Abstract
Objective
To determine the rate of adverse events associated with endotracheal intubation in newborns and modifiable factors contributing to these events.
Study design
We conducted a prospective, observational study in a 100-bed, academic, level IV Neonatal Intensive Care Unit (NICU) from September 2013 through June 2014. We collected data on intubations using standardized data collection instruments with validation by medical record review. Intubations in the delivery or operating rooms were excluded. The primary outcome was an intubation with any adverse event. Adverse events were defined and tracked prospectively as non-severe or severe. We measured clinical variables including number of attempts to successful intubation and intubation urgency (elective, urgent or emergent). We used logistic regression models to estimate the association of these variables with adverse events.
Results
During the study period, 304 intubations occurred in 178 infants. Data were available for 273 intubations (90%) in 162 patients. Adverse events occurred in 107 (39%) intubations with non-severe and severe events in 96 (35%) and 24 (8.8%) intubations, respectively. Increasing number of intubation attempts (odds ratio [OR] 2.1, 95% confidence intervals [CI], 1.6–2.6) and emergent intubations (OR 4.7, 95% CI, 1.7– 13) were predictors of adverse events. The primary cause of emergent intubations was unplanned extubation (62%).
Conclusion
Adverse events are common in the NICU, occurring in 4 of 10 intubations. The odds of an adverse event doubled with increasing number of attempts and quadrupled in the emergent setting. Quality improvement efforts to address these factors are needed to improve patient safety.
Keywords: patient safety, tracheal intubation, NICU
Infants in the Neonatal Intensive Care Unit (NICU) are among the highest risk groups for adverse events in the hospital setting (1, 2). In adult and pediatric intensive care units, adverse events related to endotracheal or tracheostomy tubes comprise a substantial proportion of total adverse events and lead to significant patient harm (3–6). Little is documented about airway safety in the NICU.
In pediatric intensive care units (PICU), 19–41% of all endotracheal intubation procedures are associated with adverse events (7–10). Studies from the National Emergency Airway Registry for Children (NEAR4Kids) report that in children beyond the newborn period, these adverse events are associated with patient (9), provider (10) and practice factors (11). Studies of endotracheal intubation in the NICU have focused primarily on proficiency, mainly of trainees, and use of premedications (12–17). Few studies have reported rates and types of adverse events associated with endotracheal intubation in critically ill newborns and potentially modifiable factors associated with these complications (18). As a result, evidence-based interventions to improve airway safety in this vulnerable population are lacking. We hypothesized that in critically ill newborns in the NICU, adverse events associated with intubation would match or exceed the rate in children or adults. Our objectives were to describe the rate and types of adverse events associated with endotracheal intubation in the NICU and identify potentially modifiable factors associated with these events.
Methods
We conducted a prospective, observational study in the 100-bed, academic, Level IV (regional) NICU of the Vanderbilt University Medical Center. Endotracheal intubations are performed by pediatric residents, neonatal fellows, neonatal nurse practitioners/hospitalists, neonatologists, anesthesiologists and otolaryngologists. Premedication for intubation was commonly used and consisted of an opiate and a benzodiazepine, though no formal protocol existed at the time of this study. The Vanderbilt Institutional Review Board approved the study with waiver of consent for infants and providers.
All intubations that occurred in the NICU from September 1, 2013, through June 30, 2014, were eligible for inclusion. To ensure high quality data collection, we excluded intubations that occurred in areas outside of the NICU such as the delivery room, operating room or during transport. Study personnel completed daily medical record review of all infants in the NICU to determine eligible intubations.
We developed two standardized data collection tools. Our primary tool was a voluntary Post-Intubation Provider Survey that the intubating clinician completed after an intubation encounter and that documented the presence of any of the a priori defined adverse events (Appendix 1; available at www.jpeds.com). Our secondary tool was an Intubation Procedural Record that the bedside nurse completed during the intubation. The study PI (LDH) also validated three adverse events (chest compressions, hypotension receiving treatment, mainstem bronchial intubation) through standardized medical record review. We defined intubation encounters, courses and attempts as described by Nishisaki et al for the NEAR4Kids investigators (9). Briefly, an encounter was defined as one completed episode of airway management and could involve multiple courses. Courses were one approach (oral, nasal or bronchoscopy) to secure an airway and could include multiple attempts. An attempt began when the laryngoscope entered the mouth and ended with laryngoscope removal.
