Abstract
A major challenge to the study and treatment of neurogenetic syndromes is accessing live neurons for study from affected individuals. Although several sources of stem cells are currently available, acquiring these involve invasive procedures, may be difficult or expensive to generate and are limited in number. Dental pulp stem cells (DPSC) are multipotent stem cells that reside deep the pulp of shed teeth. To investigate the characteristics of DPSC that make them a valuable resource for translational research, we performed a set of viability, senescence, immortalization and gene expression studies on control DPSC and derived neurons. We investigated the basic transport conditions and maximum passage number for primary DPSC. We immortalized control DPSC using human telomerase reverse transcriptase (hTERT) and evaluated neuronal differentiation potential and global gene expression changes by RNA-seq. We show that neurons from immortalized DPSC share morphological and electrophysiological properties with non-immortalized DPSC. We also show that differentiation of DPSC into neurons significantly alters gene expression for 1305 transcripts. Here we show that these changes in gene expression are concurrent with changes in protein levels of the transcriptional repressor REST/NSRF, which is known to be involved in neuronal differentiation. Immortalization significantly altered the expression of 183 genes after neuronal differentiation, 94 of which also changed during differentiation. Our studies indicate that viable DPSC can be obtained from teeth stored for ≥72hrs, these can then be immortalized and still produce functional neurons for in vitro studies, but that constitutive hTERT immortalization is not be the best approach for long term use of patient derived DPSC for the study of disease.
Keywords: dental pulp stem cells, shed teeth, immortalization, RNA-seq, senescence
INTRODUCTION
In order to understand the molecular and physiological changes in neurons of the brains of individuals with disorders ranging from intellectual disability to autism, it is essential to study live neurons that accurately represent the conditions in the brain in the disease state. A significant challenge to the study of both rare and common neurogenetic syndromes is the inability to access live neurons for study in the laboratory setting. One recent approach to overcome this problem has been the collection of skin biopsies from individuals with neurogenetic disease in order to create fibroblast cell lines, which are then induced into pluripotency using viral constructs to become pluripotent stem cells (iPSCs), before finally being differentiated down neuronal lineages in culture [1]. This approach is limited by the fact that iPSC can be difficult to generate, are often restricted in their downstream differentiation potential and can take a long time to differentiate into representative neurons [2]. One solution to these problems is to obtain biospecimens of multipotent neuronal precursor cells, which are already destined to become neurons and therefore do not need reprogramming. Mammalian dental pulp is a neural crest-derived tissue and has been shown to contain a potent population of stem cells with neurogenic potential both in vitro and in vivo [3, 4]. These stem cells can respond to local microenvironment cues in the mammalian brain to become a variety of central nervous system cell types [5] and have been shown to respond to neuronal differentiation signals in culture [6]. Normally exfoliated or extracted primary teeth (baby teeth) are a good source for dental pulp stem cells (DPSC), and are easy to collect even from remote locations. This is an important practical consideration for the study of rare disorders, where the logistics required to create a single collection of cell lines from a variety of remote locations can be prohibitive, and more so when generating enough samples to overcome the normal genetic heterogeneity and gene expression variation found in human populations.
The process of generating neurons from tooth pulp neural precursors is now well tested in control samples [5, 7-9], but it has never been used before to establish a large repository of samples for the study of neurogenetic syndromes. Currently, there are no standardized protocols for long-term storage and transportation of extracted teeth for the production of DPSC. The evaluation of factors such as storage conditions, transport time and cellular senescence of primary DPSC is crucial to the development of DPSC as a resource for the broader study of neurogenetic disease. In this study, DPSC have been grown under various conditions and immortalized with a human telomerase reverse transcriptase (hTERT) retrovirus soon after processing. Here, we assess how storage conditions, processing time, and the timing of immortalization affect the success of the immortalization process. In addition, immortalized DPSC were evaluated for their ability to efficiently form DPSC neurons that electrophysiologically and morphologically resemble non-immortalized DPSC neurons. Finally, using whole genome RNA-seq analysis, we established that the immortalized DPSC and DPSC neurons are molecularly similar to non-immortalized primary DPSC at the gene expression level. These studies indicate that teeth can be obtained from distant locations as much as 72hr away and still produce viable DPSC which, when immortalized using hTERT, are similar in many ways to non-immortalized DPSC. These are the first steps in the development of a protocol to successfully obtain DPSC from any individual with any neurogenetic syndrome anywhere in the world with the eventual goal of creating a repository of immortalized DPSC lines that can be used to study nervous system disorders at the molecular and physiologic level.
MATERIALS AND METHODS
Generation of DPSC lines
Teeth were obtained through the Department of Pediatric Dentistry at the University of Tennessee Health Science Center (UTHSC). The UTHSC Institutional Review Board approved this study and informed consent was obtained from the parent or legal guardian of all participants. Participants did not receive a dental cleaning prior to tooth donation in the clinic. Immediately following extraction of a loose tooth in the clinic, the tooth was placed in transportation media (DMEM/F12 50/50 mix with HEPES (Fisher Scientific) and 100 U/mL penicillin, 100μg/mL streptomycin). DPSC were isolated and culture as previously described with slight modifications [8]. Briefly, the pulp was minced and digested in a solution of 3 mg/mL Collagenase type I and 4 mg/mL Dispase II for 1 hr at 37°C. Cells were seeded in poly-D-Lysine 12-well dishes and maintained under standard conditions (37°C, 5% CO2) in DMEM/F12 1:1, 10% fetal bovine serum (Fisher Scientific), 10% newborn calf serum (NCS) (Fisher Scientific) and 100 U/mL penicillin, 100μg/mL streptomycin (Pen/Strep). Sub-confluent cultures were passaged regularly with 0.1μM HyQTase (HyClone).
