Abstract
Background
β-adrenergic receptors (βARs) play paradoxical roles in the heart. On one hand, βARs augment cardiac performance to fulfill the physiological demands, but on the other hand, prolonged activations of βARs exert deleterious effects that result in heart failure. The signal transducer and activator of transcription 3 (STAT3) plays a dynamic role in integrating multiple cytokine signaling pathways in a number of tissues. Altered activation of STAT3 has been observed in failing heart in both the human patients and animal models. Our objective is to determine the potential regulatory roles of STAT3 in cardiac βAR-mediated signaling and function.
Methods and Results
We observed that STAT3 can be directly activated in cardiomyocytes by β-adrenergic agonists. To follow up this finding, we analyzed βAR function in cardiomyocyte-restricted STAT3 knockouts and discovered that the conditional loss of STAT3 in cardiomyocytes markedly reduced the cardiac contractile response to acute βAR stimulation, and caused disengagement of calcium coupling and muscle contraction. Under chronic β-adrenergic stimulation, Stat3cKO hearts exhibited pronounced cardiomyocyte hypertrophy, cell death, and subsequent cardiac fibrosis. Biochemical and genetic data supported that Gαs and Src kinases are required for βAR-mediated activation of STAT3. Finally, we demonstrated that STAT3 transcriptionally regulates several key components of βAR pathway, including β1AR and PKA, and T-type Ca2+ channels.
Conclusions
Our data demonstrates for the first time that STAT3 has a fundamental role in βAR signaling and functions in the heart. STAT3 serves as a critical transcriptional regulator for βAR-mediated cardiac stress adaption, pathological remodelling and heart failure.
Keywords: GPCR, βAR signaling, STAT3, heart failure
β-adrenergic receptors (βARs) belong to G protein-coupled receptor (GPCR) superfamily and are essential to cardiac physiology.1, 2 The human heart mainly expresses β1-adrenergic receptors (β1AR) and β2-adrenergic receptors (β2AR) at a ratio of 7:3.3 Under physiological conditions, βAR signaling regulates myocardial contraction and relaxation mainly through activation of Gαs-dependent mechanisms, such as the activation of adenylyl cyclase and protein kinase A (PKA).4 βARs are closely associated with the progression of heart failure.5 The hallmarks of heart failure include the specific down-regulation of β1AR expression by up to 50%, uncoupling of β1AR from Gαs, and an increase of Gαi coupling to antagonize Gαs signaling. However, the sustained activation of βAR/Gαs-dependent adenylyl cyclase signaling has also been suggested to exert deleterious effects on heart remodeling, leading to cardiac hypertrophy, cardiomyocyte death, and subsequent cardiac fibrosis, especially in failing hearts.1, 6, 7 Therefore, regulation of the homeostasis of βAR signaling has a strong impact on both physiological and pathophysiological functions of the heart. In addition, βARs can exert their biological functions via signaling pathways independent of the second messenger cAMP-generating mechanisms.8–10 Recent evidence has demonstrated that βARs are able to couple directly with other effectors and activate the ERK MAPK pathway, which has been suggested to promote the activation of a cardiac protective program to counteract the effect of catecholamine toxicity.11, 12
STAT (signal transducer and activator of transcription) is a family of transcriptional regulators that mediate a wide range of biological functions primarily in response to extracellular signaling molecules such as cytokines and growth factors.13, 14 In general, STAT activation is through the phosphorylation of a single tyrosine residue that results in Src homology 2 (SH2) domain-mediated dimerization, nucleus localization, DNA binding, and ultimately transcription activation or repression. Recently, it has been also suggested that STAT3 (and probably other STATs as well) can exert its unique transcriptional activity without tyrosine phosphorylation.15 STAT3 plays a convergent role in integrating multiple signaling pathways that are involved in a wide variety of physiological processes.13, 14 Alterations in STAT3 activation and expression are associated with various pathophysiological adaptations in the heart, such as heart failures in humans and in a mouse model of dilated cardiomyopathy.16–18 Cardiomyocyte-restricted ablation of STAT3 (Stat3cKO) in mice produces normal cardiac morphology and histology, suggesting that STAT3 is not critical to ventricular wall development, but develops cardiac hypertrophy and eventual heart failure in adult mice (> 6-month old).19, 20 However, the underlying mechanism associated with this progressive heart failure has not been carefully characterized.
