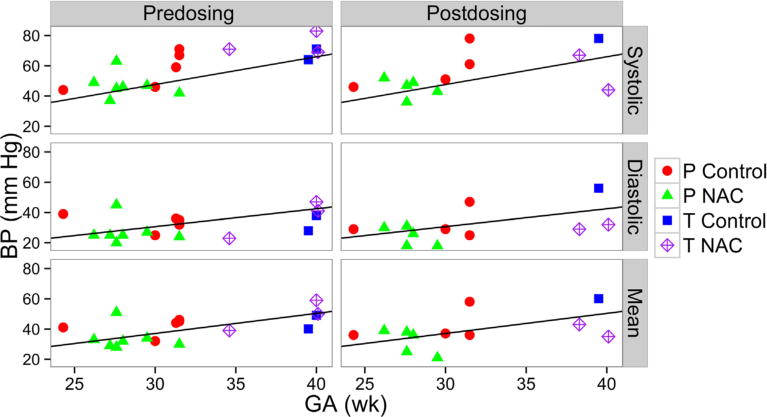
Systolic, diastolic, and mBPs before and after first NAC/saline dose (0–6 HOLs) by GA. Reference line represents normal BP measure for first week of life by GA.23 P, preterm infants; T, term infants.
Infant CBF Velocity and Vascular Resistance
Mean CBF in cerebral vessels were not significantly different before or after dosing or between treatment groups in either GA cohort (Figure 3). However, CBF velocity increased and resistance decreased significantly very early in the MCA (0 hour to 12–24 hours) in all preterm and term infants exposed to chorioamnionitis, and by 24 hours in the ACA in preterm infants. The timing of the changes were earlier than previous reports in normal24 and preterm infants exposed to chorioamnionitis (24–40 hours), and similar to preterm infants who developed IVH.25 Male sex had a significant effect on MCA (P = .014) and BA resistance (P = .032), which varied by GA (Figure 4; available at www.jpeds.com). CBF and cardiac measures were not significantly different in preterm infants with (n = 4) and without IVH (n = 7) (Figure 5; available at www.jpeds.com).
Figure 3.
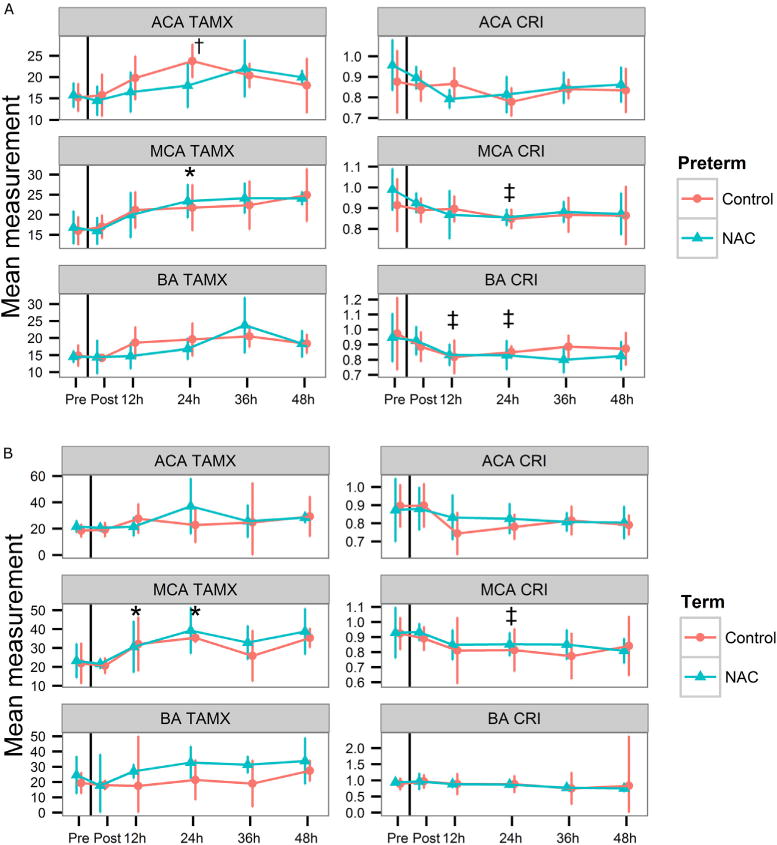
A, Infant CBF velocity (TAMX) and resistive indices (corrected resistive index [CRI]) in the ACA, MCA, and BA before and after initial dose of NAC or saline in preterm (n = 6 NAC, n = 5 control), and B, term (n = 5 NAC, n = 4 control) cohorts (mean, 95% CI). No significant differences in TAMX or CRI between NAC and control infants in any vessel. *P < .016; †P < .007; ‡P < .05, all versus predosing (0 hour).
Figure 4.
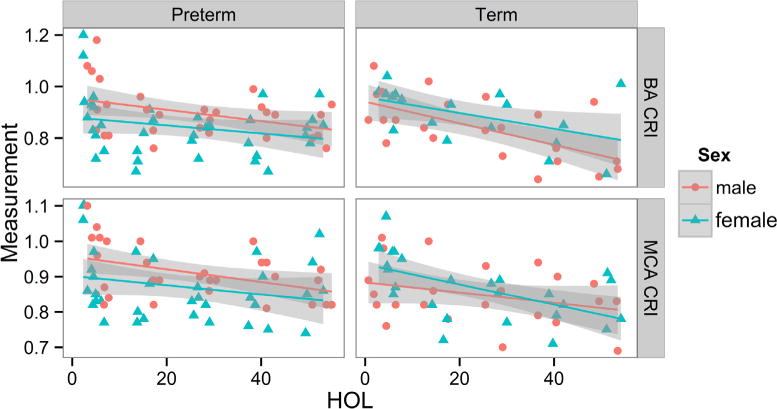
CRIs in BA and MCA over 48 HOLs by sex for individual preterm and term infants, regardless of treatment (preterm: n = 6 females, n = 5 males; term: n = 5 females, n = 4 males). Preterm males have higher CRI than females, whereas term males have lower CRI than females, as previously reported by Koch et al.26 CRI, corrected resistive index.
Figure 5.
