Abstract
Surfactant protein B (SP-B) is essential for lung function. Previous studies have indicated that a SP-B 1580C/T polymorphism (SNP rs1130866) was associated with lung diseases including pneumonia. The SNP causes an altered N-linked glycosylation modification at Asn129 of proSP-B, e.g. the C allele with this glycosylation site but not in the T allele. This study aimed to generate humanized SP-B transgenic mice carrying either SP-B C or T allele without a mouse SP-B background and then examine functional susceptibility to bacterial pneumonia in vivo. A total of 18 transgenic mouse founders were generated by the DNA microinjection method. These founders were back-crossed with SP-B KO mice to eliminate mouse SP-B background. Four founder lines expressing similar SP-B levels to human lung were chosen for further investigation. After intratracheal infection with 50μl of P. aeruginosa solution (1×107 CFU/mouse) or saline in SP-B-C, SP-B-T mice the mice were sacrificed 24 hours post-infection and tissues were harvested. Analysis of surfactant activity revealed differential susceptibility between SP-B-C and SP-B-T mice to bacterial infection, e.g. higher minimum surface tension in infected SP-B-C versus infected SP-B-T mice. These results demonstrate for the first time that human SP-B C allele is more susceptible to bacterial pneumonia than SP-B T allele in vivo.
Keywords: Allele, humanized transgenic mice, Pneumonia, Psedomonas aeruginosa, Surfactant protein B, Surface tension
1. Introduction
Surfactant protein B (SP-B), a hydrophobic protein, is essential for normal lung function, which is expressed by alveolar type II epithelial cells in the lung. SP-B protein is critical for the formation of the pulmonary surfactant film at the surface of alveoli that lowers the surface tension and prevents the collapse of alveoli in the lung [1]. Human SP-B (hSP-B) is encoded by sftpb gene (approximately 9.5 kb containing 11 exons) on chromosome 2 [2,3]. The mature SP-B product is an 8 kDa protein (79 residues) which is derived from a SP-B precursor (pro-SP-B) by a complex protein processing pathway [4,5].
hSP-B genetic variation is associated with various lung diseases, like respiratory distress syndrome in pre-term neonates (RDS), congenital alveolar protein deposition disease (CAP), bronchopulmonary dysplasia (BPD) [6], and the interstitial lung disease [7]. Critical mutations of hSP-B gene result in SP-B deficiency which is lethal for newborn infants [8]. For example, a two-base-insertion in codon 121 of hSP-B cDNA causes SP-B deficiency and neonatal alveolar proteinosis [9] and a 1-bp deletion (1553delT) in exon 4 causes a reading frame shift and the premature translational termination in exon 6. Infants carrying these homozygous mutants died shortly after birth because of a lack of mature SP-B protein [10].
A common SP-B single nucleotide polymorphism, SP-B 1580 C/T (SNP, rs11130866), causes a change in the amino acid residue from Threonine (Thr) for the C allele to Isoleucine (Ile) for the T allele at position 131 of SP-B precursor. This altered residue located at a glycosylation recognition sequence results in the C allele containing a glycosylation modification at Asn129 which is not present in the T allele [11]. Patients-based genotyping studies demonstrate that this SP-B SNP (rs11130866 C/T) is associated with several pulmonary diseases including pneumonia [12] and pneumonia-induced ARDS [13], but its effects on functional susceptibility to pulmonary pathogens induced pneumonia have never been tested. We hypothesized that the change of glycosylation status at the Asn129 in SP-B precursor has an impact on SP-B processing and physiological/pathophysiological function.
In the current study, to test our hypotheses we generated humanized SP-B transgenic (hTG) mice carrying either SP-B C or T allele but without a mouse SP-B (mSP-B) gene background, and then examine the functional susceptibility of SP-B variants on surfactant activity in response to P. aeruginosa pneumonia in the hTG SP-B-C and SP-B-T mice.
2. Materials and methods
2.1. Mice
Wild type (WT) FVB/N mice used in the present study were purchased from the Jackson laboratory and maintained in the animal core facility at SUNY Upstate Medical University. The hSP-B transgenic mice carrying either hSP-B C or T allele without a mouse SP-B gene background were generated in this study. Mice were housed in pathogen-free conditions and the animal protocols (IACUC# 236 and 380) in this study were approved by Institutional Animal Care and Use Committee at SUNY Upstate Medical University, they also meet the National Institutes of Health and ARRIVE guidelines on the use of laboratory animals.
