Abstract
Avoiding high opioid doses may reduce chronic opioid therapy (COT) risks, but the feasibility of reducing opioid doses in community practice is unknown. Washington State and a health plan's group practice implemented initiatives to reduce high dose COT prescribing. The group practice physicians were exposed to both initiatives, while its contracted physicians were exposed only to statewide changes. Using interrupted time series analyses, we assessed whether these initiatives reduced opioid doses among COT patients in group practice (N=16,653) and contracted care settings (N=5,552). From 2006 through June 2014, the percent of COT patients receiving 120 or more milligrams morphine equivalent dose declined from 16.8% to 6.3% in the group practice versus 20.6% to 13.6% among COT patients of contracted physicians. The proportion receiving excess opioid days supplied declined from 24.0% to 10.4% among group practice COT patients and from 20.1% to 14.7% among COT patients of contracted physicians. Reductions in prescribing of high opioid dose and excess opioid days supplied followed state and health plan initiatives to change opioid prescribing. Reductions were substantially greater in the group practice setting that implemented additional initiatives to alter shared physician expectations regarding appropriate COT prescribing, compared to the contracted physicians’ patients.
Keywords: Opioids, Chronic pain, Practice change
INTRODUCTION
Increased prescribing of opioids for chronic pain has been accompanied by large increases in drug overdose and addiction involving prescription opioids [3,4,14,18]. In 2011, the federal government called for action to decrease prescription drug misuse [13]. There is growing evidence that the risks of opioid overdose and addiction among chronic opioid therapy (COT) patients increase with opioid dose [2]. Avoiding COT at higher doses might reduce the risks of COT.
A Washington State guideline, initially published in 2007 and enacted into state law in 2010, recommended caution in prescribing COT at higher doses, defined as a daily morphine equivalent dose (MED) of 120 milligrams or greater [24]. Under this guideline, fewer worker's disability compensation recipients received high doses of opioids and there was a subsequent decline in the number of opioid-related deaths in this patient population [7,9]. However, it is not known whether statewide guidance produced widespread changes in opioid dose levels among COT patients in community practice.
After Washington State published its COT guideline, Group Health Cooperative (a Washington State insurance plan and health care delivery system) implemented additional initiatives in its group practice to alter physician expectations regarding COT prescribing. In contrast, Group Health's contracted physicians, who provide care outside of the group practice setting, were exposed to statewide COT guideline changes, but not to these additional group practice initiatives.
Davidoff [5] observes that, “Once established, clinical practices can be extraordinarily hard to abandon if subsequent evidence and experience find them to be ineffective, disruptive, or the cause of net-harm”. Biller-Andorno and Lee [1] propose that the shared purpose arising from focusing attention on goals broadly accepted within a health care organization can change physician practices. Relative to adoption of medical innovations, changing clinical practices deemed ineffective or unsafe has rarely been studied [5,10]. This paper compares the rates of high opioid doses among COT patients after changes in guidance regarding COT, undertaken both statewide and within a health plan. We compare opioid prescribing trends in a group practice setting which sought to alter shared physician expectations and practices for COT patients to trends among the same health plan's contracted physicians, who were exposed to the new statewide guideline but not the added group practice initiatives. We assess whether these changes reduced the average daily opioid dose received by COT patients, and whether the changes reduced the percent of COT patients receiving high-dose COT or excess days supplied of opioids.
We hypothesized that starting in 2008, following efforts to alter prescribing expectations of primary care physicians, the rate of reduction in average daily opioid dose and in the percent of COT patients receiving high opioid doses would be greater in the group practice setting relative to trends among contracted physicians who were exposed only to the Washington State guideline and legislation. We also hypothesized that any observed reduction in opioid dose would be accompanied by a reduction in the percent of COT patients receiving excess opioid days supplied. Our evaluation also assessed whether a multi-faceted opioid risk reduction initiative implemented later in 2010 in the group practice further influenced prescribing of higher opioid doses beyond any prescribing changes achieved through efforts to alter shared expectations of physicians regarding appropriate opioid prescribing.
METHODS
Setting
Group Health Cooperative [16] is a large insurance plan and care delivery system in Washington State with both group practice and contracted care settings. Providers in the group practice deliver care at Group Health's own facilities to about two-thirds of the plan's enrollees. The remaining enrollees make up Group Health's contracted care setting. They receive care from community physicians in diverse clinical settings not operated by Group Health. There was a substantially greater potential for Group Health Cooperative to change COT prescribing expectations and practices in the group practice than in the contracted care setting.
Opioid risk reduction initiatives in the group practice
The state first published its COT guideline in April 2007. In 2010, Washington State enacted legislation mandating use of the guideline for long-term opioid prescribing for chronic non-cancer pain, explicitly excluding hospice care, end of life and palliative care, and management of acute pain following injury or surgery. In conformity with the guideline, prescribers were required to conduct a physical examination and check medical records to assess the appropriateness of pain treatment, and to screen for risk of drug abuse and diversion. For high-risk patients, the guideline recommended developing a treatment plan and advised use of a written agreement outlining patient responsibilities including urine drug screening. The guideline called for periodic patient monitoring at least every six months, unless the patient was on a stable dose of less than 40 milligrams MED in which case annual review was sufficient. Periodic review was intended to assess compliance with the treatment plan, and assess the patient's condition. Physicians prescribing long-acting opioids or methadone were required to complete at least 4 hours of relevant continuing medical education. Of particular relevance to this research, physicians prescribing an average daily dose of 120 milligrams or greater MED were asked to consult with a pain specialist (through a patient visit, remote evaluation of the patient by the specialist, or a telephone consultation between the prescriber and the specialist) unless the patient was on a stable dose and the patient's pain and functional status were also stable. Situations in which there was a short-term increase in dose to manage an exacerbation were exempted.