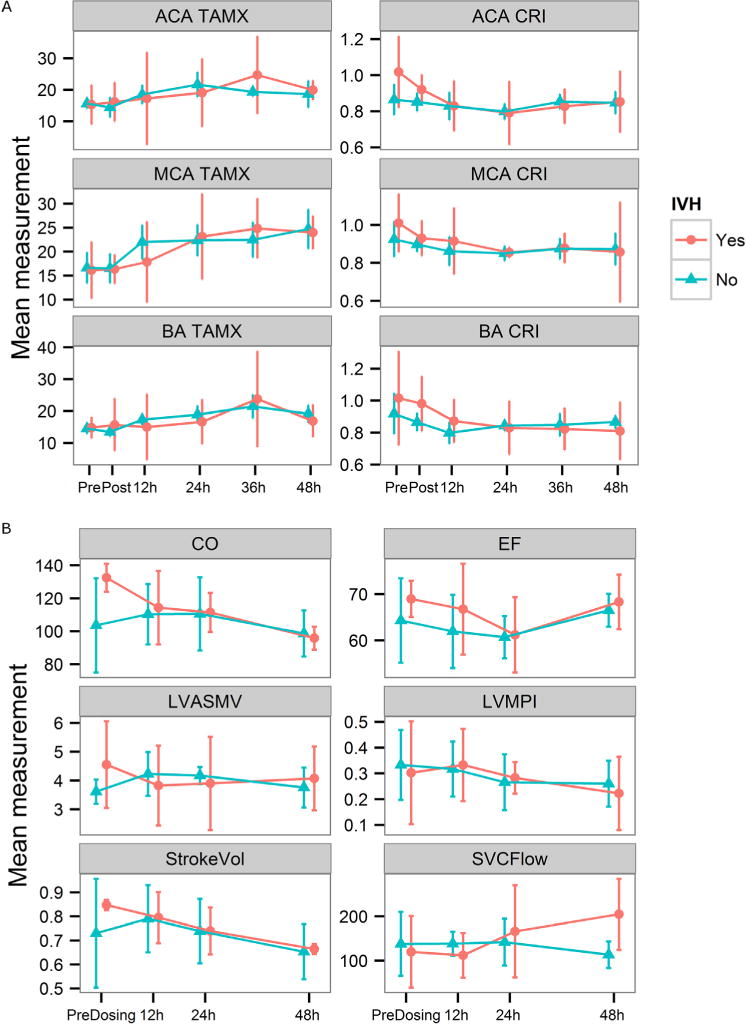
A, CBF and B, cardiac function in preterm infants with IVH in the first week of life vs those with no insult (mean, 95% confidence intervals, CI). A, CBF velocity (TAMX) and resistance (CRI) in ACA, MCA, and BA before and after treatment with NAC or saline (n = 4 IVH, n = 7 control). B, Echocardiographic measurements before and after NAC/saline (n = 4 IVH, n = 7 control). All were not significant. CO, cardiac output index (ml/kg/min); EF, ejection fraction; LVASMV (mL/s); StokeVol, stroke volume index (mL/kg); SVCFlow, superior vena cava flow velocity (mL/kg/min).
NAC Maintains Normal Cerebral Arterial Relationships despite Chorioamnionitis
Resistive indices and blood flow velocities are normally tightly correlated in the major cerebral vessels of an individual.24 Chorioamnionitis disrupts this tight correlation in term infants,26 and blood flow in different regions of the brain may be affected by neuroinflammatory factors and increased metabolic demand. NAC can restore cerebral autoregulation and nitric oxide (NO) responsiveness.27,28 We investigated the pattern of CBF correlation in ACA, MCA, and BA in individual infants, for beneficial effects of NAC on neuroinflammation-induced disruption of CBF. CBF velocities in the MCA were strongly associated with those in ACA (P = .0003) and BA (P = .003) in the group treated with NAC from 0–48 hours of the study, but not in the control group. Resistance in the ACA was also significantly associated with MCA (P = .001) and BA (P = .0004) in the group treated with NAC, but marginally associated with MCA (P = .018) in the control group over time. With Bonferroni correction for 4 mixed model comparisons within treatment groups (MCA and ACA, TAMX and corrected resistive index, P < .0125), all NAC associations remained significant.
Cardiac Function and Cerebral Oxygenation
Mean echocardiographic measurements were not significantly different between treatment groups before and after the first dose of NAC or at 12, 24, and 48 hours in either GA cohort (Figure 6; available at www.jpeds.com) and did not correlate with NAC serum concentrations. NAC administration had no main effect on left ventricular output myocardial performance index or velocity, ejection fraction, or stroke volume.29 Importantly, male sex had a significant negative effect on ejection fraction in these infants exposed to chorioamnionitis (P = .0027).
Figure 6.
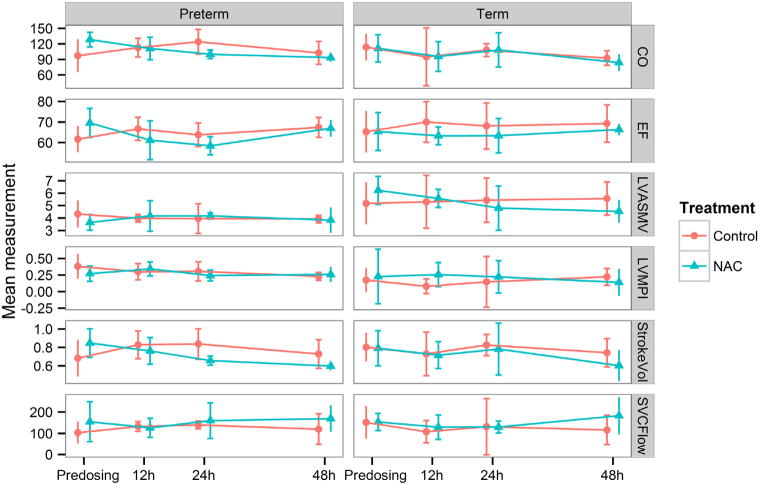
Echocardiographic measures before and after dosing of NAC or saline in preterm and term infants. Cardiac output (mL/kg/min), EF, LVASMV (mL/s,) and LVMPI, stroke volume (mL/kg), and SVC flow (mL/kg/min) were not significantly different before or after dosing of NAC or saline (mean, 95% CI; preterm: n = 6 NAC, n = 5 control; term: n = 4 NAC, n = 4 control).
Near-infrared spectroscopy data were adequate in 7 term and 10 preterm infants, and there were no differences in oxygenation before, during, or after NAC dosing or over time compared with controls. Mean RcSO2 was marginally associated with cardiac output (r = 0.24, P = .05), but not with other indices of cardiac function. In preterm infants exposed to chorioamnionitis, higher RcSO2 in the frontal lobes in the first 6 hours was associated with lower resistance in the ACA (P = .031), adjusting for HOL and GA. The duration of maternal fever prior to birth also correlated with higher RcSO2 in their infants for the first 12 hours, regardless of GA (r ≥ 0.67, P < .007, n = 15–16). Taken together, our findings suggest that neuroinflammation may determine local vasodilation and RcSO2.30
EEG
No electrographic or clinical seizures were recorded in either group. Nine infants treated with NAC and 7 controls had adequate serial EEG tracings for dosing effects. Three infants treated with NAC had abnormal background activity (low amplitude) before NAC dose, which normalized in 2 infants during the NAC infusion. One infant treated with NAC showed persistent low amplitude.
MRI
MRI at term age equivalent had low apparent diffusion coefficients in the posterior limb of the internal capsule in 1 preterm infant treated with NAC and 1 control. Both had normal outcomes. The term infant with HIE treated with NAC had abnormal signal intensities in basal ganglia (BG) bilaterally, but normal Bayley-III scores at 12 months and normal development at 2 years of age.
MRS of myoinositol (mI)/N-acetylaspartate (NAA) ratio in the BG showed a weak trend, with control infants having higher mI/NAA ratios than infants treated with NAC, controlling for GA at birth (P = .08, n = 16). In the preterm group, BG mean mI/NAA ratios were higher in control (1.4 ± 0.23; n = 6) than preterm infants treated with NAC (0.92 ± 0.30; n = 4) exposed to chorioamnionitis (P = .026). Higher mI ratios are associated with astrogliosis, and lower NAA ratios are associated with neuronal injury.31 There were no significant differences in fractional anisotropy in the corpus callosum or internal capsule in infants scanned at term age equivalent, controlling for GA at birth and scan.
Cytokines
Median and IQRs for cytokines from maternal serum at delivery, infant cord serum, and infant CSF samples are reported by treatment group (Table II; available at www.jpeds.com). Serum fibroblast growth factor 2 (FGF2) concentrations were significantly lower in mothers treated with NAC compared with the saline group over time (Figure 7, A), and interleukin (IL)-17 decreased after dosing only in the group treated with NAC (P = .01). FGF2 is produced by activated endothelial cells, and potentiates leukocyte recruitment and extravasation at sites of inflammation.32 IL-17 is secreted by a subset of T helper cells-17, which are responsive to bacterial infection and inflammation.33
Table II.
