Abstract
Objective
To assess the effect of delayed cord clamping (DCC) versus immediate cord clamping (ICC) on intraventricular hemorrhage (IVH), late onset sepsis (LOS), and 18-month motor outcomes in preterm infants.
Study design
Women (n=208) in labor with singleton fetuses (≤ 32 weeks gestation) were randomized to either DCC (30–45 seconds) or ICC (< 10 seconds). The primary outcomes were IVH, LOS, and motor outcomes at 18–22 months corrected age. Intention-to-treat was used for primary analyses.
Results
Cord clamping time was 32 ± 16 seconds (DCC) vs. 6.6 ± 6 (ICC). Infants in the DCC and ICC groups weighed 1203 ± 352 grams and 1136 ± 350 and mean gestational age was 28.3 ± 2 and 28.4 ± 2 weeks respectively. There were no differences in rates of IVH or LOS between groups. At 18–22 months, DCC was protective against motor scores below 85 on the Bayley Scales of Infant Development-III (Bayley-III) (OR 0.32, 95% CI 0.10 – .0.90, p = 0.03). There were more women with preeclampsia (PEC) in the ICC group (37% vs 22%, p = 0.02) and more women in the DCC group with premature rupture of membranes/ preterm labor (PROM/PTL) (54% vs 75%, p = 0.002). PEC halved the risk of IVH (OR 0.50, 95% CI 0.2 – 1.0) and PROM/PTL doubled the risk of IVH (OR 2.0, 95% CI 1.2–4.3).
Conclusions
Although DCC did not alter the incidence of IVH or LOS in preterm infants, it improved motor function at 18–22 months corrected age.
Keywords: delayed cord clamping, cord milking, developmental follow-up, motor outcomes
When cord clamping is delayed at birth or the cord is milked, infants receive a placental transfusion of 10 to 15 mL/kg during the first few minutes of life.1 This additional blood improves hemodynamic stability and may reduce the risk of intraventricular hemorrhage (IVH)2, 3 and the vulnerability of infants to inflammatory processes.4–6 This blood also contains stem cells that are important in repairing tissue and building immunocompetence.7 Recently, the American College of Obstetricians and Gynecologists recommended delayed cord clamping (DCC) for preterm infants when feasible but the current obstetrical practice at birth remains immediate clamping.8–10
Prior studies on DCC in preterm infants have shown benefits without harm. The most recent meta-analysis by Rabe et al on infants under 37 weeks found reduced rates of neonatal transfusion (OR 0.61, 95% CI 0.46 – 0.81, p < 0.0005), less IVH (OR 0.59, 95% CI 0.41 – 0.85, p < 0.005) and no difference in late onset sepsis (LOS).11 Backes et al reported similar findings in infants less than 32 weeks adding a finding of lower mortality (OR 0.42, 95% CI 0.19–0.95, p =0.04).12 Previously, we reported reduced rates of IVH and LOS13 and some protection of very low birth weight (VLBW) male infants against motor disability at 7 months corrected age.14
The current randomized controlled trial prospectively tested the effects of DCC for 30 to 45 seconds followed by one cord milking with the aim of confirming our prior work and providing long term follow-up. Our a priori hypotheses were that DCC would reduce the incidence of IVH, LOS, and result in better motor function at 18–22 months.
Methods
This trial was conducted at Women and Infants’ Hospital (WIH) of Rhode Island with WIH and the University of Rhode Island Institutional Review Board approval. Thirty patients (14%) were enrolled prior to trial registration, but no data were examined prior to trial registration. An independent data safety and monitoring committee reviewed the data after 36, 100, and 150 infants were randomly assigned and identified no concerns. Women with a singleton pregnancy estimated at 24 to 31.6 weeks gestation by obstetrical evaluation were eligible irrespective of mode of delivery. Exclusion criteria included multiple gestation, prenatally diagnosed major congenital anomalies, severe or multiple maternal illnesses, and mothers who were at risk for loss to follow-up. Blocked stratified randomization of subjects born before or after 28 weeks to the immediate cord clamping (ICC) or DCC group was used to assign the groups with a prespecified equal probability. Sequenced and sealed envelopes identifying the stratification and group assignment on cards were prepared by a statistician not involved in the trial and kept in a locked file box in the Labor and Delivery Unit. All participants were screened, consented and enrolled by study nurses following written informed consent. Study nurses randomized mothers based on the next study card designation when women went into active labor.