In the second half of 2006 and thereafter, Group Health's group practice sought to change shared expectations regarding COT prescribing in primary care. Primary care leadership, together with consulting Rehabilitation Medicine specialists, encouraged greater caution in prescribing opioids for chronic pain and discouraged dose escalation and use of higher opioid doses with COT patients [17]. Over several years, the medical director of rehabilitation medicine delivered occasional voluntary educational presentations about managing chronic pain and opioid prescribing. Typically about one-fourth of group practice primary care physicians (PCPs) attended these presentations. Group Health established a clinical policy making PCPs responsible for overall opioid management of their COT patients. PCPs and clinic medical directors received lists of their COT patients which flagged those receiving high opioid doses, defined by the Washington State guideline as 120 milligrams or greater MED. Physicians with unusually large numbers of COT patients taking high opioid doses received feedback and supervisory guidance from clinic medical directors. These efforts sought to change shared expectations of prescribers about appropriate management of patients using opioids long-term.
Neither the state guideline and legislation or the Group Health initiatives sought to reduce the percent of patients receiving COT. Clinical decisions about whether and how long to prescribe COT for chronic pain were left to physician discretion. While we report trends in the percent of adult patients receiving COT in the group practice and contracted care settings, we did not attempt to evaluate the impact of the interventions on the prevalence of COT in the study populations.
In September 2010, Group Health implemented a multi-faceted opioid risk reduction initiative in its group practice clinics. This initiative included: a standard guideline establishing minimum standards for risk-stratified COT monitoring (including urine drug testing), documentation of standard care plans in the medical record describing the treatment regimen and the PCP responsible for COT management, periodic monitoring visits, and modifications to the prescription refill process to prevent urgent refill requests. [20] The multi-faceted opioid risk reduction initiative sought to increase the conformity of opioid prescribing and management for chronic non-cancer pain with the recently enacted state legislation.
To support implementation, the initiative employed practice tools such as patient education materials, a care plan template, and an online calculator for estimating MED; performance measures tracking the development of COT care plans in the electronic health record; medical staff leader advocacy; expert consultation for physicians in each primary care clinic; and financial incentives for completing COT care plans. Medical staff leadership mandated a 90-minute online continuing medical education course about chronic pain management and opioids, which 87% of group practice PCPs completed. After taking the course, clinicians in each clinic met for a one-hour discussion. Prior reports have shown that the implementation of the multi-faceted risk reduction initiative resulted in near universal documentation of COT care plans in electronic health records [20] and a dramatic increase in rates of urine drug screening among COT patients [21] in the group practice setting.
None of these Group Health initiatives were implemented in the health plan's contracted care settings. Contracted physicians providing care to Group Health enrollees practiced in diverse settings statewide not operated by Group Health, with medical staff leaders not employed by Group Health, and not using Group Health's electronic health care records employed in implementing the initiatives. Group Health staff implementing opioid quality improvement initiatives were not able to work with the contracted physicians to change opioid prescribing and management practices. Typically, Group Health members represented a small minority of the patients cared for by contracted physicians; most of their patients were insured by plans other than Group Health. For these reasons, Group Health's initial efforts (starting in second half of 2006) to change shared expectations regarding appropriate opioid prescribing practices, and the multi-faceted opioid risk reduction initiative (begun in late 2010) were not implemented with the contracted care physicians. Thus, the group practice physicians were exposed to the Group Health initiatives and the statewide guideline and legislation, whereas the contracted care physicians were exposed only to the statewide guideline and legislation. While this evaluation is able to describe changes in the contracted care settings that took place as the guideline was disseminated and legislation enacted, the COT patients in the contracted care settings served as controls for evaluating the incremental effects of the more extensive efforts to change opioid prescribing practices implemented in the group practice setting.
Study design
This research was approved by the Group Health Institutional Review Board (IRB). Since participants were not contacted for this research, and data analyzed were from electronic health records, the IRB granted a waiver of individual informed consent.
We used interrupted time series analyses [8,22] to compare trends in high-dose opioid prescribing among COT patients in the group practice to trends among COT patients in the contracted care setting from 2006 through mid-2014. Of key interest were three time periods corresponding to the different phases of opioid risk reduction initiatives. The baseline time period was January 1, 2006 through December 31, 2007. The initial intervention phase, hereafter referred to as the altered prescribing expectations phase, was from January 1, 2008 through September 30, 2010 and included efforts to alter physician expectations regarding appropriate opioid prescribing through statewide guideline dissemination alone in the contracted care setting, and through the augmented educational and medical staff supervision interventions in the group practice setting. The multi-faceted opioid risk reduction initiative was implemented in the group practice setting starting in October 2010; therefore, the third phase of our evaluation, the multi-faceted initiative phase, spans the period from October 1, 2010 through June 30, 2014. Within each phase, we examined COT prescribing patterns on a quarterly basis.
We used 2006-7 as a baseline for comparison because, although the Washington State guidelines were disseminated in April 2007, group practice steps to implement the guideline were initially gradual, informal, and voluntary. We expected negligible changes in opioid prescribing during the two baseline years in both the group practice and the contracted care settings. By comparing prescribing trends in group practice to contracted care settings in later implementation periods, we assessed whether enhanced efforts to change opioid prescribing in the group practice achieved larger reductions in the use of high opioid doses among COT patients than occurred in the contracted care setting under the statewide changes alone.