In this study, we have identified STAT3 as a key signaling element in βAR functions. STAT3 plays critical roles in regulating cardiac physiology via its transcriptional regulation of the homeostasis of βAR signaling components. By examining Stat3cKO hearts at young age, we discovered that the cardiac inotropic and lusitropic response to acute βAR stimulation was severely impaired. On the other hand, chronic βAR stimulation markedly enhanced hypertrophic response, cardiomyocyte death, and cardiac fibrosis in Stat3cKO mice. Biochemical analyses further demonstrated that STAT3 is an immediate signaling mediator of βARs, and that STAT3 acts as a master transcriptional regulator for several key signaling and functional components in βAR signaling pathway, including β1AR, PKA, voltage gated L-type Ca2+ channel, and T-type Ca2+ channel subunits. Most importantly, STAT3 regulates these components under both basal and βAR activated conditions, and provides multi-phase control of cardiac physiology. This study reveals, for the first time, that STAT3 is a pivotal element of βAR signaling in the heart.
METHODS
Mice
Mice with deletion of STAT3 specifically in cardiomyocytes using MHC-cre (Stat3cKO) and their control littermates were maintained in C57 BL/6 genetic background.19 Both Stat3f/f mice (MHC-Cre negative) and Stat3f/+; MHC-Cre+ mice were used as control mice, as they are undistinguishable in all aspects of histological and physiological measurements. MHC-Cre was kept as heterozygotes. For consistency, only 8 week-old young adult Stat3cKO male mice were used for experiments unless otherwise indicated. To effectively delete STAT3 in cardiomyocytes under inducible condition, Stat3f/f mouse were crossed with doxycycline-inducible Tnnt2-rtTA;Tre-cre mouse line (Stat3icKO) 21, 22. The STAT3 deletion was thus carried out by feeding doxycycline-containing chow (Doxycycline 200mg/Kg, Bio-Serv) for 10 days. Cardiac hypertrophy induction by isoproterenol (ISO) was performed as previously described.23 All animal protocols were approved by Fuwai Hospital and Indiana University School of Medicine Institutional Animal Care and Research Advisory Committee.
Echocardiography
Mice were lightly anesthetized with 1.5% isoflurane until the heart rate stabilized at 400 to 500 beats per minute. Two-dimensional short-axis images were obtained with a high resolution Micro-Ultrasound system (Vevo770, VisualSonics Inc, Toronto, Canada) equipped with a 40-MHz mechanical scan probe as previously described. 24
Single cell calcium imaging
Myocytes were loaded with Fluo-4 AM (5μM, 20 min.) at room temperature. After de-esterification, the cells were perfused with normal Tyrode solution (1.8 mM Ca2+) at 36°C ± 0.5. Confocal Ca2+ imaging was performed with a laser scanning confocal microscope (LSM 510 Meta, Carl Zeiss) equipped with a NA=1.35, 63x lens. Line scan measurements of Ca2+ transients, SR Ca2+ content and Ca2+ sparks were acquired at a sampling rate of 1.93 ms/line along the longitudinal axis of the myocytes. Ca2+ Sparks were measured under resting conditions. Steady state Ca2+ transients were achieved by a 30 sec pacing at 1 Hz. SR Ca2+ content was measured as Ca2+ release induced by 20 mM caffeine. All digital images were processed offline with IDL 6.0 (Research System Inc).
Isolated heart perfusion system (Langendorff)
Langendorff experiments were performed in isolated mouse hearts as described previously.25, 26 Detailed protocol is described in supplemental materials.
Cell line
The MEF cells deficient of Gαs (Gαs−/− cells) were derived from Gαs (exon 2) knockout mice.27, 28 The MEF cells deficient in both β1AR and β2AR (β1−/−β2−/− cells) were derived from β1-AR and β2-AR double knockout mouse embryos.28 The MEF cells deficient in Src-family tyrosine kinases (SYF cells) were purchased from ATCC.
Statistical analysis
All values are presented as mean ± SEM. Statistical significance was determined by t test (for groups of two), or by one-way ANOVA t (for groups of three or more) using Sigma plot software package (Sigman). In the case that the results failed to pass the normality test, Wilcoxon Signed Rank T-test (for group of two), or Kruskal-Wallis methods for one-way ANOVA (for group three or more) were used to adjust the analysis and to determine the statistical significance. As the experiments analyzing calcium properties used multiple cells per animal, the experiment results were analyzed using a mixed-effect model with a “proc mixed” in the statistical software SAS 9.4 (details see: Supplemental materials and methods).