Women were randomized to either DCC followed by one milking of the cord (intervention group) or ICC (control group). For all births, research personnel used a stopwatch to record the exact timing of cord clamping. Once the cord was cut, the infant was moved to the warmer and placed on a warming mattress. All subsequent care was managed by the neonatal team in attendance. For the DCC group, the obstetrician placed the infant in a sterile warm towel or blanket and held the infant approximately 10–15 inches below the mother’s introitus at vaginal delivery or below the level of the placenta at cesarean delivery. Care was taken to avoid traction on the cord. Suctioning was at the discretion of the obstetrician. The research nurse, using a stopwatch, counted out the time elapsed in ten second intervals to the obstetrician. At 30 to 45 seconds, the obstetrician was asked to milk the infant’s cord once then clamp and cut the umbilical cord. If unable to carry out the DCC protocol as planned, the cord was milked quickly 2 to 3 times before clamping when possible (n = 11). In the event that the timing of the cord clamping was less than 30 seconds with no cord milking and the baby was randomized to the DCC group, a protocol violation report was completed and the infant remained in the DCC group for primary intention-to-treat analyses (n = 15). The ICC group received routine care of cord clamping in less than 10 seconds. Cranial ultrasounds were obtained at one, seven, and 28 days. Formal developmental follow-up assessments at seven and 18 – 22 months corrected age were completed.
IVH was defined as bleeding in the brain assessed by cranial ultrasound readings using the criteria of Papile.15 The staff pediatric radiologist read the ultrasound initially followed by a collaborating pediatric radiologist masked to the infants’ grouping. Discrepancies were adjudicated by consensus through discussion among radiologists. LOS was defined as a positive bacterial blood culture after 72 hours of age. The Bayley Scales of Infant Development Motor Scale (Bayley-III) was used to assess cognitive, language and motor function.16 The motor composite score and subscores for fine motor and gross motor skills were analyzed. The Bayley-III composite score has a mean ± SD of 100 ±15.
Secondary outcome variables included safety variables (Apgar scores, initial temperature upon admission, peak bilirubin in the first week of life), initial blood pressure, initial hematocrit, necrotizing enterocolitis (NEC), bronchopulmonary dysplasia (BPD), and retinopathy of prematurity (ROP) as diagnosed by attending clinicians. NEC was diagnosed based on Bell criteria, BPD was defined as requiring oxygen therapy at 36 weeks postmenstrual age or death, and ROP was identified by an ophthalmologist per routine eye examinations.
The study could not be blinded because of the obvious nature of the intervention. Our institutional policy requires the presence of a neonatology staff member at delivery because of early gestation. Staff who attended each birth were asked not to reveal the infant’s grouping in the infant’s medical records. Personnel collecting on-going clinical data and the follow-up staff completing the developmental assessment remained blinded.
The power analysis was based on our Phase 1 study13 and expected treatment effects for a simple test of proportions of group differences (ICC vs. DCC) for the primary study outcome of IVH. We conservatively estimated an odds ratio of about 2.5 for this new study, which yielded a sample size of 92 per group for power of 0.80. Given the need for follow-up, we allowed for an attrition rate of about 15%, resulting in a total baseline sample size of 212 VLBW infants. The use of block-stratified randomization enhanced the study design sensitivity.
The diagnoses of preterm labor (PTL) and premature rupture of the membranes (PROM) were not discrete among the subjects. Consequently, we combined them into one variable PROM/PTL that represented women who had either diagnoses. Both conditions can be associated with intrauterine inflammation.17
