Abstract
Background
Conventional benzodiazepines bind non-selectively to GABAA receptors containing α1, α2, α3, and α5 subunits (α1GABAA, α2GABAA, α3GABAA, and α5GABAA receptors, respectively), and the role of these different GABAA receptor subtypes in the reinforcing effects of benzodiazepines has not been characterized fully. We used a pharmacological antagonist approach with available subtype-selective ligands to evaluate the role of GABAA receptor subtypes in the reinforcing effects of the non-selective conventional benzodiazepine, triazolam.
Methods
Rhesus monkeys (n = 4) were trained under a progressive-ratio schedule of intravenous midazolam delivery and dose–response functions were determined for triazolam, in the absence and presence of flumazenil (non-selective antagonist), βCCT and 3-PBC (α1GABAA-preferring antagonists), and XLi-093 (α5GABAA-selective antagonist).
Results
Flumazenil, βCCT and 3-PBC shifted the dose–response functions for triazolam to the right in a surmountable fashion, whereas XLi-093 was ineffective. Schild analyses revealed rank orders of potencies of flumazenil = βCCT > 3-PBC. Comparison of potencies between self-administration and previous binding studies with human cloned GABAA receptor subtypes suggested that the potencies for βCCT and 3-PBC were most consistent with binding at α2GABAA and α3GABAA receptors, but not α1GABAA or α5GABAA receptor subtypes.
Conclusions
Our findings were not entirely consistent with blockade of α1GABAA receptors and are consistent with the possibility of α2GABAA and/or α3GABAA subtype involvement in antagonism of the reinforcing effects of triazolam. The α5GABAA receptor subtype likely does not play a substantial role in self-administration under these conditions.
Keywords: GABA, Benzodiazepine, Antagonist, Self-administration, Progressive-ratio, Rhesus monkey
1. Introduction
Benzodiazepines bind to an allosteric site on γ-aminobutyric acid type A (GABAA) receptors, producing a conformational change in the receptor leading to an enhancement in the ability of GABA to increase chloride conductance. It is through this receptor mechanism that benzodiazepines produce behavioral effects that can be beneficial therapeutically (e.g., anxiolysis). These same receptors also mediate other characteristic effects that limit the use of benzodiazepines, such as daytime drowsiness, impairment of motor coordination, and deficits in memory (for review, see Rudolph and Knoflach, 2011). In addition and perhaps of most concern is that benzodiazepines have reinforcing properties that may contribute to their having abuse liability (Griffiths and Weerts, 1997; Licata and Rowlett, 2008).
Previous molecular biological studies have revealed the existence of multiple subtypes of the GABAA receptor (McKernan and Whiting, 1996; Olsen and Sieghart, 2008; Pritchett et al., 1989; Rudolph et al., 2001). Subsequent reports have postulated that the diverse behavioral effects of benzodiazepine-type drugs may reflect actions at different subtypes of GABAA receptors (e.g., Knabl et al., 2008; Löw et al., 2000; McKernan et al., 2000; Rowlett et al., 2005; Rudolph et al., 1999; Tan et al., 2010). These observations suggest the possibility for a pharmacological dissociation between the clinically advantageous effects and unwanted side-effects of these compounds.
Most benzodiazepine ligands bind to GABAA receptors containing α1, α2, α3, and α5 subunits, but not α4 and α6 subunits (Rudolph and Knoflach, 2011). GABAA receptors containing α1 subunits (α1GABAA receptors) are located ubiquitously throughout the CNS, and have been implicated in the sedative effects of benzodiazepines as well as in effects related to physical dependence and abuse (Engin et al., 2014; Fischer et al., 2013; Mirza and Nielsen, 2006; Rudolph et al., 1999; Tan et al., 2010). In contrast, GABAA receptors containing α2 and α3 subunits (α2GABAA and α3GABAA receptors, respectively) are anatomically distributed in the cortex, limbic system and spinal cord (Rudolph and Knoflach, 2011) and have been associated with the anxiolytic effects of benzodiazepines (Fischer et al., 2010; Löw et al., 2000; McKernan et al., 2000; Rowlett et al., 2005). Finally, GABAA receptors containing α5 subunits (α5GABAA receptors) are preferentially expressed within the hippocampus and are thought to play a role in certain memory processes impacted by benzodiazepines (Atack, 2011; Collinson et al., 2002; Crestani et al., 2002).
The precise roles of α1GABAA, α2GABAA, α3GABAA and α5GABAA receptors in the reinforcing properties of benzodiazepines are unclear at present. A recent hypothesis suggests that α1GABAA receptors, specifically those expressed in the ventral tegmental area, underpin the reinforcing properties of benzodiazepines (Tan et al., 2011). According to Tan et al. (2011), benzodiazepines are proposed to decrease activity of GABAergic interneurons through activation of α1GABAA receptors, resulting in a disinhibition of dopaminergic signaling and a net increase of dopamine release in the nucleus accumbens. This hypothesis is consistent with the finding that benzodiazepines are not self-administered in mice rendered benzodiazepine-insensitive at α1GABAA receptors (Engin et al., 2014; Tan et al., 2010), and the observation that baboons do not self-administer the α1GABAA receptor-sparing (i.e., low-to-zero intrinsic efficacy at α1GABAA receptors) compound TPA023 up to doses that maximally occupy CNS benzodiazepine binding sites (Ator et al., 2010). However, we have demonstrated that α1GABAA receptor-sparing compounds are reliably self-administered in rhesus monkeys trained with GABAA positive modulators (midazolam, methohexital) but not the monoamine transport blocker cocaine (Rowlett et al., 2005; Shinday et al., 2013). Overall, these findings suggest that α1GABAA receptors are critical for the reinforcing effects of benzodiazepines only under certain conditions (e.g., history of cocaine exposure), but are not necessary for a benzodiazepine to have reinforcing effects when the monkeys are experienced with a GABAA positive modulator. The relevance of this observation to human drug abusers is unclear at present, although considerable literature suggests that a human subject’s prior drug experiences are predictors of benzodiazepine consumption (for review, see Griffiths and Weerts, 1997).