RESULTS
STAT3 activation by βAR in cardiomyocytes
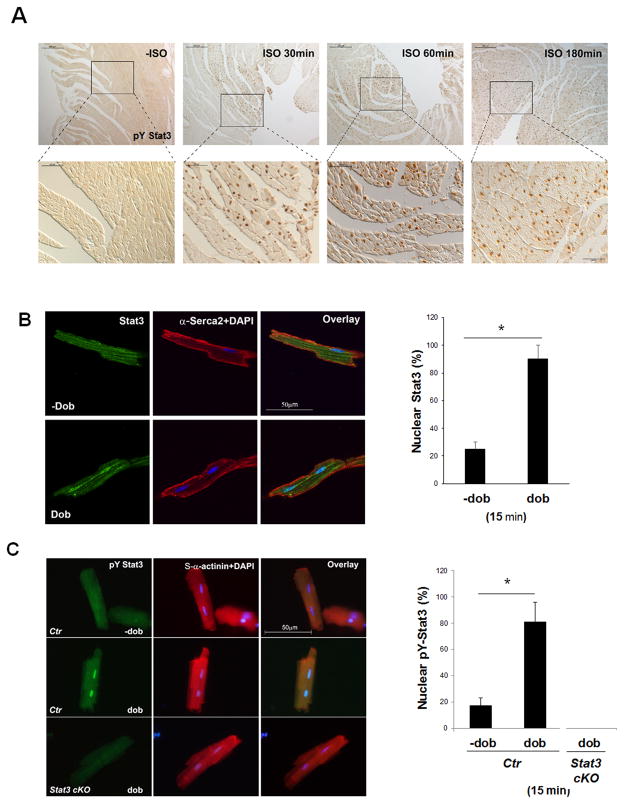
As βAR-initiated signaling played critical roles in cardiac hypertrophy and heart failure and altered STAT3 activation was observed in failing hearts,16–18 we tested if βAR agonists could activate STAT3 in the hearts. 2-month old male mice were administrated with isoproterenol (ISO) by single injection [1 μg/g bodyweight, intrapretoneal (ip)] and the levels of activated STAT3 (pY705-STAT3) were assessed by immunohistochemical (IHC) analyses. Activated STAT3 were detected within 30 minutes of ISO-administration in a small portion of cardiomyocytes. By 3 hours, almost all ventricular cardiomyocytes are positive for nuclear localized pY705-STAT3 (Figure 1A). Consistent with this finding, Western blot was able to detect pY705-STAT3 at around 1 hour after ISO-administration (Supplemental Figure 1A).
Figure 1.
Activation of STAT3 by βARs in cardiomyocytes. A, Representative images of immunohistochemical staining of pY-STAT3 in wild-type mouse hearts treated with ISO or saline (−ISO) for indicated amount of time via ip injection (1μg/g bodyweight.). B, Representative fluorescence images (left panel) and quantification (right panel) of nuclear STAT3 in isolated wild-type cardiomyocytes with or without dobutamine stimulation (dob) (1μM, for 15 minutes). Positive immune reactivity to anti-STAT3 antibody is shown in green and to anti-Serca2 antibody is shown in red. The experiments were repeated independently 3 times (N > 300 cells/each group counted). C, Representative fluorescence images (left panel) and quantification (right panel) of pY-STAT3 staining in isolated wild-type cardiomyocytes treated with or without dob (1μM, for 15 minutes). Positive immune reactivity to anti-pY-STAT3 antibody is shown in green and to anti-α–actinin (sarcomere) antibody is shown in red. Stat3cKO cardiomyocytes were used as a negative control for immune reactivity of the anti-pY-STAT3 antibody. The experiments were repeated independently 3 times (N > 300 cells/each group).
To exclude non-cell autonomous effect on STAT3 activation by βAR agonist, we isolated cardiomyocytes from wild-type adult mice and stimulated with dobutamine (1 μM) in vitro. Dobutamine treatment could rapidly induce nuclear localization of STAT3 (Figure 1B) and pY705-STAT3 (Figure 1C) in cardiomyocytes within 15 minutes, demonstrating that STAT3, in addition to its well-defined roles in cytokine receptor signaling, is likely a down-stream signaling component of βARs.