Study Outcomes
The primary safety outcome of our study was an intubation encounter with one or more adverse events. We defined these adverse events (Table I) a priori based on literature review and local expert opinion. We used strict operational definitions for each adverse event to minimize bias (Appendix 2; available at www.jpeds.com). To allow comparison with available pediatric data (9), we classified these events as either non-severe or severe. Secondary outcomes were severe hypoxemia (oxygen saturation < 60%) and bradycardia (heart rate less than 60 beats per minute for 5 seconds) during the intubation encounter. We tracked these serious events as a measure of infant stability during intubation, although to allow comparison with available pediatric data, they were not classified as adverse events and were not included in our primary analyses (8–11, 18).
Table 1.
Intubation Associated Adverse Events by Severity (n=273 neonatal intubation encounters)
| Non-severe Events- n (%) | Severe Events- n (%) | ||
|---|---|---|---|
| Any | 96 (35) | Any | 24 (8.8) |
| Esophageal intubation with immediate recognition |
58 (21.4) | Hypotension receiving treatmenta | 10 (3.7) |
| Oral/airway bleeding | 26 (9.5) | Transition to emergent | 9 (3.3) |
| Difficult bag-mask ventilation | 20 (7.3) | Chest compressionsb | 8 (2.9) |
| Mainstem bronchial intubation (CXR confirmed) |
19 (7) | Code medications | 2 (0.7) |
| Emesis | 6 (2.2) | Pneumothorax | 1 (0.4) |
| Chest wall rigidityc | 3 (1.1) | Direct airway trauma | 1 (0.4) |
| Death | 1 (0.4) | ||
| Esophageal intubation with delayed recognition |
0 | ||
Two infants were receiving treatment for hypotension prior to intubation but had escalation of treatment after intubation.
Four infants were receiving chest compressions at the time of the first intubation attempt that continued during the intubation attempts.
2 of 3 infants were treated with emergent intubation. No infant received pharmacologic treatment for chest wall rigidity.
Independent Variables
We measured clinical variables that have either been associated with adverse events in older patients or which we hypothesized would be pertinent in neonates. Intubations were defined as elective, urgent or emergent. Intubations were defined as urgent when an artificial airway was needed imminently (within 4 hours) but time was available for premedication and pre-procedural patient stabilization. An intubation was classified as emergent if establishment of an airway was considered immediately necessary due to vital sign instability and time was not available for premedications or effective bag-mask ventilation/pre-oxygenation could not be sustained. All other intubations were classified as elective.
Statistical Analyses
We used both univariable and multivariable logistic regression models to examine the associations between clinical variables and adverse events. Our multivariable model included 4 variables that we hypothesized a priori would be associated with adverse events: postmenstrual age, premedication use, first-attempt proceduralists’ clinical role and intubation urgency. Generalized estimating equations were used to fit marginal logistic regression models with clustering within patients to account for multiple intubation encounters per patient (19). Our primary analysis included only intubations with a completed Post-Intubation Provider Survey. To test the implications of missing data, we performed sensitivity analyses by coding all excluded intubations as either having an adverse event or not having an adverse event. We then re-estimated each logistic regression model to evaluate if our odds ratio (OR) estimates changed enough to alter our final conclusions. To attempt to estimate under- or over-reporting of adverse events, we compared medical record review with our voluntary data collection instrument and calculated percent agreements and kappa statistics for three adverse events. All analyses were performed in R version 3.1.2 using a 2-sided significance level of 0.05.