2.2. Constructs
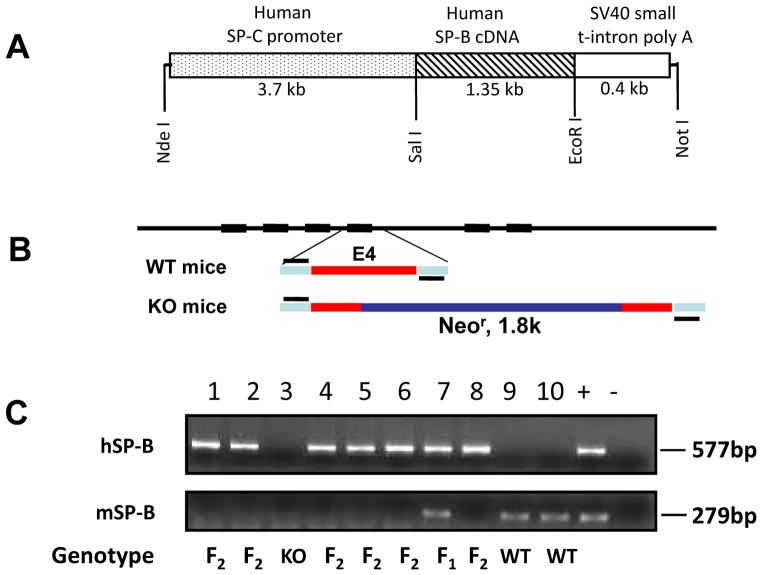
A 5.4-kb DNA fragment (Fig. 1) used for DNA microinjection was excised from a recombinant plasmid by restriction enzymes Nde I and Not I. The DNA fragment consisted of a human SP-C promoter (3.7-kb), a human SP-B cDNA (1.3-kb), and a SV40 small t-intron poly (A) sequence (0.4-kb). The basic 3.7-hSP-C/SV40 vector was kindly provided by Drs. Jeffrey A. Whitsett and Stephan W. Glasser (Cincinnati Children’s Research Foundation, Cincinnati, OH) [14]. The recombinant DNA processes were performed using standard methods of molecular cloning. The cDNA of human SP-B was cloned into a basic 3.7-hSP-C/SV40 vector and recombinant construct was verified by DNA sequencing. A 5.4 kb DNA fragment was microinjected into fertilized FVB/N oocytes from WT mice.
Fig. 1.
Recombinant construct and genotyping analysis of hSP-B transgenic mouse offspring. A diagram of recombinant DNA construct is shown in Panel A. A DNA fragment consisting of a human SP-C promoter, a human SP-B cDNA and a SV40 small t-intron poly A sequence was cloned into the basic 3.7-hSP-C/SV40 vector by restriction enzymes Nde I and Not I (A). The transgenic mice were genotyped by PCR amplification of both human SP-B and mouse SP-B fragment. hSP-B was amplified with primers which locate on hSP-C promoter and hSP-B cDNA (+421 bp), and the PCR product is 577 bp. mSP-B was amplified with primers which locate on the 3′ end of mSP-B gene intron 3 and 5′ end of intron 4. The WT mSP-B product is 279 bp, but the SP-B fragment in SP-B KO mice could not be amplified with the used PCR conditions due to a neomycine resistance gene (1.8 kb) in mouse SP-B gene exon 4 (Panel B). Panel C depicts the results of genotyping analysis of mouse offspring: the mice containing only hSP-B gene were defined as F2 (lanes 1, 2, 4–6, 8); the mice with both hSP-B and mSP-B were defined as F1 (lane 7), the mice carrying only mSP-B were defined as WT (lane 9, 10). The recombinant plasmid was used as positive control and the KO mouse genomic DNA as negative control.
