Abstract
Background
Given the high level of homology between nonhuman primates and humans in regard to anatomy, physiology and ethanol drinking patterns, nonhuman primates represent an unparalleled preclinical model for examining the neurobiological basis of ethanol abuse.
Methods
Here we examined the neurochemical consequences of chronic daily ethanol use using fast-scan cyclic voltammetry in brain slices containing the nucleus accumbens core or dorsolateral caudate taken from male cynomolgus macaques following ethanol drinking.
Results
We found that in both regions the ability of ethanol to decrease dopamine release was unchanged, indicating that ethanol self-administration does not produce tolerance or sensitization to ethanol effects on dopamine release at the dopamine terminal at this time point. We also found that in the nucleus accumbens core, autoregulation of dopamine release was shifted from equal D2 and D3 receptor involvement in control animals to primarily D2 receptor-mediated in drinkers. Specifically, the effect quinpirole, a D2/D3 receptor agonist, on dopamine release was equal across groups; however, dopamine signals were reversed to a greater extent by the selective D3 receptor antagonist SB-277,011A in control animals, indicating a greater contribution of D2 receptors in quinpirole-induced inhibition following ethanol self-administration. In the dorsolateral caudate, the effects of quinpirole and reversal with SB-277,011A was not different between ethanol and control slices.
Conclusions
This work provides novel insight into the dopaminergic adaptations resulting from chronic ethanol use in nonhuman primates and indicates that alterations in D2/D3 dopamine autoreceptor signaling may be an important neurochemical adaptation to ethanol consumption during early use.
Keywords: Autoreceptor, nucleus accumbens, macaque, monkey, D3, caudate
1. Introduction
Alcohol use disorders are a major public health concern and, as such, determining the neurobiological basis of alcoholism in search of pharmacotherapeutic targets has been an area of much research. Due to the physiological, genetic and behavioral similarities between nonhuman primates and humans, nonhuman primate models of human disease states are thought to possess high translational validity (Grant et al., 2014). Similarities between nonhuman primates and humans are particularly relevant in the study of voluntary ethanol drinking as nonhuman primates consume ethanol in a similar pattern to humans (Grant et al., 2008; Majchrowicz and Mendelson, 1970).
Alcohol abuse and addiction are thought to be mediated in part by ethanol-induced adaptations to dopaminergic signaling in the striatum (Koob, 2013). The striatum is composed of multiple distinct subregions that exert divergent control over behavioral outputs. Specifically, ventral regions of the striatum, such as the nucleus accumbens (NAc), encode associations between discrete cues, drug availability and previously learned contingencies. Conversely, dorsal regions, such as the putamen and dorsolateral caudate (DLC), mediate habitual and compulsive behaviors that emerge after repeated drug use (Porrino et al., 2004; Graybiel, 1995, 2008; Everitt and Robbins, 2013). The NAc and DLC both express D2 and D3 dopamine receptors, though distribution of these receptors is differential with the DLC preferentially expressing D2 and the NAc preferentially expressing D3 receptors (Murray et al., 1994). It is hypothesized that alterations to dopamine receptors contribute to pathological drinking behaviors, and in both rodents and humans, chronic ethanol exposure has been associated with decreased sensitivity of dopamine D2/D3 receptors in striatal regions (Volkow et al., 1996; Lucchi et al., 1988). However, the majority of these studies have not differentiated between post synaptic dopamine receptors located on striatal medium spiny neurons and presynaptic dopamine autoreceptors located on dopamine terminals originating from the ventral midbrain (Ford, 2014).
Here we sought to examine the effects of daily ethanol self-administration in male cynomolgus macaques on two important facets of dopamine terminal function: 1) the sensitivity of D2 and D3 autoreceptors and 2) the ability of ethanol to decrease dopamine release. These measures were assessed using fast scan cyclic voltammetry (FSCV), which allows for the isolated assessment of presynaptic dopamine autoreceptors, in brain slices containing the NAc core or the DLC.
2. Methods and Materials
2.1 Subjects
All procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the Oregon National Primate Research Center Institutional Animal Care and Use Committee. Eleven male cynomolgus monkeys (Macaca fascicularis) between the ages of 5.9–6.9 years were used for the current study (Daunais et al., 2014).