Results
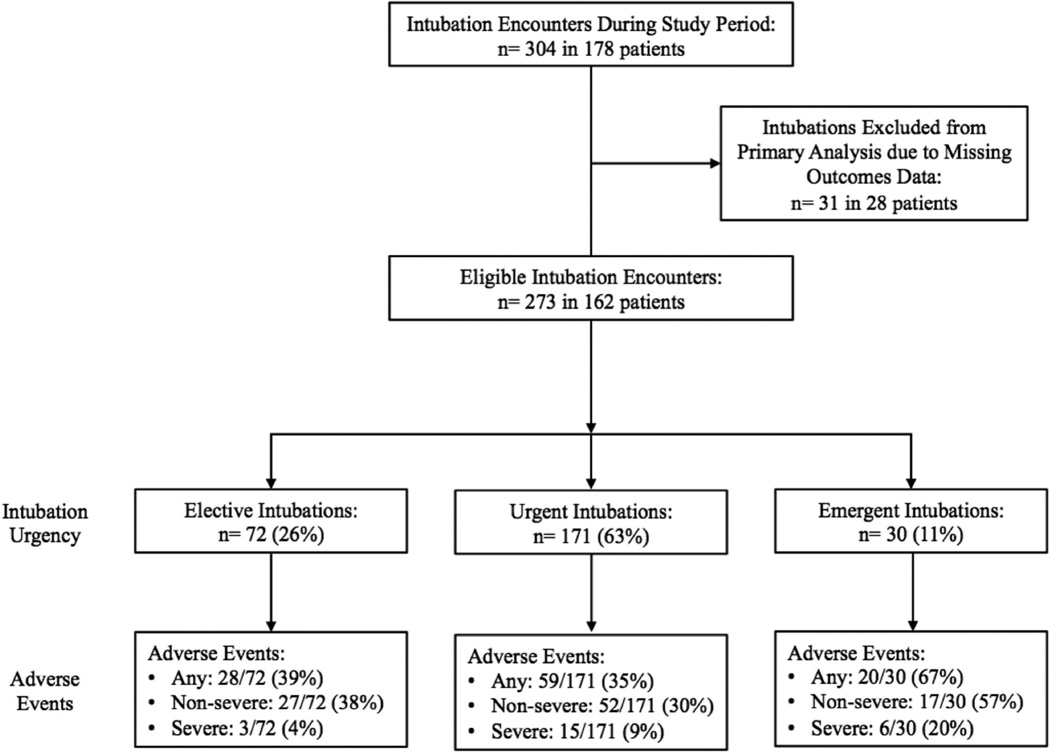
During the 10-month study period, clinicians performed 304 endotracheal intubation encounters in 178 infants, an average of 1 intubation encounter per day. All intubations were performed orally, and no intubation encounter required more than one course. Adverse event data were available for 273 intubation encounters (90%) in 162 patients. One or more adverse events occurred in 107 of 273 intubations (39%). Non-severe and severe events occurred in 96 (35%) and 24 (8.8%) intubations, respectively (Table I). Secondary outcomes of hypoxemia and bradycardia were reported in 121 (44%) and 66 (24%) intubations, respectively. Rates of both non-severe and severe adverse events were higher in emergent intubations versus urgent and elective intubations (Figure 1; available at www.jpeds.com).
Figure 1.
Flow diagram of neonatal intubation encounters, intubation urgency and adverse events during the study period.