βAR-initiated STAT3 activation requires GαS and Src kinase
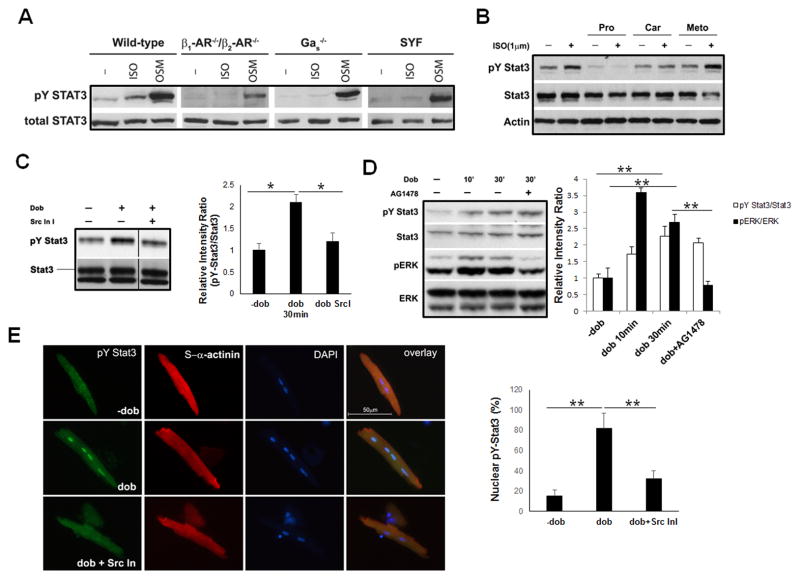
To investigate the molecular mechanism underlying βAR-mediated STAT3 activation, we took the advantage of the availability of established mouse embryonic fibroblast (MEF) cell lines with deletions of β1AR/β2AR, Gαs, or Src-family tyrosine kinases, respectively. Oncostatin M (OSM), a cytokine of the IL-6 family that activates STAT3 via dimerization of the IL-6 receptor and Gp130, was used as a positive control. Figure 2A demonstrate that ISO-induced STAT3 activation is completely abolished in β1AR/β2AR deficient (β1AR−/−/β2AR−/−) cells, while STAT3 activation by OSM treatment is not affected, indicating that STAT3 activation by ISO is a βAR-dependent event. Furthermore, ISO-induced STAT3 activation was similarly abolished in Gαs-deficient MEF cells (Figure 2A), suggesting GαS-dependence for STAT3 activation by βARs. Consistently, while specific β1AR antagonists (β-blockers) (metoprolol and bisoprolol) and specific β2AR antagonist (ICI-181,551) failed to block ISO-induced STAT3 activation in neonatal mouse cardiomyocytes (NMCs) (Figure 2B and supplemental Figure 1D), the non-selective βAR antagonists (propranolol and carvedilol) completely abolished βAR-mediated STAT3 activation in NMCs (Figure 2B and supplemental Figure 1D).
Figure 2.
Activation of STAT3 by βARs requires G αs and Src-family kinases. A, Western blot analysis of STAT3 activation in response to ISO (1 μM, 30 minutes) in β1AR/β2AR-compound deficient, G αs-deficient, and Src family kinases (SYF)-deficient mouse embryonic fibroblast (MEF) cells. OSM treatments were used as a positive control for pY-STAT3. B, Western blot analysis of STAT3 activation in neonatal cardiomyocytes in response to ISO (1 μM, 30 minutes) that pre-incubated with different βARs antagonists for 1 hour. Pro, propranolol; Car, carvedilol; Meto, metoprolol. C, Western blot analysis of pY-STAT3 in mouse neonatal cardiomyocytes with or without pre-incubation of Src kinase inhibitor I (1 μM) for 15 minutes, followed by dobutamine (1 μM) treatment. The relative intensity ratios between pY-STAT3 and total STAT3 are shown in the right panel, *P<0.05. D, Western blot analysis of pY-STAT3 and pERK activation in mouse neonatal cardiomyocytes with or without pre-incubation of EGFR inhibitor AG1478 (1 μM) followed by dobutamine (1 μM) treatment. The relative intensity ratios of pY-STAT3 vs total STAT3 and pERK1/2 vs total ERK1/2 are shown in right panel, **P<0.01 E, Representative fluorescence images of immune reactivity to anti-pY-STAT3 antibody (in green) in isolated wild-type cardiomyocytes treated with dobutamine (1 μM) for 30 minutes with or without pre-incubation of Src kinase inhibitor I (1 μM). α-actinin, in red; DAPI, in blue. The experiments were independently repeated 3 times (N >300 cells/each group) and results are expressed as positive cell number over total cell number (Right panel), **P<0.01.