In the present study, a pharmacological-antagonist approach was used to assess further the role of GABAA receptors containing different subunits in the reinforcing effects of benzodiazepines. Rhesus monkeys were trained to self-administer the non-selective benzodiazepine midazolam under a progressive ratio (PR) schedule of reinforcement. For antagonism studies, we chose the short-acting, non-selective triazolobenzodiazepine triazolam, which readily maintains self-administration in monkeys in our hands (e.g., Fischer and Rowlett, 2011). Dose–response determinations of triazolam were obtained and then re-assessed following the administration of a non-selective or a selective benzodiazepine receptor antagonist. At present, GABAA receptor subtype selective antagonists are available that show preferential binding at α1GABAA receptors or α5GABAA receptors. We evaluated the antagonists (see Table 1) βCCT (α1GABAA-preferring, Huang et al., 2000); 3-PBC (α1GABAA-preferring; Harvey et al., 2002); and XLi-093 (α5GABAA-selective; Li et al., 2003). When rightward shifts in the triazolam self-administration dose–effect functions were evident, these results were analyzed using in vivo apparent pA2 analysis (Rowlett et al., 2005; Tallarida, 2000; Woods et al., 1992). This analysis enabled us to quantitatively analyze the potency of the antagonists and to draw conclusions or hypotheses about a role for particular receptor subtypes in the reinforcing effects of benzodiazepines.
Table 1.
Benzodiazepine site antagonists used in the present study.
| Subtype | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ki (nM) | pKi (−log Ki [in M]) | ||||||||
| Ligand | Structure | α1 | α2 | α3 | α5 | α1 | α2 | α3 | α5 |
| Flumazenil | 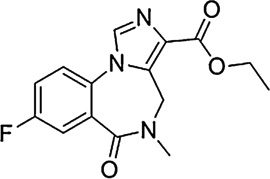 |
0.79 | 0.89 | 1.05 | 0.60 | 9.10 | 9.05 | 8.98 | 9.22 |
| βCCT | 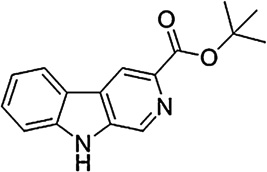 |
0.72 | 15 | 19 | 111 | 9.14 | 7.82 | 7.72 | 6.96 |
| 3-PBC | 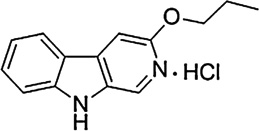 |
5.2 | 52 | 69 | 589 | 8.28 | 7.28 | 7.16 | 6.23 |
| XLi-093 | 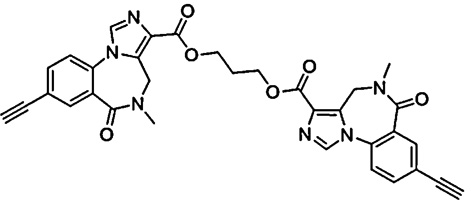 |
>1000 | >1000 | 858 | 15 | – | – | 6.07 | 7.82 |
2. Materials and methods
2.1. Animals
Subjects were 4 male adult rhesus monkeys (Macaca mulatta), individually housed and maintained on a 12-h lights-on/12-h lights-off cycle (lights on at 7:00 AM), with water available continuously. Monkeys received Teklad monkey diet, supplemented with fruits and vegetables, at least 1 h after the end of the daily session, in quantities that allowed them to gain no more than 1 kg during the 100+ days of the study. Initial weights were 8–9 kg, with no significant changes noted over the course of the experiment. Three of the four monkeys had experience self-administering benzodiazepines and/or compounds that bind to benzodiazepine sites; the fourth monkey was experimentally naïve. Animals were maintained in accordance with the guidelines of the Committee on Animals of Harvard Medical School and the Guide for Care and Use of Laboratory Animals (8th edition, 2011). Research protocols were approved by the Harvard Medical School Institutional Animal Care and Use Committee.
Monkeys were prepared with a chronic indwelling venous catheter (polyvinyl chloride, i.d.: 0.64 mm; o.d.: 1.35 mm) according to previously described procedures (Platt et al., 2011). Monkeys were anesthetized initially with 10–20 mg/kg i.m. of ketamine. Throughout surgery, anesthesia was maintained by an isoflurane/oxygen mixture. Under aseptic conditions, a catheter was implanted in the femoral, brachial, or jugular vein and passed to the level of the right atrium. The distal end of the catheter was passed subcutaneously and exited in the mid-scapular region. The external end of the catheter was fed through a fitted jacket and tether system (Lomir Biomedical, Toronto, Canada) and attached to a fluid swivel mounted to the animal’s cage. The catheters were flushed daily with heparinized saline (150–200 U/ml).
2.2. Self-administration
Daily drug self-administration sessions occurred in each monkey’s home cage. Monkeys were trained to self-administer the benzodiazepine midazolam (0.03 mg/kg/infusion) under a PR schedule of i.v. drug injection (Shinday et al., 2013). At the beginning of each session, a set of two white stimulus lights above a response lever was illuminated (Med Associates, St Albans, VT). Upon completion of a response requirement, the white lights were extinguished and a set of two red stimulus lights were illuminated for 1-s, coinciding with a 1-s infusion. Each trial ended with either an injection or the expiration of a 30-min limited hold. Trials were separated by a 30-min timeout period, during which all lights were extinguished and responding had no programmed consequences.
Experimental sessions consisted of 5 components made up of 4 trials each. The response requirement remained constant for each of the 4 trials within a component, and doubled during each successive component. The session ended when a monkey self-administered a maximum of 20 injections or when the response requirement was not completed for two consecutive trials. The PR schedule consisted of a sequence of response requirements: 40, 80, 160, 320, and 640 responses per injection. Once performance was stable under these conditions (no increasing or decreasing trend in the number of injections per session for three consecutive sessions), midazolam or saline was made available on alternating days.
Once self-administration was again stable (low levels of responding during saline availability and stable self-administration during drug availability), test sessions (T) were added to the alternating sequence of midazolam (M) and saline (S) sessions according to the following sequence: MTSMTSTMST, etc. During test sessions, a dose of triazolam was made available either alone or following a 5-min pretreatment with an i.v. dose of benzodiazepine receptor antagonist. After an initial determination of the triazolam dose–effect function, the antagonists were evaluated in the following order: flumazenil, βCCT, 3-PBC, XLi-093. Doses of antagonist were evaluated in a balanced order, except that an antagonist was finished first prior to moving to the second antagonist. After completing tests with flumazenil and βCCT, the dose–effect function for triazolam was re-determined to ensure that no changes in triazolam’s potency had occurred.