Data analyses included Pearson Chi-Square tests, t-tests, Wilcoxon rank-sum tests for non-normally distributed variables, and multiple logistic and linear regression models to adjust for possible confounders. No correction was used to adjust for multiple tests. For outcomes, odds ratios were calculated along with appropriate 95% confidence intervals. Primary analyses were conducted using intention-to-treat. Post-hoc sensitivity analyses using actual treatment were indicated and performed to assess the robustness of the findings.18
Results
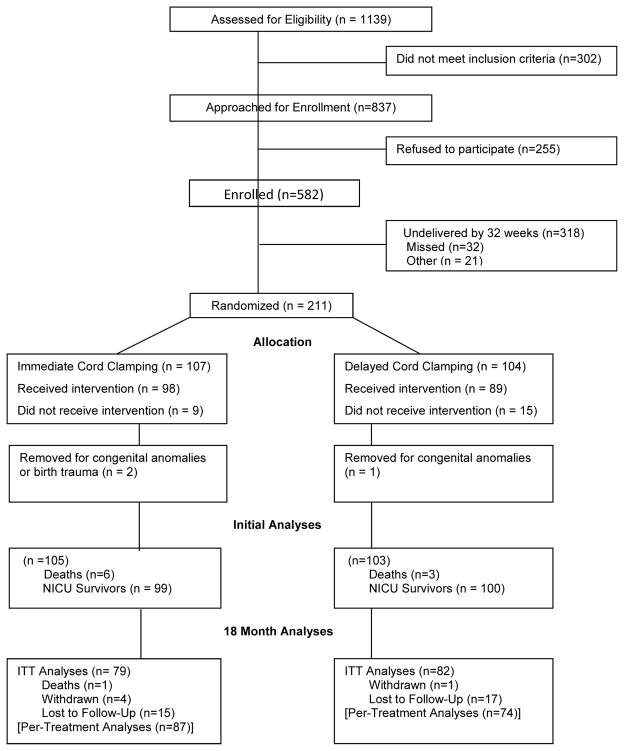
Pregnant women (n=211) between 24 and 31.6 weeks gestation were randomized from May 15, 2008, through August 31, 2014 (Figure 1; available at www.jpeds.com). There were 107 infants in the ICC and 104 in the DCC group. Three infants were withdrawn (congenital anomalies and birth injury which precluded randomization), leaving a total sample of 208 mother- infants dyads. Maternal and infant demographic and clinical variables were analyzed for the remaining randomized subjects. Neonatal morbidities were analyzed for the 199 infants who survived the Neonatal Intensive Care Unit (NICU) course. There were 24 (12%) protocol violations (9 ICC and 15 DCC group) that remained in their assigned groups for primary intention-to-treat analyses. One infant died after discharge, five refused follow-up, and 32 were lost to follow-up. At 18–22 months, 82% (DCC) and 81% (ICC) were evaluated.
Figure 1.
Flow chart of patients.
Table I shows the maternal and neonatal demographics and clinical characteristics. There were significantly more women with PROM/PTL in the DCC group in both cohorts, and more women with PEC at admission in the ICC group. There were no significant differences between the two groups in parity, public insurance, marital status, antenatal steroids, intrauterine growth restriction or cesarean delivery rate. Per protocol, cord clamping time was significantly increased in the infants in the DCC group. All other neonatal variables, including those used to assess protocol safety, did not differ between groups. There were no differences in numbers of infants who received phototherapy, days of phototherapy, delivery room resuscitation, or Score for Neonatal Acute Physiology (SNAP) scores at 12 hours of age (data not shown).
Table 1.
Maternal and Neonatal Demographics, Clinical, and Safety Variables
| Characteristic | Initial Cohort | 18 Month Cohort | ||
|---|---|---|---|---|
|
| ||||
| DCC (n =103) | ICC (n =105) | DCC (n=82) | ICC (n=79) | |
|
| ||||
| Mother’s age (yrs) | 28 ± 7 | 29 ± 6.5 | 29 ± 7 | 30 ± 6 |
|
| ||||
| Race / Ethnicity | ||||
| Black | 20 (20) | 13 (12) | 16 (20) | 7 (9) |
| White | 56 (55) | 69 (66) | 44 (54) | 56 (71) |
| Other | 26 (25) | 23 (22) | 21 (26) | 16 (20) |
|
| ||||
| Maternal Education- ≤ high school | --- | --- | 37 (47) | 24 (33) |
|
| ||||
| PROM (hrs) | 98 ± 190* | 72 ± 190 | 112 ± 209* | 80 ± 212 |
|
| ||||
| Reasons for preterm birth | ||||
| Preeclampsiaa | 23 (22)* | 39 (37) | 19 (23) | 29 (37) |
| PROM/Preterm labora | 77 (75)** | 57 (54) | 60 (73)** | 42 (53) |
| Clinical Chorioamnionitis | 23 (22)** | 10 (9.5) | 18 (22)* | 6 (8) |
|
| ||||
| Gestational age, wks | 28.3 ± 2 | 28.4 ± 2 | 28.1 ± 2 | 28.2 ± 2 |
| 24 to 276 wks | 35 | 36 | 31 | 28 |
| 28 to 316 wks | 68 | 69 | 51 | 51 |
|
| ||||
| Cord Clamp Time (seconds) Range, median | 32 ± 16*** (0–60) | 6.6 ± 6 (1–45) | 31 ± 6*** (0–60) | 6.6 ± 5.2 (0–36) |
|
| ||||
| Birth weight, g | 1203 ± 352 (430–1955) | 1136 ± 350 (390–2455) | 1178 ± 341 (480–1885) | 1101 ± 354 (390–2455) |
| Male/female ratio | 52/51 | 62/43 | 42/40 | 47/32 |
|
| ||||
| Apgar score, median (range) | ||||
| 1 min | 6 (1–9) | 6 (1–9) | 6 (1–9) | 6 (1–9) |
| 5 min | 8 (2–9) | 8 (1–10) | 8 (2–9) | 8 (1–10) |
| <4 at 1 min, | 16 (16) | 20 (19) | 13 (16) | 14 (18) |
| Cord Blood Gas, Arterial | 7.4 ± 0.1* | 7.3 ± 0.1 | 7.3 ± 0.1 | 7.3 ± 0.1 |
|
| ||||
| NICU Admission Temperature CO (Range) | 36.5 ± 0.8 (32–38) | 36.3 ± 0.8 (33–39) | 36.6 ± 0.8* (34–38) | 36.3 ± 0.9 (33–38) |
|
| ||||
| Initial Hematocrit (%) | 47.6 ± 7 (31–63) | 46.4 ± 7 (32–59) | 47.2 ± 7 (31–63) | 46.2 ± 7 (32–59) |
|
| ||||
| 1st Mean Blood Pressure | 35 ± 8 | 35 ± 8 | 35± 8 | 34± 8 |
|
| ||||
| Peak bilirubin (mg/dl), First week, Range | 8 ± 2 (5–16) | 8 ± 2 (4–14) | 8 ± 2 (5 –16) | 7.7 ± 2 (4–14) |
Mean ± SD or n (%);
p<0.05;
p<0.01;
p<0.001.