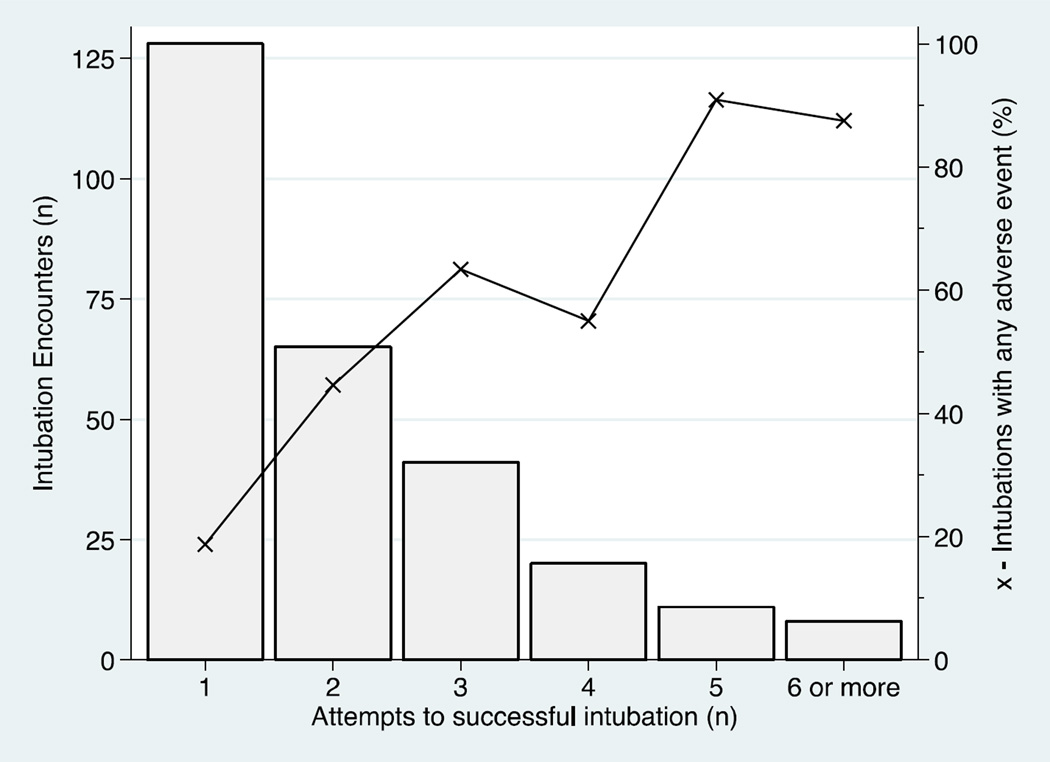
In univariable analysis, number of attempts and intubation urgency were associated with adverse events (Table II). Rates of adverse events increased with increasing number of intubation attempts (Figure 2; available at www.jpeds.com). Our multivariable analysis found that after controlling for postmenstrual age, premedication use and first attempt proceduralists’ clinical role, emergent intubations were strongly associated with adverse events compared with elective procedures (OR 4.67, 95% CI 1.68–12.95; Table III). The most common cause of emergent intubations in our cohort was unplanned extubations, accounting for 62% of those procedures.
Table 2.
Unadjusted Associations of Patient, Provider and Practice Characteristics with Adverse Events
| Variable | No Adverse Event# (n=166) |
Adverse Event# (n=107) |
Odds ratio [95% CI] |
P- value |
|---|---|---|---|---|
| Postnatal age- median days [IQR] |
14 [1,44] | 17 [2,46] | 1.1 [0.7, 1.7]a | 0.93 |
| Postmenstrual age- median weeks [IQR] |
33 [29,38] | 32 [29,38] | 1.0 [0.8,1.3]b | 0.81 |
| Weight- median grams [IQR] |
1600 [1022,2878] | 1560 [998,2815] | 0.9 [0.6, 1.5]c | 0.72 |
| Sex (male) | 105 (63) | 65 (61) | 0.9 [0.5, 1.5] | 0.68 |
| Craniofacial anomaly | 3 (2) | 7 (7) | 3.8 [1, 14.4] | 0.05 |
| Premedication use | 124 (75) | 77 (72) | 0.9 [0.5, 1.5] | 0.63 |
| Opiate use | 120 (72) | 72 (67) | 0.8 [0.5, 1.3] | 0.38 |
| Benzodiazepine use | 76 (46) | 47 (44) | 0.9 [0.6, 1.5] | 0.76 |
| Muscle relaxant use | 11 (7) | 5 (5) | 0.7 [0.2, 2] | 0.5 |
| Number of attempts- median [IQR] |
1 [1,2] | 3 [2,4] | 2.1 [1.6, 2.6]d | <0.005 |
| Proceduralist- 1st attempt | ||||
| Resident | 20 (12) | 16 (15) | Reference | 0.24* |
| Neonatology Fellow | 72 (43) | 50 (47) | 0.9 [0.2, 1.8] | |
| NNP/Hospitalist | 70 (42) | 34 (32) | 0.6 [0.3, 1.3] | |
| Other | 4 (3) | 7 (6) | 2.2 [0.5, 8.8] | |
| Self-reported experience level of first attempt proceduralists | ||||
| <10 attempts | 20 (12) | 21 (20) | Reference | 0.09* |
| 10–40 attempts | 60 (36) | 44 (41) | 0.7 [0.3, 1.5] | |
| >40 attempts | 85 (52) | 42 (39) | 0.47 [0.2, 1] | |
| Intubation urgency- no. (%) | ||||
| Elective | 44 (27) | 28 (27) | Reference | 0.01* |
| Urgent | 112 (67) | 58 (55) | 0.8 [0.4, 1.5] | |
| Emergent | 10 (6) | 19 (18) | 3 [1.4, 6.4] | |
| Device used for first attempt | ||||
| Direct laryngoscope | 149 (90) | 96 (90) | Reference | 0.95* |
| Video laryngoscope | 16 (9) | 10 (9) | 1 [0.4, 2.2] | |
| Bronchoscopy1 | 1 (1) | 1 (1) | 1.5 [0.5, 25.1] | |
Unless otherwise noted, values represent n (%).