Population and eligibility
Using pharmacy data recording prescriptions filled, we identified COT patients as those receiving 70 days or more supply of opioids in a 90-day period. The 70-day threshold was consistent with Group Health's administrative definition of COT. The study sample for each quarter in our analyses consisted of Group Health enrollees who: received care from group practice or contracted physicians, met the Group Health COT definition for the quarter, were 18 years of age or older, and were enrolled in the health plan for the entire quarter and for at least one year prior (to allow assessment of covariates). Because our focus was on non-cancer chronic pain, we excluded patients who received an opioid prescription from an oncologist, or had two or more visits with cancer diagnoses (excluding non-melanoma skin cancer), or had been admitted to a hospice during the study period.
Patient characteristics
Group Health enrollment files provided patient age, gender, and residence in Eastern or Western Washington. Electronic data indicated patient history of mental health diagnoses, opioid and non-opioid drug use disorder diagnoses, alcohol substance use disorder diagnoses, and current tobacco use status. We gathered these variables for the year prior to the first quarter of the study period in which a patient met the study criteria for receiving COT (a patient's index quarter).
The group practice patients receive their care in Group Health clinics located predominately in Western Washington and the greater Seattle area, but also in larger cities in Eastern Washington such as Spokane. The contracted care patients receive care from physicians practicing in settings that are not owned or operated by Group Health. More of these patients reside in Eastern Washington. We assessed the comparability of the two cohorts by describing salient characteristics (e.g. age, gender, history of drug and alcohol abuse, tobacco use).
Study measures
We examined three aspects of opioid prescribing for COT patients using electronic pharmacy data available for both group practice and contracted care patients:
-
(1)
The mean daily morphine equivalent dose (MED) received by each COT patient over a 90-day period. We calculated the average daily MED dispensed to each COT patient in each quarter by adding the morphine equivalents for the prescriptions dispensed during the 90 days and then dividing by 90, using methods and conversion factors described elsewhere [25]. We counted morphine equivalents dispensed prior to the quarter with a run-out date within the quarter, and prescriptions within the quarter with a run-out date after the quarter ended, on a pro-rata basis.
-
(2)
The percent of COT patients in each quarter who received an average daily opioid dose of 120 milligrams or greater MED, and the percent of COT patients in each quarter who received an average daily opioid dose of 50 milligrams or greater MED.
-
(3)
The percent of COT patients in each quarter who received more than 20% excess opioid days supplied in the quarter, defined as 109 or more days supply. This was determined separately for short-acting and long-acting opioids, so that patients using long-acting opioids with short-acting opioids prescribed for supplemental use on an as-needed basis would not be counted as receiving excess opioid days supplied on that basis alone. Only patients who received at least 109 days supply of long-acting opioids or at least 109 days supply of short-acting opioids in a quarter were classified as receiving excess opioid days supplied. We counted days supply of prescriptions filled before the quarter started but with a run-out date within the quarter, and prescriptions filled within the quarter but with a run-out date after the quarter ended, on a pro-rata basis. Prior research has shown that receiving an excess opioid days supplied is associated with opioid abuse. [6,15,19]
Analyses
We described our study sample of COT patients and how overall prevalence of COT use changed over time during the study period. We also compared differences in characteristics between COT patients in the group practice and contracted physician settings of the health plan. We used regression models to estimate case-mix adjusted trends in opioid prescribing outcomes among the group practice and contracted care COT patients. Linear regression was used for the average daily MED outcome and separate logistic regression models were used for the two high-dose opioid outcomes and the excess opioid days supplied outcome. In the models we included main effects for the health plan setting (group practice vs. contracted care) and included calendar time, measured quarterly, using linear splines [11] with knots at the first quarter of 2008 and fourth quarter of 2010 to allow for different linear temporal trends in outcomes across the three periods of interest. Further, we included interactions between calendar time and health plan setting to permit estimation and comparison of potentially different outcome trends for the two COT populations of interest. We adjusted models for patient-level covariates based on information from the year prior to each patient's index quarter: gender, age, location (Eastern/Western Washington), smoking status (current or not), mental health diagnoses, and separate diagnoses of opioid and non-opioid drug use disorders and alcohol substance use disorder. To estimate models we used generalized estimating equations (GEE) with a working correlation matrix and robust standard errors estimated via the sandwich estimator to account for correlations between observations (i.e. patients) across time. [26]
We present graphs showing the raw, unadjusted mean opioid dose among COT patients or the percent of COT patients receiving high doses or excess opioid days supplied in each quarter of the study period in the two health plan settings (depicted with square and triangle markers). Additionally, to provide a visual comparison of rates controlled for case-mix differences between the two populations, we provide plots of estimated adjusted trends (depicted with lines) based on the fitted multivariate linear or logistic regression model and standardized to a common distribution of patient characteristics. Below each figure we provide estimates of the annual rate of change in the outcome (with 95% confidence intervals (CIs)) for the group practice and contracted care COT patients within each of the three time periods of interest. For average daily opioid dose, this entails an estimated change (Δ) per year in mean daily opioid MED (in milligrams) received. For the binary outcomes (i.e. high dose use or receiving excess opioid days supplied), this entails an estimated annual change in odds of the outcome (using adjusted odds ratios (ORs)). In these tables we also present Wald-based p-values [23] for two-sided tests evaluating whether these rates of change are significantly different between the two health plan settings in each period (that is, a “difference in differences” approach). We additionally tested for differences in the rates of change within each health plan setting between the time periods of the three implementation phases. These results are described in the text for the average daily dose outcome only, as similar differences were observed for the other outcomes and can be inferred from the figures.
