Abstract
Objective
To determine the associations of adiposity and insulin resistance with measures of vascular structure and function in children.
Study design
A cross-sectional study included 252 children (age 15.1±2.4 yrs; body mass index (BMI)-percentile 68.2±26.5%; Tanner 2–5). Measurements of body fat percentage (BF%) were obtained with dual-energy X-ray absorptiometry (DXA) and visceral fat (VAT) with computed tomography (CT). Insulin resistance was measured with hyperinsulinemic euglycemic clamp. Vascular measurements for endothelial function (brachial artery flow-mediated dilation [FMD]), vascular structure (carotid intima-media thickness [cIMT]), vascular stiffness (carotid incremental elastic modulus [cIEM]), and pulse wave velocity (PWV) were analyzed by tertiles of adiposity and insulin resistance. Additional analyses with ANCOVA and linear regression, were adjusted for Tanner, sex, race, and family relationship; FMD was also adjusted for baseline artery diameter.
Results
FMD was positively associated with high adiposity (BMI, BF%, and VAT) (p<0.01 all). Insulin resistance was not associated with FMD. cIMT was significantly, positively related to obesity, VAT, and insulin resistance (p<0.05 all). No differences in cIEM and PWV were observed in relation to adiposity or insulin resistance.
Conclusions
The findings suggest that adiposity is associated with higher FMD, and insulin resistance and VAT are associated with higher cIMT in children. Further research is needed to clarify the progression of these relations.
Keywords: Adiposity, Obesity, Insulin Resistance, Pediatric, Flow-mediated Dilation, Carotid Intima-Media Thickness, Cardiovascular Disease Risk
Studies in adults have shown adiposity and insulin resistance to be associated with vascular dysfunction and adverse thickening of the vascular wall, measured by carotid intima-media thickness (cIMT)1–5. Although these relations are less clear in children, several studies have reported the same adverse relations for adiposity with vascular measures 6–12, and others observed no significant relations.13–15 However, a recent population based cohort of pre-pubertal children provided data suggesting that adiposity tends to be associated with both an increase in brachial artery flow mediated dilation (FMD) and decrease in arterial stiffness as measured by pulse wave velocity (PWV).15 Thus, the relationship between adiposity with FMD, cIMT, and arterial stiffness (PWV) remains to be fully defined.
Insulin resistance is associated with obesity and cardiovascular risk factors, beginning in childhood.16 Studies are needed to examine the relation between insulin resistance, and FMD, cIMT, and arterial stiffness in children. Given that total body and visceral adipose tissue (VAT) play significant, and potentially different roles in relation to insulin resistance and the pathophysiology of cardiovascular disease (CVD),17 body fat measurements will improve our understanding of the role it plays in the early changes in FMD, cIMT, and arterial stiffness. Furthermore, studying the associations of insulin resistance and adiposity with measures of vascular structure and function, while controlling for pubertal maturation, may yield information toward the understanding of the complex relations between obesity and vascular function and structure in children.
The purpose of this study was to examine the relationship of FMD, cIMT, and arterial stiffness with multiple measures of adiposity and insulin resistance measured by hyperinsulinemic euglycemic clamp in a cohort of healthy children.
Methods
The study protocol was approved by the University of Minnesota Institutional Review Board, and consent/assent was obtained from parents/participants. Data for this study were collected from participants recruited from the Minneapolis - St. Paul Metro area, for two longitudinal studies conducted at the University of Minnesota: 1) a community-based study evaluating cardiometabolic risk in healthy children (2006–2011; n=141; age 9–18 years); and 2) healthy siblings serving as a control group for a cohort of childhood cancer survivors (2007–2012; n=111; age 8–20 years). Participants were included if they were Tanner stage 2–5, normotensive, non-diabetic, free from chronic diseases, and were not taking medications known to influence vascular function and/or glucose metabolism. Both studies were conducted in the Clinical Research Center using similar personnel, equipment, and protocols.
All testing was performed in the morning after an overnight fast (including no caffeine consumption) of at least 8 hours. Height and weight were measured using a wall-mounted stadiometer and an electronic scale, respectively. BMI was calculated as body weight in kilograms divided by the height in meters squared. BMI-percentiles were determined using age and sex based Centers for Disease Control definitions.18 Normal-weight was defined as >5th to <85th percentile, overweight ≥85th to < 95th percentile, and obesity ≥ 95th percentile. Tanner staging for pubertal maturation was performed by a trained nurse or physician.19, 20 Blood pressure was measured twice on the right arm after participants were sitting in a quiet room for at least five minutes using a digital blood pressure monitor and the average of the two values was reported for systolic (SBP) and diastolic blood pressure (DBP). A fasting lipid profile [total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglycerides (TG)], and fasting insulin were measured using standard procedures at the Fairview Diagnostic Laboratories at the Fairview-University Medical Center (Minneapolis, MN), a Centers for Disease Control and Prevention–certified laboratory.