Cytokine concentrations by treatment group (median, IQR, pg/mL)
| NAC | Control | |
|---|---|---|
| Maternal cytokines at delivery | ||
| CKine | 446 (212–912) | 181 (77–317) |
| Eotaxin | 46 (20–81) | 37 (31–44) |
| FGF2 | 7.6 (7.6–26) | 26 (9.2–93) |
| Fractalkine | 23 (23–23) | 41 (25–69) |
| GCSF | 1345 (357–3932) | 729 (218–2050) |
| GMCSF | 7.8 (7.5–9.3) | 11 (8.4–25) |
| GRO | 1574 (1520–2083) | 944 (787–1566) |
| IL-1a | 9.4 (9.4–9.4) | 9.4 (9.4–17) |
| IL-1Ra | 8.3 (8.3–11) | 8.3 (8.3–8.3) |
| IL-6 | 81 (53–407) | 35 (17–157) |
| IL-7 | 4.4 (1.4–12) | 4.5 (2.5–8.8) |
| IL-8 | 13 (6.3–35) | 38 (17–65) |
| IL-10 | 48.89 (30–352) | 132 (38–198) |
| IL-17 | 0.7 (0.7–3.0) | 20 (9.9–161) |
| IL-23 | 37 (32–51) | 45 (32–305) |
| IP10 | 386 (226–564) | 245 (192–506) |
| LIF | 6.0 (5.8–7.2) | 6.8 (6.3–17) |
| MCP1 | 626 (371–1640) | 1018 (333–1114) |
| MCP2 | 34 (30–41) | 21 (16–34) |
| MCP4 | 51 (37–84) | 21 (14–58) |
| MDC | 923 (790–953) | 699 (500–864) |
| MIP1a | 3.4 (2.9–5.5) | 3.8 (2.9–7.2) |
| MIP1b | 43 (33–64) | 48 (25–103) |
| MIP1d | 5835 (4266–7965) | 6074 (3062–7351) |
| sCD40L | 18 703 (9597–29 332) | 5000 (2630–11 719) |
| sDF1ab | 1950 (1141–2643) | 831 (173–1724) |
| sIL-2RA | 11 (11–11) | 11 (11–36) |
| TPO | 201 (178–446) | 116 (77–730) |
| VEGF | 40 (26–129) | 209 (26–468) |
| Cord serum cytokines | ||
| CKine | 1562 (991–1948) | 689 (100–846) |
| Eotaxin | 41 (19–80) | 57 (42–71) |
| FGF2 | 26 (14–77) | 22 (15–76) |
| Fractalkine | 42 (24–60) | 28 (24–40) |
| GCSF | 3995 (821–14 423) | 827 (176–3618) |
| GMCSF | 7.5 (7.5–12) | 7.5 (7.5–7.9) |
| GRO | 2092 (1253–2521) | 1151 (966–1570) |
| IL-1a | 9.4 (9.4–22) | 9.4 (9.4–9.4) |
| IL-1Ra | 745 (251–1302) | 8.3 (8.3–448) |
| IL-6 | 125 (53–212) | 71 (9.9–195) |
| IL-7 | 1.4 (1.4–2.1) | 1.4 (1.4–6.4) |
| IL-8 | 114 (40–392) | 96 (11–139) |
| IL-10 | 27 (4.0–107) | 4.7 (2.2–32) |
| IL-17 | 0.7 (0.7–0.7) | 0.7 (0.7–3.4) |
| IL-23 | 32 (32–32) | 32 (32–32) |
| IP10 | 294 (176–589) | 255 (194–320) |
| LIF | 8.4 (5.8–14) | 15 (7.8–19) |
| MCP1 | 887 (725–2882) | 1034 (728–1124) |
| MCP2 | 39 (33–48) | 47 (44–69) |
| MCP4 | 85 (49–108) | 52 (46–86) |
| MDC | 1517 (946–1988) | 1223 (1022–2327) |
| MIP1a | 30 (9.7–88) | 11 (9.6–25) |
| MIP1b | 105 (86–143) | 88 (85–98) |
| MIP1d | 11 234 (9796–12 754) | 11 693 (9968–13 114) |
| sCD40L | 30 619 (20 426–56 028) | 18 430 (15 865–40 696) |
| sDF1ab | 2247 (1034–2546) | 2582 (383–2981) |
| sIL2RA | 187 (57–230) | 11 (11–193) |
| TPO | 269 (89–420) | 224 (120–593) |
| VEGF | 69 (26–233) | 241 (234–293) |
| Infant CSF cytokines | ||
| Cathepsin D | 34 577 (29 711–38 967) | 33 890 (28 619–44 022) |
| Complement C4 | 1419 (1295–1448) | 1618 (1162–1981) |
| CRP | 54 (16–121) | 48 (12–179) |
| FGF2 | 7.6 (7.6–9.1) | 11 (7.9–14) |
| Flt3L | 36 (30–43) | 38 (30–41) |
| Fractalkine | 49 (45–59) | 56 (51–67) |
| GCSF | 36 (24–224) | 46 (35–199) |
| GRO | 36 (32–105) | 67 (38–137) |
| IL-1b | 0.8 (0.8–0.8) | 0.8 (0.8–0.8) |
| IL-1Ra | 8.7 (8.3–26) | 8.3 (8.3–8.9) |
| IL-2 | 1.0 (1.0–1.0) | 1.1 (1.0–1.2) |
| IL-6 | 6.2 (0.9–18) | 3.5 (1.5–4.5) |
| IL-7 | 3.7 (2.3–5.9) | 4.9 (3.9–6.1) |
| IL-8 | 403 (243–2239) | 876 (408–1735) |
| IL-10 | 4.9 (3.0–5.2) | 6.2 (2.9–7.7) |
| IL-17 | 0.7 (0.7–0.7) | 0.7 (0.7–0.7) |
| IP10 | 262 (219–357) | 333 (266–1535) |
| MCP1 | 4359 (2962–5688) | 5360 (4504–6168) |
| MDC | 3.6 (3.6–5.5) | 3.6 (3.6–3.6) |
| MIP1a | 6.8 (5.5–12) | 4.4 (3.5–13) |
| MIP1b | 29 (18–31) | 25 (16–41) |
| MIP4 | 0.2 (0.1–0.2) | 0.1 (0.1–0.2) |
| NCAM | 199 685 (17 8413–269 305) | 171 580 (148 688–240 162) |
| PAI1 total | 5221 (2009–16 333) | 4053 (2998–4883) |
| PDGF AA | 65 (37–82) | 53 (48–104) |
| PEDF | 845 (754–931) | 796 (752–955) |
| S100b | 471 (427–652) | 453 (395–808) |
| sCD40L | 5.1 (5.1–38) | 5.1 (5.1–5.1) |
| sICAM1 | 1257 (1071–2443) | 1130 (1029–2088) |
| sIL-2Ra | 11 (11–11) | 11 (11–11) |
| sVCAM1 | 358 857 (261 649–428 756) | 353 097 (276 063–373 702) |
| VEGF | 26 (26–26) | 26 (26–26) |
CKine, secondary lymphoid-tissue chemokine; FGF2, fibroblast growth factor 2; GCSF, granulocyte colony stimulating factor; GM-CSF, granulocyte monocyte colony stimulating factor; GROa, (CXCL1) growth regulated protein alpha; IP10, interferon gamma inducible protein 10; LIF, leukemia inhibitory factor; MCP, monocyte chemotactic factor; MIP, macrophage inflammatory protein; sCD, soluble cluster of differentiation antige; sDF, stromal cell-derived factor; TPO, thyroid peroxidase; CRP, C-reactive protein; FLT3L, FMS-like tyrosine kinase 3 ligand; MCD, macrophage-derived chemokine; NCAM, neural cell adhesion molecule; sICAM1, soluble intercellular adhesion molecule; sVCAM, soluble vascular cell adhesion molecule; PAI1, plasminogen activator inhibitor 1; PDGF AA, Platelet-derived growth factor -AA; PEDF, pigment epithelium-derived factor; S100b, S100 calcium binding protein B.