Src-family protein tyrosine kinases are non-receptor kinases and have been known as major mediators for cytokine and various extracellular stimuli-induced STAT3 activation. Src-family kinases are known to be activated by βARs in GαS-dependent or -independent manners, and promote the activation of a second wave of signaling cascades including the ERK MAPK pathway.8, 10 To test whether Src kinases were involved in βAR-initiated STAT3 activation, we used SYF cells which were Src−/−/Yes−/−/Fyn−/− MEF cells (Src, Yes and Fyn are the main Src-family kinases in MEF cells). As shown in Figure 2A, ISO-initiated STAT3 activation was abolished in SYF cells. These data suggest that βAR activation of STAT3 depends upon Src-family tyrosine kinases in MEF cells. To extend these observations, we examined STAT3 activation in cardiomyocytes treated with dobutamine with or without pre-incubation of Src kinase inhibitor I. Both Western blot and immunofluorescence staining confirmed that Src kinase inhibitor I inhibited βAR-initiated STAT3 phosphorylation (Figure 2C and 2E).
As several studies indicated that β1AR is capable of trans-activating epidermal growth factor receptor (EGFR) to elevate MAPK signaling via a β-arrestin dependent pathway,11, 12 we tested if STAT3 activation induced by βAR was also in part dependent upon the activation of EGFR. We pre-incubated neonatal cardiomyocytes with EGFR inhibitor AG1478. This treatment completely blocked the dobutamine-induced ERK activation, but had no effect on dobutamine-induced STAT3 activation (Figure 2D). Collectively, our data demonstrate that βAR-mediated STAT3 activation is via Gαs and Src kinase.
Impaired βAR induced cardiac contractile function in Stat3cKO hearts
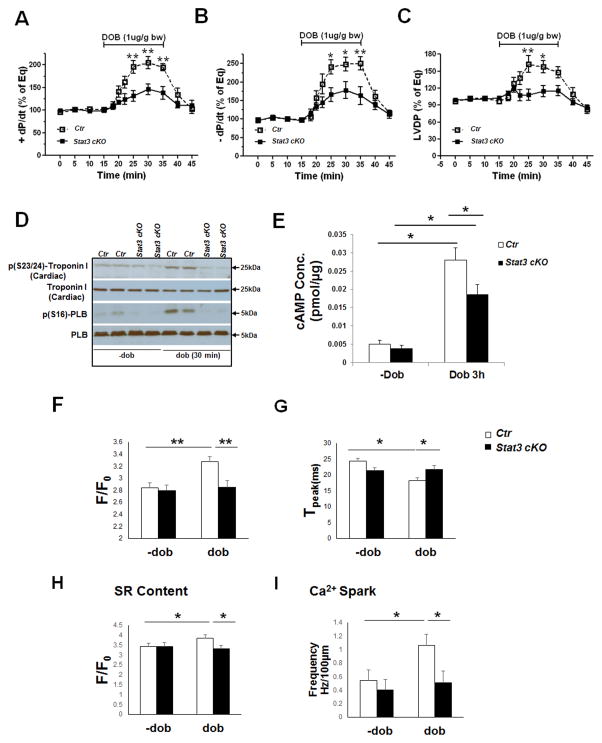
Next, we asked whether Stat3cKO hearts had an altered response to β-adrenergic stimulation. To exclude potential secondary effects from other exocrine systems on cardiac performance, we used the Langendorff system to directly measure and compare the contractile function of Stat3cKO and control hearts. As Stat3cKO mice did not have obvious altered cardiac morphology, histology and cardiac function under echocardiographic analysis before 2 months of age (Supplemental Figure 2), we used 2-month old hearts in the analyses. Of note, the treatment of β1AR agonist dobutamine could dramatically stimulate LV contractile [dP/dt (max) and LV developed pressure – LVDP] and relaxation function [dP/dt (min)] in control hearts. However, we observed that this effect of dobutamine on LV function was markedly diminished in Stat3cKO hearts (Figure 3A–C), suggesting that STAT3 is critical for β-adrenergic signaling-initiated cardiac functions. As Stat3cKO hearts showed a comparable inotropic response to Na+/K+ ATPase inhibitor ouabain, indicating the inotropic reserve in STAT3 mutant hearts was at least partially maintained, and the impaired inotropic response to βAR agonists was unlikely due to the collapse of inotropic reserve in Stat3cKO hearts (Supplemental Figure 3A), As the phosphorylation of troponin I [p(S23/24)-troponin I] and the phosphorylation of the phospholamban [p(S16)-PLB] by PKA are two of the major events underlying β-adrenergic mediated signaling,29 the levels of p-troponin I and p-PLB following 30 minutes of dobutamine stimulation were assessed by Western blots. We found that the base-line levels and the induction levels of p-troponin I and p-PLB were both significantly down-regulated in Stat3cKO hearts (Figure 3D). Consistently, up-regulation of second messenger cAMP induced by dobutamine was attenuated in Stat3cKO hearts when compared with controls (Figure 3E), suggesting that βAR-mediated signaling is impaired in Stat3cKO hearts. To further confirm that down-regulation of βARs/PKA signaling was not due to a secondary myocardium adaption resulted