Insulin resistance was measured by hyperinsulinemic euglycemic clamp, as previously described.21 Insulin was infused at a constant rate of 1 μU/kg/min for 3 hours, and glucose was infused at a variable rate to maintain euglycemia (100mg/dl). Insulin resistance (M) was expressed as the amount of glucose required to maintain euglycemia in the last 40 minutes of the clamp (mg/kg/min of glucose) with adjustment for lean body mass (Mlbm). Lower Mlbm represents greater insulin resistance.
Body fat percentage (BF%) was measured using dual-energy X-ray absorptiometry (DXA) (Lunar Prodigy, General Electric Medical Systems) and analyzed using its enCoreTM software (platform version 13.6, GE Healthcare, Madison, WI, USA). Estimates of abdominal VAT were obtained by CT using a Siemens Sensation 16 (Siemens Medical Solutions, Malvern, PA, USA) with two separate 10 mm slices obtained at the L4–L5 interspace. The two images were subdivided into 5 mm slices and the first and third 5 mm slices were combined and analyzed for VAT. The upper limit of adipose tissue density was −30 Hounsfield units and the lower limit was −190 Hounsfield units. Image slices were individually analyzed by one trained technician using a computer program (Fat Scan version 3.0; N2 System, Osaka, Japan).
Endothelial function was evaluated using brachial artery flow-mediated dilation (FMD) measured with standard ultrasound using a 8–15 MHz linear array transducer held at a constant pressure on the skin and at a fixed point over the imaged artery by a stereotactic arm to obtain B-mode images (Siemens, Sequoia 512, New York, NY) following current guidelines.22 An electronic wall-tracking software program (Medical Imaging Applications, Coralville, IA) was used for the measurement of brachial artery diameter and blood flow. Following baseline measurements, a blood pressure cuff was placed on the forearm (distal to the imaged area) and inflated to a suprasystolic level (>200mmHg) for 5 minutes. After 5 minutes, cuff occlusion was released and B-mode ultrasound images were captured for approximately three minutes after release. The maximum diameter recorded following reactive hyperemia was reported relative to baseline vessel diameter (FMD = peak diameter – baseline diameter/baseline diameter). An area under the curve (AUC) for FMD was calculated using the trapezoidal method. All measurements were conducted by the same group of sonographers, under the supervision of the same laboratory director. Our laboratory has previously documented satisfactory FMD reproducibility with the same individual tested one week apart having a mean difference of 0.53±0.28%.23
Vascular structure was evaluated using carotid intima-media thickness (cIMT). Images for determining cIMT were obtained at end-diastole (gated by R wave on ECG) using B-mode images of the far wall of the left common carotid artery. Measurements were obtained at the distal 10 mm of the common carotid artery as recommended by pediatric guidelines.24 An electronic wall-tracking software program was used for the analysis of cIMT. Our laboratory has previously documented satisfactory reproducibility for measurement of cIMT, with the mean difference for repeated measurements on separate days in the same subjects of 0.02 ± 0.03 mm.25
Carotid arteries also were imaged to capture the left common carotid artery diastolic and systolic lumen diameters to determine carotid incremental elastic modulus (cIEM, mmHg), a measure of carotid artery stiffness. Systolic and diastolic blood pressures were recorded with an automated blood pressure sphygmomanometer (Colin Medical Instruments Corp., San Antonio, TX, USA) during the 10-sec measurements. The ultrasound scanning system was interfaced with a standard computer, with images collected at 20 frames/sec for 10-sec (200 frames) to ensure the capture of full arterial diameter change during a cardiac cycle and calculated using a standard formula.26 Arterial stiffness also was measured by carotid-radial pulse wave velocity (PWV) (SphygmoCor® system, Sydney, Australia). PWV was calculated as distance (m)/transit time (s). The distance was measured between the carotid and radial sites and the sternal notch.