2.3. Drugs
Triazolam and flumazenil were purchased from Sigma-Aldrich (St. Louis, MO, USA) and dissolved in 50% propylene glycol, 50% sterile water. βCCT (β-carboline-3-carboxylate-tert-butyl ester; Huang et al., 2000; June et al., 2003), 3-PBC (3-propoxy-β-carboline hydrochloride; Harvey et al., 2002) and XLi-093 (1,3-bis(8-ethynyl-5,6-dihydro-5-methyl-6-oxo-4H-imidazo-[1,5a][1,4]benzodiazepine-3-carboxy)propyl diester; Li et al., 2003) were synthesized at the Department of Chemistry and Biochemistry at the University of Wisconsin–Milwaukee. βCCT, 3-PBC, and XLi-093 were dissolved in 20% ethanol, 60% propylene glycol, and 20% sterile water. All doses of triazolam and antagonists were chosen based on previous work in our laboratory using rhesus monkeys and the i.v. route of administration.
2.4. Data analysis
During day-to-day sessions and testing, the primary dependent measure was the number of injections self-administered per session. Differences from vehicle or maximum number of injections/session maintained by triazolam were determined by Bonferroni t-tests (alpha level constrained to p ≤ 0.05). In order to obtain potency estimates, the self-administration data were analyzed as percent of maximum for individual subjects with maxima being the highest number of injections/session obtained for an individual monkey with triazolam alone. Potency values (dose engendering a 50% maximum effect; ED50) were calculated in individual monkeys by log-linear regression when at least three data points were available on the linear portion of the dose–effect curve or by interpolation when only two data points (one above and one below 50%) were available. These values were obtained by converting the maximum number of injections per session of triazolam alone to 100% for individual monkeys. For each monkey, dose ratios were calculated as the ED50 of triazolam in the presence of some dose of antagonist divided by the ED50 of triazolam alone.
Dose ratios also were used to calculate in vivo apparent pA2 values and to construct Schild plots for flumazenil, 3-PBC, and βCCT antagonism of the reinforcing effects of triazolam. In vivo apparent pA2 values were defined as the negative logarithm of the molar dose of antagonist required to produce a 2-fold rightward shift in the triazolam dose–effect function, and these values provide an in vivo estimate of the affinity of the antagonist for the receptor that mediates the effects of triazolam (Rowlett and Woolverton, 1996; Tallarida, 2000; Woods et al., 1992). Schild analysis was conducted by plotting the logarithm of the dose ratio minus one (log DR − 1) as a function of the dose of the negative logarithm of the molar dose of antagonist. Here, the slope of the Schild plot was statistically compared to −1 as an evaluation of the assumption of unity (Tallarida, 2000) and to zero as an evaluation of a significant relationship between log (DR − 1) and dose of antagonist, in both cases by comparing 95% confidence limits (CIs). If slopes were equal to −1.0 but different from zero, the regression analysis was repeated with the slope of the regression line set at −1.0 (constrained method). In theory, this latter approach should improve estimation of pA2 values, based on the assumption that unity was achieved and slight deviations from −1.0 were due to random sampling error.
For all three antagonists, the in vitro potency at each GABAA receptor subtype was available from experiments with human cloned receptors in HEK cells (Harvey et al., 2002; Huang et al., 2000). We compared the potencies of antagonism in self-administration to the potencies based on binding affinities obtained in the cloned human GABAA receptor subtypes, in order to determine if in vivo apparent pA2 values could accurately predict relative potencies among compounds and binding sites. The binding affinities for all antagonists in cloned receptors were converted to pKi values. Apparent pA2 (constrained) and pKi values were compared using linear regression analysis, with the prediction being that the slope for α1GABAA receptor subtypes would be closest to 1.0 relative to the other receptor subtypes.
3. Results
3.1. Triazolam self-administration
Under training conditions, presentation of saline engendered low rates of responding in each monkey (range = 2–4 injections/session), whereas presentation of midazolam resulted in a significantly greater number of injections/session (midazolam range = 13–15 injections/session), consistent with this drug functioning as a positive reinforcer. When substituted for midazolam during test sessions, triazolam alone functioned as a reinforcer, producing dose–dependent increases in self-administration behavior, with break points (i.e., last response requirement completed) of a maximum of 320 responses/injection (data not shown). Doses of 0.001 to 0.01 mg/kg/injection maintained mean number of injections/session above vehicle levels (Bonferroni t-tests, p < 0.05).
3.2. Antagonism of triazolam self-administration: Rightward shifts in dose–response functions
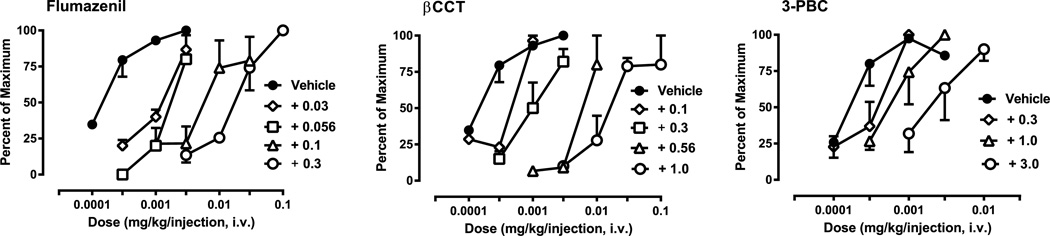
Fig. 1 shows the self-administration of triazolam alone and following pretreatment with flumazenil (left panel), and the α1GABAA receptor-preferring antagonists βCCT (middle panel) and 3-PBC (right panel). These data were converted to percent of the maximum effect engendered by triazolam in order to calculate ED50 values. For the α5GABAA-selective antagonist XLi-093, no antagonism was evident at any of the doses tested (0.3–3.0 mg/kg, i.v., N = 3; data not shown). To summarize the results with XLi-093, we have provided the ED50 values and dose ratios in Table 2. For all antagonists, it is important to note that self-administration sessions had the potential to last approximately 9.5 h, i.e., longer than the antagonist duration of action. However, the contingency in the PR schedule that the sessions end with 2 consecutive limited holds without completing a response requirement limited the session duration. Although non-consecutive trials could occur (i.e., a monkey could skip trials, which in turn would result in self-administration once the antagonist was eliminated), there were no instances during the study in which non-consecutive trials were completed.
Fig. 1.
Blockade of triazolam self-administration by the non-selective benzodiazepine site antagonist flumazenil and the α1GABAA subtype-preferring antagonists βCCT and 3-PBC under a progressive-ratio schedule of i.v. midazolam self-administration. Data are expressed as the average percent of maximum (±SEM), with maxima being the highest number of injections/session obtained for an individual monkey for triazolam alone (N = 4 per antagonist). Doses for each antagonist (administered i.v., 5-min pre-session) are shown in the figure legends. Note that the same triazolam dose–effect function was used for flumazenil and βCCT and was re-determined for 3-PBC.