2.2 Drinking Procedure
Monkeys (8 ethanol and 3 control) were trained to obtain fluids and their meals from an operant panel that replaced one of the walls of their home cage, as previously described (Vivian et al., 2001; Grant et al., 2008). Briefly, the panels had two spouts, one on each side of a 15″ video display screen. Near each spout the display showed a set of three stimulus lights (white, red, and green) that indicated an active session, food or fluid availability, respectively. A recessed dowel activated the fluid spouts and an infrared finger-poke detector activated the pellet dispenser (env-203-1000, Med Associates, St. Albans City, VT). Each spout was connected via Nalgene tubing to a 1-L fluid reservoir set on a digital scale (Ohaus Adventurer Pro Balances AV4101C, Ohaus Corporation, Pine Brook, NJ). Schedule-induced polydipsia was used to induce ethanol self-administration in daily 16-h sessions as previously described (Vivian et al., 2001; Grant et al., 2008). Following completion of ethanol drinking induction, animals began daily 22-h sessions during which water and ethanol were concurrently available. Animals were allowed ethanol access until the morning of necropsy, but sessions where the monkey was anesthetized for experimental (e.g., MRI imaging) or veterinary interventions (e.g., health checkups) are not included in average ethanol intake calculations. The actual number of 22hr sessions used for intake measures exceeds 6 months and ranged between 201-205 days for controls and 204-214 days for ethanol drinking subjects. Intakes during the induction of ethanol self-administration are not included in the daily intake averages. Monkeys were anesthetized with ketamine (10mg/kg), maintained on isoflurane, and perfused with ice-cold oxygenated monkey perfusion solution, as previously described (Daunais et al., 2010). The brain was removed approximately 3.5-6.5 hours after the last ethanol access period.
2.3 In Vitro Voltammetry
A vibrating tissue slicer was used to prepare 250 μm thick coronal brain sections containing either the DLC or NAc core, as previously described (Siciliano et al., 2014). The tissue was immersed in oxygenated artificial cerebrospinal fluid (aCSF) containing (in mM): NaCl (126), KCl (2.5), NaH2PO4 (1.2), CaCl2 (2.4), MgCl2 (1.2), NaHCO3 (25), glucose (11), L-ascorbic acid (0.4) and pH was adjusted to 7.4. Once sliced, the tissue was transferred to testing chambers containing bath aCSF (32°C), which flowed at 1 ml/min. A carbon fiber microelectrode (100–200 μM length, 7 μM diameter) and bipolar stimulating electrode were placed in close proximity on the tissue. Extracellular dopamine was recorded by applying a triangular waveform (−0.4 to +1.2 to −0.4V vs Ag/AgCl, 400 V/s) to the recording electrode and scanning every 100 ms. Dopamine release was evoked by 1 pulse stimulations (350 μA, 4 msec, monophasic) applied to the tissue every 5 minutes until a stable baseline was established (3 collections within 10% variability), followed by bath application of ethanol (80 mM, 120 mM cumulatively), quinpirole (30 nM, 100 nM, cumulatively) or SB-277,011A (10 μM) to the slice (Austin et al., 2000; Rose et al., 2013). Signals were allowed to stabilize before subsequent concentrations were added to the bath. Quinpirole (100 nM) remained in the bath during SB-277,011A application. Demon Voltammetry and Analysis software was used for all analysis of FSCV data (Yorgason et al., 2011).
3. Results
3.1 Autoregulation of Dopamine Release Shifts From D2/D3-mediated to Primarily D2-mediated Following Ethanol Exposure
Dopamine release and uptake kinetics as well as behavioral data from these animals were previously published in Siciliano et al. (2015a). The average amounts of ethanol consumed daily varied across individuals and ranged from 0.7 to 3.0 g/kg (Figure 1A; group average: 1.79 ± 0.27 g/kg/day). Average BECs ranged from 6 to144 mg/dl (Figure 1B; group average: 62.38 ± 16.59). Ethanol self-administration increased dopamine release (control: 0.49 ± 0.16 μM, drinkers: 0.97 ± 0.11 μM) and uptake (control: 1.74 ± 0.16 μM/s, drinkers: 2.16 ± 0.08 μM/s) in the NAc core (depicted in Figure 1C). Additionally, ethanol self-administration had the opposite effect in the DLC, where dopamine release (control: 0.85 ± 0.06 μM, drinkers: 0.41 ± 0.10 μM) and uptake (control: 2.09 ± 0.26 μM/s, drinkers: 1.31 ± 0.18 μM/s) were decreased in ethanol drinking animals (data not shown).