RESULTS
The total number of COT patients included in the analyses over the 8.5-year study period was 22,205 (16,653 in group practice; 5,552 in contracted care). In the first quarter of the study period, the sample included 3745 COT patients cared for by group practice physicians and 1003 COT patients cared for by contracted care physicians. In the last quarter, these counts were 4969 and 1444, respectively. From 2006 through 2014 the number of adult enrollees receiving COT increased in both the group practice and the contracted care settings. When standardized for age and gender, the percent of group practice enrollees receiving COT increased from 1.9% (95% CI 1.85, 1.96) at the beginning of 2006 to 2.7% (2.67, 2.80) in June 2014. By comparison, in the contracted care setting the percent receiving COT increased from 1.4% (1.29, 1.45) at the beginning of 2006 to 2.8% (2.68, 2.89) in June 2014.
Comparisons of COT patients in our study sample in the group practice and contracted care settings showed some similarities as well as notable differences (see Table 1). The percent of COT patients residing in Western Washington was markedly higher among group practice patients (88%) than among contracted care patients (45%). Risk factors for misuse of prescription opioids were somewhat more common in the group practice than the contracted care setting. For example, 17% of COT patients were current tobacco users in the group practice compared to 12% in contracted care, while the percent with a recent opioid use disorder diagnosis was only slightly higher in the group practice (1.6% versus 1.1%).
Table 1.
Chronic opioid therapy (COT) patient characteristics based on the year prior to each patient's index COT quarter.
| All COT Patients | Group Practice COT Patients | Contracted Physician COT Patients | ||||
|---|---|---|---|---|---|---|
| N | Percent | N | Percent | N | Percent | |
| Total | 22,205 | -- | 16,653 | -- | 5,552 | -- |
| Western Washington | 17,203 | 77.5 % | 14,709 | 88.3 % | 2,494 | 44.9 % |
| Female | 13,858 | 62.4 % | 10,482 | 62.9 % | 3,376 | 60.8 % |
| Age 18-39 | 3,133 | 14.1 % | 2,384 | 14.3 % | 749 | 13.5 % |
| 40-49 | 4,488 | 20.2 % | 3,131 | 18.8 % | 1,357 | 24.4 % |
| 50-59 | 6,467 | 29.1 % | 4,757 | 28.6 % | 1,710 | 30.8 % |
| 60-69 | 3,953 | 17.8 % | 3,031 | 18.2 % | 922 | 16.6 % |
| 70-79 | 2,171 | 9.8 % | 1,740 | 10.5 % | 431 | 7.8 % |
| 80 or older | 1,933 | 9.0 % | 1,610 | 9.7 % | 383 | 6.9 % |
| Current tobacco use | 3,438 | 15.5 % | 2,752 | 16.5 % | 686 | 12.4 % |
| Mental disorder diagnosis | 8,587 | 38.7 % | 6,570 | 39.5 % | 2,017 | 36.3 % |
| Opioid abuse/dependence | 332 | 1.5 % | 273 | 1.6 % | 59 | 1.1 % |
| Non-opioid drug abuse/dependence | 560 | 2.5 % | 467 | 2.8 % | 93 | 1.7 % |
| Alcohol abuse/dependence | 872 | 3.9 % | 723 | 4.3 % | 149 | 2.7 % |
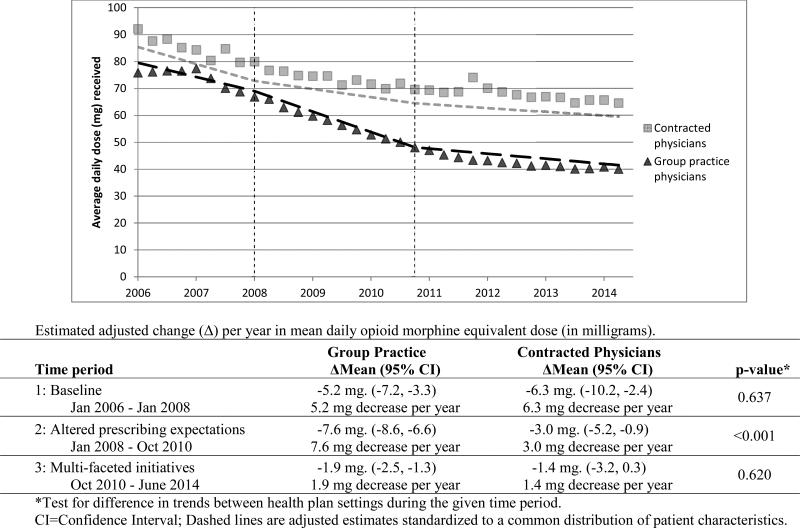
From the first quarter of 2006 to June 2014, the average daily morphine equivalent dose declined from 75.8 milligrams to 40.0 milligrams among group practice COT patients (47% lower), compared to a drop from 92.1 milligrams to 64.6 milligrams among COT patients of contracted physicians (30% lower), as shown in Figure 1. Average daily opioid dose was decreasing in the baseline period among COT patients of both group practice and contracted care physicians. In 2006-7, there was an average decrease of 5.2 milligrams per year in the group practice, and 6.3 milligrams per year in contracted care. The difference in the rate of decrease between the group practice and contracted care was non-significant (p=0.637) during this time. During the altered prescribing expectations phase of 2008 through October 2010, when shared expectations regarding appropriate opioid prescribing were systematically targeted in the group practice setting, the annual rate of reduction in average daily opioid dose was substantially greater in the group practice than it was in contracted care (average decline of 7.6 milligrams per year vs. 3.0 milligrams per year, p<0.001). In the multi-faceted initiatives risk reduction phase (October 2010 through June 2014), the rate of reduction in average daily opioid dose was substantially diminished in both the group practice (a reduction of only 1.9 milligrams per year) and contracted care settings (a reduction of 1.4 milligrams per year), with the rates of decline no longer significantly different between groups (p=0.620) during that time period.