Statistical Analyses
Demographic, anthropometric, and cardiometabolic differences across sex were compared by paired t-test. Data were examined using both continuous and categorical analysis. Data for BF%, VAT, insulin resistance (Mlbm), and fasting insulin were split into categorical variables by tertile. The relation between obesity status (normal-weight, overweight, obese), BF%, VAT, insulin resistance, and fasting insulin as categorical variables and both FMD and FMD AUC were assessed by ANCOVA using linear mixed models to adjust for Tanner stage, sex, race, family relationship (ie, siblings), and baseline artery diameter with post-hoc testing for between group differences conducted using Bonferroni correction. Relations to cIMT, cIEM, and PWV were evaluated by ANCOVA using linear mixed models adjusting for Tanner stage, sex, race, and family relationships (siblings). Multiple linear regression analysis was used to examine the association of Mlbm, fasting insulin, and BF% on FMD and FMD AUC as continuous variables. Covariates in the analysis were Tanner stage, sex, race, family relationship (siblings), and baseline artery diameter. cIMT, cIEM, and PWV were evaluated similarly using multiple linear regression models adjusting for Tanner stage, sex, race, and family relationships (siblings). All models accounted for sibling relationships (17 families with 2 siblings; 2 with 3 siblings; and 1 with 4 siblings. It has been proposed that simple adjustment for baseline artery diameter may not be sufficient to account for the confounding relationship of artery diameter on FMD, therefore we conducted allometric scaling in addition to more traditional analysis in order to confirm our findings.27, 28 Analysis was conducted using SPSS version 22.0 (IBM, Armonk, NY). Data are presented as mean ± SD (except where noted). Significance was set at an alpha level of p<0.05.
Results
Demographic and clinical characteristics of the study population divided by sex are presented in the Table. A total of 252 children, age 8–20 years (mean age = 15.1 ± 2.4 yrs; M=121; mean BMI-percentile = 68.2 ± 26.5) were included. No sex-specific differences were observed for age, BMI, BMI – percentile, total cholesterol, LDL-C, HDL-C, TG, DBP, VAT, Mlbm, Fasting insulin, cIMT, cIEM, or PWV. Males were taller, had higher SBP, and had larger brachial artery diameter than females. Females had a greater Tanner stage, BF%, FMD, and FMD AUC than males. However, after adjustment for baseline artery diameter, no differences were present between males and females for FMD (P = 0.58) and FMD AUC (P= 0.84). Combined data for males and females examining the relations of adiposity with vascular measures are presented in Figure 1 and 2.
Table 1.
Descriptive and clinical characteristics split by sex.
| Male | Female | Difference | |
|---|---|---|---|
| Overall n = 252 | n = 121 | n = 131 | |
| Age (years) | 14.9 ± 2.3 | 15.3 ± 2.5 | 0.199 |
| Tanner | 3.9 ± 1.2 | 4.2 ± 0.9 | 0.004 |
| Height (cm) | 168.6 ± 12.1 | 161.7 ± 8.6 | <0.001 |
| Weight (kg) | 66.3 ± 21.2 | 61.8 ± 17.4 | 0.061 |
| BMI (kg/m2) | 22.9 ± 5.1 | 23.4 ± 5.5 | 0.433 |
| BMI - percentile (%) | 67.5 ± 27.9 | 68.7 ± 25.1 | 0.721 |
| Cholesterol (mg/dL) | 147 ± 26 | 148 ± 24 | 0.778 |
| LDL-c (mg/dL) | 83 ± 23 | 83 ± 21 | 0.918 |
| HDL-c (mg/dL) | 48 ± 11 | 49 ± 10 | 0.347 |
| TG (mg/dL) | 77 ± 40 | 80 ± 39 | 0.482 |
| SBP (mmHg) | 112 ± 9 | 107 ± 9 | <0.001 |
| DBP (mmHg) | 58 ± 8 | 59 ± 7 | 0.376 |
| BF% | 21.6 ± 9.9 | 32.5 ± 9.0 | <0.001 |
| Visceral Fat (cm2) | 21.4 ± 13.6 | 20.8 ± 8.5 | 0.654 |
| Mlbm (mg/kgLBM/min) | 12.7 ± 4.3 | 12.4 ± 4.1 | 0.527 |
| Fasting insulin (μU/L) | 10.2 ± 7.8 | 11.1 ± 6.6 | 0.346 |
| Brachial artery diameter (mm) | 3.6 ± 0.6 | 3.1 ± 0.4 | <0.001 |
| cIMT (mm) | 0.45 ± 0.04 | 0.44 ± 0.04 | 0.079 |
| cIEM | 1018 ± 560 | 969 ± 314 | 0.390 |
| PWV (m/s) | 6.9 ± 1.2 | 7.1 ± 1.1 | 0.223 |
| FMD% | 7.0 ± 3.3 | 8.3 ± 3.8 | 0.002 |
| FMD (AUC) | 669 ± 406 | 774 ± 433 | 0.048 |
Data are Mean ± SD
Group difference determined by paired t-test.