Figure 1. Ethanol Self-Administration Shifts Autoregulation of Dopamine Release from Primarily D3-Mediated to Primarily D2-Mediated.
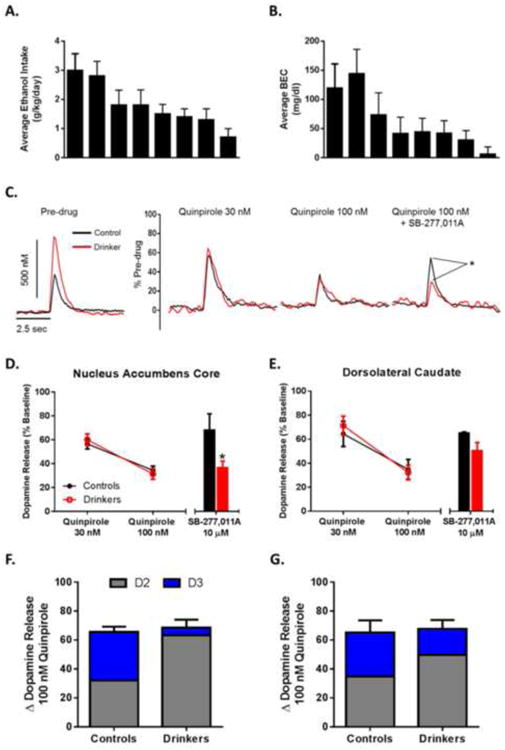
(A) Average (± SD) daily ethanol intake for each animal. (B) Average (± SD) BEC taken every 5-7 days over the 6-month access period. Samples were collected 7 hours after session onset. Animals are presented in the same order between graphs A and B. Data from graphs A and B were previously published by Siciliano et al. (2015). (C) Representative traces demonstrating increased dopamine release and uptake in ethanol self-administration animals (left) and the effects of quinpirole and SB-277,011A on dopamine release between groups (right). (D) Group data (n=2 for controls; n=6 for drinkers) indicating no difference in sensitivity to D2/D3 agonist quinpirole between groups in the nucleus accumbens core. Application of the selective D3 antagonist SB-277,011A revealed a shift from a predominantly D3 mediated effect of quinpirole in controls to a predominantly D2 mediated effect in drinkers. (E) Group data (n=2 for controls; n=6 for drinkers) indicating no difference in sensitivity to D2/D3 agonist quinpirole between groups and a trend towards a more D2 mediated effect of quinpirole in drinkers. (F) Total inhibition induced by quinpirole (whole bar) as well as the percent reversal induced by SB-277,011A (blue) in the NAc core. Inhibition present after application of SB-277,011A was presumed to be due to D2 receptor activation (gray). (G) Relative contribution of dopamine autoreceptor subtypes in the DLC. Total inhibition induced by quinpirole is depicted by the whole bar. Calculation of the contribution of D2 and D3 subtypes are represented in gray and blue respectively. *, p < 0.05 vs control.
Given the implication of dopamine autoreceptors in regulating the rewarding properties of both natural and drug rewards (Bello et al., 2011), the effects of ethanol self-administration on D2/D3 autoreceptor sensitivity were examined. Quinpirole, a selective D2/D3 receptor agonist, was bath applied (30 and 100 nM) to slices containing the NAc core or DLC to determine differences in sensitivity between drinkers and control animals (Figure 1C). Following application of quinpirole in the NAc core, a two-way repeated measures ANOVA revealed a main effect of concentration (F1,7 = 11.91, p = 0.01), but no effect of group (F1,7 = 0.13, p = 0.73) indicating that D2/D3 autoregulation of dopamine release was not changed by ethanol use (Figure 1D). The selective D3 antagonist SB-277,011A was bath applied (10 μM) to the slice to determine the proportion of the quinpirole-induced decrease in dopamine release that was mediated through D3 receptors. A Student's t-test revealed that the quinpirole-induced decrease in dopamine release was restored by SB-277,011A to a greater extent in the control animals than in the ethanol exposed group (Figure 1D) (t6 = 2.62, p = 0.02), indicating that there is a reduced contribution of D3 receptors to autoregulation of dopamine release following ethanol use.