p-value for the entire covariate.
Odds change for each 40 postnatal days change.
Odds change for each 10 weeks of postmenstrual age change.
Odds change for each 2000 grams weight change.
Odds change for each 1 attempt change.
Performed by Pediatric Otolaryngologists for 1) selective mainstem intubation in an infant with severe pulmonary interstitial emphysema and 2) to confirm placement of endotracheal tube in an infant requiring multiple attempts on a prior encounter.
Figure 2. Adverse events are related to number of intubation attempts.
Bars represent the number of intubation courses that required each number of attempts. Connected dots represent the adverse event rate for intubations requiring each number of attempts.
Table 3.
Multivariable Logistic Regression Model with Generalized Estimating Equations
| Variable | Odds Ratio [95% CI] | P-value |
|---|---|---|
| Postmenstrual age at intubation | 1.04 [0.64, 1.67]a | 0.88 |
| Any premedication used | 1.58 [0.78, 3.23] | 0.21 |
| Proceduralist- 1st attempt | ||
| Resident | Ref. | 0.33 |
| Neonatology fellow | 0.79 [0.36, 1.76] | |
| NNP/Neonatal Hospitalist | 0.58 [0.26, 1.28] | |
| Intubation urgency | ||
| Elective | Reference | <0.005 |
| Urgent | 0.95 [0.5, 1.81] | |
| Emergent | 4.67 [1.68, 12.95] |
Odds change for each 10 weeks of postmenstrual age change.
Overall and first attempt success rates were lower for residents than neonatal nurse practitioners/hospitalists and neonatal-perinatal medicine fellows. As expected, success rates were higher for experienced practitioners (Table IV; available at www.jpeds.com). Attending neonatologists intubated infrequently in our NICU as trainees and nurse practitioners performed most procedures.
Table 4.
First Attempt and Overall Success Rates by First Intubation Attempt Provider
| First attempt Successful/total (%) |
All attempts Successful/total (%) |
|
|---|---|---|
| All providers | 129/273 (47) | 273/567 (48) |
| Clinical Role | ||
| Resident (PGY 1–4) | 8/36 (22) | 11/64 (17) |
| Neonatal Fellow (PGY 4–7) | 68/123 (55) | 130/230 (57) |
| NNP/Hospitalist | 48/103 (47) | 103/218 (47) |
| Self-Reported Experience | ||
| Novice (<10 attempts) | 9/41 (22) | 14/77(18) |
| Intermediate (10–40 attempts) | 47/104 (45) | 90/194 (46) |
| Experienced (>40 attempts) | 73/127 (57) | 169/296 (57) |
Our sensitivity analyses showed that our multivariable model was robust even after accounting for missing data. After imputing extremes of outcomes, emergent intubations remained significantly associated with adverse events.
Our data collection instrument had high agreement with medical record review for chest compressions (agreement= 99.3%, κ= 0.89). Hypotension receiving intervention (agreement= 97.4%, κ= 0.45) and mainstem bronchial intubation (agreement= 94.5%, κ= 0.33) showed lower agreement because medical record review detected more events than our voluntary data collection instrument reported. Low sensitivity for 2 of 3 validated adverse events suggests that our data collection instrument may under-report adverse events overall.