2.3. Generation of hTG SP-B mice
Human SP-B positive transgenic founders carrying either hSP-B C or T allele (hSP-B+, mSP-B +/+) were bred with conditional SP-B KO mice (mSP-B −/−) for several generations to eliminate mSP-B gene background. Then homozygous hTG SP-B-C and SP-B-T mice (hSP-B +/+, mSP-B −/−) were generated by the self-breeding of hemizygous mice (hSP-B +/−, mSP-B −/−). All mice were genotyped using DNA from tail samples by PCR genotyping with Primer pair 1458/189 for hSP-B and primer pair 75/76 for mSP-B. To verify hSP-B in hTG mice, primer pair 1458/1401 was used to amplify the whole fragment of hSP-B gene and the PCR products were analyzed by DNA sequencing.
2.4. Western blotting analysis
To examine the expression of hSP-B protein level in hTG SP-B mice, Bronchoalveolar lavage fluid (BALF) from hTG mice was analyzed by Western blotting with anti-SP-B antibody (Hycult Biotech, Plymouth Meeting, PA) at 1:200 dilutions as previously described [15,16].
2.5. Analysis of histology and immunohistochemistry (IHC)
Mouse lungs from hTG mice (8 to 12 weeks) were fixed by 10% formalin solution at about 25cm of water pressure for at least 24 hr, and then processed into paraffin blocks. The sections of lung tissue were approximately 5μm in thickness. Lung sections were stained with hematoxylin and eosin, or used for IHC analysis using the ABC kit (Vector Laboratories, Burlingame, CA) as described previously [15,16].
2.6. Pseudomonas aeruginosa infection
hTG SP-B-C and SP-B-T mice were infected by intratracheal inoculation of 50μl of P. aeruginosa PA01 (1×107 CFU/mouse) or sterile saline (sham control). Mice were sacrificed 24 hrs post infection. Tissues were harvested and prepared as described previously [15,16].
2.7. Electron microscopy analysis
Lung tissues were prepared as previously described [17]. The samples were fixed by 4% glutaraldehyde, 2.5% paraformaldehyde for 24 h, then stained with osmium tetroxide, 1.5% potassium ferrocyanide and embedded in Embed812 resin (Electron microscopy science). The tissues were cut into ultrathin sections (90 nm) and stained with lead citrate and 2% aqueous uranyl acetate before electron microscopy analysis. Each sample was examined at the magnification varied from ×10, 000 to ×40, 000, and at least 100 fields were examined from several sections.
2.8. Surfactant large and small aggregates
Mouse lungs were lavaged for 3 times each with 0.7 ml saline solution. BALFs from 6 mice of each group were prepared and centrifuged at 150xg, 4°C for 10 min. and the supernatant was then centrifuged for 15 min at 40,000xg, 4°C. After centrifugation, the pellet containing surfactant large aggregates was resuspended in 0.3 ml of saline for surface tension study as previous description [18,19]. The phospholipid concentration of the large aggregates was determined using phosphate assays to be around 1 mg/mL.
2.9. Analysis of Surfactant activity
Surface activity of the mouse surfactants was determined with a constrained drop surfactometer (CDS; BioSurface Instruments, HI) [20]. A droplet of the mouse surfactant of ~10 μL was dispensed onto the CDS drop holder. After the equilibrium surface tension was established by rapid adsorption, the surfactant film was compressed and expanded at a rate of 3 seconds per cycle with a compression ratio controlled to be less than 40% of the initial surface area. At least five compression-expansion cycles were studied for each droplet. Cycles were quantified with the minimum surface tension (γmin) at the end of compression and the maximum surface tension (γmin) at the end of expansion. Drop images were taken at 10 frames per second. The surface tension and surface area were determined with Axisymmetric Drop Shape Analysis (ADSA) [21].
2.10. Statistical analysis
All the experiments were repeated at least 3 times in this study. Western blot bands were quantified by software Quantity One (version 4.6.1). Statistics were performed by SigmaStat version 3.5 software. Significant difference in statistics among groups was considered when p<0.05 by t-test or ANOVA.