Following application of quinpirole in the DLC (Figure 1E), a two-way repeated measures ANOVA revealed a main effect of concentration (F1,7 = 70.58, p = 0.0001), but no effect of group (F1,7 = 0.04, p = 0.85). In the DLC there was no difference in SB-277,011A-induced reversal of quinpirole effects between controls and drinkers (Figure 1E) (t6 = 1.15, p = 0.15). Given the differential effect of SB-277,011A between drinkers and control animals, we calculated the relative contribution of D2 and D3 receptors to the total inhibition induced by quinpirole in the NAc core (Figure 1F) and DLC (Figure 1G). The percent change induced by SB-277,011A was plotted as D3 receptor contribution, while any remaining inhibition by quinpirole was assumed to be D2 receptor contribution.
3.2 Ethanol Sensitivity at the Dopamine Terminal is Unchanged Following Voluntary Ethanol Drinking
To determine if ethanol sensitivity at the dopamine terminal was altered by ethanol self-administration, 80 and 120 mM ethanol was bath applied to brain slices containing the DLC or NAc core. In the NAc core (Figure 2A), a two-way repeated measures ANOVA revealed a main effect of concentration (F1,8 = 33.34, p = 0.0004), but no effect of ethanol self-admiration (F1,8 = 0.15, p = 0.71). In the DLC (Figure 2B), a two-way repeated measures ANOVA revealed a main effect of concentration (F1,8 = 21.23, p = 0.009) whereby ethanol decreased dopamine release in a concentration-dependent manner. There was no effect of ethanol history on sensitivity to ethanol (F1,8 = 0.04, p = 0.83).
Figure 2. Ethanol Sensitivity at the Dopamine Terminal is Unchanged by Ethanol Self-Administration.
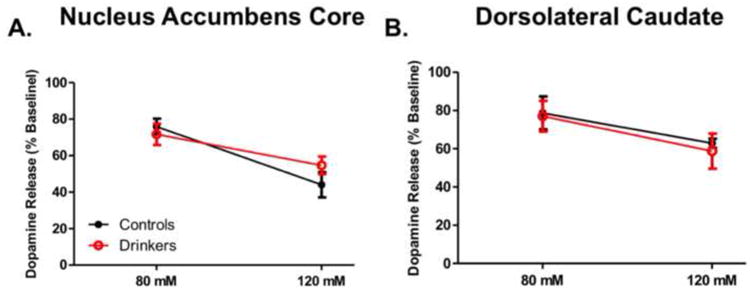
Ethanol was bath applied to slice containing either the nucleus accumbens core (A) or dorsolateral caudate (B). (A) Group data (n=3 for controls; n=7 for drinkers) indicating no difference in ethanol sensitivity between groups in the nucleus accumbens core. (B) Group data (n=3 for controls; n=7 for drinkers) indicating no difference in ethanol sensitivity between groups in the dorsolateral caudate.
4. Discussion
We found that following free-access ethanol self-administration in male cynomolgus macaques there is a marked shift in the relative contribution of D2 versus D3 type autoreceptors in regulating dopamine release from presynaptic terminals in the nucleus accumbens core. Indeed, in control animals, autoregulation of dopamine release was roughly equally D2 and D3 receptor mediated, while in ethanol self-administration animals autoregulation was primarily D2 receptor mediated. Additionally, we found that the overall sensitivity of autoreceptors, when D2 and D3 receptors were measured together, was not different between control and ethanol slices. Finally, we found that following a history of chronic ethanol self-administration did not alter the ability of ethanol to decrease electrically stimulated dopamine release at this time point. These results give novel insights into specific, long-term dopaminergic adaptations induced by daily, voluntary ethanol drinking and suggest that ethanol-induced alterations in autoreceptor regulation of presynaptic dopamine release may be a consequence of chronic ethanol use.