Evaluation of Cellular Senescence in DPSC
Senescence testing was performed following the Senescence Cell Histochemical Staining Kit protocol (Sigma-Aldrich). The assay is based on a histochemical stain for senescence-associated β-galactosidase (β-gal) activity at pH 6. Each DPSC line was washed twice in 1X PBS, 400-μl-fixation buffer was added to each DPSC line and incubated for 7 minutes at room temperature. The staining solution had 250μl of stock X-gal solution, and 400μL of staining solution was added to each sample according to the manufacturers protocol. The samples were incubated at 37°C without CO2 until the cells appeared blue (approximately 18 hr). After the incubation the cells were rinsed twice in 1X PBS and counterstained with Eosin (Sigma-Aldrich) for 8 minutes. Cells were viewed under a light microscope and cells staining blue were counted as senescent. For each time point, at least 500 cells were counted to determine the percentage of senescent cells.
Immortalization of Primary DPSC
Immortalization was performed as described previously [10]. 1×104 cells were plated in a poly-D-lysine coated 12-well dish and infected with pBABE-hTERT-PuroR retrovirus at a multiplicity of infection of 20 plus 6 μg/mL polybrene for 24 hr. The virus was removed and replaced with growth media for a 72-90 hour period. After this period, the infected DPSC were selected by adding 1μg/mL puromycin for 7-10 days. The DPSC that survived this process were immortalized DPSC and were ready for differentiation into neurons.
Neural Differentiation
DPSC were converted to neurons according to a previously published protocol [8]. 20,000 cells/cm2 were seeded in poly-D-lysine coated 12 well plates or T-25 flasks in DMEM/F12 (1:1), 2.5% fetal calf serum (FCS), 100 U/mL penicillin and 100 μg/l streptomycin, and cultured for 24 hr. Epigenetic reprogramming was performed by exposing the DPSC to 10 μM 5-azacytidine (Acros Scientific) in DMEM/F12 containing 2.5% fetal calf serum (Fisher Scientific) and 10 ng/mL bFGF for 48 hr. Neural differentiation was induced by exposing the cells to 250 μM IBMX, 50 μM forskolin, 200 nM TPA (Sigmat Aldrich), 1 mM db-cAMP, 10 ng/mL bFGF, 10 ng/mL NGF(Invitrogen), 30 ng/mL NT-3 (Peprotech), and 1% insulin-transferrin-sodium selenite premix (ITS) in DMEM/F12 for 3 days. At the end of the neural induction treatment, the cells were washed with 1X PBS. Neuronal maturation was performed by maintaining the cells in Neurobasal A media (Invitrogen) supplemented with 1 mM dbcAMP, 1% N2, 1% B27, and 30 ng/mL NT-3 and 1X Glutamax for 3 weeks.
Western Blot Analysis
Protein was extracted from DPSC and neurons using radioimmunoprecipitation assay (RIPA) lysis buffer for DPSC or neuronal protein extraction reagent for neurons (Thermo Fisher Scientific). Samples were resolved on a NuPage 1.5 mm 4–12% Bis-Tris or a 3-8% Tris-Acetate gel according to manufacturers instructions (Invitrogen) and transferred to Immobilon-FL PVDF membrane (Millipore). The membrane was blocked with 5% BSA, and 0.2% Tween-20 in Phosphate Buffered Saline (PBS). Primary antibodies used include α-GAPDH (dilution 1:5000 from Novus Biologicals catalog#: IMG-3073), α-REST (dilution 1:2500 from Bethyl laboratories Inc. catalog#: A300-540A), α-MAP2 (dilution 1:10,000 from Abcam catalog#: ab5392) and α-GFAP (dilution 1:1500 from Abcam catalog#: ab7260). Infrared (IR) labeled secondary antibodies purchased from Li-Cor (Lincoln, NE) and used in a 1:5000 dilution (α-rabbit 680, α-goat 800 and α-chicken 680). The blot was imaged and analyzed using the Odyssey Infrared Imaging System (LiCor, Lincoln, NE). After adjustment for background fluorescence, lanes were normalized using the signal from the α-GAPDH loading control as the reference channel and signal intensity was then adjusted based on the calculated normalization factor assigned to each lane in the channel.
Electrophysiology Analysis
Whole-cell patch recordings
Patch electrodes were fabricated from soda lime capillary glass (Scientific Products, B4416-1) using a Narishige 2-stage vertical puller, coated with Sylgard (Dow Corning) to about 200 μm from the tip, and fire polished to a final resistance of 0.8-2.0 MΩ, using a Narishige microforge. The patch electrodes were filled with a solution containing: 140 mM CsF, 1 mM MgCl2, 10 mM NaCl, 11 mM ethylene glycol-bis (β-aminoethylether)-N,N,N',N',-tetraacetic acid (EGTA), and 10 mM (N-[2-hydroxyethyl]piperazine-N’-[2-ethanesulfonic acid]) (HEPES), adjusted to pH 7.2 with CsOH. Gig-ohm seals and recordings were obtained in Tyrode's solution containing 140 mM NaCl, 4 mM KCl, 2 mM MgCl2, 2 mM CaCl2, 10 mM glucose, 10 HEPES, adjusted to pH 7.4 with NaOH.
Data acquisition
Data acquisition was carried out using Clampex 8.2 (Axon Instruments) commanding an Axopatch 2A interfaced to the computer with a 1322A series Digidata (Axon Instruments). The amplifier was tuned to null whole-cell capacitance and optimize clamp speed and series resistance (Rs) compensation. Prediction and Rs compensation were set to 80-85%. Data were filtered at 5 kHz, and all Na+ currents were leak subtracted using the P/4 method. I-V relationships were generated by holding the DPSC cells at −80 mV and giving 20 ms test potentials to −75 through +70 mV.
RNA Sequencing
Total RNA was extracted from ~3 week cultured Neurons and DPSC using the miRNeasy Mini kit (Qiagen). Total RNA was checked for quantity and quality on an Agilent Bioanalyzer 6000 pico chip and determined to have an RNA Integrity Number (RIN) of ≥8.0. NuGen amplified material was sheared on a Covaris S2 with a duty cycle of 10%, intensity of 5100 cycles/burst, and 6 × 60 sec cycles (total processing time 6 min). 500ng of this sheared double strand DNA was then used to prepare libraries for sequencing using the Ion plus Core Library Module for AB Library Builder System. Libraries were used from this point without amplification. Before sequencing, small aliquots of this material were pooled and sequenced on an Ion Torrent PGM 314 chip. Barcode quantification data from the PGM were used to balance the samples for final pooling before sequencing. Following this final pooling the library pools were sized to a target size of 260 bp on a Pippin Prep instrument. The sized libraries were examined on an Agilent High Sensitivity DNA chip, quantified using real-time PCR, and used to prepare spheres using a One-Touch 2 device. These spheres were then sequenced on an Ion Torrent Proton sequencer with a P1 chip. On average, ~30 million reads were produced per sample for downstream analysis resulting in ≥7.6 million mapped reads per sample.