Figure 1.
Case-mix adjusted trends in mean daily opioid dose received in morphine equivalents (milligrams) for COT patients of group practice and contracted physicians.
Within the group practice setting, the rate of decline in average daily opioid dose was significantly larger in the 2008-10 altered prescribing expectations phase than in the 2006-7 baseline phase (decline of 7.6 mg. per year versus 5.2 mg per year, p=0.048). However, the rate of decline in the group practice setting was markedly attenuated in the 2010-14 multi-faceted initiatives risk reduction phase (decline of 1.9 mg. per year).
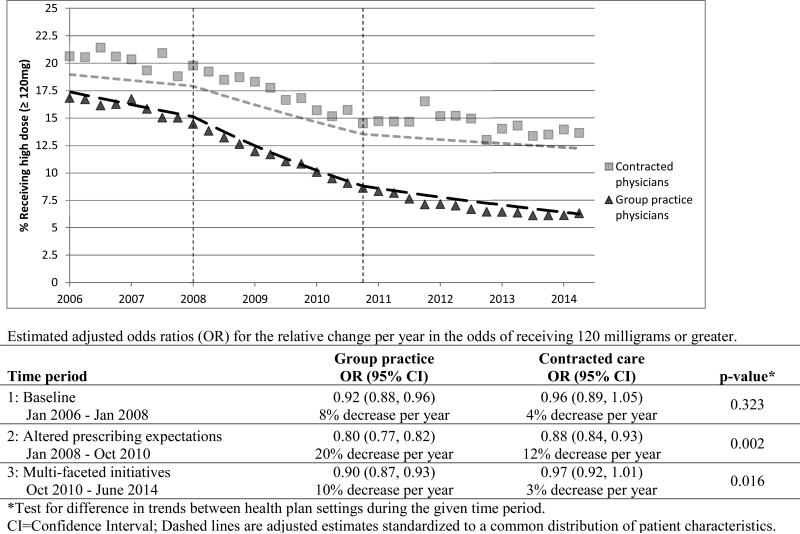
For the percent of COT patients receiving an average daily dose of 120 milligrams or greater MED, the group practice setting showed markedly larger reductions than the contracted care setting (Figure 2). Over the study period, the percent of COT patients receiving opioid doses of 120 mg. MED or greater in the group practice declined from 16.8% to 6.3% (63% lower) compared to a reduction from 20.6% to 13.6% (34% lower) in contracted care. During the baseline phase, the odds of COT patients in the group practice using high opioid doses decreased an average of 8% per year during the baseline phase (OR 0.92; 95% CI 0.88-0.96, adjusted for patient characteristics) (Figure 2). This decline in odds accelerated during the altered prescribing expectations phase (2008-10) to about a 20% reduction per year (OR 0.80; 95% CI 0.77-0.82), followed by a slower, but still significant, rate of decline during the multi-faceted initiatives risk reduction phase (OR 0.90; 95% CI 0.87-0.93). In contrast, the contracted care setting showed a statistically significant annual rate of decline only during the altered prescribing expectations phase (OR 0.88; 95% CI 0.84-0.93). The rate of decline was significantly larger in the group practice than in the contracted care setting during the altered prescribing expectations phase (p=0.002) and the multi-faceted initiatives phase (p=0.016).
Figure 2.
Case-mix adjusted trends in percent receiving average daily opioid dose of 120 milligrams morphine equivalents or greater for COT patients of group practice and contracted physicians.
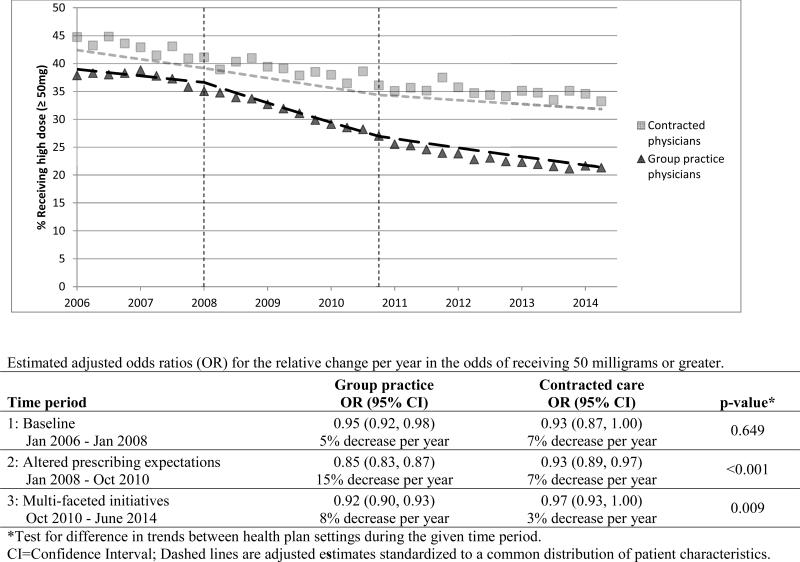
The percent of COT patients receiving an average daily dose of 50 milligrams or greater MED also showed larger reductions in the group practice than in the contracted care setting (Figure 3).
Figure 3.
Case-mix adjusted trends in percent receiving average daily opioid dose of 50 milligrams morphine equivalents or greater for COT patients of group practice and contracted physicians.