Table 2.
Potency values (ED50) and dose ratios of triazolam alone and following pretreatment with the α5GABAA-selective antagonist, XLi-093.
| Antagonist dose (mg/kg, i.v.) |
N | ED50 (SEM) | Dose ratio (SEM) |
|---|---|---|---|
| 0 | 3 | 0.00046 (0.00027) | – |
| 0.1 | 3 | 0.00047 (0.00018) | 1.34 (0.27) |
| 0.3 | 3 | 0.00058 (0.00023) | 1.64 (0.48) |
| 1.0 | 3 | 0.00047 (0.00027) | 1.07 (0.31) |
In general, flumazenil administration resulted in blockade of triazolam self-administration that was overcome by increasing the triazolam dose (Fig. 1, left panel). In most cases, increasing the triazolam dose in the presence of flumazenil resulted in a percent maximum obtained that was at or near 100% (i.e., surmountable antagonism); and we obtained 4 rightward shifts in the triazolam dose–response function. Similarly, βCCT administration resulted in rightward shifts in the dose–response function consistent with surmountable antagonism (Fig. 1, middle panel). As with flumazenil and βCCT, 3-PBC administration resulted in surmountable antagonism (Fig. 1, right panel), however, we had only 3 rightward shifts in the triazolam dose–response function for all monkeys due to catheter failure in one animal.
3.3. In vivo apparent pA2 analyses
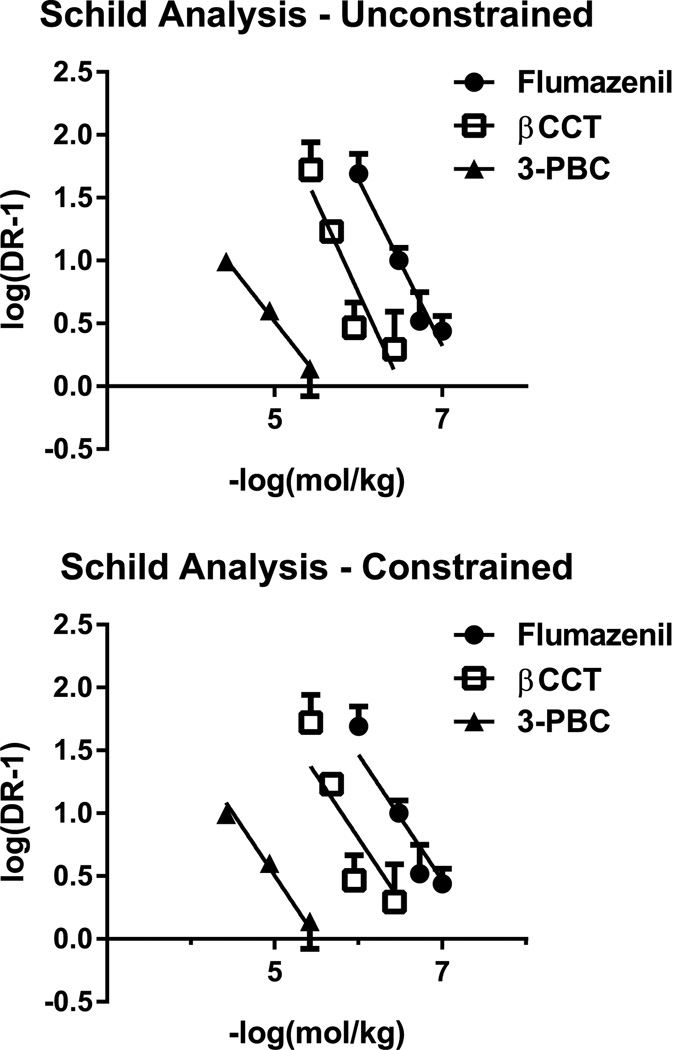
Fig. 2 shows Schild plots, either unconstrained (i.e., all variables free to vary in the linear regression; top panel) or constrained (i.e., slope constrained to −1.0; bottom panel). Table 3 shows the results of Schild analyses using the unconstrained and constrained slope approaches for the 3 antagonists. The unconstrained slope analysis (shown in the left columns of the table) revealed average slopes that ranged from −0.85 to −1.45 and did not differ statistically from unity (−1.0) but did differ significantly from zero, based on 95% CIs. The average in vivo apparent pA2 values showed a rank order of potency of flumazenil = βCCT > 3-PBC (comparison of 95% CIs) based on constrained values.
Fig. 2.
In vivo apparent pA2 analyses of antagonism of the reinforcing effects of triazolam in rhesus monkeys (N = 4) trained under a progressive-ratio schedule of i.v. midazolam injection. Top panel: Schild plots for the 3 antagonists with Schild regressions calculated under conditions in which all parameters were free to vary (i.e., “unconstrained”). Bottom panel: Schild plots in which the parameter of slope of the regression was set at −1.0 (i.e., “constrained”).
Table 3.
In vivo apparent pA2 analyses of antagonism of the reinforcing effects of triazolam.
| Schild analyses, unconstrained slopeA | Schild analyses, constrained slopeB | |||
|---|---|---|---|---|
| Antagonist | pA2 (95% CIs) | Slope (95% CIs) | pA2 (95% CIs) | Slope (95% CIs) |
| Flumazenil | 7.24 (6.91, 8.51) | − 1.32 (−2.16, −0.48) | 7.40 (7.18, 7.75) | − 1.0 |
| βCCT | 6.51 (6.01, 7.43) | − 1.45 (−2.47, −0.40) | 6.80 (6.31, 7.29) | − 1.0 |
| 3-PBC | 5.61 (5.18, 5.70) | − 0.85 (−1.61, −0.09) | 5.55 (5.30, 5.71) | − 1.0 |
Individual data points for the antagonists were averaged for each monkey and Schild regression conducted on the grouped data. For the regression analysis, all parameters were free to vary.
Individual data points for the antagonists were averaged for each monkey and Schild regression conducted on the grouped data. For the regression analysis, the slope values were constrained to −1.0.