RNA-seq Analysis
Short transcript fragments were mapped to the reference human genome (GRCh38; accession number GRCh38.77) using TopHat v2.0.13 [11] with Bowtie v2.1.0 [12] and Samtools v0.1.19. The complete sequencing read dataset is available at the NCBI Gene Expression Omnibus (GEO) site (http://www.ncbi.nlm.nih.gov/geo) using the GEO:GSE67124 accession number. These alignments used reference annotation based alignments against the GENCODE v21 transcriptome. Mapped reads were indexed to known genes using HTseq v0.6.1 [13]. Differentially expressed transcripts were identified using the DESeq2 bioconductor package [14]. Heatmaps were generated using the R software package (v 3.1.1)[15]. Venn diagrams were generated online using Gene List Venn Diagram software (http://genevenn.sourceforge.net/) Additional descriptive analysis was performed using Gene Set Enrichment Analysis (GSEA) using a rank ordered gene list based on fold change to identify pathways and transcription factor binding motifs enriched in particular data sets [16]. Statistical significance for GSEA analysis was set at q≤0.25 as per the developer's suggestion. These gene sets were obtained from MSigDB version 4.0. Gene sets that were found to be significantly up or down regulated (q≥0.05) were also analyzed as a gene list with default stringency settings using The Database for Annotation, Visualization and Integrated Discovery (DAVID v6.7) (http://david.abcc.ncifcrf.gov/).
RESULTS
Teeth were collected from 10 female and 6 male subjects. The subject ages ranged from 6-13 years old, with an average age of 9. All subjects were typically developing children with no suspicion of undiagnosed neurogenetic disorders. The teeth included in the study were non-carious, had no previous restorations, and had no reports of prior facial or dental trauma. At the time of collection in the clinic, there was a general assessment of oral hygiene from poor to excellent. A total of 16 healthy extracted teeth were processed.
Evaluations of storage and processing time from tooth to cell line
To investigate the optimal conditions for transportation and process time of the teeth, we stored and processed them at 3 different time intervals. Teeth were randomly assigned to a group for viability studies (Table 1). Cells were considered viable if they grew to confluence after 4 weeks in culture. Viability and morphology were checked using a light microscope to determine which transportation conditions and process times yielded the best results. On average there was a 56% success rate in culturing DPSC with no significant differences among the three groups (Fisher's Exact Test Pvalue≥0.05). Those teeth that failed to grow DPSC were contaminated or did not grow after 4 weeks. Two samples from group 1 became contaminated. One sample from group 2 became contaminated and two samples did not grow one month after processing. In group 3, two samples did not grow and one sample became contaminated. In all cases these contaminants appear to have come from the mouth despite the use of both penicillin and streptomycin in the transport media. A total of 8 samples failed to grow in this study: 3 from subjects with good oral hygiene and 5 from subjects with fair oral hygiene. Good oral hygiene is defined as no visible plaque and no bleeding on probing. Fair oral hygiene is demonstrated by generalized plaque not covering more than 25% of the teeth with or without bleeding on probing. There were almost twice as many failures from samples with fair oral hygiene verses subjects with good oral hygiene. These experiments indicate that teeth stored at RT for >72 hrs appear to make viable DPSC just as efficiently as those stored under more favorable conditions.
Table 1.
Groups Used for Storage Studies.
| Group | Tooth | Stored | Processed |
|---|---|---|---|
| 1 | Extracted | 4°C | < 24 hours |
| 2 | Extracted | 4°C | > 72 hours |
| 3 | Extracted | RT | > 72 hours |
Longevity of non-immortalized DPSC lines
In order to develop DPSC as a model system in the lab for long term studies we investigated the growth potential of primary DPSC. To determine the rate of cellular senescence and the passage when primary DPSC stop dividing we used a senescence-associated β-galactocidase assay to identify the percentage of senescent cells in four DPSC lines from passage 2 to passage 15 in culture (Figure 1A). Cellular senescence increased at a steady rate until passage 13 when percentage of senescent cells peaked for all cell lines (Figure 1B). Two cell lines (TP-037A and TP-030A) completely stopped dividing by passage 14, while two other lines (TP-040 and TP-023) survived this crisis stage and began to divide again, decreasing the percentage of senescent cells to as low as 40% in both cell lines in subsequent passages. These two cell lines appear to have spontaneously immortalized and their tumorgenic potential was evaluated in mice in a different study [17]. The mean senescence rate for the four samples at passage 2 was 22.8% and at passage 13 was 93.6%. These studies indicate that unless the lines become spontaneously immortalized, which most likely has genome wide effects on their characteristics, the primary DPSC will not continue to grow in culture for more than 13 passages and therefore can not be used long term for laboratory studies without further modification.
Figure 1.
Senescence–associated β-galactosidase assay. A) At each passage cells were stained with β-gal (Blue) and counterstained with Eosin (Red). Only blues cells were counted as a measure of senescence. Arrows point to definitively blue cells. B) 4 DPSC (TP-023, TP-030A, TP-037A and TP-040) underwent senescence testing from passage 2-15. At each passage there was an increase in the number of senescent cells. After passage 13 >93% of the cells were senescent except for TP-023, which appears to have immortalized in culture and continued to grow well past passage 25.