BMI = Body Mass Index; BF = Body Fat; LDL-C = Low-density lipoprotein cholesterol; HDL-C = High-density lipoprotein cholesterol; TG= Triglycerides; SBP = systolic blood pressure; DBP = diastolic blood pressure; Mlbm = insulin sensitivity/resistance (adjusted for lean body mass); cIMT = Carotid intima-media thickness; cIEM = carotid incremental elastic modulus; PWV = Pulse wave velocity; FMD = Flow-mediated dilation.
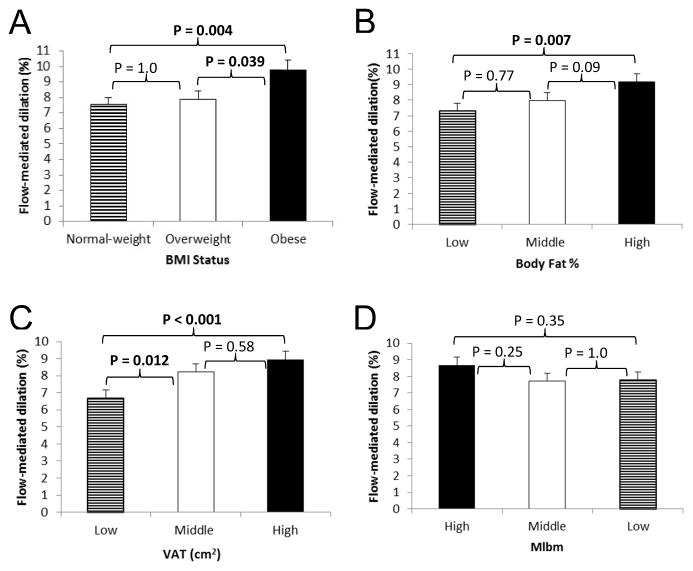
Figure 1.
Association between BMI Status (A), Body Fat (B), VAT (C), and Mlbm (D; Lower Mlbm equals greater insulin resistance) with Flow-mediated Dilation in Children.
Data are means ± SE adjusted for Tanner stage, sex, race, family relationship (siblings), and baseline artery diameter using ANCOVA; p-values determined using Bonferroni post-hoc testing.
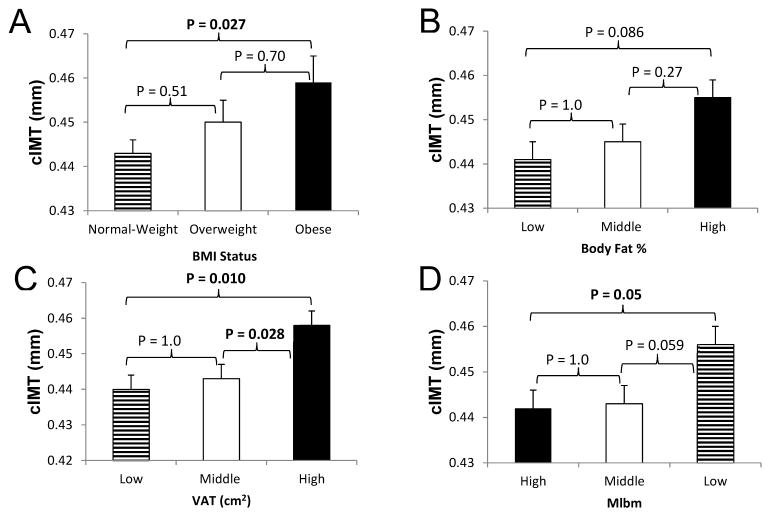
Figure 2.
Association between BMI Status (A), Body Fat % (B), VAT (C), and Mlbm (D; Lower Mlbm equals greater insulin resistance) with cIMT in Children.
Data are means ± SE adjusted for tanner stage, sex, race, and family relationship (siblings) using ANCOVA; p-values determined using Bonferroni post-hoc testing.
Obese children had significantly greater FMD (Figure 1, A) and FMD AUC (p=0.01) compared with overweight and normal-weight children. Data analyzed across tertiles of BF% and VAT showed significantly higher FMD (Figure 1, B and C) and FMD AUC (p=0.003 and p<0.001, respectively) in the highest compared with the lowest tertile. There were no significant differences in FMD or FMD AUC between normal weight and overweight children or between low and mid BF% tertiles. No differences in FMD or FMD AUC were seen by tertiles of Mlbm (Figure 1, D). Children in the highest tertile of fasting insulin (>12 μU/L) had significantly higher FMD (p=0.029) than the mid (7–12 μU/L) or lowest (<7 μU/L) tertiles. When allometric scaling was conducted the results for any of the measurements and FMD did not meaningfully differ.