3.4. Comparison of in vivo and in vitro potencies
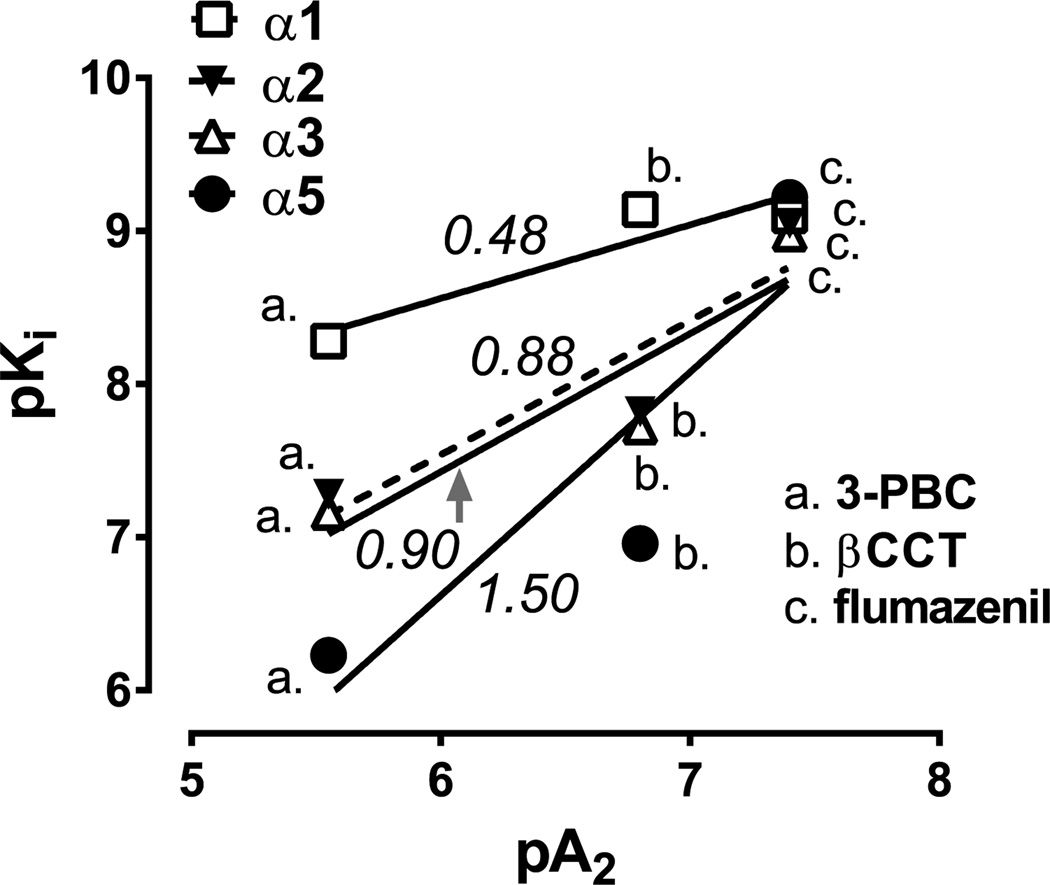
The primary purpose for computing apparent pA2 values was to calculate relative potencies that, in turn, could be used to compare with relative potencies based on binding affinities across GABAA receptor subtypes obtained from cloned human receptors in vitro. As shown in Fig. 3, linear relationships were evident for the antagonists across the four binding sites, with R2 values that were relatively high (0.78–0.87), though not statistically significant (p’s = 0.23–0.31). The lack of statistical significance likely was due to the low sample size (i.e., calculations based on 3 antagonists) and therefore preclude strong conclusions regarding a role for any receptor subtype in antagonism of triazolam self-administration. However, we hypothesized that the slope for α1GABAA receptors would be 1.0, i.e., a change in antagonist binding affinity for α1GABAA sites in vitro predicts the equivalent change in antagonist potency in vivo. In contrast to our hypothesis, the predicted slope of 1.0 was approached more closely for regressions of antagonist potency with α2GABAA and α3GABAA binding affinities (slopes = 0.88 and 0.90, respectively) than for α1GABAA binding affinities (slope = 0.48) or α5GABAA binding affinities (slope = 1.50).
Fig. 3.
Linear regression analyses of pKi values for 3-PBC, βCCT, and flumazenil for GABAA receptors containing α1, α2, α3, and α5 subunits, as a function of in vivo apparent pA2 values obtained from self-administration studies. Numbers in italics represent the slopes of the indicated functions. Small-case letters represent the individual symbols associated with a particular antagonist. Data are from n = 4 monkeys (pA2) or previously published data with cloned human receptor subtypes (pKi; Huang et al., 2000; Harvey et al., 2002).
4. Discussion
Conventional benzodiazepines bind non-selectively to α1GABAA, α2GABAA, α3GABAA, and α5GABAA, receptors, and the role of these different GABAA receptor subtypes in the reinforcing effects of benzodiazepines has not been characterized fully. In the present study, the conventional benzodiazepine triazolam demonstrated reinforcing effectiveness similar to previously-reported findings from our laboratory (e.g., Fischer and Rowlett, 2011), and this effect was antagonized by the non-selective benzodiazepine antagonist flumazenil in a dose-dependent and surmountable fashion. Pretreatments with the α1GABAA receptor-preferring antagonists βCCT and 3-PBC also produced predominantly rightward shifts in the triazolam dose–effect function. In contrast, the α5GABAA receptor antagonist XLi-093 did not alter self-administration of triazolam. Collectively, these data suggest that non-selective and α1GABAA-preferring antagonists, but not an α5GABAA-selective antagonist, can block the reinforcing effects of a benzodiazepine in a manner consistent with competitive antagonism.
Schild analysis was conducted to determine potencies, as well as in vivo estimates of affinity, for the antagonists that blocked the reinforcing effects of triazolam. The slopes for the Schild plots for flumazenil, βCCT, and 3-PBC antagonism of triazolam self-administration were not statistically different from −1. Therefore, apparent pA2 values could be calculated, and these affinity estimates indicated a rank order of potency of flumazenil = βCCT > 3-PBC, based on comparisons of 95% CIs. To our knowledge, this is the first study to determine in vivo affinity estimates for the reinforcing effects of a benzodiazepine following antagonist administration, providing an experimental framework for using this pharmacological approach to exploring mechanisms of action underlying benzodiazepine self-administration.
The observation that the Schild plot slopes for flumazenil, βCCT, and 3-PBC included the value −1 suggests that the reinforcing effects of triazolam were mediated by a single population of pharmacologically similar receptors. For flumazenil, these pharmacologically similar receptors may include the α1GABAA, α2GABAA, α3GABAA and/or α5GABAA receptors, as flumazenil is known to bind non-selectively across these GABAA receptor subtypes (see Table 1). The finding that the Schild plot slope for flumazenil included −1 is in contrast to a previous study which assessed flumazenil antagonism of the discriminative stimulus effects of triazolam and in which the slope of the Schild plot was different from unity (Lelas et al., 2001, 2002). One possible contributor to this departure from unity is that the discriminative stimulus effects of triazolam may involve a receptor population other than benzodiazepine-sensitive GABAA receptors. Taken together, these dual findings raise the possibility that the reinforcing properties and discriminative stimulus properties of triazolam may be mediated by distinct receptor populations.