Optimal Immortalization of DPSC lines with telomerase reverse transcriptase
It has previously been shown that DPSC can be immortalized using a viral vector containing human telomerase reverse transcriptase (hTERT) [10]. We wanted to validate these results and then further investigate morphological, electrophysiological and gene expression changes in immortalized vs. non-immortalized DPSC and DPSC derived neurons. Seven different DPSC cell lines were immortalized between P2 to P5. After P6 all cells lines died during Puromycin treatment, meaning that infection of these cells with the hTERT virus failed in DPSC ≥P6 (Table 2). Cells at ≤P2 were the most likely to become stably infected with the virus with 86% of cell lines successfully immortalized. We confirmed the cells were immortalized by qRT-PCR for hTERT. hTERT levels were high in immortalized DPSC cell lines, but hTERT transcripts could not be detected in non-immortalized DPSC by qRT-PCR (data not shown). More extensive gene expression analysis was performed by RNA-seq that clearly confirmed these results (see below).
Table 2.
Transduction of DPSC with hTERT in different passages.
| DPSC ID | P2 | P3 | P4 | P5 | P6 |
|---|---|---|---|---|---|
| TP-016 | + | - | - | - | n/a |
| TP-030B | - | - | - | - | n/a |
| TP-039 | - | - | - | - | n/a |
| TP-024A | + | + | - | - | n/a |
| TP-024B | + | + | - | - | n/a |
| TP-027A | + | + | + | + | - |
| TP-027B | + | + | + | + | - |
| 86% | 57% | 28% | 28% | 0% | |
(+) = Immortalized; (−) = cell die; n/a= not available.
Neural Differentiation of Immortalized DPSC and Non-Immortalized DPSC
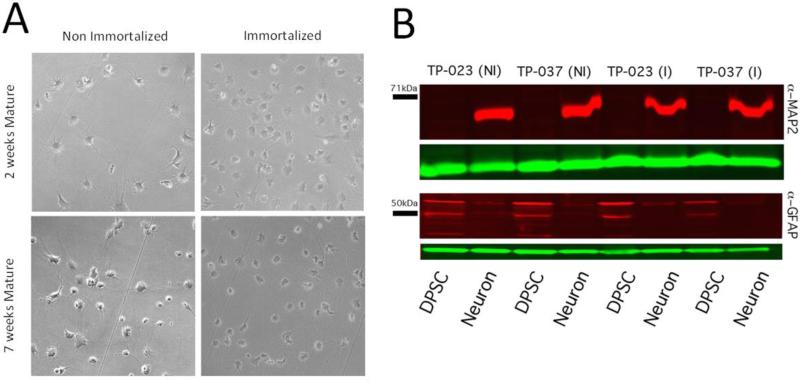
3 Immortalized DPSC and 3 Non-immortalized DPSC were converted successfully to neurons. Immortalized DPSC-neurons and Non-immortalized DPSC-neurons were compared at 3 weeks and 7 weeks of maturation by light microscopy and appear morphologically identical (Figure 2A). In addition, there was a substantial increase in the protein levels of the neuron specific marker MAP2 in neurons versus DPSC concurrent with a decrease in the amount of the astroglial specific GFAP protein marker detected in differentiated neurons (Figure 2B). Previous studies have shown that DPSC neurons not only morphologically appear like neurons, but also exhibit Na+ currents [5, 8] when stimulated with a sharp electrode. We were able to detect Na+ currents in both non-immortalized and immortalized DPSC at roughly equal frequency (Figure 3A-C). Both non-immortalized and immortalized neurons displayed similar apparent activation thresholds averaging −33 mV and −38 mV, respectively, and peak amplitudes averaging 539 pA and 738 pA, respectively, with no significant difference between the two groups (Supplemental Figure 1). These currents demonstrate that immortalization does not substantially affect the ability of DPSC to differentiate into cultured neurons with measurable activity.
Figure 2.
Immortalized DPSC neurons are grossly indistinguishable from non-immortalized DPSC neurons. A) Morphology of immortalized DPSC neurons viewed at 4X magnification by bright-field. Note the long neuronal projections in both immortalized and non-immortalized DPSC neurons. B) Neuron and astroglial specific marker analysis in DPSC neurons from two different DPSC lines (TP-023 and TP-037). The neuronal specific marker MAP2 was detected by IR Western blot in neurons using α-MAP2 (red signal, top), but not DPSC, regardless of the immortalization state (top, red band). Shown in green is an α-GAPDH loading control that shows the presence of protein in each lane. The astroglial specific marker GFAP was detectable in undifferentiated DPSC using α-GFAP (red signal, lower blot) but decreased substantially in differentiated neurons.
Figure 3.
Electrophysiological analysis of immortalized DPSC neurons. A) Family of Na+ currents evoked by test potentials to −40mV through −5mV from a holding potential of −80mV. Note that the Na+ currents evoked from immortalized DPSC Neurons (B) did not differ from those evoked from non-immortalized DPSC Neurons (A). Immortalized and non-immortalized DPSC neurons did not differ in peak amplitude, threshold or frequency. (C) I-V plot of Na+ currents evoked by test potentials to −75mV through +70mV in the same neuron depicted in (A). All recordings were carried out with 140mM Na+ outside and CsF or CsCl in the pipette.
Additional experiments were performed to determine if immortalized DPSC could differentiate into both osteocytes and adipocytes as previously reported [18, 19]. In a supplemental publication we demonstrate that DPSC can efficiently differentiate into osteocytes, but that osteogenesis is ~60% less efficient using immortalized DPSC [20]. Adipogenesis was sufficiently inhibited in both non-immortalized and immortalized DPSC as well, but these cells were not pre-sorted for adipogenic markers in a previous study [19]. These data suggest that constitutive expression of hTERT to immortalize DPSC causes a significant reduction in the ability of these stem cells to differentiate into osteocytes and adipocytes [20].
RNA-seq Analysis of Differentiation and Immortalization of DPSC Neurons
Typical control DPSC lines were immortalized with hTERT as previously described [21]. Both the immortalized and non-immortalized versions of these cell lines were grown to confluence for total RNA extraction. Early passages (P2 or P3) of these lines were also differentiated into neurons along side the immortalized versions of these lines. Total RNA was extracted from 3 cell lines in each group: DPSC, DPSC immortalized, 3 week old neurons, and 3 week old immortalized neurons. Principle component analysis indicated that differentiation of the DPSC into neurons is the main dividing factor differentiating the gene expression in these cell lines since all DPSC clustered together and all neurons derived from DPSC clustered together regardless of immortalization status (Figure 4A).