Only PEC and PROM/PTL are mutually exclusive categories.
Table II shows that in initial analysis of the cohort there were no significant differences in the primary outcome variables of IVH and LOS at NICU discharge. Nine infants (3 = DCC and 6 = ICC) were removed from this analysis as they died before discharge. For each week of gestational age (GA) gained, the odds of IVH were reduced by 15% (OR .85, 95% CI .7 – .98) (data not shown). The diagnosis of PROM/PTL in the mother doubled the risk of IVH (OR 2, 95% CL 1, 4.3), and PEC lowered the risk by one-half (OR .5, 95% CL .2–1). When both PEC and PROM/PTL were included in a regression model, they balanced each other out leaving only GA to predict IVH.
Table 2.
Primary and Neonatal Outcomes By Intention-to-Treat and Sensitivity Analyses (Actual Treatment)
| Intention-to-Treat Analyses | Sensitivity Analyses | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Outcome | Initial Cohort | 18 Month Cohort | Initial Cohort | 18 Month Cohort | ||||
|
| ||||||||
| DCC (n =100) | ICC (n =99) | DCC (n=82) | ICC (n=79) | DCC (n =97) | ICC (n =111) | DCC (n=74) | ICC (n=87) | |
|
| ||||||||
| IVH – all | 31 (31) | 23 (23) | 26 (32) | 17 (22) | 25 (26) | 27 (24) | 20 (27) | 23 (26) |
|
| ||||||||
| Confirmed LOS | 14 (14) | 13 (13) | 13 (16) | 9 (11) | 14 (14) | 13 (12) | 13 (18) | 9 (10) |
|
| ||||||||
| Deaths | 3 | 6 | 3 | 7 | 3 | 6 | 3 | 7 |
|
| ||||||||
| Motor Scorea | -- | -- | 92.1 ± 15 | 91.2 ± 12 | -- | -- | 93 ± 15 | 90 ± 13 |
| <85 | 14 (19) | 18 (24) | 9 (13)** | 23 (28) | ||||
|
| ||||||||
| <70 | -- | -- | 8 (11) | 4 (5) | -- | -- | 6 (9) | 6 (7) |
|
| ||||||||
| Fine Motor Score | -- | -- | 9.4 ± 2.2 | 9.4 ± 3 | -- | -- | 9.7 ± 2.9 | 9.1 ± 2.4 |
|
| ||||||||
| Gross Motor Score | -- | -- | 8.1 ± 2.6 | 7.7 ± 2.6 | -- | -- | 8.1 ± 2.6 | 7.7 ± 2.6 |
p 0.01;
Bayley III Composite Motor Score; There were no differences between the groups in ROP, NEC, death or IVH, death or LOS, oxygen use at 36 weeks. Range on Bayleys (46-121) for both groups.
Infants with LOS were younger at birth (mean of 26.7 versus 28.7 weeks, p <0.001); 22% (n = 15) of infants less than 28 weeks had LOS, and 9% (12) of those greater than 28 weeks had LOS (p = 0.008). Using regression analyses to predict LOS, we found no effect of PEC or PROM/PTL on LOS. Only GA predicted LOS with younger infants more affected as expected (OR .63, 95% CI .5 – .79) (data not shown).
There were no differences between the groups at seven-month corrected age assessments (data not shown). At 18–22 months, primary bivariate analysis by intention-to-treat showed no significant differences between groups on the Bayley-III motor assessment (Table II).