Schild analysis also revealed that the slopes for βCCT and 3-PBC did not differ from unity, again suggesting that the effects of triazolam were mediated by a single population of receptors. As βCCT and 3-PBC are α1GABAA-preferring antagonists, it would be logical to assume that the reinforcing properties of triazolam in rhesus monkeys may involve the α1GABAA receptor subtype. In support of this conclusion, the rank order of potencies calculated from the in vivo apparent pA2 values were most similar to the rank order of potencies for the antagonists based on in vitro pKi values at the cloned α1GABAA receptor, with flumazenil and βCCT equipotent and 3-PBC significantly less potent. Collectively, these findings are suggestive of a role for α1GABAA receptor in the reinforcing effects of benzodiazepines, although due to the current lack of availability of α2GABAA and α3GABAA receptor-preferring antagonists, we are unable to directly assess α2GABAA and α3GABAA receptor involvement in benzodiazepine reinforcement.
To explore the role of GABAA receptor subtypes and the blockade by βCCT and 3-PBC further, we calculated pKi values from experiments in which the binding of the 3 antagonists to cloned GABAA receptor subtypes was assessed (Huang et al., 2000; Harvey et al., 2002). We then regressed the pKi values with the apparent pA2 values obtained from self-administration. We hypothesized that if the relative potencies were similar across antagonists, then the slope closest to 1.0 would be for α1GABAA binding, consistent with the statistical comparison of rank order of potency at α1GABAA receptors (flumazenil = βCCT > 3-PBC). Interestingly, the opposite was observed. That is, the slopes for α2GABAA and α3GABAA binding approached 1.0, whereas for α1GABAA binding the slope was 0.48 and α5GABAA binding the slope was 1.50. While strong conclusions are precluded because of the underpowered regression analyses (and consequent lack of statistical significance), these findings raise the possibility that the binding sites that 3-PBC, βCCT and flumazenil antagonized were more likely to be the α2-and/or α3GABAA receptor sites than either α1- or α5GABAA sites. This possibility is bolstered by our previous work with subtype-selective agonists, which implicated the α3GABAA, and potentially the α2GABAA, receptor subtype in the reinforcing effects of benzodiazepines (Rowlett et al., 2005; Shinday et al., 2013).
Although our previous studies combined with the present report cast doubt on a sole role for α1GABAA receptor subtypes in the reinforcing effects of benzodiazepines, there are other lines of evidence that do suggest modulation of benzodiazepine reinforcement by this subtype. First, α1GABAA subtype-preferring agonists are self-administered robustly by non-human primates, often to a degree greater than other benzodiazepine-type drugs (e.g., Griffiths et al., 1992; Rowlett et al., 2005; Rowlett and Lelas, 2007). Second, mice with a point mutation that rendered the α1GABAA receptor insensitive to benzodiazepines had a reduced preference for a benzodiazepine in a two-bottle choice procedure, in contrast to wild-type mice (e.g., Engin et al., 2014). Finally, α1GABAA receptors appear to play a key role in the self-administration of benzodiazepines in monkeys with a history of cocaine self-administration (Shinday et al., 2013). Given these observations, the precise role of α1GABAA receptors in the reinforcing effects of benzodiazepines remains unclear at present. We have proposed that this subtype can play a modulatory role on reinforcing effects of benzodiazepines, even though α1GABAA subtypes may not be necessary for self-administration per se.
In contrast to the effects observed with flumazenil, βCCT and 3-PBC, the α5GABAA-selective antagonist XLi-093 did not produce significant shifts in the triazolam dose–effect function. The dose range (0.3–3.0 mg/kg, i.v.) for XLi-093 used in the present study was the same as used previously, in which this ligand dose-dependently reversed triazolam-induced, but not zolpidem-induced, attenuation of performance by rhesus monkeys on a cognitive task (Makaron et al., 2013). Because zolpidem does not bind to α5GABAA receptors, the results of Makaron et al. (2013) provide support for the idea that XLi-093 has selectivity for this receptor subtype over the dose range tested. Therefore, our current findings provide evidence that α5GABAA receptors may play a limited role (if any) in the reinforcing properties of benzodiazepines. Of all the benzodiazepine-sensitive GABAA receptor subtypes, the α5GABAA subtype is one of the more discretely localized anatomically in the brain, as it is expressed primarily in hippocampal regions. The hippocampal formation has been linked extensively with memory processes and likely plays a role in drug taking (e.g., Schwabe et al., 2014); however, our findings suggest that this brain region does not play a critical role in benzodiazepine taking, at least under the conditions of this study.
There are alternate possibilities and/or factors that must be considered when interpreting the findings in our paper. In particular, although preliminary behavioral work in our laboratories suggested that the onset and durations of action among the 3 antagonists are similar, pharmacokinetics of the antagonists may have contributed to the differences in relative potency. In this regard, differences in CNS penetration and/or metabolism among the antagonists could alter in vivo potencies, and this pharmacokinetic information is not available at this time for 3-PBC or βCCT in rhesus monkeys.
The findings from the present study demonstrated competitive antagonism of the reinforcing effects of triazolam under a PR procedure, confirming a role for GABAA receptors in behavior maintained by a conventional benzodiazepine-type drug. However, although βCCT and 3-PBC have selectivity for α1GABAA receptor subtypes, our findings do not provide robust evidence for antagonism via this subtype. Instead, these results point to α2GABAA and/or α3GABAA receptors being critically involved in antagonism of triazolam’s effects, based on relative potencies of the antagonists. Taken together with our previous findings (Rowlett et al., 2005; Shinday et al., 2013), the reinforcing effects of benzodiazepines may involve α3GABAA receptors specifically, since the α3GABAA-prefering agonist TP003 was self-administered, although α2GABAA receptors also have been implicated in a recent study using transgenic mouse technology (Engin et al., 2014). Finally, our results suggest that α5GABAA receptors play little-to-no role in benzodiazepine reinforcement. These hypotheses should provide an important framework for studying the role of different GABAA receptor subtypes in the behavioral effects of benzodiazepine-type drugs, which in turn should help guide development of improved therapeutic agents for treating anxiety-related disorders.