from an early stage ablation of STAT3 in myocardium, we used a doxycycline-inducible cre transgenic line (Tnnt2-rtTA;Tre-cre)21 to ablate myocardial STAT3 in 2-month old mice (Stat3icKO). We found that similar to what we observed in Stat3cKO hearts in response to acute dobutamine treatment, the phosphorylation levels of troponin I [p(S23/24)] and PLB [p(S16)] were both reduced in the hearts of doxycycline-administered Stat3icKO mice when compared to Stat3f/f;Tnnt2-rtTA;Tre-cre mice without doxycycline treatment (Supplemental Figure 3D). Taking together, this series of work demonstrate that STAT3 plays a key role in βAR signaling. Furthermore, as intracellular Ca2+ handling plays a central role in coordinating cardiac contraction and relaxation in response to β-adrenergic activation,5 we measured the steady Ca2+ transient dynamics, SR Ca2+ content, and Ca2+ sparks in response to β-adrenergic stimulation in single cardiomyocytes isolated from control and Stat3cKO ventricles. At quiescent state, the Ca2+ transient amplitude and activation kinetics (Tpeak) were comparable in control and Stat3cKO cardiomyocytes (Figure 3F and Supplemental Figure 4). Dobutamine stimulation significantly elevated the amplitude and accelerated the upstroke kinetics (Tpeak) of Ca2+ transients in control cardiomyocytes, but not, or to a significantly lesser degree, in Stat3-deficient cardiomyocytes (Figure 3G). Similarly, the increases of SR Ca2+ content and the frequency of Ca2+ sparks in Stat3cKO cardiomyocytes induced by dobutamine were diminished when compared with control cardiomyocytes (Figure 3H and I). Together, these data clearly demonstrate a critical role for STAT3 in βAR-mediated physiological function in cardiomyocyte.
Figure 3.
Impaired βAR agonist-induced cardiac contractile function and blunted calcium coupling in STAT3-deficient hearts and cardiomyocytes. A–C, Evaluation of acute inotropic (A), lusitropic (B) responses, and LV developed pressure (LVDP) (C) in Stat3cKO and control hearts in response to dobutamine stimulation using a Langendorff perfusion system. Dobutamine was infused for 20 minutes. (bw: body weight ; Mean ± SEM; N=6/each group; *p<0.01 and **p<0.001). D, Western blots show that the levels of phosphorylated troponin I and phospholamban (PLB) are reduced in Stat3cKO hearts when compared to controls after 30 minutes of dobutamine stimulation. E, cAMP levels in Stat3cKO hearts are significantly lower compared to controls treated with dobutamine stimulation (N = 3/each group; *p<0.05). F–G, Comparison of steady-state Ca2+ transients from control and Stat3cKO cardiomyocytes. Prior to dobutamine treatment, Ca2+ transient amplitudes (F) and Tpeak (activation kinetics) (G) are comparable in control and Stat3cKO cardiomyocytes. (Control: N = 17 cells/4 mice; Stat3cKO: N = 17 cells/3 mice). Dobutamine stimulation increases the amplitude and speeds the kinetics (Tpeak) of control cardiomyocytes. In contrast, the amplitude and Tpeak of Ca2+ transient in Stat3cKO cells are not affected by dobutamine stimulation. H, Assessment of SR Ca2+ content of control and Stat3cKO ventricular cardiomyocytes. Prior to dobutamine treatment, SR Ca2+ content is similar between control and Stat3cKO ventricular myocytes. In response to dobutamine treatment, SR Ca2+ content is increased in control, but not in Stat3cKO cardiomyocytes (Control: N = 15 cells/3 mice; Stat3cKO: N = 18 cells/3 mice). I, Measurement of spontaneous Ca2+ spark frequency in control and Stat3cKO ventricular cardiomyocytes. Prior to dobutamine treatment, Ca2+ spark frequency is comparable in control and Stat3cKO cardiomyocytes. In response to dobutamine treatment, Ca2+spark frequency increases significantly in control cells, but not in Stat3cKO cells. (Control: N = 20 cells/3 mice; Stat3cKO: N = 21 cells/3 mice). *P<0.05, **P<0.01.