We completed sensitivity analyses because of the 12% rate of protocol violations and the symmetrically unequal groups on two major confounding variables. There were no differences between the two groups of infants with protocol violations on the 1- or 5-minute Apgar scores (data not shown). Using sensitivity analyses, no differences were found in the incidence of IVH or LOS (Table II). However, more than twice the number of children who received ICC scored less than 85 on the Bayley-III motor composite versus those who received DCC using the bivariate sensitivity analysis.
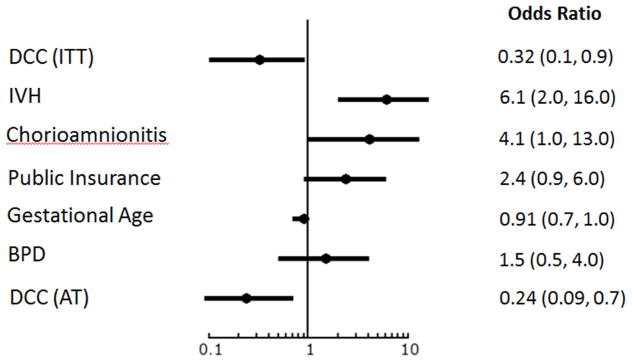
In Figure 2, multiple logistic regression analysis shows that if the child had DCC, the child’s chance of scoring less than 85 on the Bayley-III motor composite score was reduced by 68% after adjusting for known risk factors using intention-to-treat analysis and 76% using actual treatment (sensitivity analysis). If the mother had a history of clinical chorioamnionitis, her infant had 4-fold greater odds of scoring below 85. If the child had IVH, he had 6-fold increased odds of scoring less than 85 on the motor composite. Having public insurance more than doubled the child’s chances of scoring less than 85 on the motor composite. Scores under 70 points were predicted only by GA in all models (data not shown). The last bar represents the sensitivity analysis showing that the effect of DCC on Bayley-III motor scores less than 85 was stronger among the infants who actually received the intervention.
Figure 2.
Logistic Regression Analyses showing Risk Factors for Motor Composite Score <85. DCC, delayed cord clamping; ITT, intention-to-treat analysis; IVH, intraventricular hemorrhage; BPD, bronchopulmonary dysplasia; AT, actual treatment used for the sensitivity analysis.
Eligible women delivering < 32 weeks (n = 121) not enrolled differed from the women randomized within the study on antenatal steroid use (ANS) (93% vs 98%, p = 0.009), rates of one ANS dose only (73% vs 27%, p <0.001), MgSO4 (13% vs 39%, p = <0.0001), hours of PROM (60% vs 76%, p = 0.002), chorioamnionitis (5% vs 15%, p = 0.0001), PROM (30% vs 39% p = 0.03), and births by cesarean (51% vs 66%, p = 0.005). Infants of these women differed from enrolled infants only on median temperatures on NICU admission (median 36.3 vs 36.5 degrees centigrade, p = 0.02). There were no differences in the incidence of IVH (18% vs 25%, p = 0.35).
Discussion
This study of VLBW preterm infants randomized to ICC versus DCC (plus milking) reports the motor outcomes at 18–22 months corrected age. Unbalanced groups with respect to two powerful confounders, PEC and PROM/PTL, complicated the analyses. Nevertheless, the main finding from our study was that a brief delay in cord clamping was associated with better motor outcomes at 18–22 months when controlling for NICU morbidities and public health insurance as proxy for socioeconomic status.