Obese children had significantly greater cIMT than normal weight children, and similar results were found in high VAT compared with low VAT tertiles (Figure 2, A and C). No significant differences were seen by BF% tertile (Figure 2, B). Children in the most insulin resistant tertile (low Mlbm) had higher cIMT than those in the high Mlbm tertile (Figure 2, D). No differences in cIEM or PWV were found for the adiposity or Mlbm measures.
Analyses by sex showed some differences between females and males. FMD% in females was significantly higher in the high BF% tertile (p=0.04) and high VAT tertile (p=0.002), cIMT was significantly greater in the highest VAT tertile (p=0.02), but no differences were found by tertiles of Mlbm (p=0.35). FMD% in males was significantly higher only in the highest VAT tertile (p=0.046), there were no differences by tertiles of BF% (p=0.29) or Mlbm (p=0.38), and there were no differences in cIMT in relation to BF%, VAT or BMI status.
The associations between FMD, cIMT, cIEM, and PWV and BF%, VAT, insulin resistance, and fasting insulin were also assessed in a continuous fashion using multiple linear regression analysis. Insulin resistance was not a predictor of FMD% or FMD AUC. BF% was positively associated with FMD% (β (SE) = 0.07 ± 0.02, p=0.001, R2 = 0.197) and FMD AUC (β (SE) = 8.3 ± 2.7, p=0.002, R2 = 0.155). Fasting insulin was positively associated with FMD% (β (SE) = 0.08 ± 0.03, p=0.016, R2 = 0.218), independent of BF%. VAT was positively associated with FMD% (β (SE) = 0.09 ± 0.02, p<0.001, R2= 0.226), FMD AUC (β (SE) = 12.1 ± 2.3, p<0.001, R2=0.224) and cIMT (β (SE) = 0.01 ± 0.01, p=0.002, R2= 0.083). There were no significant associations between cIMT, cIEM, or PWV with insulin resistance or fasting insulin. BF% was positively associated with cIMT (β (SE) = 0.01 ± 0.01, p=0.028, R2= 0.048), but no significant associations were observed between cIEM or PWV and BF%.
When BMI and BMI-percentile replaced BF%, in the ANCOVA and linear regression model(s) the results did not differ. To determine if differences were present between pubertal and post-pubertal children, we compared Tanner stage 2–4 (n=136) versus Tanner stage 5 (n=116). No statistically significant differences were observed between the 2 pubertal groups for any of the outcomes.
Discussion
The findings of this cross-sectional study of children indicate that: (1) obesity, regardless of method used to define it is associated with higher FMD; (2) obesity (BMI) and higher VAT are associated with higher cIMT; (3) insulin resistance measured by hyperinsulinemic euglycemic clamp, within the most insulin resistant tertile group, was associated with higher cIMT, but not with lower FMD or arterial stiffness; (4) fasting insulin was associated with higher FMD independent of BF%; and (5) sex-specific differences were present, however, the current sample size is insufficient to draw firm conclusions about the role of sex.
We acknowledge that these findings for FMD do not agree with a body of literature suggesting that obesity in childhood is associated with reduced FMD 6, 7, 9, 11, 29, or with some studies suggesting no relationship between obesity and FMD.13, 14 Differences between the present and previous studies should be noted. Our study has a larger sample size, wider age range, and accounts for potential confounding variables (i.e. baseline artery diameter). The lack of controlling for baseline artery diameter highlights a potential serious flaw in other previous studies which found a relationship between lower FMD and obesity without this adjustment. This issue has been raised extensively in other publications as FMD is often partly a function of baseline artery diameter.15, 27, 28, 30
Although it is unclear why these findings differ, our data are in agreement with a prior study, showing obesity to be associated with higher FMD: in a large group (n>6000) of pre-pubertal children, it was shown that obese children had larger brachial artery diameters, increased blood flow, and marginally increased FMD compared with normal-weight and overweight children and also showed a significant, positive association of DXA trunk fat with FMD.15 These findings were replicated in our data (DXA trunk fat vs FMD data not shown), and further expanded upon through our more sensitive and specific VAT data and in a cohort that included pubertal and post-pubertal children.
Our insulin resistance findings are incongruent with previous studies using the homeostasis model assessment index of insulin resistance (HOMA-IR).29 A recent study in 150 children (10.4 ± 3.1yr; BMI-percentile = 83.0 ± 23.3%), showed greater HOMA-IR, BMI, and waist circumference to be associated with lower FMD. However, this study did not account for the important contribution of pubertal maturation in their models and the potential confounding contribution of baseline artery diameter, which has been shown to be larger in obese children.11, 15, 31 Moreover, our measurement of insulin resistance with hyperinsulinemic euglycemic clamp offers a more robust estimation of peripheral insulin sensitivity than HOMA-IR which more relates to basal insulin levels.32 A number of in vivo and in vitro studies support a role for insulin in the regulation of vascular tone.5, 33, 34 In association with insulin sensitivity, both fluid shear stress and insulin preferentially stimulate a signaling pathway that induces downstream nitric oxide (NO),5, 34–36. However, in the presence of insulin resistance the signaling pathway induces downstream production of endothelin-1 (ET-1), a potent vasoconstrictor.5, 37 Therefore, it seems reasonable to suggest that the apparent paradoxical relation between adiposity and endothelial function results from still insulin sensitive endothelial cells not yet altered by obesity related insulin resistance.