3. Results
3.1. Generation of hTG SP-B-C and SP-B-T mice
To generate hTG SP-B-C and SP-B-T mice, the cDNA of hSP-B gene was cloned into a basic 3.7-hSP-C/SV40 vector (Fig. 1A) and A 5.4 kb DNA fragment was microinjected into fertilized FVB/N oocytes from WT mice. A total of 18 hSP-B positive founders were identified by PCR. To eliminate the mSP-B background, the conditional SP-B knockout (KO) mice by inserting Neomycin resistant gene into the gene (Fig. 1B) were used to breed with hSP-B positive founders. The hSP-B positive F1 were bred with conditional SP-B KO mice to generate F2 hSP-B positive mice (hSP-B +/mSP-B −/−) (Fig. 1C). Finally, four homozygous hTG SP-B-C and SP-B-T mouse lines, e.g. (hSP-B +/+, mSP-B −/−), were obtained by the self-breeding of hemizygous mice (hSP-B +/−, mSP-B −/−) and showed healthy status suggesting that hSP-B protein functions well in the transgenic mice.
3.2. Expression of transgene SP-B-T or SP-B-C in hTG mice
The histology of lung in the hTG SP-B-c and SP-B-T mice from four founders was assessed using H/E staining sections (Fig. 2A). The results demonstrated hTG SP-B-C founder line and SP-B-T founder lines displayed normal alveolar structure and septal thickness. The chord length (mean linear intercepts: Lm) as a measure of the acinar air space complex was measured, no significant difference was found between hTG SP-B-C and SP-B-T mice. Furthermore, hSP-B expression was analyzed in the lung by IHC method (Fig. 2B) and western blotting (data shown in 3.4). The results indicated that only alveolar type II epithelial cells showed positive staining for SP-B expression in the lung tissue of both hTG SP-B-T and SP-B-C mice (Fig. 2B).
Fig. 2.
Histology and hSP-B expression in the lung of hTG SP-B-C and SP-B-T mice. Lung tissue was fixed by formalin for 24 hrs and then processed into paraffin blocks. About 5-μm-thick sections were prepared and slides were stained using hematoxylin and eosin (H/E) method. hTG SP-B-C and SP-B-T mice exhibited normal lung histology (A). To identify SP-B expression in the lung the sections of lung were analyzed by IHC with anti-SP-B antibody. The results showed positive in the type II alveolar cells (pointed by arrows) (B).
3.3. Bacterial pneumonia
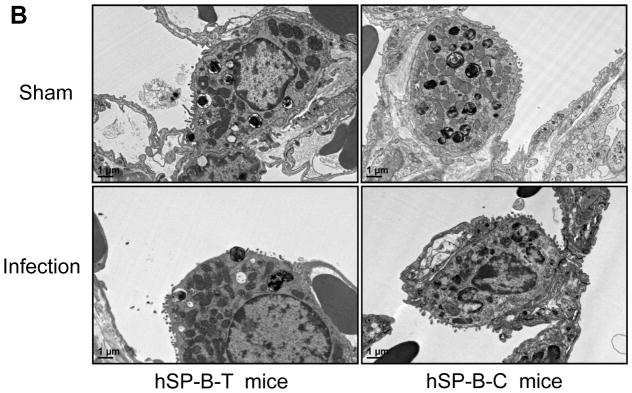
To examine the functional variation of hSP-B genetic variants under infectious conditions, a bacterial pneumonia model was developed and applied for the assessment of functional effects of hSP-B genetic variants in vivo. hTG mice were infected intratracheally by 50μl of P. aeruginosa PA01 (107 CFU/mouse) or same amount of saline solution (Sham). The infected mice were sick but survival for 24 hrs. The lung pathohistology displayed pneumonia pathogenic characteristics and lung injury in the infected mice but not in Sham control (Fig. 3A). A large amount of neutrophils were observed in the BALF of infected mice but not in the control. Ultrastructural analysis revealed significant decrease of lamellar bodies in the type II cells and remarkable less microvilli on the surface of type II cells in infected mice compared to Sham (Fig. 3B, p<0.05), suggesting bacterial infection decreased activation of type II cells and surfactant/protein expression.
Fig. 3.
Lung injury caused by bacterial pneumonia. Mice were infected intratracheally with 50 μl of bacterial solution (1×107 CFU/mouse) or saline (sham) and sacrificed 24 hrs after infection. Lung tissues were fixed with 10% Formalin solution. The results showed that infected mice had severe lung injury with a large amount of inflammatory cells e.g. PMN and macrophages in the alveoli and pathogenic changes of lung tissues but not in uninfected mice (A). For analysis of lung ultrastructure after infection lung tissues from infection and sham group of hSP-B-T and hSP-B-C mice were fixed stained and embedded, and then analyzed by electron microscopy as described in the methods. The results showed decreased lamellar bodies in the type II alveolar cells from infected mice compared with sham mice (B). The hSP-B-T and hSP-B-C mice showed a normal ultrastructure of type II alveolar cells in health mice.