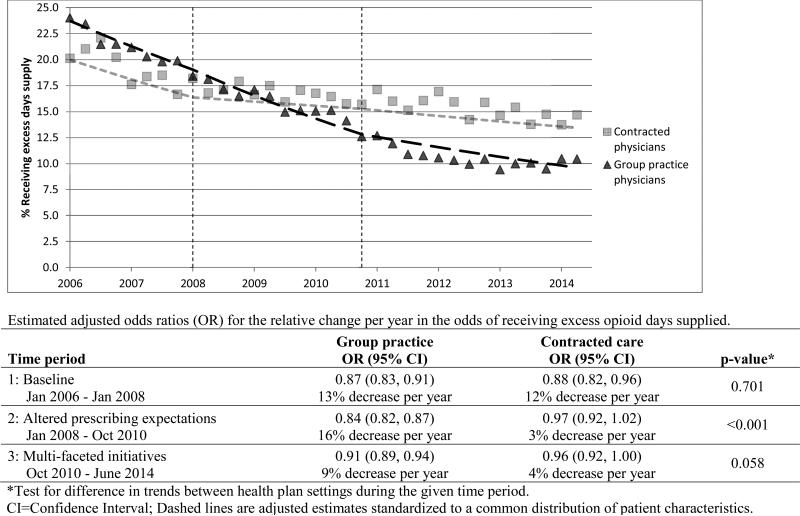
As shown in Figure 4, the percent receiving excess opioid days supplied declined from 24.0% to 10.4% in the group practice (57% lower) compared to a decline from 20.1% to 14.7% among COT patients of contracted physicians (27% lower). Adjusted for differences in patient characteristics, the odds of COT patients receiving excess opioid days supplied decreased at similar rates in both settings during the baseline phase (Figure 4), with odds declining approximately 12-13% per year (group practice patients OR 0.87; 95% CI 0.83-0.91, and contracted care patients OR 0.88; 95% CI 0.82-0.96). While this rate of decline continued in the group practice during the 2008-10 phase of altered prescribing expectations (OR 0.84; 95% CI 0.82-0.87) and to a lesser extent during the 2010-14 multi-faceted initiatives risk reduction phase (OR 0.91; 95% CI 0.89-0.94), we observed no such significant rates of decline in prescribing an excess days supply in the contracted care setting during both these periods (ORs 0.97 and 0.96 for the two phases from 2008 through 2014, with confidence intervals overlapping 1.0).
Figure 4.
Case-mix adjusted trends in percent receiving more than 20% excess opioid days supplied for COT patients of group practice and contracted physicians.
DISCUSSION
In Washington State, use of high opioid dose and dispensing excess opioid days supplied for COT patients declined after dissemination of a state COT guideline. These reductions were substantially larger in a health plan's group practice setting which devoted additional effort and resources to changing shared expectations regarding COT prescribing among its PCPs compared to reductions among contracted physicians of the same health plan exposed only to the statewide changes. While dissemination of the statewide guideline may have initiated changes in opioid prescribing among Washington State physicians, more intensive group practice efforts to change shared expectations resulted in greater reductions in high-dose opioid prescribing. Exploratory analyses, reported elsewhere [17], suggest that these differences may have been due to lower rates of opioid dose escalation and increased rates of partial-dose reduction in the group practice setting. The divergence was not due to differences in rates of large reductions in opioid dose among COT patients.
Contrary to our initial expectations, a decline in prescribing higher average doses, prescribing of high opioid doses, and dispensing excess opioid days supplied appeared to be underway during the baseline period. Inspection of trends suggests that some prescribing changes may have started in both the group practice and the contracted care settings before the state guideline was released and before the group practice initiatives were implemented. However, prescribing of higher doses and excess opioid days supplied plateaued at a higher level among the COT patients of contracted care physicians than among the COT patients of group practice physicians. These observations are consistent with the augmented efforts to change clinician expectations of appropriate opioid prescribing in the group practice setting changing opioid doses among COT patients. The differences in opioid dose and excess opioid days supplied achieved by the end of the study period were both clinically and statistically significant in the group practice setting.
This evaluation did not have access to COT patients from outside Washington State who were not affected by the Washington State guideline and legislation. For that reason, we are unable to determine whether the Washington State guideline and legislation played a role in the reductions in average daily dose and high dose prescribing observed in the contracted care setting. However, national data on opioid prescribing indicates that aggregate quantities of opioids prescribed in the United States increased dramatically from 2007 through 2011, and that opioid prescribing by family medicine and internal medicine physicians were also increasing from 2007 through 2012 [12]. This suggests that the reductions observed in Washington State were attributable, at least in part, to the State guideline and legislation.
It is not possible to determine whether the initiatives implemented in the group practice setting would have had similar impact in the absence of the statewide guideline and legislation. Both the initial efforts to alter shared clinician expectations regarding appropriate opioid prescribing of group practice physicians and the multi-faceted opioid risk reduction initiative were influenced by the statewide guideline and state legislation. There was synergy between the statewide guideline and legislation and the efforts to change opioid prescribing practices in the group practice setting. While the Washington State guideline and legislation were controversial nationally, the development and implementation of the guideline and legislation were less controversial among physicians practicing medicine within Washington State, who strongly supported the guideline provisions. Key leaders in pain medicine from the University of Washington were actively involved in the development of the guidelines and supported their dissemination.
It is noteworthy that the number of patients receiving COT continued to increase over the study period in both the group practice and contracted care settings. Neither the statewide guideline nor the group practice initiatives proposed changes intended to reduce the prevalence of long-term opioid prescribing per se. In the absence of specific guidance that could be expected to reduce the prevalence of use of COT, it is not surprising that the long-term trends in both the group practice and contracted care settings were towards a larger percentage of the population using COT over time.