The findings did not support our hypothesis that infants with DCC would have less IVH or LOS. Exploration of the data showed that conditions antecedent to birth had a powerful confounding effect on IVH. PEC, higher in the ICC group, reduced an infant’s odds of experiencing IVH by half. PROM/PTL, more prevalent among mothers randomized to the DCC group, doubled the infant’s odds of experiencing IVH. Recent research suggests probable mechanisms of action for the two opposite effects.19, 20
The mechanism for protection against IVH in infants born to women with PEC is not known. It is possible that intrauterine “stress” from maternal PEC protects the preterm infant against IVH by “maturing” the brain blood vessels especially those in the fragile germinal matrix (GM).20 Before 32 weeks gestation, high levels of vascular endothelial growth factor (VEGF) and angiopoietin stimulate the rapid growth in the vascular GM. Like indomethacin, PEC in the mother may suppress these important growth factors in the fetus allowing more time for the pericytes to cover and protect the vessels.20 Slowing the rapid growth may reduce the chance of rupture of these vessels into the GM when the infant is stressed by preterm birth.20
Conversely, PROM/PTL increased the infant’s chance of IVH after preterm birth twofold through inflammatory processes. There is robust evidence linking IVH with intrauterine inflammation.19, 21, 22 Inflammation with and without infection is known to cause release of pro-inflammatory cytokines into the amniotic fluid and surrounding tissues. These enter the fetus where they disrupt vascular tight junctions and cross the blood brain barrier. In the brain, this action continues especially in the rapidly growing vessels in the GM rendering them more fragile especially when the infant is stressed by preterm birth.23 Although the association between chorioamnionitis and PROM is well known, new evidence suggests that inflammation/infection may be a cause rather than a result of PROM and PTL.23 Bacteria release proteolytic enzymes that reduce the tensile strength and elasticity of the membranes. Even when the membranes remain intact, there is evidence that infection is associated with an increased production of cytokines known to inhibit progesterone synthesis, stimulate prostaglandin synthesis by the amnion and chorion, and thereby incite uterine contractility leading to preterm labor.21, 23, 24
We did not confirm our earlier findings of less LOS in infants who had DCC at birth.13 It is well known that smaller infants are more at risk for LOS.25 There was no evidence of confounding of the LOS results by PEC or PROM/PTL. Neither of the current meta-analyses reported significant differences in the incidence of LOS.11, 12 In the Backes review which included only infants less than 32 weeks (n = 255), the odds ratio was 0.73 (CI 0.44, 1.20) suggesting that the larger trials with younger infants (less than 30 weeks GA) and longer timing for delay underway in England26 and Australia may yield more definitive answers. (Tarnow-Mordi, personal communication).
Manuck et al report that approximately one in four preterm infants (23–34 weeks) had neurodevelopmental impairment at two years of age.27 Possible mechanisms contributing to better motor outcomes at 18–22 months with DCC include increased overall blood volume (10 to 30%) offering the infant a more salutary neonatal transition and greater red cell mass for oxygenation and iron content.1, 2 Higher levels of red blood cells provide additional iron for the infant known to be neuroprotective at critical times in brain development as iron is essential for the proper maturation of the pre-oligodendrocytes.28, 29 Likewise, infants with DCC may have more hematopoietic stem cells (HSC) at birth. Infusions of HSCs soon after injury in animal models have both neuroprotective and neurorestorative effects.30–33 Therefore, we suggest that additional iron and stem cells at birth may be the mechanisms that offer infants with DCC an advantage.
The differences between the subjects and the eligible women who did not enroll were probably due to the fact that the latter were more likely to deliver sooner after admission, allowing less time for the various procedures.
The sensitivity analyses demonstrates that the protocol violations in this study had only a small influence on the conclusions from the primary intention to treat analyses. Thabane et al suggest that sensitivity analyses are underused and recommend their use in every clinical trial.18
The major limitation of this study is the lack of a normal preterm control group. There may be confounders of which we remain unaware. There was some loss of equipoise in our institution but our sensitivity analyses showed that it had only a small impact on our results. It is not possible to assess the infants’ vital signs and breathing during such a short delay. Lastly, a delay of 30 to 45 seconds is very brief and would accordingly limit the magnitude of the placental transfusion. In spite of our conservative protocol, even this brief delay in cord clamping appears to benefit very preterm infants at 18–22 months corrected age. Current studies are underway that will provide transitional support and resuscitative measures with the cord intact for at least one to two minutes.26, 34 Further studies should provide some answers as to the value of longer placental support during neonatal transition.
Our protocol of a brief delay in cord clamping followed by one milking of the cord is safe. DCC may provide an advantage in reducing rates of poor motor performance at 18 to 22 months corrected age. Although there were no group differences in rates of IVH and LOS in very preterm infants in this study, controlling for the major confounding factors of PEC and PROM/PTL revealed their significant and opposite impact on IVH. Further studies are needed to validate these findings.
Acknowledgments
The main study was funded by the National Institute for Nursing Research (RO1 NR100015), and the 18–22 month follow-up was funding by the Thrasher Research Fund (9185).
Abbreviations
- Bayley-III
Bayley Scales of Infant Development III
- BPD
Bronchopulmonary dysplasia
- DCC
Delayed cord clamping
- GA
Gestational age
- ICC
Immediate cord clamping
- IVH
Intraventricular hemorrhage
- LOS
Late onset sepsis
- PEC
Pre-eclampsia
- PTL
Preterm labor
- PROM
Premature rupture of membranes
Footnotes
Clinical trial registration ClinicalTrials.gov: NCT00818220 and NCT01426698.
The authors declare no conflicts of interest.