3.4. Differential surfactant activity between SP-B-C and SP-B-T in response to bacterial infection
Surfactant activity of large aggregates of BALF from infected and sham mice was analyzed by the Constrained Drop Surfactometer (CDS) [18,19,20]. The results indicated that uninfected hSP-B-T and hSP-B-C mice (sham) had similar surface activity, i.e., minimum surface tension (γmin) about 1.8–2.2 (mN/m) (Fig. 4A). After infection, the minimum surface tension in infected SP-B-C mice increased significantly by two times compared to uninfected control (Fig. 4A, p<0.05), suggesting that surfactant activation has been inhibited in the infected SP-B-C mice. But the minimum surface tension of infected SP-B-T mice (hSP-B-T) showed less change compared to uninfected SP-B-T mice (Fig. 4A). These data indicate that the SP-B-T mice had less susceptibility on surfactant activity to bacterial infection when compared to SP-B-C mice (p<0.05) (Fig. 4A). Analysis of Western blotting demonstrated decreased SP-B levels of both infected SP-B-C and SP-B-T mice compared to their uninfected mice (Fig. 4B, p<0.01). Infected SP-B-C mice had lower SP-B tendency vs. infected SP-B-T mice.
Fig. 4.
Different susceptibility of surfactant activity in hTG SP-B-C and SP-B-T mice to bacterial infection. Surfactant large aggregates were prepared from the BALF samples as described in the methods. Minimum surface tension (γmin) was measured. The results showed that there are similar minimum surface tension (γmin) in the uninfected SP-B-C and SP-B-T mice, but the level of minimum surface tension (γmin) was increased significantly in infected SP-B-C mice compared to infected SP-B-T mice, as well as uninfected SP-B-C mice (Panel A). Western blot analysis showed that the hSP-B expression in BALF 20μg of total protein from the BALF of sham or infection group samples was subjected to 12% SDS-PAGE under non-reducing condition. Human BALF was used as a positive control. The hSP-B protein was detected by a rabbit anti-pig SP-B antibody (at 1:200X). The dimer (16 kDa) of hSP-B was detected on the blot. The data show that samples from infection group express a decreased level of hSP-B protein by comparing with sham group (B). There is a significant difference between sham and infection groups (p<0.01) (C).
Discussion
Pulmonary surfactant consists of about 90% phospholipids and 10% surfactant-associated proteins, including SP-B, an essential protein for normal lung function [1]. The SP-B precursor is about 42 kDa peptide containing three sapsin-like protein domains, e.g. domain N, M, and C. Mature 8-kDa SP-B protein is derived from sapsin-like domain M, which plays a key role in lowering surface tension in the alveolar space [22]. Decreased levels of mature SP-B significantly influence lung function and oxygenation [23] and cause disease exacerbation [24]. The SP-B SNP (rs11130866 C/T) was identified to be associated with several pulmonary diseases including pneumonia [12], in which the individuals carrying the C allele of SP-B are more susceptible than those with the T allele [13]. However, the detailed mechanisms are unclear. The humanized SP-B transgenic mouse model provides a powerful in vivo tool for studying the mechanistic role of human SP-B genetic variants in the pathogenesis of pulmonary diseases [25].
Previous studies found that the production of mature SP-B protein is completed by complex SP-B precursor processing and trafficking [26]. SP-B was secreted to alveoli or stored in the lamellar bodies in the alveolar type II cells of lung [26]. Several proteases are involved in the protein processing, including protease Napsin and Cathepsin H [26]. Differentially posttranslational modifications of SP-B precursor caused by genetic variation may have an impact on the efficiency of SP-B processing and trafficking in the type II cells. The C allele of human SP-B has an additional protein glycosylation site at the residue Asn129 compared to the T allele [11,27]. SP-B C and T alleles are two common genetic alleles in human population and no obviously abnormal SP-B expression was observed in the healthy individuals with either the C or T allele, indicating the additional Asn129 glycosylational modification in SP-B precursor may not significantly influence physiological function under healthy conditions. The similar levels of the SP-B expression in the hTG SP-B-C and SP-B-T mice in this study also confirmed the observation in human beings. However, it is unknown whether the additional glycosylation site at the As129 in the SP-B-C variant has an negative impact on pro-SP-B processing and trafficking under disease conditions, like bacterial pneumonia.