Figure 4.
A) Principle component analysis (PCA) of gene expression data from DPSC and neurons both immortalized and non-immortalized. Light blue and light green are primary (not immortalized) and dark blue and dark green are immortalized cells. Note that all DPSC grouped to one side and all Neurons grouped to the other side regardless of the state of immortalization, suggesting that differentiation state is the primary component that distinguishes these samples. B) Venn diagram of significant gene expression changes during both differentiation and immortalization. Note that 94 transcripts changed both during differentiation and were different between immortalized and non-immortalized neurons. Immortalization in DPSC appeared to have little effect with the exception of increased hTERT and decreased GAS7 transcript (two genes shared by immortalized DPSC and immortalized Neurons.
Transcript abundance was evaluated based on three comparisons: DPSC vs. Neurons, DPSC immortalized vs. non-immortalized and neurons immortalized vs. non-immortalized. 1305 transcripts were differentially regulated (577 up and 731 down at a normalized Pvalue≤0.05) during the process of differentiation from DPSC to neurons (Figure 4B). Among these transcripts, 94 genes showed differential expression both during the differentiation process from DPSC to neurons and were significantly different between immortalized and non-immortalized neurons (Figure 4B). By contrast, only 5 transcripts were differentially regulated in immortalized vs non-immortalized DPSC. Two of these genes were in common between both immortalized neurons and immortalized DPSC: the human TERT (telomerase reverse transcriptase) gene which was inserted into the cell lines for immortalization which increased in both DPSC and neurons; and the GAS7 (growth arrest specific gene 7) which is thought to play a role in neurite outgrowths and neurotransmission. This gene was decreased by immortalization in both DPSC and neurons. A complete list of differentially expressed genes is available in Supplemental Tables 1-3.
A heatmap of gene expression changes that occur during differentiation indicates that more transcripts (55.9%) were down regulated than up regulated (44.1%) during the transition from DPSC to neurons (Figure 5A). Gene set enrichment analysis (GSEA) of the transcripts that changed during differentiation indicates that many of these genes are involved in the biological process of cell cycle progression (decreased) or neuronal differentiation (increased). Specifically, for the down regulated gene set of 731 genes the normalized enrichment scores (NES) were ≤−2 for groups of genes related to cytokinesis, M-phase, regulation of cell cycle, cell cycle progression, interphase, DNA replication for a total of 25 cell cycle related categories that were significantly down regulated during the transition to neurons at an FDR ≤ 0.01 (Supplemental Table 5). GSEA of the positively regulated gene sets indicated that genes involved in nervous system development, synaptic transmission, CNS development, synaptogenesis and regulation of neurotransmitters were all enriched by ≥1.5 NES at an FDR≤0.13 (Supplemental Table 5). DAVID analysis of the 577 up regulated gene set that was up regulated during differentiation confirmed an enrichment for genes that code for ion channel integral membrane proteins and neural adhesion proteins (Enrichment Score = 4.72) as well as glycoproteins and signal peptides (Enrichment Score=20.56) (Supplemental Table 8).
Figure 5.
Heatmaps of transcripts that changed during differentiation into neurons. A) Heatmap representing the 1305 transcripts that changed in primary DPSC that differentiated into Neurons. The three control DPSC lines are listed above each lane. Note that approximately equal number of transcripts were up regulated as down regulated. B) Heatmap for a sub-set of 94 transcripts that changed during both differentiation and immortalization. Note that immortalization of DPSC dampened gene expression changes for these transcripts in differentiated neurons, but that expression was still not back to DPSC levels. A complete list of genes in this diagram is available in Supplemental Table 4.
To investigate the molecular mechanisms driving the neuronal differentiation, we performed GSEA using predicted transcription factor binding site data. Genes with an upstream binding site for the Neuron-Restrictive Silencer Factor (NRSF)/RE1-silencing transcription factor (REST) binding site were significantly up regulated during differentiation by NES≥1.9 at FDR≤0.05 (Figure 6). In fact, 16 out of 26 genes that made up the core enrichment group were significantly up-regulated, including ATP2B2, a gene expressed in neurons associated with autism spectrum disorder [22, 23]; and DRD2, which encodes a D2 type dopamine receptor that has been associated with various nervous system disorders, including schizophrenia [24] (Supplemental Table 5). Based on these results, the driving force during differentiation from DPSC to neurons appears to be regulated by the REST transcription factor. Expression analysis did not show significant changes in the REST transcript during differentiation (log2Fc=+0.018,Pvalue=0.94 Supplemental Tables 5-7). However, REST is known to be post-translationally regulated through ubiquitination and degradation during the process of differentiation from stem cells to neurons [25], so we performed Western blot analysis to detect REST protein levels in DPSC and differentiated neurons. Figure 6C shows that there is a dramatic decrease in REST protein levels in neurons versus DPSC. In fact, REST protein isoforms could only be detected in the undifferentiated DPSC and not in neurons. This protein expression was not affected at all by hTERT immortalization (Figure 6C) and implies that the process of differentiation is driven by down regulation of REST protein levels in neurons.
Figure 6.
A) GSEA enrichment plot for transcripts with NSRF/REST transcription factor binding sites. Note that the leading edge subset of genes in this particular group containing 26 differentially regulated transcripts contributed to a peak positive enrichment score >0.53, a normalized enrichment score of 1.9 at an FDR<0.005. B) Gene expression changes during differentiation for genes with REST transcription factor binding motifs. These genes were identified as enriched in the elevated gene set during the transition from DPSC to neurons by GSEA transcription factor motif analysis. These same genes were elevated in hTERT immortalized DPSC derived neurons, but to a lesser degree than in the non-immortalized DPSC neurons. C) Western blot for REST protein expression in DPSC and neurons. The red signal is α-REST and the green signal is a loading control (α-GAPDH). REST protein could be detected in both immortalized and non-immortalized DPSC, but not in the neurons derived from these cells, despite the detection of REST transcript in both cell types. These results imply that REST dependent transcriptional changes observed may be driven by changes in REST protein levels during the process of differentiation into neurons.