Discussion
We report that adverse events associated with endotracheal intubation in a large academic NICU were common, occurring in 4 of 10 intubations. Emergent intubations are associated with more than four-fold increased odds of an adverse event. The most common cause for an emergent intubation in our cohort was unplanned extubation. In addition, higher numbers of intubation attempts were associated with increasing rates of adverse events, with each additional attempt doubling the odds of an adverse event.
Our study is similar to a single-center study that evaluated tracheal intubation associated events in a large, academic NICU using the NEAR4Kids registry (18). Foglia et al showed an adverse event rate of 22% compared with our finding of 39%. Several reasons may explain this discrepancy. First, premedication practices differed between our units. Although muscle relaxants were rarely used in our cohort, they were used in the majority of intubations in the study by Foglia et al and were associated with decreased odds of an adverse event (18). The infrequent use of muscle relaxants likely explains our finding that premedication use alone did not decrease the odds of an adverse event. The majority of American neonatologists use premedication but do not use muscle relaxants for intubation (20). The addition of muscle relaxants to analgesia for neonatal intubation has been shown to decrease the number of attempts (17). Our finding that increased intubation were associated with adverse events, coupled with the findings from the study by Foglia et al suggesting that the careful addition of muscle relaxants for intubation will improve the safety of this procedure. Another possible reason for the difference in adverse event rates is how adverse events were measured. Although we used adverse events similar to the NEAR4Kids investigators, we also captured events such as chest wall rigidity that may be more specific to a neonatal population. Variation in definitions of adverse events makes it difficult to compare single center studies and highlights the need for multi-center neonatal studies with common definitions and outcomes.
Our findings suggest that adverse events may be more common during endotracheal intubation in newborns than in older children, although proportions of events are similar. In the NEAR4Kids multi-center cohort, adverse events occurred in 20% of intubations, approximately half of our rate. Similar to our study, esophageal intubation was the most commonly reported adverse event. Also similar to our findings, hypotension receiving intervention was the most common severe event in children, occurring in approximately 3% of intubations (9).
We found that emergent intubations were strongly associated with adverse events, a finding similar to a retrospective study of older children (7). In our cohort, the most common cause of emergent intubation was re-intubation after an unplanned extubation. In a study using a focused trigger tool to identify harm in the NICU, accidental extubation requiring re-intubation was the fourth most common adverse event in North American NICUs and accounted for 8.3% of the total adverse events detected (21). Avoiding unplanned extubations is an important and potentially modifiable area of patient safety and would be expected to decrease the rate of intubation related adverse events as well.
Our study has several limitations. First, as with any observational study, we can only show associations and not causality. Some of the adverse events, such as difficulty with bag-mask ventilation, may reflect the underlying patient pathology necessitating intubation rather than a complication of the procedure. Although we included difficulty with bag-mask ventilation to capture unrecognized laryngospasm, bronchospasm or chest wall rigidity, future studies should describe whether any difficulty with bag-mask ventilation was present initially or began during the intubation encounter. Second, we used a voluntary reporting system combined with medical record review to measure the rates of adverse events. Even though voluntary reporting systems are important in patient safety research (22), adverse events may be under- or over reported and lead to potential misclassification bias. For the three adverse events validated in the medical record, our analysis indicated a tendency for underreporting of adverse events. As a result, our findings likely underestimate the number of adverse events that actually occur. Another potential limitation is that our single center study may not be generalizable to other NICUs. Substantial variation exists in rates of adverse events among PICU sites in the NEAR4Kids registry (11) and it is likely that similar variation exists among NICUs. We were able to capture adverse event data on 90% of intubations during our study period. Although the missing data may be different, our sensitivity analyses suggest these missing intubations would have had no impact on our observed associations. Also, given our study size, in our multivariable model we were only able to adjust for those exposures we hypothesized a priori would be associated with adverse events.