Previous studies demonstrate that there are decreased expressions of SP-B after a variety of infectious conditions [28]. In the present study we observed a significant decrease of SP-B level in the BALF of infected mice, and SP-B level in infected SP-B-C mice was lower than infected SP-B-T mice, suggesting that altered glycosylation modification at the Asn129 in the SP-B-C mice influenced SP-B processing and trafficking under bacterial pneumonia. Although decreased levels of other surfactant proteins (SP-A, SP-C and SP-D) in the BALF were also observed in the infected mice compared to uninfected mice (data not shown), but no differences of these protein expressions were detected in infected SP-B-C and SP-B-T mice in the infectious condition. Furthermore, ultrastructural analysis of the lung tissues indicated lower density of LB in type II cells suggesting decreased activation of alveolar type II cells in infected mice. These pathological changes in the cellular and molecular levels in the lung of infected SP-B-C and SP-B-T mice might cause lung dysfunction in mouse pneumonia.
The SNP (rs11130866 C/T) studied in the present work has been found to be associated with several pulmonary diseases in several independent groups [13] [7,12]. For instance, individual with C/C genotype was found more susceptible to RDS [29] while individual with T/T genotype was found to protect the patients with SSC against the development of ILD[7]. The individuals carrying one or more C allele had more severe lung injury and required MV compared to those with T/T genotype [30]. These patients-based genotyping analyses demonstrate that the C allele of SP-B is more susceptible to various pulmonary diseases. This hTG model may provide a unique tool to study the mechanisms of human SP-B genetic variants on functional variation in various pulmonary diseases. Indeed, the results observed in this work revealed differential susceptibility of human SP-B genetic variants in response to bacterial pneumonia. Decreased surfactant activity of BALF in the lung might lead to worse respiratory function and disease severity in pneumonia. However, it is warrant to explore detailed mechanisms how the altered glycosylational modification at the Asn129 influence pro-SP-B processing, folding and trafficking in the alveolar type II cells in vivo in various diseases in the future.
Highlights.
Generated the first humanized SP-B transgenic mice with the C or T allele.
Examined the functional variation of human SP-B genetic variants in vivo.
Found different susceptibility of SP-B-C and SP-B-T allele to bacterial pneumonia.
Differential surface tension of human SP-B-C and SP-B-T in bacterial pneumonia.
Acknowledgments
We would like to thank Drs. Jeffrey A. Whitsett and Stephan W. Glasser (Cincinnati Children’s Research Foundation, Cincinnati, OH) for kindly providing SP-B KO mice and the basic 3.7-hSP-C/SV40 vector. This work was supported in part by NIH grant HL096007, Michael Connolly Endowment Fund and Hendricks Foundation.