Figure 7.
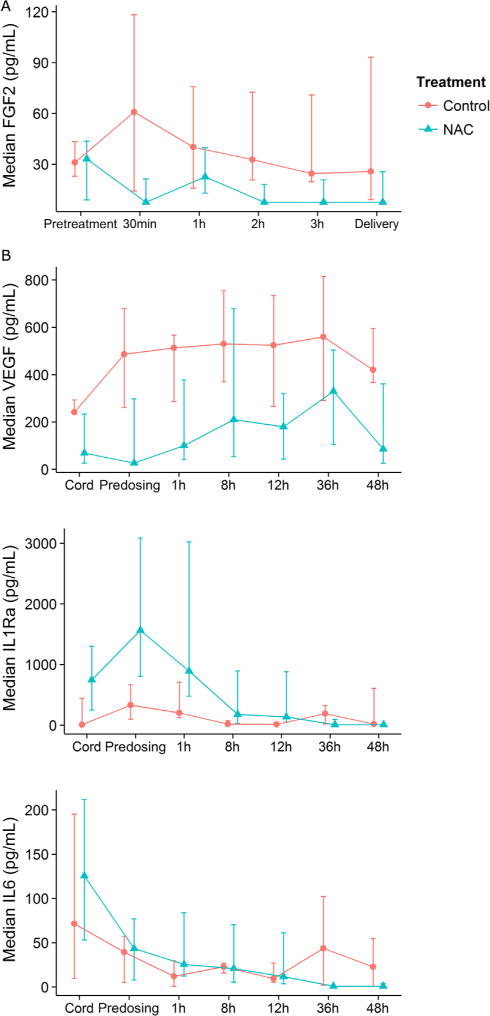
Median, IQR of serum cytokine concentrations (pg/mL): A, FGF-2 before and after NAC/saline dosing in mothers (NAC n = 11; saline n = 7, P = .02); B, VEGF and IL-1Ra in infants over 0–48 hours after delivery with GA and time in mixed model (n = 12 NAC, n = 9 saline; P ≤ .014). For IL-6, there was a significant NAC treatment*time interaction effect (P = .014), from 36–48 HOL. FGF-2, fibroblast growth factor 2.
Infants treated with NAC had significantly lower serum proinflammatory vascular endothelial growth factor (VEGF) and higher anti-inflammatory IL-1 receptor antagonist (IL-1Ra) over time compared with controls (Figure 7, B). For IL-1Ra and IL-6, treatment-time interactions were evident, and IL-6 was lower at 36 and 48 hours in infants treated with NAC vs controls. Our infant serum IL-6 concentrations were similar to published values in preterm infants exposed to chorioamnionitis with fetal inflammatory response and early sepsis (>40 pg/mL)34 and in preterm infants with white matter injury.35 VEGF contributes to early brain injury as a permeability factor associated with blood brain barrier disruption, hemorrhage, and ischemia.36 Anti-inflammatory IL-1Ra provides significant neuroprotection in animal models of chorioamnionitis and HI injury, by blocking IL-1b activity, leukocyte infiltration, and microglial activation.37 Consistent with these data, lower serum IL-1Ra was associated with higher mI/creatinine in the white matter in our infants (n = 13, r = −0.73; P = .01). There were no significant differences between treatment groups in CSF cytokines obtained within 12 hours of birth, controlling for GA (n = 16).
Serum IL-6, IL-8, VEGF, and monocyte chemotactic protein-1 were also significantly related to MCA CBF velocity over the 48 hours of the study in a mixed model with HOL, treatment, and GA (all P ≤ .03, n = 20 subjects, 91 paired CBF/cytokine observations from 0–48 hours). These data are consistent with the roles of these cytokines as biomarkers of endothelial activation and injury.
Developmental Outcomes
Twenty-one infants exposed to chorioamnionitis were available for follow-up to 3–4 years of age (9 controls and 12 infants treated with NAC). Two out of 9 control infants had developmental delay; 1 preterm infant with IVH had speech delay at 3 years, and 1 preterm infant with NEC had fine motor delay at 4 years. Two of the 12 infants treated with NAC had delays; 1 preterm infant with late NEC, grade 4 IVH, and PVL, had spastic quadriplegia and cognitive impairment, and 1 preterm infant had fine motor delay and autism spectrum disorder at 4 years.
Discussion
In this pilot study of neuroprotection in preterm and term infants exposed to chorioamnionitis, NAC administration antenatally in the mother and postnatally to their infants, resulted in no significant adverse effects on CBF, cerebral oxygenation, cardiac function, clotting measurements, or BP. Infants treated with NAC did show beneficial effects, as NAC restored normal cerebrovascular coupling between major cerebral vessels, decreased proinflammatory VEGF, and increased anti-inflammatory IL-1Ra compared with infants who received saline. Mothers treated with NAC also had lower cytokines associated with endothelial activation and leukocyte recruitment during inflammation. Fetal systemic inflammatory response with elevated circulating IL-6 and clotting abnormalities occurred in the majority of infants, strongly suggesting the presence of fetal endothelial activation and neuroinflammation.