Enhanced cardiomyocyte death in response to chronic β-adrenergic stimulation in Stat3cKO hearts
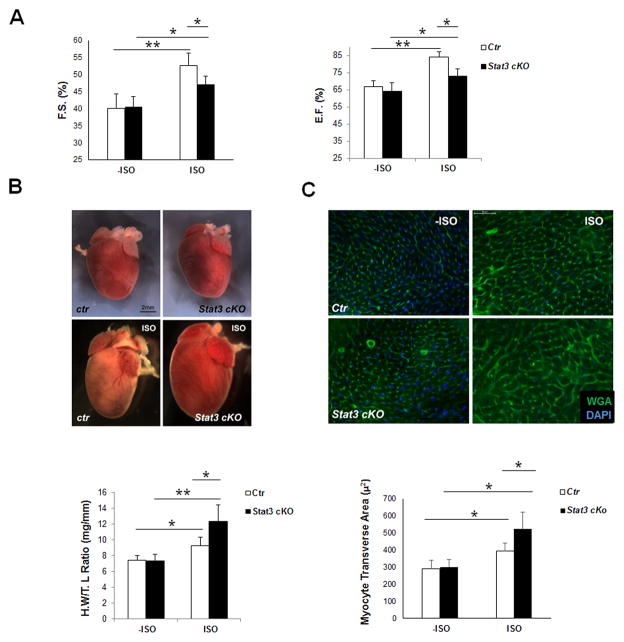
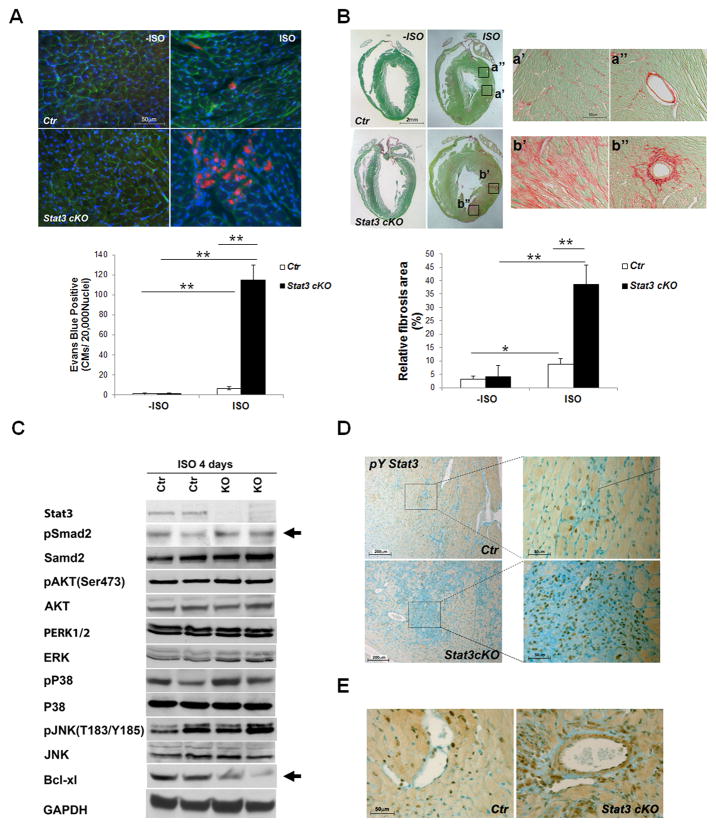
To investigate the function of STAT3 in response to chronic β-adrenergic stimulation, Stat3cKO mice and their control littermates were subjected to a 7-day isoproterenol administration using mini-osmotic pumps (1μg/g bodyweight/hour, 7 days). Cardiac function was assessed by echocardiographic analysis prior to and after ISO treatment. The 7-day β-adrenergic stimulation significantly enhanced cardiac systolic function, but simultaneously caused cardiac hypertrophy, cardiomyocyte death and cardiac fibrosis. As shown in Figure 4A, Stat3cKO mice showed an appreciable increase of cardiac contractile function in response to the chronic β-adrenergic stimulation, but the response was significantly attenuated when compared with that of littermate controls [FS (%): control mice, −ISO versus ISO: 40.18±4.1 to 52.6±3.1, n = 10; Stat3cKO mice, −ISO versus ISO: 40.54±3.7 to 47.7±2.5, n = 7)] (also see Supplemental Figure 5). Importantly, Stat3cKO mice developed a much more severe cardiac hypertrophy when compared with hearts from control littermates as demonstrated by the heart size, heart weight (normalized by tibia length) (Figure 4B), and cardiomyocyte transverse area (Figure 4C). Excessive cardiomyocyte necrosis and apoptosis were found in Stat3cKO hearts as compared to controls (Figure 5A and Supplemental Figure 6) which were accompanied by an increased level of perivascular and interstitial collagen deposition (Figure 5B). Consistent with the enhanced levels of cell death and fibrosis in Stat3cKO hearts, Western blot analyses demonstrated a significant reduction of Bcl-xl and an increase of TGFβ-signaling (i.e., an increased level of pSmad2) in Stat3cKO hearts after 4 days of continuous ISO stimulation when compared with controls (Figure 5C). Additionally, we found that deletion of STAT3 in cardiomyocytes greatly increased STAT3 activation in vasculature and cardiac fibroblast cells under prolonged ISO stimulation (Figure 5D and E), which strongly suggests the role of STAT3 in regulating collagen synthesis in myofibroblast cells. The protective function of STAT3 in cardiomyocyte against chronic ISO stimulation was further confirmed in the doxycycline treated Stat3icKO hearts; there were increased levels of cardiomyocyte necrosis and myocardial fibrosis in in the doxycycline treated Stat3icKO hearts after prolonged ISO administration (Supplemental Figure 7).