Differences in the percent of patients on high opioid dose regimens and the percent receiving excess opioid days supplied in the group practice setting were significant from both a clinical and public health perspective. At the end of the study period, the percent of COT patients on high opioid dose regimens was 54% lower among group practice COT patients than among COT patients of contracted physicians. Since the risks of COT increase with dose [2], these differences may reduce risks of opioid-related adverse events. Further research is needed to determine whether sustained differences in opioid dose among similar patient populations affect rates of opioid-related adverse events, and to assess implications for pain outcomes. Since there are significant risks of opioid adverse effects at low as well as high doses, the research reported in this paper does not establish that dose reductions observed in the group practice setting will necessarily reduce COT risks of overdose, addiction, or other potential harms.
This evaluative research has important limitations. We were not able to compare opioid prescribing trends in the group practice and contracted care settings to trends among primary care physicians and COT patients not exposed to the Washington State guideline. It is possible that the reductions in high dose prescribing observed in the contracted care setting reflected broader trends toward reduced use of high opioid doses in other states. We think this is unlikely because the trends toward reduced use of high opioid doses were observed shortly after the dissemination of the Washington State guideline in April, 2007, and national trends toward increased prescribing of opioids in terms of the total volume of opioid medications prescribed continued through at least 2011 [12]. While we were able to control for some key patient characteristics in our analyses, we cannot exclude residual confounding as a potential explanation of the divergence in trends between the group practice and contracted care settings. It is also possible that the divergence in trends was explained by unmeasured differences in the two care settings other than the Group Health initiatives to change opioid prescribing. However, the timing of changes in opioid prescribing was generally consistent with the timing of the initiatives intended to reduce high dose prescribing.
We conclude that large reductions in use of high opioid doses among COT patients can be achieved and sustained. The changes observed appeared to be initiated, in part, by dissemination of a statewide guideline regarding use of high opioid doses for COT patients. Changes in average daily opioid dose, high dose prescribing and dispensing of excess opioid days supplied were appreciably greater in a group practice setting which took additional steps to change shared expectations of its physicians regarding appropriate COT prescribing when compared to a contracted care setting exposed only to the Washington State guideline and legislative changes.
PERSPECTIVE.
Washington State and a health plan's group practice implemented initiatives to reduce high dose COT prescribing. Group practice physicians were exposed to both initiatives, while the health plan's contracted physicians were exposed to only the statewide changes. Reductions in prescribing of high opioid dose, average daily dose, and excess opioid days supplied followed state and health plan initiatives to change opioid prescribing. Reductions were substantially greater in the group practice setting that implemented additional initiatives to alter shared physician expectations regarding appropriate COT prescribing, compared to the contracted physicians’ patients.
HIGHLIGHTS.
Using interrupted time series analyses, we assessed whether the Washington State chronic opioid therapy guideline and complementary health plan initiatives to change opioid prescribing reduced opioid doses among COT patients in a health plan's group practice (N=16,653) compared to its contracted care settings (N=5,552) exposed to only state guideline changes.
From 2006 through June 2014, the percent of COT patients receiving 120 or more milligrams morphine equivalent dose declined from 16.8% to 6.3% in the group practice versus 20.6% to 13.6% among COT patients of contracted physicians.
The proportion receiving excess opioid days supplied declined from 24.0% to 10.4% among group practice COT patients and from 20.1% to 14.7% among COT patients of contracted physicians.
Acknowledgments
We gratefully acknowledge the contributions of members of the Patient Advisory Committee guiding this research, including Catherine Cartwright, Penny Cowen, David Duhrkoop (chairperson), Mariann Farrell, Ada Giudice-Tompson, Kathryn Guthrie, Catherine Lippincott, Max Sokolnicki, and Betts Tully.
This research was supported by grants to Group Health Research Institute (GHRI) from Pfizer Inc. (Von Korff, Principal Investigator), the Patient-Centered Outcomes Research Institute (IHS-1306-02198, Von Korff, Principal Investigator), and the National Institute on Aging (AG034181, Von Korff, Principal Investigator).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Von Korff is the Principal Investigator of grants to GHRI from Pfizer Inc. that concern opioids. These grants also support work on this project by Dr. Shortreed, Ms. Saunders, and Mr. Walker. Dr. Shortreed has received funding from research grants awarded to GHRI by Bristol-Myers Squibb and Pfizer Inc. Ms. Saunders owns stock in Merck. The remaining authors report no conflicts.