In human infants and animal models of chorioamnionitis, FIRS and neuroinflammation cause injury to the CNS and other organs by direct toxicity of cytokine mediators, NO dysregulation, and alterations in perfusion and cerebral autoregulation, which occur very early after infection. Vascular endothelial injury results in dysregulation of fetal and neonatal CBF,8,38 as we saw with decreased cerebrovascular resistance and increased CBF within 12–24 hours, particularly in males. Furthermore, the immediate loss of correlation of CBF and resistance between the 3 major cerebral vessels after birth is consistent with intrauterine onset of neuroinflammation and vascular dysfunction.26,39 Although we did not include a control group of preterm and term infants without chorioamnionitis exposure, another report has found uncoupling of CBF and resistance in term neonates exposed to chorioamnionitis, compared with term infants not exposed to chorioamnionitis.26 Also, abnormal CBF has been documented in early onset sepsis and in preterm infants who develop IVH.25,30 Fetal inflammatory cascades also predispose term and preterm infants to significant morbidities of IVH, HIE, white matter injury, and NEC, in which vascular insufficiency may be a contributing factor.25,30
Antenatal NAC can counteract fetal neuroinflammation associated with chorioamnionitis by several mechanisms, including scavenging oxygen free radicals, restoring intracellular glutathione levels, and decreasing inflammatory cytokine production.10–13 NAC stabilizes CBF, enhances autoregulation, and re-establishes normal vascular reactivity, which depends on endothelial synthesis of NO.27,40 NAC inhibits conversion of NO to peroxynitrite, thereby preserving the bioavailability of NO for normal vascular responsiveness under oxidative stress.27,41
NAC also had no untoward effects on cerebral or systemic perfusion in the fetus or infant when started within 4 hours of clinical diagnosis of chorioamnionitis and administered for the first 48 hours after birth. In a similar time frame in adult patients with endotoxic shock, NAC increased cardiac output index, oxygen delivery, and systemic vascular resistance.42 However, NAC effects are more variable when started later than 24 hours in sepsis.43 Furthermore, high dose NAC has been associated with reduced left ventricular stroke work in a small number of adult patients, when given more than 24 hours from onset of septic shock.44 If present in excess, NAC sulfhydryl groups may react with NO to form S-nitrosothiols, a stable, stored form of NO that can cause vasodilation.45
Although treatment was instituted within 4 hours of chorioamnionitis in our study, we considered that NAC could interact with specific vulnerabilities of fetal and neonatal physiology to produce hypotension. With extensive physiologic monitoring before and after NAC dosing, we found no adverse hemodynamic changes with 100 mg/kg in mothers or 12.5–25 mg/kg/dose NAC administered to their infants compared with saline treatment. Fetuses had significant NAC plasma concentrations, but umbilical and CBF velocities were stable before and after maternal NAC dosing.19
We found no differences in infant BP or RcSO2 over 2 days of dosing between NAC and saline-treated infants, contrary to a report in fetal sheep.18 In this model of septic shock, daily lipopolysaccharide (LPS) administration was followed by a 5-hour infusion of 50, 100, or 200 mg/kg NAC for 5 days (n = 2 per dose),18 which causes significant hypoxemia and hypotension for 3–24 hours in this model.46 When comparing effect of NAC, most of the significant hemodynamic differences were between sham and LPS animals in the mixed model. In post hoc analyses, NAC-LPS animals showed small changes of questionable clinical significance in partial pressure of oxygen in arterial blood, pH, lactate, and mBP compared with LPS-saline animals.18
NAC plasma concentrations and cerebral perfusion were not measured in the fetal sheep, which inhibits a PD comparison with our human study, but NAC dose did not correlate with the changes.18 In our infants, NAC did not adversely affect systemic perfusion, cardiac function, or CBF before and after NAC dosing, or over 48 hours compared with control infants exposed to chorioamnionitis. None of the PD measurements of hemodynamic, cerebral perfusion, or cardiac variables correlated with concurrent NAC plasma concentrations. This indicates that these PD measures are not influenced by NAC at the doses used in infants exposed to chorioamnionitis.
The small sample size in this intensive safety study is a potential limitation. However, other studies have documented no AEs in fetuses of mothers who received NAC for acetaminophen overdose, or in very preterm infants who received NAC continuously for the first 6 DOLs to prevent bronchopulmonary dysplasia.16,17 Our incidence of NEC and IVH after chorioamnionitis is similar to other reports, but our sample size is not large enough to evaluate effects of NAC treatment on these complications. Infants treated with NAC had no greater adverse outcomes related to early events than control infants.
More than one-half of our infants with clotting studies had significant DIC within the first 24 hours after birth, independent of treatment. Coagulopathy has been noted in other reports in 25%-30% of infants exposed to chorioamnionitis, and provides laboratory evidence of systemic inflammatory response with endothelial activation.47,48 In addition, these findings suggest that clotting abnormalities and early increases in CBF may contribute to greater incidence of IVH and neurodevelopmental delays in infants exposed to chorioamnionitis.25
Although our sample size is limited, we did not see threshold or concentration-dependent adverse effects of NAC on CBF, cardiac function, or cerebral oxygenation. Neuroprotective compounds that can be used in this population are rare, and these data support further evaluation of NAC for antenatal neuroprotection.
For antenatal drug administration to be effective in future treatment trials for chorioamnionitis, consideration should be given to length of the consent process in laboring mothers and the short duration of labor in term mothers with chorioamnionitis. However, our PK data show that even a short infusion of NAC rapidly crosses the placenta and can be measured in the cord blood of the neonate, making antenatal administration of NAC feasible for fetal neuroprotection in chorioamnionitis.19
Acknowledgments
Supported by the National Institute of Neurological Disorders and Stroke (NS52448)
We are grateful for the expertise of Donna Roberts, MD, in reading the T2 MRI scans.
Glossary
- ACA
Anterior cerebral artery
- AE
Adverse event
- BA
Basilar artery
- BG
Basal ganglia
- BP
Blood pressure
- CBF
Cerebral blood flow
- CNS
Central nervous system
- CSF
Cerebrospinal fluid
- CUS
Cranial ultrasound
- DIC
Disseminated intravascular coagulopathy
- DOL
Day of life
- EEG
Electroencephalogram
- FIRS
Fetal inflammatory state
- GA
Gestational age
- HI
Hypoxic ischemic
- HIE
HI encephalopathy
- HOL
Hour of life
- IL
Interleukin
- IL-1Ra
IL-1 receptor antagonist
- IVH
Intraventricular hemorrhage
- LPS
Lipopolysaccharide
- mBP
Mean BP
- MCA
Middle cerebral artery
- mI
Myoinositol
- MRI
Magnetic resonance imaging
- MRS
Magnetic resonance spectroscopy
- NAA
N-acetylaspartate
- NAC
N-acetylcysteine
- NEC
Necrotizing enterocolitis
- NO
Nitric oxide
- PD
Pharmacodynamic
- PK
Pharmacokinetics
- PT
Prothrombin time
- PVL
Periventricular leukomalacia
- RcSO2
Regional cerebral oxygenation
- SAE
Serious adverse event
- TAMX
Time average maximum velocity
- VEGF
Vascular endothelial growth factor
Appendix
Methods
Consent was initially obtained within 4 hours of onset of fever, but delivery frequently occurred before study procedures could be completed. With Institutional Review Board approval, we obtained prequalifying consent from women in preterm labor or with ruptured membranes.
Enrollment/randomization was initiated only if inclusion criteria were met. Enrollment was constrained by the time to obtained informed consent in actively laboring women, late presentation/diagnosis of chorioamnionitis in term mothers, and by emergent delivery. Of 111 patients approached but not enrolled, reasons included inadequate time between the qualifying temperature and infant delivery (n = 56); temperature elevation <38°C (n = 16); met exclusion criteria (bronchodilator dependent asthma, participation in another trial, n = 17); or declined participation (n = 22). Seventeen mothers had epidural anesthesia: in 2, fever onset preceded epidural placement, and in 6, fever onset was 9–24 hours after epidural placement. Preterm mothers received magnesium for neuroprotection as standard of care. Obstetrical decisions regarding delivery were not altered for study procedures.
For mothers, criteria to immediately stop N-acetylcysteine (NAC) infusion included seizure, maternal hypotension (blood pressure [BP] <90/60, after fluid bolus), bronchospasm requiring inhaled beta-agonists, angioedema requiring diphenhydramine, anaphylaxis, or clinical bleeding episode with disseminated intravascular coagulopathy (DIC) requiring fresh frozen plasma (FFP).
For infants, criteria to immediately stop NAC infusion included seizures, refractory hypotension, bronchospasm, or DIC (prothrombin time [PT] ≥19; fibrinogen <100 mg/dL) unresponsive to FFP.
Electroencephalograms (Electrocap international Inc, Eaton, Ohio) were recorded during the first dose of NAC/saline, and read by a single pediatric neurologist.
Cerebral blood flow (CBF) studies obtained pulsatility index, time averaged maximum velocity, and resistive index (systolic velocity-diastolic velocity/systolic velocity), corrected for heart rate, were averaged over 5-beat intervals. A single pediatric radiologist verified quality and analysis of Doppler spectra.