Next, we looked at the direction of gene expression changes for the 94 transcripts that changed both during differentiation from DPSC into neurons and were significantly different between immortalized and non-immortalized neurons (Figure 4B). A heatmap view of these genes during differentiation reveals that the majority of transcripts were up regulated in non-immortalized neurons, but then expression levels reverted for many of these genes almost to original DPSC levels in immortalized neurons (Figure 5B). Further investigation of this gene set revealed several genes involved in cell motility and adhesion. The core sets of these genes centered around the FN1 protein and a cluster of interacting collagen proteins (COL45A, COL181A, COL25A1, and COL23A1) (Supplemental Figure 3). In addition, for the 189 up-regulated transcription factor networks identified by GSEA, 129 of them were decreased with immortalization and 60 were increased further supporting the hypothesis that at least for TFT networks there was a significant reversion of putative REST regulated differentiation pathways as a result of hTERT immortalization (Figure 6B).
DISCUSSION
The objective of the current study was to characterize growth, physiological and molecular aspects of human DPSC for the in vitro study of patient derived DPSC neurons. We chose to evaluate DPSC from primary teeth because studies have shown that they have the potential for long-term cultivation, research, and possible tissue banking in the future [26]. Numerous studies have shown that DPSC can be differentiated into a variety tissue types, including neurons [7-9]. We are now at the early stages of this field with regards to neural differentiation and it is therefore critical to understand the basic properties of DPSC and their differentiation into neurons if we want to use this system as a surrogate for disease associated changes in the brains of individuals with these syndromes. Unfortunately, based on the current study, we must conclude that constitutive expression of hTERT results in less that fully differentiated neurons, although they appear morphologically normal and have electrophysiological properties of neurons. The global gene expression changes found for 94 transcripts in this study indicate constitutive immortalization of DPSC with hTERT is less than ideal for our purposes.
Primary teeth collected from individuals with neurogenetic disorders are regularly mailed to our laboratory from all around the country in an ongoing study of neurogenetic disorders associated with autism spectrum disorder. The temperature and time before processing can vary greatly among samples because of this remote collection approach. Analysis of storage conditions in this study indicates contamination by Pen/Strep resistant bacteria was the primary reason for failure of the DPSC to grow and not the time after exfoliation or temperature that the tooth was stored at prior to processing. Since initiating these studies in the laboratory, our technical abilities have improved and our success rate in culturing non-contaminated DPSC is now much closer to 80% of the control teeth we receive. We achieved a slightly lower rate of non-contaminated DPSC culture for teeth from subjects with various genetic syndromes, possibly due to general oral hygiene issues in children with developmental disabilities. These results indicate that we can receive teeth from remote locations that may take days to transport, even if they are kept at room temperature during shipping.
It should be noted that bacterial growth in DPSC cultures appeared to loosely correlate with poor oral hygiene of our subjects. The mouth has hundreds of species of bacteria [27], all of which may have the potential to contaminate the media during storage even with the addition of antibiotics to the storage media [28, 29]. It is also well known that as oral hygiene gets worse, more pathologic flora appear in the mouth [29]. Therefore, it is not surprising that poor oral hygiene would lead to more DPSC growth failures due to contamination, but further investigation is needed to validate this theory.
Previous studies have shown that DPSC can be cultured for several passages without cellular senescence, but until the current study the longevity of primary DPSC was unknown [30, 31]. Our results indicate that primary DPSC are >93% senescent by passage 13, after this crisis point these lines may spontaneously immortalize in culture or they die. Of note, Suchanek et al. reported culturing DPSC lines to the 9th passage with a 90% viability and continued culturing to passage 20, but it appears that the determination of their viability rate was not formally measured in a senescence-associated β-galactocidase assay, nor was their definition of viability made clear [30]. We also noted that as the number of passages increased, cell morphology became poor and the density of cells while culturing decreased, which is also contrary to their findings [30].
We have now demonstrated that the hTERT retrovirus is quite effective at immortalizing DPSC in early passages (Table 2). These immortalized DPSC can be efficiently differentiated into neurons that are morphologically and electrophysiologically indistinguishable to non-immortalized DPSC neurons. They also express neuron specific markers like MAP2 and show some residual expression of the astroglial marker GFAP (Figure 2B), which would be expected in a non-homogeneous culture derived from stem cells without sorting for particular lineages. However, our molecular analysis revealed that some of the transcripts that are elevated during the differentiation process are suppressed by immortalization with hTERT in differentiated neurons (Figure 3B). We can only assume, however, that since the immortalized DPSC can be converted to neurons, display Na+ currents and show appropriate down regulation of the REST transcriptional repressor, that these particular transcripts may not be absolutely critical to the differentiation process or may not require extremely high levels of expression for DPSC derived neurons to be produced.
The gene expression changes that occurred during the differentiation process were consistent with our expectations of changes that must occur during neurogenesis. For example, there was a positive enrichment for genes involved in synaptogenesis and a negative enrichment for genes involved in progression of the cell cycle (see Supplemental Table 8 – DAVID Analysis). This is completely expected of any cell that is transitioning from a proliferative state to a quiescent neuronal state. We must be careful, however, not to assume that hTERT immortalization produces DPSC derived neurons that are functionally indistinguishable to neurons from primary DPSC. In fact, our GSEA analysis indicates that many transcripts with NSRF/REST transcription factor binding sites were significantly up regulated during the process of differentiation of primary DPSC to neurons (Figure 5A and Supplemental Table 5), despite the fact that REST transcript levels did not increase significantly. We can now show that REST protein is indeed down regulated in both immortalized and non-immortalized DPSC during the process of differentiation, which supports our finding of an enrichment in REST regulated transcripts during the differentiation process (Figure 6).