Dopamine D2 and D3 autoreceptors regulate dopamine neurotransmission through inhibitory feedback that decreases dopamine release, nerve-terminal excitability and dopamine synthesis (Ford, 2014). Autoregulation of dopamine neurotransmission is important for modulating the rewarding and reinforcing aspects of natural rewards and drugs of abuse, and D2 autoreceptor knockout mice have greatly augmented cocaine reward and reinforcing efficacy of food (Bello et al., 2011). Here we show that the ability of the nonselective D2/D3 agonist quinpirole to decrease dopamine release is not different from controls in the NAc core and DLC following of monkeys given >200 consecutive days of ethanol drinking, which is a fairly short-term exposure in this monkey model (Grant et al., 2008). However, it is likely that as ethanol use persists, the sensitivity of autoreceptors is altered by ethanol, as previous work from our lab has demonstrated supersensitivity of autoreceptors in monkeys following 18 months of ethanol self-administration (Budygin et al., 2003). These findings are in contrast to reports of ethanol-induced decreases in D2/D3 receptor sensitivity, however this may be a result of measuring global dopamine receptor sensitivity (e.g. Volkow et al., 1996; Lucchi et al., 1988) as opposed to specifically measuring presynaptic autoreceptors (Budygin et al., 2003; current study). Further, the contingency of administration may be a critical factor in the development of ethanol-induced adaptations to dopamine receptors, and future work will aim to determine if these effects are a purely pharmacological consequence of ethanol exposure, or a product of voluntary consumption.
Additionally, we found that quinpirole-induced deceases in dopamine release were reversed with application of a selective D3 antagonist to a greater extent in controls animals. Further investigations are needed to 1) more fully characterize this effect by examining the effects of SB-277,011A across a concentration-response curve and 2) determine the behavioral relevance of ethanol-induced alterations to presynaptic dopamine receptor subtype regulation of dopamine neurotransmission. Another potential caveat of the current study is the low number of subjects, especially in the control group, thus limiting statistical power. However, these results indicate that ethanol self-administration decreased the contribution of D3 autoreceptors while maintaining the same overall effect of quinpirole, presumably by increasing the contribution of D2 receptors. One possibility is that increased D2 receptor regulation of dopamine release may produce long-lasting decreases in dopamine levels, due to reduced receptor desensitization as compared to D3 receptors (O'Hara et al., 1995), during withdrawal to produce hypofunction of the dopamine system. Moreover, the effects of ethanol self-administration on autoregulation of dopamine release were divergent across regions, which may be a function of greater D3 receptor availability in the NAc compared to the DLC (Murray et al., 1994). It may be a promising avenue of future research to explore other striatal subregions. The NAc shell is of particular interest given its role in assigning motivational value to reinforcers, including abused drugs (Saddoris et al., 2013).
Many theories of addiction propose that following extensive use of a drug, the user adapts (becomes tolerant or sensitized) to the effects of the drug and that the neural basis of these adaptations is integral to the addiction process (Robinson and Berridge, 1993; MacRae et al., 1987; Koob, 2003; Siciliano et al., 2015b). We found that the inhibitory effects of ethanol on dopamine neurotransmission at the nerve terminal were unchanged by ethanol self-administration. However, it is important to note that the ability of ethanol to augment dopamine levels is dependent on interactions with the dopaminergic cell bodies projecting from the ventral midbrain to increase cell firing (Brodie et al., 1990; Mrejeru et al., 2015; Gessa et al., 1985). Thus, while we show that the inhibitory effects of ethanol at the dopamine terminal are unchanged, ethanol's excitatory effects on dopamine firing are unknown for primates. Together, the current findings give novel insight into the neurobiological basis of ethanol abuse and indicate that alterations in relative function of dopamine autoreceptor subtypes may contribute to aberrant drinking behaviors during early ethanol use.
Highlights.
Dopamine D2 autoreceptors are supersensitive following ethanol self-administration
Dopamine D3 autoreceptors are subsensitive following ethanol self-administration
Ethanol self-administration does not alter ethanol effects on dopamine release
Acknowledgments
This work was funded by NIH grants U01 AA014091, P01 AA021099 (SRJ), F31 DA031533 (ESC), F31 DA037710, T32 AA007565 (CAS), F31 AA020439 (JTY), U01 OD011092, R24 AA019431, P60 AA10760 (KAG), Division of Intramural Clinical and Biomedical Research NIAAA (DML), Integrative Neuroscience Initiative on Alcoholism AA 13510 (KAG) and OHSU Administration Core AA 13641 (KAG).