Portions of the study were presented as an abstract at the meeting of the Pediatric Academic Societies, San Diego, CA, Apri 25–28, 2015, as well as an oral presentation at the Joint European Neonatal Societies, Budapest, Hungary, September 16–20, 2015
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aladangady N, McHugh S, Aitchison TC, Wardrop CA, Holland BM. Infants’ blood volume in a controlled trial of placental transfusion at preterm delivery. Pediatrics. 2006;117:93–8. doi: 10.1542/peds.2004-1773. [DOI] [PubMed] [Google Scholar]
- 2.Bhatt S, Alison BJ, Wallace EM, Crossley KJ, Gill AW, Kluckow M, et al. Delaying cord clamping until ventilation onset improves cardiovascular function at birth in preterm lambs. The Journal of Physiology. 2013;591:2113–26. doi: 10.1113/jphysiol.2012.250084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sommers R, Stonestreet BS, Oh W, Laptook A, Yanowitz TD, Raker C, et al. Hemodynamic effects of delayed cord clamping in premature infants. Pediatrics. 2012;129:e667–72. doi: 10.1542/peds.2011-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molitoris BA, Sutton TA. Endothelial injury and dysfunction: role in the extension phase of acute renal failure. Kidney Int. 2004;66:496–9. doi: 10.1111/j.1523-1755.2004.761_5.x. [DOI] [PubMed] [Google Scholar]
- 5.Rajnik M, Salkowski CA, Thomas KE, Li YY, Rollwagen FM, Vogel SN. Induction of early inflammatory gene expression in a murine model of nonresuscitated, fixed-volume hemorrhage. Shock. 2002;17:322–8. doi: 10.1097/00024382-200204000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Makley AT, Goodman MD, Belizaire RM, Friend LA, Johannigman JA, Dorlac WC, et al. Damage control resuscitation decreases systemic inflammation after hemorrhage. The Journal of surgical research. 2012;175:e75–82. doi: 10.1016/j.jss.2011.11.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tolosa JN, Park DH, Eve DJ, Klasko SK, Borlongan CV, Sanberg PR. Mankind’s first natural stem cell transplant. J Cell Mol Med. 2010;14:488–95. doi: 10.1111/j.1582-4934.2010.01029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.ACOG. Committee Opinion No.543: Timing of umbilical cord clamping after birth. Obstet Gynecol. 2012;120:1522–6. doi: 10.1097/01.AOG.0000423817.47165.48. [DOI] [PubMed] [Google Scholar]
- 9.Jelin AC, Kuppermann M, Erickson K, Clyman R, Schulkin J. Obstetricians’ attitudes and beliefs regarding umbilical cord clamping. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2014;27:1457–61. doi: 10.3109/14767058.2013.864275. [DOI] [PubMed] [Google Scholar]
- 10.AAP. Timing of Umbilical Cord Clamping After Birth. Pediatrics. 2013;131:e1323. [Google Scholar]
- 11.Rabe H, Diaz-Rossello JL, Duley L, Dowswell T. Effect of timing of umbilical cord clamping and other strategies to influence placental transfusion at preterm birth on maternal and infant outcomes. Cochrane Database Syst Rev. 2012;8:Cd003248. doi: 10.1002/14651858.CD003248.pub3. [DOI] [PubMed] [Google Scholar]
- 12.Backes CH, Rivera BK, Haque U, Bridge JA, Smith CV, Hutchon DJ, et al. Placental transfusion strategies in very preterm neonates: a systematic review and meta-analysis. Obstet Gynecol. 2014;124:47–56. doi: 10.1097/AOG.0000000000000324. [DOI] [PubMed] [Google Scholar]
- 13.Mercer JS, Vohr BR, McGrath MM, Padbury JF, Wallach M, Oh W. Delayed cord clamping in very preterm infants reduces the incidence of intraventricular hemorrhage and late-onset sepsis: a randomized, controlled trial. Pediatrics. 2006;117:1235–42. doi: 10.1542/peds.2005-1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mercer JS, Vohr BR, Erickson-Owens DA, Padbury JF, Oh W. Seven-month developmental outcomes of very low birth weight infants enrolled in a randomized controlled trial of delayed versus immediate cord clamping. J Perinatol. 2010;30:11–6. doi: 10.1038/jp.2009.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papile LA, Rudolph AM, Heymann MA. Autoregulation of cerebral blood flow in the preterm fetal lamb. Pediatr Res. 1985;19:159–61. doi: 10.1203/00006450-198502000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Bayley N. Bayley Scales of Infant and Toddler Development, Administrative Manual and Technical Manual. San Antonio, TX: Harcourt Assessment; 2006. [Google Scholar]