Finally, a potential limitation is our classification of adverse events as non-severe or severe. It is possible that under certain circumstances, non-severe events could have resulted in significant patient harm similar to severe events. For example, chest wall rigidity and difficulty with bag-mask ventilation in the presence of multiple attempts at intubation could be catastrophic. Although our classification of nonsevere or severe events allows comparison with existing pediatric data, we performed our statistical analysis with all adverse events treated equally.
Despite these limitations, our study comprehensively documents adverse safety events associated with endotracheal intubation in the NICU. Our prospective design, strict definitions of adverse events, data validation procedures and high intubation capture rate improve the internal validity of our study and allow us to develop evidence-based quality improvement interventions to decrease the rate of adverse events in our NICU. Interventions such as the increased use of premedication with muscle relaxants to decrease the number of intubation attempts, checklists utilizing prospective criteria to identify infants at highest risk of a difficult airway and selective criteria for infants who may be intubated by trainees will likely improve the safety of this common procedure.
In conclusion, adverse events associated with endotracheal intubation are common in the NICU and associated with emergent intubations and increasing number of attempts. Future studies are needed to document the burden of these adverse events in clinical practice and test quality improvement interventions to decrease their frequency.
Supplementary Material
Acknowledgments
Use of the Research Electronic Data Capture program was supported by National Center for Advancing Translational Sciences/National Institutes of Health (NIH; UL1 TR000445). L.H. was supported by the NIH (5T32HD068256-02) and the John and Leslie Hooper Neonatal-Perinatal Endowment Fund.
Abbreviations
- NICU
Neonatal Intensive Care Unit
- OR
odds ratio
- CI
confidence intervals
- PICU
Pediatric Intensive Care Unit
- NEAR4Kids
National Emergency Airway Registry for Children
- PMA
postmenstrual age
- NNP
Neonatal nurse practitioner
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
Portions of this study have been presented as abstracts and posters at the Vermont Oxford Network Quality Congress, <city, state>, <dates>, and the meeting of the Pediatric Academic Societies, San Diego, CA, April 25–28, 2015.
References
- 1.Kaushal R, Bates DW, Landrigan C, McKenna KJ, Clapp MD, Federico F, et al. Medication errors and adverse drug events in pediatric inpatients. JAMA. 2001;285:2114–2120. doi: 10.1001/jama.285.16.2114. [DOI] [PubMed] [Google Scholar]
- 2.Snijders C, van Lingen RA, Molendijk A, Fetter WP. Incidents and errors in neonatal intensive care: a review of the literature. Arch Dis Child Fetal Neonatal Ed. 2007;92:F391–F398. doi: 10.1136/adc.2006.106419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Needham DM, Thompson DA, Holzmueller CG, Dorman T, Lubomski LH, Wu AW, et al. A system factors analysis of airway events from the Intensive Care Unit Safety Reporting System (ICUSRS) Crit Care Med. 2004;32:2227–2233. doi: 10.1097/01.ccm.0000145230.52725.6c. [DOI] [PubMed] [Google Scholar]
- 4.Rivera R, Tibballs J. Complications of endotracheal intubation and mechanical ventilation in infants and children. Crit Care Med. 1992;20:193–199. doi: 10.1097/00003246-199202000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Stambouly JJ, McLaughlin LL, Mandel FS, Boxer RA. Complications of care in a pediatric intensive care unit: a prospective study. Intensive Care Med. 1996;22:1098–1104. doi: 10.1007/BF01699236. [DOI] [PubMed] [Google Scholar]
- 6.Skapik JL, Pronovost PJ, Miller MR, Thompson DA, Wu AW. Pediatric safety incidents from an intensive care reporting system. J Patient Saf. 2009;5:95–101. doi: 10.1097/PTS.0b013e3181a70c68. [DOI] [PubMed] [Google Scholar]