Acknowledgments
This work was supported by USPHS grants DA011792 (JKR), DA033795 (JKR), AA016179 (DMP), RR00168/OD011103 (BDF, DMP, JKR), and MH046851 (SR, OAN, JMC). We thank Kristen Jordan for technical assistance and Dr. Kevin Freeman for comments on the manuscript.
Role of funding source
The research described in this report was supported financially by grants awarded by the National Institutes of Health, U.S. Department of Health and Human Services. The grants provided financial support for the conduct of the research and the preparation of the article. The National Institutes of Health played no direct role in the study design; collection, analysis and interpretation of data; writing of the report; and decision to submit the article for publication.
Footnotes
Author disclosures
Conflict of interest statement
The authors have no conflicts of interest to declare concerning the data presented in this report.
Contributors
| Author | Contribution |
|---|---|
| Bradford D. Fischer | Dr. Fischer prepared the manuscript and incorporated edits from co-authors. Dr. Fischer was directly responsible for conducting the experiments and aided in the design of the studies |
| Donna M. Platt | Dr. Platt edited the manuscript and wrote parts of the Section 4. Dr. Platt contributed to the original conceptualization and design of the studies |
| Sundari K. Rallapalli | Dr. Rallapalli edited the manuscript and helped in the construction of the table with chemical structures. Dr. Rallapalli aided in the conceptualization, design, and synthesis of the novel compounds used in the experiments |
| Ojas A. Namjoshi | Dr. Namjoshi edited the manuscript and took the lead on preparing the table with chemical structures. Dr. Namjoshi aided in the conceptualization, design, and synthesis of the novel compounds used in the experiments |
| James M. Cook | Dr. Cook is the Director of the laboratory that supplied the novel compounds for these studies. Dr. Cook edited the manuscript, and contributed to the original conceptualization and design of the studies |
| James K. Rowlett | Dr. Rowlett is the Director of the laboratory that conducted the self-administration studies. Dr. Rowlett edited the manuscript and helped prepare the figures, and contributed to the original conceptualization and design of the studies |
References
- Atack JR. GABAA receptor subtype-selective modulators. II. α5-selective inverse agonists for cognition enhancement. Curr. Top. Med. Chem. 2011;11:1203–1214. doi: 10.2174/156802611795371314. [DOI] [PubMed] [Google Scholar]
- Ator NA, Atack JR, Hargreaves RJ, Burns HD, Dawson GR. Reducing abuse liability of GABAA/benzodiazepine ligands via selective partial agonist efficacy at alpha1 and alpha2/3 subtypes. J. Pharmacol. Exp. Ther. 2010;332:4–16. doi: 10.1124/jpet.109.158303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinson N, Kuenzi FM, Jarolimek W, Maubach KA, Cothliff R, Sur C, Smith AIFM, Howell O, Atack JR, McKernan RM, Seabrook GR, Dawson GR, Whiting PJ, Rosahl TW. Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the alpha 5 subunit of the GABAA receptor. J. Neurosci. 2002;22:5572–5580. doi: 10.1523/JNEUROSCI.22-13-05572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L, Blüthmann H, Möhler H, Rudolph U. Trace fear conditioning involves hippocampal alpha5 GABA(A) receptors. Proc. Natl. Acad. Sci. U.S.A. 2002;99:8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engin E, Bakhurin KI, Smith KS, Hines RM, Reynolds LM, Tang W, Sprengel R, Moss SJ, Rudolph U. Neural basis of benzodiazepine reward: requirement for α2 containing GABAA receptors in the nucleus accumbens. Neuropsychopharmacology. 2014;39:1805–1815. doi: 10.1038/npp.2014.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer BD, Licata SC, Edwankar RV, Wang ZJ, Huang S, He X, Yu J, Zhou H, Johnson EM, Jr, Cook JM, Furtmüller R, Ramerstorfer J, Sieghart W, Roth BL, Majumder S, Rowlett JK. Anxiolytic-like effects of 8-acetylene imidazobenzodiazepines in a rhesus monkey conflict procedure. Neuropharmacology. 2010;59:612–618. doi: 10.1016/j.neuropharm.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer BD, Rowlett JK. Anticonflict and reinforcing effects of triazolam + pregnanolone combinations in rhesus monkeys. J. Pharmacol. Exp. Ther. 2011;337:805–811. doi: 10.1124/jpet.111.180422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer BD, Teixeira LP, van Linn ML, Namjoshi OA, Cook JM, Rowlett JK. Role of gamma-aminobutyric acid type A (GABAA) receptor subtypes in acute benzodiazepine physical dependence-like effects: evidence from squirrel monkeys responding under a schedule of food presentation. Psychopharmacology. 2013;227:347–354. doi: 10.1007/s00213-013-2975-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Sannerud CA, Ator NA, Brady JV. Zolpidem behavioral pharmacology in baboons: self-injection, discrimination, tolerance and withdrawal. J. Pharmacol. Exp. Ther. 1992;260:1199–1208. [PubMed] [Google Scholar]
- Griffiths RR, Weerts EM. Benzodiazepine self-administration in humans and laboratory anims—implications for problems of long-term use and abuse. Psychopharmacology. 1997;134:1–37. doi: 10.1007/s002130050422. [DOI] [PubMed] [Google Scholar]
- Harvey SC, Foster KL, McKay PF, Carroll MR, Seyoum R, Woods JE, 2nd, Grey C, Jones CM, McCane S, Cummings R, Mason D, Ma C, Cook JM, June HL. The GABA(A) receptor alpha1 subtype in the ventral pallidum regulates alcohol-seeking behaviors. J. Neurosci. 2002;22:3765–3775. doi: 10.1523/JNEUROSCI.22-09-03765.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, He X, Ma C, Liu R, Yu S, Dayer CA, Wenger GR, McKernan R, Cook JM. Pharmacophore/receptor models for GABAA/BzR subtypes (α1β3γ2, α5β3γ2, and α6β3γ2) via a comprehensive ligand mapping approach. J. Med. Chem. 2000;43:71–95. doi: 10.1021/jm990341r. [DOI] [PubMed] [Google Scholar]
- June HL, Foster KL, McKay PF, Seyoum R, Woods JE, Harvey SC, Eiler WJ, Grey C, Carroll MR, McCane S, Jones CM, Yin W, Mason D, Cummings R, Garcia M, Ma C, Sarma PV, Cook JM, Skolnick P. The reinforcing properties of alcohol are mediated by GABA(A1) receptors in the ventral pallidum. Neuropsychopharmacology. 2003;28:2124–2137. doi: 10.1038/sj.npp.1300239. [DOI] [PubMed] [Google Scholar]