- 17.McElrath TF, Hecht JL, Dammann O, Boggess K, Onderdonk A, Markenson G, et al. Pregnancy disorders that lead to delivery before the 28th week of gestation: an epidemiologic approach to classification. American journal of epidemiology. 2008;168:980–9. doi: 10.1093/aje/kwn202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thabane L, Mbuagbaw L, Zhang S, Samaan Z, Marcucci M, Ye C, et al. A tutorial on sensitivity analyses in clinical trials: the what, why, when and how. BMC medical research methodology. 2013;13:92. doi: 10.1186/1471-2288-13-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chau V, McFadden DE, Poskitt KJ, Miller SP. Chorioamnionitis in the pathogenesis of brain injury in preterm infants. Clinics in perinatology. 2014;41:83–103. doi: 10.1016/j.clp.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Ballabh P. Pathogenesis and prevention of intraventricular hemorrhage. Clinics in perinatology. 2014;41:47–67. doi: 10.1016/j.clp.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galinsky R, Polglase GR, Hooper SB, Black MJ, Moss TJ. The consequences of chorioamnionitis: preterm birth and effects on development. Journal of pregnancy. 2013;2013:412831. doi: 10.1155/2013/412831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leviton A, Dammann O. Coagulation, inflammation, and the risk of neonatal white matter damage. Pediatr Res. 2004;55:541–5. doi: 10.1203/01.PDR.0000121197.24154.82. [DOI] [PubMed] [Google Scholar]
- 23.Spinillo A, Iacobone AD, Calvino IG, Alberi I, Gardella B. The role of the placenta in feto-neonatal infections. Early Hum Dev. 2014;90 (Suppl 1):S7–9. doi: 10.1016/S0378-3782(14)70003-9. [DOI] [PubMed] [Google Scholar]
- 24.Holzman C, Lin X, Senagore P, Chung H. Histologic chorioamnionitis and preterm delivery. American journal of epidemiology. 2007;166:786–94. doi: 10.1093/aje/kwm168. [DOI] [PubMed] [Google Scholar]
- 25.Shane AL, Stoll BJ. Neonatal sepsis: Progress towards improved outcomes. Journal of Infection. 2014;68(Supplement 1):S24–S32. doi: 10.1016/j.jinf.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 26.Pushpa-Rajah A, Bradshaw L, Dorling J, Gyte G, Mitchell EJ, Thornton J, et al. Cord pilot trial - immediate versus deferred cord clamping for very preterm birth (before 32 weeks gestation): study protocol for a randomized controlled trial. Trials. 2014;15:258. doi: 10.1186/1745-6215-15-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manuck TA, Sheng X, Yoder BA, Varner MW. Correlation between initial neonatal and early childhood outcomes following preterm birth. Am J Obstet Gynecol. 2014;210:426.e1–9. doi: 10.1016/j.ajog.2014.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Georgieff MK. Iron in the Brain: Its Role in Development and Injury. NeoReviews. 2006;7:e344–e52. [Google Scholar]
- 29.Connor JR, Menzies SL. Relationship of iron to oligodendrocytes and myelination. Glia. 1996;17:83–93. doi: 10.1002/(SICI)1098-1136(199606)17:2<83::AID-GLIA1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 30.Meier C, Middelanis J, Wasielewski B, Neuhoff S, Roth-Haerer A, Gantert M, et al. Spastic paresis after perinatal brain damage in rats is reduced by human cord blood mononuclear cells. Pediatr Res. 2006;59:244–9. doi: 10.1203/01.pdr.0000197309.08852.f5. [DOI] [PubMed] [Google Scholar]
- 31.Gonzales-Portillo GS, Reyes S, Aguirre D, Pabon MM, Borlongan CV. Stem cell therapy for neonatal hypoxic-ischemic encephalopathy. Frontiers in neurology. 2014;5:147. doi: 10.3389/fneur.2014.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tajiri N, Kaneko Y, Shinozuka K, Ishikawa H, Yankee E, McGrogan M, et al. Stem Cell Recruitment of Newly Formed Host Cells via a Successful Seduction? Filling the Gap between Neurogenic Niche and Injured Brain Site. PLoS ONE. 2013;8:1. doi: 10.1371/journal.pone.0074857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cotten CM, Murtha AP, Goldberg RN, Grotegut CA, Smith PB, Goldstein RF, et al. Feasibility of Autologous Cord Blood Cells for Infants with Hypoxic-Ischemic Encephalopathy. The Journal of Pediatrics. 2014;164:973–9.e1. doi: 10.1016/j.jpeds.2013.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tarnow-Mordi WO, Duley L, Field D, Marlow N, Morris J, Newnham J, et al. Timing of cord clamping in very preterm infants: more evidence is needed. Am J Obstet Gynecol. 2014;211:118–23. doi: 10.1016/j.ajog.2014.03.055. [DOI] [PubMed] [Google Scholar]