- 7.Carroll CL, Spinella PC, Corsi JM, Stoltz P, Zucker AR. Emergent endotracheal intubations in children: be careful if it’s late when you intubate. Pediatr Crit Care Med. 2010;11:343–348. [PubMed] [Google Scholar]
- 8.Nishisaki A, Ferry S, Colborn S, DeFalco C, Dominguez T, Brown CA, 3rd, et al. Characterization of tracheal intubation process of care and safety outcomes in a tertiary pediatric intensive care unit. Pediatr Crit Care Med. 2012;13:e5–e10. doi: 10.1097/PCC.0b013e3181fe472d. [DOI] [PubMed] [Google Scholar]
- 9.Nishisaki A, Turner DA, Brown CA, 3rd, Walls RM, Nadkarni VM, et al. National Emergency Airway Registry for C, A National Emergency Airway Registry for children: landscape of tracheal intubation in 15 PICUs. Crit Care Med. 2013;41:874–885. doi: 10.1097/CCM.0b013e3182746736. [DOI] [PubMed] [Google Scholar]
- 10.Sanders RC, Jr, Giuliano JS, Jr, Sullivan JE, Brown CA, 3rd, Walls RM, Nadkarni V, et al. Level of trainee and tracheal intubation outcomes. Pediatrics. 2013;131:e821–e828. doi: 10.1542/peds.2012-2127. [DOI] [PubMed] [Google Scholar]
- 11.Nett S, Emeriaud G, Jarvis JD, Montgomery V, Nadkarni VM, Nishisaki A. Site-Level Variance for Adverse Tracheal Intubation-Associated Events Across 15 North American PICUs: A Report From National Emergency Airway Registry for Children. Pediatr Crit Care Med. 2014;15:306–313. doi: 10.1097/PCC.0000000000000120. [DOI] [PubMed] [Google Scholar]
- 12.Falck AJ, Escobedo MB, Baillargeon JG, Villard LG, Gunkel JH. Proficiency of pediatric residents in performing neonatal endotracheal intubation. Pediatrics. 2003;112:1242–1247. doi: 10.1542/peds.112.6.1242. [DOI] [PubMed] [Google Scholar]
- 13.Lane B, Finer N, Rich W. Duration of intubation attempts during neonatal resuscitation. The Journal of pediatrics. 2004;145(1):67–70. doi: 10.1016/j.jpeds.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Le CN, Garey DM, Leone TA, Goodmar JK, Rich W, Finer NN. Impact of premedication on neonatal intubations by pediatric and neonatal trainees. J Perinatol. 2014;34:458–460. doi: 10.1038/jp.2014.32. [DOI] [PubMed] [Google Scholar]
- 15.Leone TA, Rich W, Finer NN. Neonatal intubation: success of pediatric trainees. J Pediatr. 2005;146(5):638–641. doi: 10.1016/j.jpeds.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 16.O’Donnell CP, Kamlin CO, Davis PG, Morley CJ. Endotracheal intubation attempts during neonatal resuscitation: success rates, duration, and adverse effects. Pediatrics. 2006;117:e16–e21. doi: 10.1542/peds.2005-0901. [DOI] [PubMed] [Google Scholar]
- 17.Roberts KD, Leone TA, Edwards WH, Rich WD, Finer NN. Premedication for nonemergent neonatal intubations: a randomized, controlled trial comparing atropine and fentanyl to atropine, fentanyl, and mivacurium. Pediatrics. 2006;118:1583–1591. doi: 10.1542/peds.2006-0590. [DOI] [PubMed] [Google Scholar]
- 18.Foglia EE, Ades A, Napolitano N, Leffelman J, Nadkarni V, Nishisaki A. Factors Associated with Adverse Events during Tracheal Intubation in the NICU. Neonatology. 2015;108:23–29. doi: 10.1159/000381252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 20.Muniraman HK, Yaari J, Hand I. Premedication Use Before Nonemergent Intubation in the Newborn Infant. Am J Perinatol. 2015 doi: 10.1055/s-0034-1543987. [DOI] [PubMed] [Google Scholar]
- 21.Sharek PJ, Horbar JD, Mason W, Bisarya H, Thurm CW, Suresh G, et al. Adverse events in the neonatal intensive care unit: development, testing, and findings of an NICU-focused trigger tool to identify harm in North American NICUs. Pediatrics. 2006;118:1332–1340. doi: 10.1542/peds.2006-0565. [DOI] [PubMed] [Google Scholar]
- 22.Kohn LT, Corrigan JM, Donaldson MS. To err is human: building a safer health system. Washington, DC: National Academy Press; 1999. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.