Near-infrared spectroscopy probe placed over the forehead (INVOS 4100, Covidien, Ltd, Mansfield, Massachusetts) recorded regional cerebral oxygenation (RcSO2) continuously after admission (preterm), or before, during and after drug administration (term infants) to allow breastfeeding and other interactions with parents. A minimum of 20 minutes continuous RcSO2 data were analyzed before, during, and after NAC/saline dosing, at 0–6, 12, 24, 36, and 48 hours of life (HOLs). Abrupt changes from baseline <60 seconds in duration were omitted.
Echocardiograms were performed before the first dose of study drug (Philips iE33; Philips Medical Systems, Andover, Massachusetts) and at 12, 24, and 48 HOLs during the drug infusion, and read by a single pediatric cardiologist. Superior vena cava (SVC) flow was measured according to the following algorithm [SVC flow = (SVC-velocity time integral * πr2 * heart rate)/weight]. Left ventricular dimensions and contractility measurements consisted of 3-dimensional ejection fraction, stroke volume index (stroke volume/weight), cardiac output index ([stroke volume*heart rate]/weight), left ventricular annular systolic myocardial velocity (LVASMV), and left ventricular myocardial performance index (LVMPI).
Serum was separated, and serum and cerebrospinal fluid samples were stored at −70°C until analysis. Cytokine analysis was performed using Milliplex bead assays (HCTOMAG, NDG4, HNDG2, HNDG3, HCYP2MAG; EMD Millipore, Billerica, Massachusetts) on a BioPlex system (BioRad; Luminex Technology, Hercules, California).
Developmental follow-up was assessed with infant neurodevelopmental screening, gross motor function, clinical auditory, and language assessments (CAT/CLAMS), Bayley III, and/or Vineland testing in neonatal follow-up clinic at 12–24 months of age. Cerebral palsy, developmental delay, autism spectrum disorder, and learning disabilities were assessed at 4 years by the infants’ general and developmental pediatricians. Sixteen infants returned for neurodevelopmental testing at neonatal follow-up clinic, and 5 were seen by their developmental specialist or pediatrician for developmental testing.
Statistical Analyses
Summary descriptive statistics were performed for frequency, severity, and duration of adverse events (AEs). Maternal heart rate and BP, fetal CBF, and infant BP were analyzed by ANOVA before and after dosing between treatment groups and by gestational age (GA) cohorts. Infant CBF, echocardiographic, and RcSO2 were analyzed over time using generalized linear mixed models with repeated measures and GA in the model, as appropriate. Spearman or Pearson correlations defined the relationship of potential pharmacodynamic variables to NAC plasma concentrations. For cytokines, median values and IQRs are reported (nonparametric data), and logarithmic transformations were performed before statistical analysis. Statistics were performed with SAS v 9.2 (SAS Institute, Cary, North Carolina) and R v 3.1.0 (R Development Core Team; designed by Ross Ihaka and Robert Gentleman).
Results
Peak NAC serum levels did not correlate with mean or diastolic BP measurements in mothers, 1–3 hours after NAC. There were no episodes of postpartum bleeding, and PT at delivery did not correlate with serum NAC concentrations in mothers. A single maternal serious AE was reported in a control mother who had rales, wheezing, and hypotension; purulent amniotic fluid and fetal heart rate abnormalities were noted during her urgent cesarean delivery for fetal heart rate abnormalities. Her 24-week GA infant died in the delivery room with severe mixed acidosis (cord pH <6.8, base deficit 24). Placental pathology demonstrated severe necrotizing chorioamnionitis and trivascular funisitis. Infant blood cultures were negative, as the mother received antibiotics.
Fifty-seven percent of the infants with clotting studies had PT >18 seconds (3/6 control; 5/8 NAC). At birth, PT was >18 seconds in 4 out of 8 preterm infants (2 infants treated with NAC and 2 controls), and 1 out of 4 term infants with clotting studies. The term infant treated with NAC had severe hypoxic ischemic (HI) encephalopathy. FFP was infused for DIC in 2 infants treated with NAC and 1 control infant.
Three preterm infants with DIC developed intraventricular hemorrhage (IVH) (2 infants treated with NAC and 1 control). In total, 4 preterm infants had IVH during first week of life. Cranial ultrasound within 24 hours of birth showed grade II IVH in 1 infant treated with NAC and grade III IVH in 1 control infant, both of whom had DIC at birth. Between day of life (DOL) 5 and 7, 1 preterm infant treated with NAC had unilateral small grade II IVH, and 1 infant treated with NAC had grade II/III IVH after requiring FFP for DIC at birth. One infant with NAC had grade I IVH bilaterally with cystic changes at 4.5 HOL, consistent with an intrauterine event prior to study drug infusion. NAC was undetectable in this infant’s cord blood, and coagulation studies clotted. Only 3 infants with IVH also had near-infrared spectroscopy data.
One term infant with NAC had shoulder dystocia requiring vacuum extraction, subgaleal and subdural hemorrhages, severe HI encephalopathy treated with hypothermia, and Erb palsy. Abnormal T1 shortening was present in thalami and the basal ganglia bilaterally, consistent with HI injury. Other serious AEs included postnatally diagnosed multicystic kidney disease (1 infant treated with NAC), and ectopic atrial tachycardia treated with propranolol (1 control).
Necrotizing enterocolitis (NEC) before 30 DOLs occurred in 2 infants. One control infant had medical NEC at DOL 14, requiring antibiotics and bowel rest for 14 days. The second NEC case occurred on DOL 6 in a 30 week GA infant, delivered with severe acidosis (pH 6.92) after rupture of membranes for 9 days with active maternal Escherichia coli urinary tract infection and chorioamnionitis. This infant treated with NAC had just been advanced to 24 calorie/ounce preterm formula on a feeding protocol. Surgical resection resulted in short gut syndrome and placement of a gastrostomy tube. Late NEC developed in 1 infant treated with NAC on DOL 35 with E coli sepsis, hypotension, and renal and respiratory failure. This infant progressed to grade IV IVH at DOL 36 with periventricular leukomalacia at DOL 45 and had cerebral palsy and cognitive deficits at 4 years.
NAC treatment had no main effect on echocardiographic measures, but treatment had an interaction effect with HOL in determining stroke volume (P = .013), with different patterns of stroke volume over time in NAC and saline infants. NAC serum concentrations (cord, predosing trough, peak, 12, or 48 hours) did not show pharmacodynamic correlations with predosing, 12-, 24-, or 48-hour cardiac variables in either GA cohort. Importantly, left ventricular output measures (LVASMV or LVMPI), take into account both diastolic and systolic periods and are independent of heart rate, BP or geometric assumptions inherent in other cardiac output measures, and were not different between treatment groups. As expected, LVASMV and LVMPI were significantly different in preterm and term infants (P < .002). Cardiac output index and stroke volume were different by GA with an interaction effect with HOL (P ≤ .02). Patent ductus arteriosus was found in all preterm infants during the first 2 DOLs, and persisted in 1 infant treated with NAC and 1 control at DOL 7, requiring indomethacin.
Using a mixed model with log transformed cytokines, GA, time, and an interaction term for GA and treatment, serum levels of fibroblast growth factor 2 were significantly lower over time in mothers treated with NAC compared with those receiving saline (Figure 6, A). IL-17 concentrations were significantly lower in mothers treated with NAC at all time points including before dosing, but decreased after dosing only in the group receiving NAC (P = .01).
Footnotes
The authors declare no conflicts of interest.