In contrast to the apparent beneficial effect of obesity on vascular function, measures of adiposity in this study (BMI and VAT) were associated with greater cIMT, but there were no significant relations between any measure of adiposity and arterial stiffness (cIEM or PWV). The association of higher levels of adiposity and greater cIMT thickening has been shown previously.6, 12, 13, 24
Sex-specific differences in vascular measures may be important in understanding the discrepancies in the literature. In this study females had higher FMD, but smaller baseline artery diameter than males. When adjustments were made for baseline artery diameter no sex differences in FMD were present. The majority of the current pediatric reports showing FMD to be lower in obese youth failed to adjust for differences in baseline artery diameter,7, 9–11 and those that did account for baseline artery diameter13, 15 found no relationship or (similar to the present study) found enhanced FMD with obesity in children.
The current study has a number of strengths. It evaluated a relatively large cohort of children (n=252) with balanced of sex distribution, used gold-standard measurements of body composition, fat depots, insulin resistance, non-invasive assessment of FMD, cIMT, and arterial stiffness (cIEM and PWV), and controlled for confounding variable of baseline artery diameter which issparsely accounted for in previous studies. Limitations of the study include the cross-sectional design, which does not allow inferences about causality. The limited sample size, did not allow a clear interpretation of the differences between males and females regarding vascular measures based upon tertiles adiposity and insulin resistance. Despite our best efforts to standardize the FMD procedure, we were not able to account for a number of other factors which may influence FMD (i.e. physical activity and fitness). We are unable to determine the role body size may play in carotid artery remodeling which may affect cIMT levels in obese youth. There are no validated pediatric thresholds for insulin resistance measured by the hyperinsulinemic euglycemic clamp; therefore, we cannot assign clinical significance to our arbitrary tertile cut-points of insulin resistance.
The findings of the present study suggest that FMD, is higher in the context of excess adiposity. Although insulin resistance was not associated with FMD, there was a significant positive relation with fasting insulin, independent of adiposity, suggesting this may explain, in part, the association with adiposity. Although this study does not provide evidence for causation, obesity, VAT, and insulin resistance were associated with thickening in the carotid vascular wall (cIMT), but no differences in arterial stiffness were observed for any adiposity or insulin resistance measure. These data suggest that obesity, insulin, and/or insulin resistance may play a pivotal role in regulating early adaptations within the vascular milieu.
Acknowledgments
Funded by the National Institutes of Health(NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (IDDK) (R01-DK072124-01A3 [to J.S.] and T32-DK083250 [to J.R.]), National Cancer Institute/NIDDK (R01CA113930-01A1 [to J.S.]), General Clinical Research Center Program (M01- RR00400), National Center for Research Resources (1UL1-RR033183), the Clinical and Translational Science Institute at the University of Minnesota-Twin Cities (UL1TR000114), and NIH/National Heart, Lung, and Blood Institute (F32-HL127851-01 [to J.R]). A.K. has served on pediatric obesity advisory boards for Takeda and Novo Nordisk (unpaid).
We would like to thank all of the children who participated in this study.