Footnotes
Disclosure
All the authors declared no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Whitsett JA, Weaver TE. Hydrophobic surfactant proteins in lung function and disease. New England Journal of Medicine. 2002;347:2141–2148. doi: 10.1056/NEJMra022387. [DOI] [PubMed] [Google Scholar]
- 2.Glasser SW, Korfhagen TR, Weaver T, Pilot-Matias T, Fox JL, Whitsett JA. cDNA and deduced amino acid sequence of human pulmonary surfactant-associated proteolipid SPL (Phe) Proceedings of the National Academy of Sciences. 1987;84:4007–4011. doi: 10.1073/pnas.84.12.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pilot-Matias TJ, Kister SE, Fox JL, Kropp K, Glasser SW, Whitsett JA. Structure and organization of the gene encoding human pulmonary surfactant proteolipid SP-B. DNA. 1989;8:75–86. doi: 10.1089/dna.1.1989.8.75. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs KA, Phelps DS, Steinbrink R, Fisch J, Kriz R, Mitsock L, Dougherty JP, Taeusch HW, Floros J. Isolation of a cDNA clone encoding a high molecular weight precursor to a 6-kDa pulmonary surfactant-associated protein. J Biol Chem. 1987;262:9808–9811. [PubMed] [Google Scholar]
- 5.Guttentag SH, Beers MF, Bieler BM, Ballard PL. Surfactant protein B processing in human fetal lung. Am J Physiol. 1998;275:L559–566. doi: 10.1152/ajplung.1998.275.3.L559. [DOI] [PubMed] [Google Scholar]
- 6.deMello DE, Lin Z. Pulmonary alveolar proteinosis: a review. Pediatr Pathol Mol Med. 2001;20:413–432. [PubMed] [Google Scholar]
- 7.Sumita Y, Sugiura T, Kawaguchi Y, Baba S, Soejima M, Murakawa Y, Hara M, Kamatani N. Genetic polymorphisms in the surfactant proteins in systemic sclerosis in Japanese: T/T genotype at 1580 C/T (Thr131Ile) in the SP-B gene reduces the risk of interstitial lung disease. Rheumatology (Oxford) 2008;47:289–291. doi: 10.1093/rheumatology/kem355. [DOI] [PubMed] [Google Scholar]
- 8.Gower WA, Nogee LM. Surfactant dysfunction. Paediatr Respir Rev. 2011;12:223–229. doi: 10.1016/j.prrv.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nogee LM, Garnier G, Dietz H, Singer L, Murphy A. A mutation in the surfactant protein B gene responsible for fatal neonatal respiratory disease in multiple kindreds. Journal of Clinical Investigation. 1994;93:1860. doi: 10.1172/JCI117173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wallot M, Wagenvoort C, Müller KM, Floros J, Roll C. Congenital alveolar proteinosis caused by a novel mutation of the surfactant protein B gene and misalignment of lung vessels in consanguineous kindred infants. European journal of pediatrics. 1999;158:513–518. doi: 10.1007/s004310051132. [DOI] [PubMed] [Google Scholar]
- 11.Wang G, Christensen ND, Wigdahl B, Guttentag SH, Floros J. Differences in N-linked glycosylation between human surfactant protein-B variants of the C or T allele at the single-nucleotide polymorphism at position 1580: implications for disease. Biochem J. 2003;369:179–184. doi: 10.1042/BJ20021376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quasney MW, Waterer GW, Dahmer MK, Kron GK, Zhang Q, Kessler LA, Wunderink RG. Association between surfactant protein B+ 1580 polymorphism and the risk of respiratory failure in adults with community-acquired pneumonia*. Critical care medicine. 2004;32:1115–1119. doi: 10.1097/01.ccm.0000124872.55243.5a. [DOI] [PubMed] [Google Scholar]
- 13.Lin Z, Pearson C, Chinchilli V, Pietschmann SM, Luo J, Pison U, Floros J. Polymorphisms of human SP-A, SP-B, and SP-D genes: association of SP-B Thr131Ile with ARDS. Clin Genet. 2000;58:181–191. doi: 10.1034/j.1399-0004.2000.580305.x. [DOI] [PubMed] [Google Scholar]
- 14.Glasser SW, Burhans MS, Eszterhas SK, Bruno MD, Korfhagen TR. Human SP-C gene sequences that confer lung epithelium-specific expression in transgenic mice. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2000;278:L933–L945. doi: 10.1152/ajplung.2000.278.5.L933. [DOI] [PubMed] [Google Scholar]