References
- 1.Yoon BH, Romero R, Park JS, Kim CJ, Kim SH, Choi JH, et al. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol. 2000;182:675–81. doi: 10.1067/mob.2000.104207. [DOI] [PubMed] [Google Scholar]
- 2.Shalak LF, Laptook AR, Jafri HS, Ramilo O, Perlman JM. Clinical chorioamnionitis, elevated cytokines, and brain injury in term infants. Pediatrics. 2002;110:673–80. doi: 10.1542/peds.110.4.673. [DOI] [PubMed] [Google Scholar]
- 3.Thaxton JE, Nevers TA, Sharma S. TLR-mediated preterm birth in response to pathogenic agents. Infect Dis Obstet Gynecol. 2010;2010 doi: 10.1155/2010/378472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D’Alquen D, Kramer BW, Seidenspinner S, Marx A, Berg D, Groneck P, et al. Activation of umbilical cord endothelial cells and fetal inflammatory response in preterm infants with chorioamnionitis and funisitis. Pediatr Res. 2005;57:263–9. doi: 10.1203/01.PDR.0000148713.48218.86. [DOI] [PubMed] [Google Scholar]
- 5.Ghosh A, Birngruber T, Sattler W, Kroath T, Ratzer M, Sinner F, et al. Assessment of blood-brain barrier function and the neuroinflammatory response in the rat brain by using cerebral open flow microperfusion (cOFM) PLoS One. 2014;9:e98143. doi: 10.1371/journal.pone.0098143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armstrong-Wells J, Donnelly M, Post MD, Manco-Johnson MJ, Winn VD, Sebire G. Inflammatory predictors of neurologic disability after preterm premature rupture of membranes. Am J Obstet Gynecol. 2015;212:212e1–9. doi: 10.1016/j.ajog.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paintlia MK, Paintlia AS, Contreras MA, Singh I, Singh AK. Lipopolysaccharide-induced peroxisomal dysfunction exacerbates cerebral white matter injury: attenuation by N-acetyl cysteine. Exp Neurol. 2008;210:560–76. doi: 10.1016/j.expneurol.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garnier Y, Coumans AB, Jensen A, Hasaart THM, Berger R. Infection-related perinatal brain injury: the pathogenic role of impaired fetal cardiovascular control. J Soc Gynecol Investig. 2003;10:450–9. doi: 10.1016/s1071-5576(03)00150-3. [DOI] [PubMed] [Google Scholar]
- 9.Eklind S, Mallard C, Leverin AL, Gilland E, Blomgren K, Mattsby-Baltzer I, et al. Bacterial endotoxin sensitizes the immature brain to hypoxic-ischaemic injury. Eur J Neurosci. 2001;13:1101–6. doi: 10.1046/j.0953-816x.2001.01474.x. [DOI] [PubMed] [Google Scholar]
- 10.Paintlia MK, Paintlia AS, Barbosa E, Singh I, Singh AK. N-acetylcysteine prevents endotoxin-induced degeneration of oligodendrocyte progenitors and hypomyelination in developing rat brain. J Neurosci Res. 2004;78:347–61. doi: 10.1002/jnr.20261. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Svedin P, Nie C, Lapatto R, Zhu C, Gustavsson M, et al. N-acetylcysteine reduces lipopolysaccharide-sensitized hypoxic-ischemic brain injury. Ann Neurol. 2007;61:263–71. doi: 10.1002/ana.21066. [DOI] [PubMed] [Google Scholar]
- 12.Beloosesky R, Gayle DA, Ross MG. Maternal N-acetylcysteine suppresses fetal inflammatory cytokine responses to maternal lipopolysaccharide. Am J Obstet Gynecol. 2006;195:1053–7. doi: 10.1016/j.ajog.2006.06.081. [DOI] [PubMed] [Google Scholar]
- 13.Buhimschi IA, Buhimschi CS, Weiner CP. Protective effect of N-acetylcysteine against fetal death and preterm labor induced by maternal inflammation. Am J Obstet Gynecol. 2003;188:203–8. doi: 10.1067/mob.2003.112. [DOI] [PubMed] [Google Scholar]
- 14.Ercal N, Oztezcan S, Hammond TC, Matthews RH, Spitz DR. High-performance liquid chromatography assay for N-acetylcysteine in biological samples following derivatization with N-(1-pyrenyl)maleimide. J Chromatogr B Biomed Appl. 1996;685:329–34. doi: 10.1016/s0378-4347(96)00196-x. [DOI] [PubMed] [Google Scholar]
- 15.Holmay MJ, Terpstra M, Coles LD, Mishra U, Ahlskog M, Oz G, et al. N-Acetylcysteine boosts brain and blood glutathione in Gaucher and Parkinson diseases. Clin Neuropharmacol. 2013;36:103–6. doi: 10.1097/WNF.0b013e31829ae713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McElhatton PR, Sullivan FM, Volans GN. Paracetamol overdose in pregnancy: analysis of the outcomes of 300 cases referred to the teratology information service. Reprod Toxicol. 1997;11:85–94. doi: 10.1016/s0890-6238(96)00200-6. [DOI] [PubMed] [Google Scholar]
- 17.Ahola T, Lapatto R, Raivio KO, Selander B, Stigson L, Jonsson B, et al. N-acetylcysteine does not prevent bronchopulmonary dysplasia in immature infants: a randomized controlled trial. J Pediatr. 2003;143:713–9. doi: 10.1067/S0022-3476(03)00419-0. [DOI] [PubMed] [Google Scholar]
- 18.Probyn ME, Cock ML, Duncan JR, Tolcos M, Hale N, Shields A, et al. The anti-inflammatory agent N-acetyl cysteine exacerbates endotoxin-induced hypoxemia and hypotension and induces polycythemia in the ovine fetus. Neonatology. 2010;98:118–27. doi: 10.1159/000280385. [DOI] [PubMed] [Google Scholar]
- 19.Wiest DB, Chang E, Fanning D, Garner S, Cox T, Jenkins DD. Antenatal pharmacokinetics and placental transfer of N-acetylcysteine in chorioamnionitis for fetal neuroprotection. J Pediatr. 2014;165:672–7e2. doi: 10.1016/j.jpeds.2014.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Willoughby RE, Jr, Nelson KB. Chorioamnionitis and brain injury. Clin Perinatol. 2002;29:603–21. doi: 10.1016/s0095-5108(02)00058-1. [DOI] [PubMed] [Google Scholar]
- 21.Redline RW. Inflammatory response in acute chorioamnionitis. Semin Fetal Neonatal Med. 2012;17:20–5. doi: 10.1016/j.siny.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Jenkins DD, Rollins LG, Perkel JK, Wagner CL, Katikaneni LP, Bass WT, et al. Serum cytokines in a clinical trial of hypothermia for neonatal hypoxic-ischemic encephalopathy. J Cereb Blood Flow Metab. 2012;32:1888–96. doi: 10.1038/jcbfm.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pejovic B, Peco-Antic A, Marinkovic-Eric J. Blood pressure in non-critically ill preterm and full-term neonates. Pediatr Nephrol. 2007;22:249–57. doi: 10.1007/s00467-006-0311-3. [DOI] [PubMed] [Google Scholar]
- 24.Evans DH, Levene MI, Shortland DB, Archer LN. Resistance index, blood flow velocity, and resistance-area product in the cerebral arteries of very low birth weight infants during the first week of life. Ultrasound Med Biol. 1988;14:103–10. doi: 10.1016/0301-5629(88)90176-7. [DOI] [PubMed] [Google Scholar]
- 25.Noori S, McCoy M, Anderson MP, Ramji F, Seri I. Changes in cardiac function and cerebral blood flow in relation to peri/intraventricular hemorrhage in extremely preterm infants. J Pediatr. 2014;164:264–70. e1–3. doi: 10.1016/j.jpeds.2013.09.045. [DOI] [PubMed] [Google Scholar]