Footnotes
The other authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Perticone F, Ceravolo R, Candigliota M, Ventura G, Iacopino S, Sinopoli F, et al. Obesity and body fat distribution induce endothelial dysfunction by oxidative stress: protective effect of vitamin C. Diabetes. 2001;50:159–65. doi: 10.2337/diabetes.50.1.159. [DOI] [PubMed] [Google Scholar]
- 2.Parikh NI, Keyes MJ, Larson MG, Pou KM, Hamburg NM, Vita JA, et al. Visceral and subcutaneous adiposity and brachial artery vasodilator function. Obesity (Silver Spring) 2009;17:2054–9. doi: 10.1038/oby.2009.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brook RD, Bard RL, Rubenfire M, Ridker PM, Rajagopalan S. Usefulness of visceral obesity (waist/hip ratio) in predicting vascular endothelial function in healthy overweight adults. Am J Cardiol. 2001;88:1264–9. doi: 10.1016/s0002-9149(01)02088-4. [DOI] [PubMed] [Google Scholar]
- 4.Deanfield JEHJ, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115:1285–95. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 5.Muniyappa R, Montagnani M, Koh KK, Quon MJ. Cardiovascular Actions of Insulin. Endocrine Reviews. 2007;28:463–91. doi: 10.1210/er.2007-0006. [DOI] [PubMed] [Google Scholar]
- 6.Woo KS, Chook P, Yu CW, Sung RYT, Qiao M, Leung SSF, et al. Overweight in children is associated with arterial endothelial dysfunction and intima-media thickening. Int J Obes Relat Metab Disord. 2004;28:852–7. doi: 10.1038/sj.ijo.0802539. [DOI] [PubMed] [Google Scholar]
- 7.Aggoun Y, Farpour-Lambert NJ, Marchand LM, Golay E, Maggio AB, Beghetti M. Impaired endothelial and smooth muscle functions and arterial stiffness appear before puberty in obese children and are associated with elevated ambulatory blood pressure. Eur Heart J. 2008;29:792–9. doi: 10.1093/eurheartj/ehm633. [DOI] [PubMed] [Google Scholar]
- 8.Tounian P, Aggoun Y, Dubern B, Varille V, Guy-Grand B, Sidi D, et al. Presence of increased stiffness of the common carotid artery and endothelial dysfunction in severely obese children: a prospective study. Lancet. 2001;358:1400–4. doi: 10.1016/S0140-6736(01)06525-4. [DOI] [PubMed] [Google Scholar]
- 9.Farpour-Lambert NJ, Aggoun Y, Marchand LM, Martin XE, Herrmann FR, Beghetti M. Physical Activity Reduces Systemic Blood Pressure and Improves Early Markers of Atherosclerosis in Pre-Pubertal Obese Children. Journal of the American College of Cardiology. 2009;54:2396–406. doi: 10.1016/j.jacc.2009.08.030. [DOI] [PubMed] [Google Scholar]
- 10.Kapiotis S, Holzer G, Schaller G, Haumer M, Widhalm H, Weghuber D, et al. A Proinflammatory State Is Detectable in Obese Children and Is Accompanied by Functional and Morphological Vascular Changes. Arteriosclerosis, Thrombosis, and Vascular Biology. 2006;26:2541–6. doi: 10.1161/01.ATV.0000245795.08139.70. [DOI] [PubMed] [Google Scholar]
- 11.Pena A, Wiltshire E, MacKenzie K, Gent R, Piotto L, Hirte C, et al. Vascular Endothelial and Smooth Muscle Function Relates to Body Mass Index and Glucose in Obese and Nonobese Children. J Clin Endocrinol Metab. 2006;91:4467–71. doi: 10.1210/jc.2006-0863. [DOI] [PubMed] [Google Scholar]
- 12.Urbina EMKT, McCoy CE, Khoury P, Daniels S, Dolan L. Youth With Obesity and Obesity-Related Type 2 Diabetes Mellitus Demonstrate Abnormalities in Carotid Structure and Function. Circulation. 2009;119:2913–9. doi: 10.1161/CIRCULATIONAHA.108.830380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naylor LH, Green DJ, Jones TW, Kalic RJ, Suriano KL, Shah M, et al. Endothelial Function and Carotid Intima-Medial Thickness in Adolescents with Type 2 Diabetes Mellitus. The Journal of pediatrics. 2011;159:971–4. doi: 10.1016/j.jpeds.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 14.Tryggestad JB, Thompson DM, Copeland KC, Short KR. Obese children have higher arterial elasticity without a difference in endothelial function: the role of body composition. Obesity (Silver Spring) 2012;20:165–71. doi: 10.1038/oby.2011.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charakida M, Jones A, Falaschetti E, Khan T, Finer N, Sattar N, et al. Childhood obesity and vascular phenotypes: a population study. J Am Coll Cardiol. 2012;60:2643–50. doi: 10.1016/j.jacc.2012.08.1017. [DOI] [PubMed] [Google Scholar]
- 16.Sinaiko AR, Caprio S. Insulin resistance. J Pediatr. 2012;161:11–5. doi: 10.1016/j.jpeds.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tchernof A, Despres JP. Pathophysiology of human visceral obesity: an update. Physiological reviews. 2013;93:359–404. doi: 10.1152/physrev.00033.2011. [DOI] [PubMed] [Google Scholar]