Role of Funding Source: Nothing declared
Footnotes
Conflict of Interest: The authors have no conflicts to report.
Contributors: Cody Siciliano: designed and performed experiments, analyzed data, wrote manuscript.
Erin Calipari: designed and performed experiments, analyzed data.
Jordan Yorgason: provided equipment/reagents.
Yolanda Mateo: provided equipment/reagents.
Christa Helms: performed experiments.
David Lovinger: provided equipment/reagents.
Kathleen Grant: designed experiments, provided equipment/reagents.
Sara Jones: designed experiments, provided equipment/reagents, edited manuscript.
All authors have approved the final article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Austin NE, Avenell KY, Boyfield I, Branch CL, Hadley MS, Jeffrey P, Johnson CN, MacDonald GJ, Nash DJ, Riley GJ, Smith AB, Stemp G, Thewlis KM, Vong AK, Wood M. Novel 2,3,4,5-tetrahydro-1H-3-benzazepines with high affinity and selectivity for the dopamine D3 receptor. Bioorg Med Chem Lett. 2000;10:2553–2555. doi: 10.1016/s0960-894x(00)00505-9. [DOI] [PubMed] [Google Scholar]
- Bello EP, Mateo Y, Gelman DM, Noaín D, Shin JH, Low MJ, Alvarez VA, Lovinger DM, Rubinstein M. Cocaine supersensitivity and enhanced motivation for reward in mice lacking dopamine D2 autoreceptors. Nat Neurosci. 2011;14:1033–1038. doi: 10.1038/nn.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchi L, Moresco RM, Govoni S, Trabucchi M. Effect of chronic ethanol treatment on dopamine receptor subtypes in rat striatum. Brain Res. 1988;449:347–351. doi: 10.1016/0006-8993(88)91051-7. [DOI] [PubMed] [Google Scholar]
- Brodie MS, Shefner SA, Dunwiddie TV. Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res. 1990;508:65–69. doi: 10.1016/0006-8993(90)91118-z. [DOI] [PubMed] [Google Scholar]
- Budygin EA, John CE, Mateo Y, Daunais JB, Friedman DP, Grant KA, Jones SR. Chronic ethanol exposure alters presynaptic dopamine function in the striatum of monkeys: a preliminary study. Synapse. 2003;50:266–268. doi: 10.1002/syn.10269. [DOI] [PubMed] [Google Scholar]
- Daunais JB, Davenport AT, Helms CM, Gonzales SW, Hemby SE, Friedman DP, Farro JP, Baker EJ, Grant KA. Monkey alcohol tissue research resource: banking tissues for alcohol research. Alcohol Clin Exp Res. 2014;38:1973–1981. doi: 10.1111/acer.12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daunais JB, Kraft RA, Davenport AT, Burnett EJ, Maxey VM, Szeliga KT, Rau AR, Flory GS, Hemby SE, Kroenke CD, Grant KA, Friedman DP. MRI-guided dissection of the nonhuman primate brain: a case study. Methods. 50:199–204. doi: 10.1016/j.ymeth.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. From the ventral to the dorsal striatum: devolving views of their roles in drug addiction. Neurosci Biobehav Rev. 2013;37:1946–1954. doi: 10.1016/j.neubiorev.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Ford CP. The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience. 2014;282C:13–22. doi: 10.1016/j.neuroscience.2014.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessa GL, Muntoni F, Collu M, Vargiu L, Mereu G. Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res. 1985;348:201–203. doi: 10.1016/0006-8993(85)90381-6. [DOI] [PubMed] [Google Scholar]
- Grant KA, Leng X, Green HL, Szeliga KT, Rogers LS, Gonzales SW. Drinking typography established by scheduled induction predicts chronic heavy drinking in a monkey model of ethanol self-administration. Alcohol Clin Exp Res. 2008;32:1824–1838. doi: 10.1111/j.1530-0277.2008.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, Ferguson B, Helms CM, McClintick MN. Drinking to dependence risk factors in nonhuman primates. In: Noronha A, Cui C, Harris RA, Crabbe JC, editors. Neurobiology of Alcohol Dependence. Elsevier; Amsterdam: 2014. pp. 412–424. [Google Scholar]