- 15.Wang G, Guo X, Diangelo S, Thomas NJ, Floros J. Humanized SFTPA1 and SFTPA2 transgenic mice reveal functional divergence of SP-A1 and SP-A2: formation of tubular myelin in vivo requires both gene products. J Biol Chem. 2010;285:11998–12010. doi: 10.1074/jbc.M109.046243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J, Abdel-Razek O, Liu Z, Hu F, Zhou Q, Cooney RN, Wang G. Role of surfactant proteins A and D in sepsis-induced acute kidney injury. Shock. 2015;43:31–38. doi: 10.1097/SHK.0000000000000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang G, Guo X, DiAngelo S, Thomas NJ, Floros J. Humanized SFTPA1 and SFTPA2 Transgenic Mice Reveal Functional Divergence of SP-A1 and SP-A2 FORMATION OF TUBULAR MYELIN IN VIVO REQUIRES BOTH GENE PRODUCTS. Journal of Biological Chemistry. 2010;285:11998–12010. doi: 10.1074/jbc.M109.046243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goetzman ES, Alcorn JF, Bharathi SS, Uppala R, McHugh KJ, Kosmider B, Chen R, Zuo YY, Beck ME, McKinney RW, Skilling H, Suhrie KR, Karunanidhi A, Yeasted R, Otsubo C, Ellis B, Tyurina YY, Kagan VE, Mallampalli RK, Vockley J. Long-chain acyl-CoA dehydrogenase deficiency as a cause of pulmonary surfactant dysfunction. J Biol Chem. 2014;289:10668–10679. doi: 10.1074/jbc.M113.540260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banaschewski BJ, Veldhuizen EJ, Keating E, Haagsman HP, Zuo YY, Yamashita CM, Veldhuizen RA. Antimicrobial and biophysical properties of surfactant supplemented with an antimicrobial peptide for treatment of bacterial pneumonia. Antimicrob Agents Chemother. 2015;59:3075–3083. doi: 10.1128/AAC.04937-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valle RP, Wu T, Zuo YY. Biophysical influence of airborne carbon nanomaterials on natural pulmonary surfactant. ACS Nano. 2015;9:5413–5421. doi: 10.1021/acsnano.5b01181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zuo Y, Ding M, Li D, Neumann A. Further development of Axisymmetric Drop Shape Analysis-Captive Bubble for pulmonary surfactant related studies. Biochimica et Biophysica Acta (BBA)-General Subjects. 2004;1675:12–20. doi: 10.1016/j.bbagen.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Yang L, Johansson J, Ridsdale R, Willander H, Fitzen M, Akinbi HT, Weaver TE. Surfactant protein B propeptide contains a saposin-like protein domain with antimicrobial activity at low pH. The journal of immunology. 2010;184:975–983. doi: 10.4049/jimmunol.0900650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nesslein LL, Melton KR, Ikegami M, Na CL, Wert SE, Rice WR, Whitsett JA, Weaver TE. Partial SP-B deficiency perturbs lung function and causes air space abnormalities. Am J Physiol Lung Cell Mol Physiol. 2005;288:L1154–1161. doi: 10.1152/ajplung.00392.2004. [DOI] [PubMed] [Google Scholar]
- 24.Cole FS. Surfactant protein B: unambiguously necessary for adult pulmonary function. Am J Physiol Lung Cell Mol Physiol. 2003;285:L540–542. doi: 10.1152/ajplung.00111.2003. [DOI] [PubMed] [Google Scholar]
- 25.Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7:118–130. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- 26.Ueno T, Linder S, Na CL, Rice WR, Johansson J, Weaver TE. Processing of pulmonary surfactant protein B by napsin and cathepsin H. Journal of Biological Chemistry. 2004;279:16178–16184. doi: 10.1074/jbc.M312029200. [DOI] [PubMed] [Google Scholar]
- 27.Taponen S, Huusko JM, Petäjä-Repo UE, Paananen R, Guttentag SH, Hallman M, Haataja R. Allele-specific N-glycosylation delays human surfactant protein B secretion in vitro and associates with decreased protein levels in vivo. Pediatric research. 2013;74:646–651. doi: 10.1038/pr.2013.151. [DOI] [PubMed] [Google Scholar]
- 28.Kerr MH, Paton JY. Surfactant protein levels in severe respiratory syncytial virus infection. Am J Respir Crit Care Med. 1999;159:1115–1118. doi: 10.1164/ajrccm.159.4.9709065. [DOI] [PubMed] [Google Scholar]
- 29.Yin X, Meng F, Wang Y, Xie L, Kong X, Feng Z. Surfactant protein B deficiency and gene mutations for neonatal respiratory distress syndrome in China Han ethnic population. Int J Clin Exp Pathol. 2013;6:267–272. [PMC free article] [PubMed] [Google Scholar]
- 30.Dahmer MK, O’Cain P, Patwari PP, Simpson P, Li SH, Halligan N, Quasney MW. The influence of genetic variation in surfactant protein B on severe lung injury in African American children. Crit Care Med. 2011;39:1138–1144. doi: 10.1097/CCM.0b013e31820a9416. [DOI] [PubMed] [Google Scholar]