- 18.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital and health statistics Series 11, Data from the national health survey. 2002:1–190. [PubMed] [Google Scholar]
- 19.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marshall WA, Tanner JM. Variations in the Pattern of Pubertal Changes in Boys. Archives of Disease in Childhood. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinaiko AR, Jacobs DR, Jr, Steinberger J, Moran A, Luepker R, Rocchini AP, et al. Insulin resistance syndrome in childhood: associations of the euglycemic insulin clamp and fasting insulin with fatness and other risk factors. J Pediatr. 2001;139:700–7. doi: 10.1067/mpd.2001.118535. [DOI] [PubMed] [Google Scholar]
- 22.Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, et al. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol. 2011;300:H2–12. doi: 10.1152/ajpheart.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelly AS, Kaiser DR, Dengel DR, Bank AJ. Comparison of B-mode and echo tracking methods of assessing flow-mediated dilation. Ultrasound in medicine & biology. 2004;30:1447–9. doi: 10.1016/j.ultrasmedbio.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 24.Urbina EM, Williams RV, Alpert BS, Collins RT, Daniels SR, Hayman L, et al. Noninvasive Assessment of Subclinical Atherosclerosis in Children and Adolescents: Recommendations for Standard Assessment for Clinical Research: A Scientific Statement From the American Heart Association. Hypertension. 2009;54:919–50. doi: 10.1161/HYPERTENSIONAHA.109.192639. [DOI] [PubMed] [Google Scholar]
- 25.Dengel DR, Jacobs DR, Steinberger J, Moran AM, Sinaiko AR. Gender differences in vascular function and insulin sensitivity in young adults. Clinical science. 2011;120:153–60. doi: 10.1042/CS20100223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marlatt KL, Kelly AS, Steinberger J, Dengel DR. The influence of gender on carotid artery compliance and distensibility in children and adults. Journal of clinical ultrasound: JCU. 2013;41:340–6. doi: 10.1002/jcu.22015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atkinson G, Batterham AM. Allometric scaling of diameter change in the original flow-mediated dilation protocol. Atherosclerosis. 2013;226:425–7. doi: 10.1016/j.atherosclerosis.2012.11.027. [DOI] [PubMed] [Google Scholar]
- 28.Atkinson G, Batterham AM, Thijssen DH, Green DJ. A new approach to improve the specificity of flow-mediated dilation for indicating endothelial function in cardiovascular research. J Hypertens. 2013;31:287–91. doi: 10.1097/HJH.0b013e32835b8164. [DOI] [PubMed] [Google Scholar]
- 29.Miniello VL, Faienza MF, Scicchitano P, Cortese F, Gesualdo M, Zito A, et al. Insulin resistance and endothelial function in children and adolescents. Int J Cardiol. 2014;174:343–7. doi: 10.1016/j.ijcard.2014.04.115. [DOI] [PubMed] [Google Scholar]
- 30.Leeson CP, Whincup PH, Cook DG, Mullen MJ, Donald AE, Seymour CA, et al. Cholesterol and arterial distensibility in the first decade of life: a population-based study. Circulation. 2000;101:1533–8. doi: 10.1161/01.cir.101.13.1533. [DOI] [PubMed] [Google Scholar]
- 31.Charakida M, Masi S, Lüscher TF, Kastelein JJP, Deanfield JE. Assessment of atherosclerosis: the role of flow-mediated dilatation. 2010 doi: 10.1093/eurheartj/ehq340. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz B, Jacobs DR, Moran A, Steinberger J, Hong C-P, Sinaiko AR. Measurement of Insulin Sensitivity in Children: Comparison between the euglycemic-hyperinsulinemic clamp and surrogate measures. Diabetes Care. 2008;31:783–8. doi: 10.2337/dc07-1376. [DOI] [PubMed] [Google Scholar]
- 33.Osanai T, Fujita N, Fujiwara N, Nakano T, Takahashi K, Guan W, et al. Cross talk of shear-induced production of prostacyclin and nitric oxide in endothelial cells. Am J Physiol Heart Circ Physiol. 2000;278:H233–8. doi: 10.1152/ajpheart.2000.278.1.H233. [DOI] [PubMed] [Google Scholar]
- 34.Ando J, Yamamoto K. Vascular mechanobiology: endothelial cell responses to fluid shear stress. Circulation journal: official journal of the Japanese Circulation Society. 2009;73:1983–92. doi: 10.1253/circj.cj-09-0583. [DOI] [PubMed] [Google Scholar]
- 35.Vanhoutte PM, Shimokawa H, Tang EH, Feletou M. Endothelial dysfunction and vascular disease. Acta physiologica. 2009;196:193–222. doi: 10.1111/j.1748-1716.2009.01964.x. [DOI] [PubMed] [Google Scholar]
- 36.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–5. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 37.Mather KJ, Steinberg HO, Baron AD. Insulin resistance in the vasculature. The Journal of clinical investigation. 2013;123:1003–4. doi: 10.1172/JCI67166. [DOI] [PMC free article] [PubMed] [Google Scholar]