- Graybiel AM. The basal ganglia. Trends Neurosci. 1995;18:60–62. [PubMed] [Google Scholar]
- Graybiel AM. Habits, rituals, and the evaluative brain. Annu Rev Neurosci. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- MacRae JR, Scoles MT, Siegel S. The contribution of Pavlovian conditioning to drug tolerance and dependence. Br J Addict. 1987;82:371–380. doi: 10.1111/j.1360-0443.1987.tb01493.x. [DOI] [PubMed] [Google Scholar]
- Majchrowicz E, Mendelson JH. Blood concentrations of acetaldehyde and ethanol in chronic alcoholics. Science. 1970;168:1100–1102. doi: 10.1126/science.168.3935.1100. [DOI] [PubMed] [Google Scholar]
- Mrejeru A, Martí-Prats L, Avegno EM, Harrison NL, Sulzer D. A subset of ventral tegmental area dopamine neurons responds to acute ethanol. Neuroscience. 2015;290:649–658. doi: 10.1016/j.neuroscience.2014.12.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray AM, Ryoo HL, Gurevich E, Joyce JN. Localization of dopamine D3 receptors to mesolimbic and D2 receptors to mesostriatal regions of human forebrain. Proc Natl Acad Sci U S A. 1994;91:11271–11275. doi: 10.1073/pnas.91.23.11271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Smith HR, Daunais JB, Nader MA. Cocaine self-administration produces a progressive involvement of limbic, association, and sensorimotor striatal domains. J Neurosci. 2004;24:3554–3562. doi: 10.1523/JNEUROSCI.5578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hara CM, Uhland-Smith A, O'Malley KL, Todd RD. Inhibition of dopamine synthesis by dopamine D2 and D3 but not D4 receptors. J Pharmacol Exp Ther. 1996;277:186–192. [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rose JH, Calipari ES, Mathews TA, Jones SR. Greater ethanol-induced locomotor activation in DBA/2J versus C57BL/6J mice is not predicted by presynaptic striatal dopamine dynamics. PLoS One. 2013;8:e83852. doi: 10.1371/journal.pone.0083852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saddoris MP, Sugam JA, Cacciapaglia F, Carelli RM. Rapid dopamine dynamics in the accumbens core and shell: learning and action. Front Biosci (Elite Ed) 2013;5:273–288. doi: 10.2741/e615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano CA, Calipari ES, Cuzon Carlson VC, Helms CM, Lovinger DM, Grant KA, Jones SR. Voluntary ethanol intake predicts kappa opioid receptor supersensitivity and regionally distinct dopaminergic adaptations in macaques. J Neurosci. 2015a;35:5959–5968. doi: 10.1523/JNEUROSCI.4820-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano CA, Calipari ES, Ferris MJ, Jones SR. Biphasic mechanisms of amphetamine action at the dopamine terminal. J Neurosci. 2014;34:5575–5582. doi: 10.1523/JNEUROSCI.4050-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano CA, Calipari ES, Ferris MJ, Jones SR. Adaptations of presynaptic dopamine terminals induced by psychostimulant self-administration. ACS Chem Neurosci. 2015b;6:27–36. doi: 10.1021/cn5002705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivian JA, Green HL, Young JE, Majerksy LS, Thomas BW, Shively CA, Tobin JR, Nader MA, Grant KA. Induction and maintenance of ethanol self-administration in cynomolgus monkeys (Macaca fascicularis): long-term characterization of sex and individual differences. Alcohol Clin Exp Res. 2001;25:1087–1097. [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS, Pappas N, Shea C, Piscani K. Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol Clin Exp Res. 1996;20:1594–1598. doi: 10.1111/j.1530-0277.1996.tb05936.x. [DOI] [PubMed] [Google Scholar]
- Yorgason JT, España RA, Jones SR. demon voltammetry and analysis software: analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J Neurosci Methods. 2011;202:158–164. doi: 10.1016/j.jneumeth.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