- 26.Koch FR, Wagner CL, Jenkins DD, Caplan MJ, Perkel JK, Rollins LG, et al. Sex differences in cerebral blood flow following chorioamnionitis in healthy term infants. J Perinatol. 2014;34:197–202. doi: 10.1038/jp.2013.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu JQ, Lee TF, Chen C, Bagim DL, Cheung PY. N-acetylcysteine improves hemodynamics and reduces oxidative stress in the brains of newborn piglets with hypoxia-reoxygenation injury. J Neurotrauma. 2010;27:1865–73. doi: 10.1089/neu.2010.1325. [DOI] [PubMed] [Google Scholar]
- 28.Ellis EF, Dodson LY, Police RJ. Restoration of cerebrovascular responsiveness to hyperventilation by the oxygen radical scavenger n-acetylcysteine following experimental traumatic brain injury. J Neurosurg. 1991;75:774–9. doi: 10.3171/jns.1991.75.5.0774. [DOI] [PubMed] [Google Scholar]
- 29.Mertens L, Seri I, Marek J, Arlettaz R, Barker P, McNamara P, et al. Targeted Neonatal Echocardiography in the Neonatal Intensive Care Unit: practice guidelines and recommendations for training. Writing Group of the American Society of Echocardiography (ASE) in collaboration with the European Association of Echocardiography (EAE) and the Association for European Pediatric Cardiologists (AEPC) J Am Soc Echocardiogr. 2011;24:1057–78. doi: 10.1016/j.echo.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 30.Basu S, Dewangan S, Shukla RC, Anupurva S, Kumar A. Cerebral blood flow velocity in early-onset neonatal sepsis and its clinical significance. Eur J Pediatr. 2012;171:901–9. doi: 10.1007/s00431-011-1643-y. [DOI] [PubMed] [Google Scholar]
- 31.Wisnowski JL, Schmithorst VJ, Rosser T, Paquette L, Nelson MD, Haynes RL, et al. Magnetic resonance spectroscopy markers of axons and astrogliosis in relation to specific features of white matter injury in preterm infants. Neuroradiology. 2014;56:771–9. doi: 10.1007/s00234-014-1380-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang H, Issekutz AC. Growth factor regulation of neutrophil-endothelial cell interactions. J Leukoc Biol. 2001;70:225–32. [PubMed] [Google Scholar]
- 33.Reynolds JM, Angkasekwinai P, Dong C. IL-17 family member cytokines: regulation and function in innate immunity. Cytokine Growth Factor Rev. 2010;21:413–23. doi: 10.1016/j.cytogfr.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ng PC, Li K, Wong RP, Chui K, Wong E, Li G, et al. Proinflammatory and anti-inflammatory cytokine responses in preterm infants with systemic infections. Arch Dis Childhood Fetal Neonatal Ed. 2003;88:F209–13. doi: 10.1136/fn.88.3.F209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Procianoy RS, Silveira RC. Association between high cytokine levels with white matter injury in preterm infants with sepsis. Pediatr Crit Care Med. 2012;13:183–7. doi: 10.1097/PCC.0b013e3182231074. [DOI] [PubMed] [Google Scholar]
- 36.van Bruggen N, Thibodeaux H, Palmer JT, Lee WP, Fu L, Cairns B, et al. VEGF antagonism reduces edema formation and tissue damage after ischemia/reperfusion injury in the mouse brain. J Clin Invest. 1999;104:1613–20. doi: 10.1172/JCI8218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Girard S, Sebire H, Brochu ME, Briota S, Sarret P, Sebire G. Postnatal administration of IL-1Ra exerts neuroprotective effects following perinatal inflammation and/or hypoxic-ischemic injuries. Brain Behav Immun. 2012;26:1331–9. doi: 10.1016/j.bbi.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yanowitz TD, Jordan JA, Gilmour CH, Towbin R, Bowen A, Roberts JM, et al. Hemodynamic disturbances in premature infants born after chorioamnionitis: association with cord blood cytokine concentrations. Pediatr Res. 2002;51:310–6. doi: 10.1203/00006450-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Okumura A, Toyota N, Hayakawa F, Kato T, Maruyama K, Kubota T, et al. Cerebral hemodynamics during early neonatal period in preterm infants with periventricular leukomalacia. Brain Dev. 2002;24:693–7. doi: 10.1016/s0387-7604(02)00083-9. [DOI] [PubMed] [Google Scholar]
- 40.Prasad A, Andrews NP, Padder FA, Husain M, Quyyumi AA. Glutathione reverses endothelial dysfunction and improves nitric oxide bioavailability. J Am Coll Cardiol. 1999;34:507–14. doi: 10.1016/s0735-1097(99)00216-8. [DOI] [PubMed] [Google Scholar]
- 41.Macarthur H, Westfall TC, Wilken GH. Oxidative stress attenuates NO-induced modulation of sympathetic neurotransmission in the mesenteric arterial bed of spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2008;294:H183–9. doi: 10.1152/ajpheart.01040.2007. [DOI] [PubMed] [Google Scholar]
- 42.Ortolani O, Conti A, De Gaudio AR, Moraldi E, Cantini Q, Novelli G. The effect of glutathione and N-acetylcysteine on lipoperoxidative damage in patients with early septic shock. Am J Respir Crit Care Med. 2000;161:1907–11. doi: 10.1164/ajrccm.161.6.9903043. [DOI] [PubMed] [Google Scholar]
- 43.Rank N, Michel C, Haertel C, Lenhart A, Welte M, Meier-Hellmann A, et al. N-acetylcysteine increases liver blood flow and improves liver function in septic shock patients: results of a prospective, randomized, double-blind study. Crit Care Med. 2000;28:3799–807. doi: 10.1097/00003246-200012000-00006. [DOI] [PubMed] [Google Scholar]
- 44.Peake SL, Moran JL, Leppard PI. N-acetyl-L-cysteine depresses cardiac performance in patients with septic shock. Crit Care Med. 1996;24:1302–10. doi: 10.1097/00003246-199608000-00006. [DOI] [PubMed] [Google Scholar]
- 45.Muller B, Kleschyov AL, Malblanc S, Stoclet JC. Nitric oxide-related cyclic GMP-independent relaxing effect of N-acetylcysteine in lipopolysaccharide-treated rat aorta. Br J Pharmacol. 1998;123:1221–9. doi: 10.1038/sj.bjp.0701737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duncan JR, Cock ML, Scheerlinck JP, Westcott KT, McLean C, Harding R, et al. White matter injury after repeated endotoxin exposure in the preterm ovine fetus. Pediatr Res. 2002;52:941–9. doi: 10.1203/00006450-200212000-00021. [DOI] [PubMed] [Google Scholar]
- 47.Andrews WW, Goldenberg RL, Faye-Petersen O, Cliver S, Goepfert AR, Hauth JC. The Alabama Preterm Birth study: polymorphonuclear and mononuclear cell placental infiltrations, other markers of inflammation, and outcomes in 23- to 32-week preterm newborn infants. Am J Obstet Gynecol. 2006;195:803–8. doi: 10.1016/j.ajog.2006.06.083. [DOI] [PubMed] [Google Scholar]
- 48.Been JV, Lievense S, Zimmermann LJ, Kramer BW, Wolfs TG. Chorioamnionitis as a risk factor for necrotizing enterocolitis: a systematic review and meta-analysis. J Pediatr. 2013;162:236–42e2. doi: 10.1016/j.jpeds.2012.07.012. [DOI] [PubMed] [Google Scholar]