However, some of the same transcripts that have REST binding site motifs and were up regulated during differentiation reverted back to the previous expression levels in immortalized DPSC neurons (Figure 6B). Furthermore, in a parallel set of experiments, we demonstrate that both osteogenesis and adipogenesis are significantly less efficient in constitutively hTERT immortalized DPSC versus non immortalized DPSC [20]. These results lead us to conclude that constitutive hTERT immortalization may not be useful for most experimental investigations of genetic disorders. Recently, Page et al. showed that a doxycycline inducible hTERT constructs could be used to maintain mesenchymal stem cells (MSC) in culture long term up to p50 with no ill effects on subsequent differentiation if the hTERT gene is turned back off [32]. One viable option for maintaining DPSC from subjects with rare neurogenetic disorders long term may be to use an inducible promoter system for hTERT expression in DPSC which can also be turned off prior to differentiation into neurons. It should also be noted that other methods, perhaps those recently used to make iPSC from DPSC [33], may also be appropriate for studies involving particular syndromes. In fact, it appears that making iPSC from DPSC may be more efficient than making iPSC from fibroblasts [34]. Nevertheless, we will always have the option of using primary DPSC for our experiments, despite the fact that this is a limited resource and especially scarce when studying rare syndromes.
However, the utility of DPSC is not just limited to disorders involving neuronal function. DPSC are able to differentiate into a variety of different cell lineages [35-37]. In the near future, DPSC may be used for tissue engineering, possible treatment of neural tissue injury, degenerative diseases, and as well as regenerative dentistry. DPSC even have the ability to integrate into host brain tissue in the mammalian brain, migrate to injured areas and express neuronal specific markers as well as voltage dependent sodium and potassium channels [1]. Understanding the culture conditions, characteristics, and potential of DPSC may prove beneficial to all fields of health care. By establishing the technical boundaries of this renewable resource, we have provided a baseline for future studies of neurogenetic syndromes at the molecular and cellular biological levels.
Supplementary Material
Highlights.
Shed teeth can be stored at RT for days in media and still produce viable DPSC.
Neurons can be made from hTERT immortalized DPSC as well as primary DPSC.
Differentiation of DPSC changes 1305 transcripts
94 transcripts are affected by immortalization and differentiation.
ACKNOWLEDGEMENTS
We would like to thank the families who contributed teeth to the study. We also thank Dr. Michael A. Dyer for his critical review of the manuscript and Dr. Amanda Preston for edits to the revised manuscript. RNAseq was performed with the assistance of the UTHSC Molecular Resource Center. These experiments were funded in part by NIH R21NS075709-02 to L.T.R. and a Dental School Alumni Fund scholarship to R.M.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- 1.Marchetto MC, Winner B, Gage FH. Pluripotent stem cells in neurodegenerative and neurodevelopmental diseases. Hum Mol Genet. 2010;19:R71–76. doi: 10.1093/hmg/ddq159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arbab M, Baars S, Geijsen N. Modeling motor neuron disease: the matter of time. Trends in neurosciences. 2014;37:642–652. doi: 10.1016/j.tins.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Abraham R, Verfaillie CM. Neural differentiation and support of neuroregeneration of non-neural adult stem cells. Progress in brain research. 2012;201:17–34. doi: 10.1016/B978-0-444-59544-7.00002-0. [DOI] [PubMed] [Google Scholar]
- 4.Achilleos A, Trainor PA. Neural crest stem cells: discovery, properties and potential for therapy. Cell research. 2012;22:288–304. doi: 10.1038/cr.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiraly M, Kadar K, Horvathy DB, Nardai P, Racz GZ, Lacza Z, Varga G, Gerber G. Integration of neuronally predifferentiated human dental pulp stem cells into rat brain in vivo. Neurochemistry international. 2011;59:371–381. doi: 10.1016/j.neuint.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Mezey E. The fate of neural crest stem cells: nature vs nurture. Mol Psychiatry. 2003;8:128–130. doi: 10.1038/sj.mp.4001315. [DOI] [PubMed] [Google Scholar]
- 7.Arthur A, Rychkov G, Shi S, Koblar SA, Gronthos S. Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells. 2008;26:1787–1795. doi: 10.1634/stemcells.2007-0979. [DOI] [PubMed] [Google Scholar]
- 8.Kiraly M, Porcsalmy B, Pataki A, Kadar K, Jelitai M, Molnar B, Hermann P, Gera I, Grimm WD, Ganss B, Zsembery A, Varga G. Simultaneous PKC and cAMP activation induces differentiation of human dental pulp stem cells into functionally active neurons. Neurochemistry international. 2009;55:323–332. doi: 10.1016/j.neuint.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 9.Nosrat IV, Smith CA, Mullally P, Olson L, Nosrat CA. Dental pulp cells provide neurotrophic support for dopaminergic neurons and differentiate into neurons in vitro; implications for tissue engineering and repair in the nervous system. Eur J Neurosci. 2004;19:2388–2398. doi: 10.1111/j.0953-816X.2004.03314.x. [DOI] [PubMed] [Google Scholar]
- 10.Egbuniwe O, Idowu BD, Funes JM, Grant AD, Renton T, Di Silvio L. P16/p53 expression and telomerase activity in immortalized human dental pulp cells. Cell cycle. 2011;10:3912–3919. doi: 10.4161/cc.10.22.18093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome biology. 2013;14:R36. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome biology. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anders S, Pyl PT, Huber W. HTSeq - A Python framework to work with high- throughput sequencing data. Bioinformatics. 2014:1–4. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-Seq data with DESeq2. BioRxIV. 2014. [DOI] [PMC free article] [PubMed]
- 15.R Core Team R. A Language and Environment for Statistical Computing. 2013.