- Knabl J, Witschi R, Hösl K, Reinold H, Zeilhofer UB, Ahmadi S, Brockhaus J, Sergejeva M, Hess A, Brune K, Fritschy J-M, Rudolph U, Möhler H, Zeilhofer HU. Reversal of pathological pain through specific spinal GABAA receptor subtypes. Nature. 2008;451:330–334. doi: 10.1038/nature06493. [DOI] [PubMed] [Google Scholar]
- Lelas S, Rowlett JK, Spealman RD. Triazolam discrimination in squirrel monkeys distinguishes high-efficacy agonists from other benzodiazepines and non-benzodiazepines. Psychopharmacology. 2001;154:96–104. doi: 10.1007/s002130000615. [DOI] [PubMed] [Google Scholar]
- Lelas S, Rowlett JK, Spealman RD, Cook JM, Ma C, Li X, Yin W. Role of GABAA/benzodiazepine receptors containing alpha 1 and alpha 5 subunits in the discriminative stimulus effects of triazolam in squirrel monkeys. Psychopharmacology (Berl.) 2002;161:180–188. doi: 10.1007/s00213-002-1037-y. [DOI] [PubMed] [Google Scholar]
- Li X, Cao H, Zhang C, Furtmuller R, Fuchs K, Huck S, Sieghart W, Deschamps J, Cook JM. Synthesis, in vitro affinity, and efficacy of a bis 8-ethynyl-4H-imidazo[1,5a]-[1,4]benzodiazepine analogue, the first bivalent α5 subtype selective BzR/GABAA antagonist. J. Med. Chem. 2003;46:5567–5570. doi: 10.1021/jm034164c. [DOI] [PubMed] [Google Scholar]
- Licata SC, Rowlett JK. Abuse and dependence liability of benzodiazepine-type drugs: GABA(A) receptor modulation and beyond. Pharmacol. Biochem. Behav. 2008;90:74–89. doi: 10.1016/j.pbb.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löw K, Crestani F, Keist R, Benke D, Brunig I, Benson JA, Fritschy JM, Rulicke T, Bluethmann H, Mohler H, Rudolph U. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- Makaron L, Moran CA, Namjoshi O, Rallapalli S, Cook JM, Rowlett JK. Cognition-impairing effects of benzodiazepine-type drugs: role of GABAA receptor subtypes in an executive function task in rhesus monkeys. Pharmacol. Biochem. Behav. 2013;104:62–68. doi: 10.1016/j.pbb.2012.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain? Trends Pharmacol. Sci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, Farrar S, Myers J, Cook G, Ferris P, Garrett L, Bristow L, Marshall G, Macaulay A, Brown N, Howell O, Moore KW, Carling RW, Street LJ, Castro JL, Ragan CI, Dawson GR, Whiting PJ. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABAA receptor α1 subtype. Nat. Neurosci. 2000;3:587–592. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- Mirza NR, Nielsen EØ. Do subtype-selective gamma-aminobutyric acid A receptor modulators have a reduced propensity to induce physical dependence in mice? J. Pharmacol. Exp. Ther. 2006;316:1378–1385. doi: 10.1124/jpet.105.094474. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: classification on the basis of subunit composition, pharmacology, and function. Pharmacol. Rev. 2008;60:243–260. doi: 10.1124/pr.108.00505. (Update). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt DM, Carey G, Spealman RD. Models of neurological disease (substance abuse): self-administration in monkeys. Curr. Protoc. Pharmacol. 2011;Chapter 10(Unit 10.5) doi: 10.1002/0471141755.ph1005s56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchett DB, Lüddens H, Seeburg PH. Type I and type II GABAA-benzodiazepine receptors produced in transfected cells. Science. 1989;245:1389–1392. doi: 10.1126/science.2551039. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Woolverton WL. Assessment of benzodiazepine receptor heterogeneity in vivo: apparent pA2 and pKB analyses from behavioral studies. Psychopharmacology. 1996;128:1–16. doi: 10.1007/s002130050103. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Platt DM, Lelas S, Atack JR, Dawson GR. Different GABAA receptor subtypes mediate the anxiolytic, abuse-related, and motor effects of benzodiazepine-like drugs in primates. Proc. Natl. Acad. Sci. U.S.A. 2005;102:915–920. doi: 10.1073/pnas.0405621102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlett JK, Lelas S. Comparison of zolpidem and midazolam self-administration under progressive-ratio schedules: consumer demand and labor supply analyses. Exp. Clin. Psychopharmacol. 2007;15:328–337. doi: 10.1037/1064-1297.15.4.328. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Benke D, Brünig I, Benson JA, Fritschy JM, Martin JR, Bluethmann H, Möhler H. Benzodiazepine actions mediated by specific gamma-aminobutyric acid(A) receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Möhler H. GABA(A) receptor subtypes: dissecting their pharmacological functions. Trends Pharmacol. Sci. 2001;22:188–194. doi: 10.1016/s0165-6147(00)01646-1. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Knoflach F. Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nat. Rev. Drug Discov. 2011;10:685–697. doi: 10.1038/nrd3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, Nader K, Pruessner JC. Reconsolidation of human memory: brain mechanisms and clinical relevance. Biol. Psychiatry. 2014;76:274–280. doi: 10.1016/j.biopsych.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Shinday NM, Sawyer EK, Fischer BD, Platt DM, Licata SC, Atack JR, Dawson GR, Reynolds DS, Rowlett JK. Reinforcing effects of compounds lacking intrinsic efficacy at α1 subunit-containing GABAA receptor subtypes in midazolam- but not cocaine-experienced rhesus monkeys. Neuropsychopharmacology. 2013;38:1006–1014. doi: 10.1038/npp.2012.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan KR, Brown M, Labouèbe G, Yvon C, Creton C, Fritschy JM, Rudolph U, Lüscher C. Neural bases for addictive properties of benzodiazepines. Nature. 2010;463:769–774. doi: 10.1038/nature08758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan KR, Rudolph U, Luscher C. Hooked on benzodiazepines: GABAA receptor subtypes and addiction. Trends Neurosci. 2011;4:188–197. doi: 10.1016/j.tins.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallarida RJ. Drug Synergism and Dose–Effect Data Analysis. Boca Raton, FL: Chapman & Hall/CRC Press; 2000. [Google Scholar]
- Woods JH, Winger G, France CP. Use of in vivo apparent pA2 analysis in assessment of opioid abuse liability. TiPS. 1992;13:282–286. doi: 10.1016/0165-6147(92)90086-l. [DOI] [PubMed] [Google Scholar]