REFERENCES
- 1.Biller-Andorno N, Lee TH. Ethical physician incentives—From carrots and sticks to chared purpose. NEJM. 2013;368:980–982. doi: 10.1056/NEJMp1300373. [DOI] [PubMed] [Google Scholar]
- 2.Chou R, Turner JA, Devine EB, Hansen RN, Sullivan SD, Blazina I, Dana T, Bougatsos C, Deyo RA. The Effectiveness and Risks of Long-Term Opioid Therapy for Chronic Pain: A Systematic Review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162:276–86. doi: 10.7326/M14-2559. [DOI] [PubMed] [Google Scholar]
- 3.Coben J, Davis S, Furbee P, Sikora RD, Tillotson RD, Bossarte RM. Hospitalizations for poisoning by prescription opioids, sedatives, and tranquilizers. Am J Prev Med. 2010;38:517–524. doi: 10.1016/j.amepre.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 4.Compton W, Volkow N. Major increases in opioid analgesic abuse in the United States: Concerns and strategies. Drug Alcohol Depend. 2006;81:103–107. doi: 10.1016/j.drugalcdep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Davidoff F. Less is More: On the undiffusion of established practices. JAMA Internal Medicine. 2015;175:809–11. doi: 10.1001/jamainternmed.2015.0167. [DOI] [PubMed] [Google Scholar]
- 6.Edlund MJ, Martin BC, Fan MY, Devries A, Braden JB, Sullivan MD. Risks for opioid abuse and dependence among recipients of chronic opioid therapy: results from the TROUP study. Drug Alcohol Depend. 2010;112:90–8. doi: 10.1016/j.drugalcdep.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franklin GM, Mai J, Turner J, Sullivan M, Wickizer T, Fulton-Kehoe D. bending the prescription opioid dosing and mortality curves: impact of the Washington State opioid dosing guideline. Am J Ind Med. 2012;55:325–31. doi: 10.1002/ajim.21998. [DOI] [PubMed] [Google Scholar]
- 8.Fretheim A, Zhang F, Ross-Degnan D, Oxman AD, Cheyne H, Foy R, Goodacre S, Herrin J, Kerse N, McKinlay RJ, Wright A, Soumerai SB. A reanalysis of cluster randomized trials showed interrupted time-series studies were valuable in health system evaluation. J Clin Epidemiol. 2015;68:324–33. doi: 10.1016/j.jclinepi.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Garg RK, Fulton-Kehoe D, Turner JA, Bauer AM, Wickizer T, Sullivan MD, Franklin GM. Changes in opioid prescribing for Washington workers’ compensation claimants after implementation of an opioid dosing guideline for chronic noncancer pain: 2004 to 2010. J Pain. 2013;14:1620–8. doi: 10.1016/j.jpain.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Greenhalgh T, Robert G, Macfarlane F, Bate P, Kyriakidou O. Diffusion of innovations in service organizations: Systematic review and recommendations. Milbank Quarterly. 2004;82:581–629. doi: 10.1111/j.0887-378X.2004.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hastie T, Tibshirani R, Friedman J. The Elements of Statistical Learning. Second edition Springer; 2009. [Google Scholar]
- 12.Jones CM. The Opioid Epidemic: Overview and a Look to the Future.. Presentation at the Washington State; Seattle, WA. June 12, 2015. [Google Scholar]
- 13.Office of National Drug Control Policy [February 19, 2014];Epidemic: Responding to America's Prescription Drug Abuse Crisis. 2011 Apr 11; [updated 2011 Apr 11; cited 2011 Jun 10]. Available at: http://www.whitehousedrugpolicy.gov/publications/pdf/rx_abuse_plan.pdf.
- 14.Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. 2010;363:1981–1985. doi: 10.1056/NEJMp1011512. [DOI] [PubMed] [Google Scholar]
- 15.Palmer R, Carrell D, Cronkite D, Saunders K, Gross DE, Masters E, Donevan S, Hylan T, VonKorff M. The prevalence of problem opioid use in patients receiving chronic opioid therapy: computer assisted review of electronic health record clinical notes. Pain. 2015;156:1208–14. doi: 10.1097/j.pain.0000000000000145. [DOI] [PubMed] [Google Scholar]
- 16.Saunders KW, Davis RL, Stergachis A. Group Health Cooperative. In: Strom B, editor. Pharmacoepidemiology. 4th ed. John Wiley and Sons; West Sussex, England: 2005. pp. 223–39. [Google Scholar]
- 17.Saunders K, Shortreed S, Thielke S, Turner JA, LeResche L, Beck R, Von Korff M. Evaluation of health plan interventions to influence chronic opioid therapy prescribing. Clin J Pain. 2015 Jan 23; doi: 10.1097/AJP.0000000000000159. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Substance Abuse and Mental Health Services Administration (SAMHSA) Results from the 2012 National Survey on Drug Use and Health: Summary of national findings. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2013. NSDUH Series H-46, HHS Publication No. (SMA) 13-4795. [Google Scholar]
- 19.Sullivan MD, Von Korff M, Banta-Green C, Merrill JO, Saunders K. Problems and concerns of patients receiving chronic opioid therapy for chronic non-cancer pain. Pain. 2010;149:345–53. doi: 10.1016/j.pain.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trescott CE, Beck RM, Seelig MD, VonKorff MR. Group Health's initiative to avert opioid misuse and overdose among patients with chronic non-cancer pain. Health Aff. 2011;30:1420–1424. doi: 10.1377/hlthaff.2011.0759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turner JA, Saunders K, Shortreed SM, Rapp SE, Thielke S, Leresche L, Riddell KM, Von Korff M. Chronic Opioid Therapy Risk Reduction Initiative: Impact on Urine Drug Testing Rates and Results. J Gen Intern Med. 2014;29:305–11. doi: 10.1007/s11606-013-2651-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27:299–309. doi: 10.1046/j.1365-2710.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- 23.Wald A. Tests of Statistical Hypotheses Concerning Several Parameters When the Number of Observations Is Large. Transactions of the American Mathematical Society. 1943;54:426–82. [Google Scholar]
- 24.Washington State Agency Medical Directors’ Group [February 19, 2014];Interagency guideline on opioid dosing for chronic non-cancer pain: an educational aid to improve care and safety with opioid therapy. 2010 update. Available at: http://www.agencymeddirectors.wa.gov/Files/OpioidGdline.pdf.
- 25.Von Korff M, Saunders K, Ray GT, Boudreau D, Campbell C, Merrill, Sullivan MD, Rutter C, Silverberg M, Banta-Green C, Weisner C. Defacto Long-term Opioid Therapy for Non-cancer Pain. Clinical Journal of Pain. 2008;24:521–527. doi: 10.1097/AJP.0b013e318169d03b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeger S L, Liang JK-Y. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30. [PubMed] [Google Scholar]