- 16.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson R, Urraca N, Skobowiat C, Hope KA, Miravalle L, Chamberlin R, Donaldson M, Seagroves TN, Reiter LT. Assessment of the Tumorigenic Potential of Spontaneously Immortalized and hTERT-Immortalized Cultured Dental Pulp Stem Cells. Stem cells translational medicine. 2015;4:905–912. doi: 10.5966/sctm.2014-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abuarqoub D, Awidi A, Abuharfeil N. Comparison of osteo/odontogenic differentiation of human adult dental pulp stem cells and stem cells from apical papilla in the presence of platelet lysate. Archives of oral biology. 2015;60:1545–1553. doi: 10.1016/j.archoralbio.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Gibellini L, De Biasi S, Nasi M, Carnevale G, Pisciotta A, Bianchini E, Bartolomeo R, Polo M, De Pol A, Pinti M, Cossarizza A. Different origin of adipogenic stem cells influences the response to antiretroviral drugs. Exp Cell Res. 2015 doi: 10.1016/j.yexcr.2015.07.031. [DOI] [PubMed] [Google Scholar]
- 20.El-Iyachi I, Goorha S, Reiter LT, Miranda-Carboni GA. Effects of hTERT immortalization on osteogenic and adipogenic differentiation of dental pulp stem cells. Data in Brief. 2015 doi: 10.1016/j.dib.2016.01.009. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Egbuniwe O, Grant AD, Renton T, Di Silvio L. Phenotype-independent effects of retroviral transduction in human dental pulp stem cells. Macromolecular bioscience. 2013;13:851–859. doi: 10.1002/mabi.201300020. [DOI] [PubMed] [Google Scholar]
- 22.Yang W, Liu J, Zheng F, Jia M, Zhao L, Lu T, Ruan Y, Zhang J, Yue W, Zhang D, Wang L. The evidence for association of ATP2B2 polymorphisms with autism in Chinese Han population. PLoS One. 2013;8:e61021. doi: 10.1371/journal.pone.0061021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carayol J, Sacco R, Tores F, Rousseau F, Lewin P, Hager J, Persico AM. Converging evidence for an association of ATP2B2 allelic variants with autism in male subjects. Biol Psychiatry. 2011;70:880–887. doi: 10.1016/j.biopsych.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 24.Liu L, Fan D, Ding N, Hu Y, Cai G, Wang L, Xin L, Xia Q, Li X, Xu S, Xu J, Yang X, Zou Y, Pan F. The relationship between DRD2 gene polymorphisms (C957T and C939T) and schizophrenia: a meta-analysis. Neurosci Lett. 2014;583:43–48. doi: 10.1016/j.neulet.2014.09.024. [DOI] [PubMed] [Google Scholar]
- 25.Huang Z, Wu Q, Guryanova OA, Cheng L, Shou W, Rich JN, Bao S. Deubiquitylase HAUSP stabilizes REST and promotes maintenance of neural progenitor cells. Nature cell biology. 2011;13:142–152. doi: 10.1038/ncb2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suchanek J, Visek B, Soukup T, El-Din Mohamed SK, Ivancakova R, Mokry J, Aboul-Ezz EH, Omran A. Stem cells from human exfoliated deciduous teeth--isolation, long term cultivation and phenotypical analysis. Acta Medica (Hradec Kralove) 2010;53:93–99. doi: 10.14712/18059694.2016.66. [DOI] [PubMed] [Google Scholar]
- 27.Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. Journal of clinical microbiology. 2005;43:5721–5732. doi: 10.1128/JCM.43.11.5721-5732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Do T, Devine D, Marsh PD. Oral biofilms: molecular analysis, challenges, and future prospects in dental diagnostics. Clinical, cosmetic and investigational dentistry. 2013;5:11–19. doi: 10.2147/CCIDE.S31005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas JG, Nakaishi LA. Managing the complexity of a dynamic biofilm. Journal of the American Dental Association. 2006;137(Suppl):10S–15S. doi: 10.14219/jada.archive.2006.0409. [DOI] [PubMed] [Google Scholar]
- 30.Suchanek J, Soukup T, Visek B, Ivancakova R, Kucerova L, Mokry J. Dental pulp stem cells and their characterization, Biomedical papers of the Medical Faculty of the University Palacky. Olomouc, Czechoslovakia. 2009;153:31–35. doi: 10.5507/bp.2009.005. [DOI] [PubMed] [Google Scholar]
- 31.Yu J, He H, Tang C, Zhang G, Li Y, Wang R, Shi J, Jin Y. Differentiation potential of STRO-1+ dental pulp stem cells changes during cell passaging. BMC cell biology. 2010;11:32. doi: 10.1186/1471-2121-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piper SL, Wang M, Yamamoto A, Malek F, Luu A, Kuo AC, Kim HT. Inducible immortality in hTERT-human mesenchymal stem cells. J Orthop Res. 2012;30:1879–1885. doi: 10.1002/jor.22162. [DOI] [PubMed] [Google Scholar]
- 33.Griesi-Oliveira K, Acab A, Gupta AR, Sunaga DY, Chailangkarn T, Nicol X, Nunez Y, Walker MF, Murdoch JD, Sanders SJ, Fernandez TV, Ji W, Lifton RP, Vadasz E, Dietrich A, Pradhan D, Song H, Ming GL, Gu X, Haddad G, Marchetto MC, Spitzer N, Passos-Bueno MR, State MW, Muotri AR. Modeling non-syndromic autism and the impact of TRPC6 disruption in human neurons. Mol Psychiatry. 2014 doi: 10.1038/mp.2014.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan X, Qin H, Qu C, Tuan RS, Shi S, Huang GT. iPS cells reprogrammed from human mesenchymal-like stem/progenitor cells of dental tissue origin. Stem cells and development. 2010;19:469–480. doi: 10.1089/scd.2009.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morad G, Kheiri L, Khojasteh A. Dental pulp stem cells for in vivo bone regeneration: a systematic review of literature. Archives of oral biology. 2013;58:1818–1827. doi: 10.1016/j.archoralbio.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 36.Verma K, Bains R, Bains VK, Rawtiya M, Loomba K, Srivastava SC. Therapeutic potential of dental pulp stem cells in regenerative medicine: An overview. Dental research journal. 2014;11:302–308. [PMC free article] [PubMed] [Google Scholar]
- 37.Young F, Sloan A, Song B. Dental pulp stem cells and their potential roles in central nervous system regeneration and repair. Journal of neuroscience research. 2013;91:1383–1393. doi: 10.1002/jnr.23250. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.