Figure 4.
Chronic administration of isoproterenol (ISO) induces excessive hypertrophy in Stat3cKO hearts. A, Echocardiographic analysis of cardiac function of control and Stat3cKO mouse (2-month old) in response to chronic ISO treatment. Left panel shows fractional shortening (FS%); Right panel shows ejection fraction (EF%) (Control: N = 10; Stat3cKO: N = 7), *P<0.05, **P<0.01. B, Upper panel shows representative images of control and Stat3cKO hearts (2-month old) prior to and after 7-day ISO perfusion. Lower panel shows heart weight vs tibia length ratios (N = 7/each group) of Stat3cKO hearts compared to controls after 7-day ISO perfusion, *P<0.05, **P<0.01. C, Upper panel shows representative fluorescent images of WGA (wheat germ agglutinin) staining (in green) of comparable left ventricular region of control and Stat3cKO hearts prior to and after 7-day ISO perfusion; DAPI, in blue. Lower panel shows the quantitation of cardiomyocyte transverse area (in μ2) of Stat3cKO and control hearts after 7-day ISO perfusion (N = 5/each group), *P<0.05, **P<0.01.
Figure 5.
Chronic administration of isoproterenol causes excessive myocyte death and cardiac fibrosis in Stat3cKO hearts. A, Chronic ISO stimulation induces excessive cardiomyocyte death in Stat3cKO hearts. Upper panel shows representative images of Evans blue (in red) uptake in control and Stat3cKO hearts with or without 7-day ISO perfusion (400x). WGA, in green; DAPI, in blue. Lower panel shows the quantitation of Evans blue positive myocytes in control and Stat3cKO hearts (N = 5/each group). B, Upper panel shows Sirius red and fast green staining to demonstrate the collagen deposition in control and Stat3cKO hearts with or without 7-day ISO perfusion (N = 5/each group). Lower panel shows the quantification of collagen contents, *P<0.05, **P<0.01. C, Representative results of Western blots show the reduced levels of Bcl-xl and the increased levels of pSmad2 in Stat3cKO hearts after 4 days of continuous ISO stimulation when compared with controls. D–E, Representative images of IHC staining of pY-STAT3 in control and Stat3cKO hearts after 7-day ISO perfusion. Activated STAT3 are found enhanced in myofibroblast cells in Stat3cKO hearts.
βAR signaling components are direct transcriptional target of STAT3
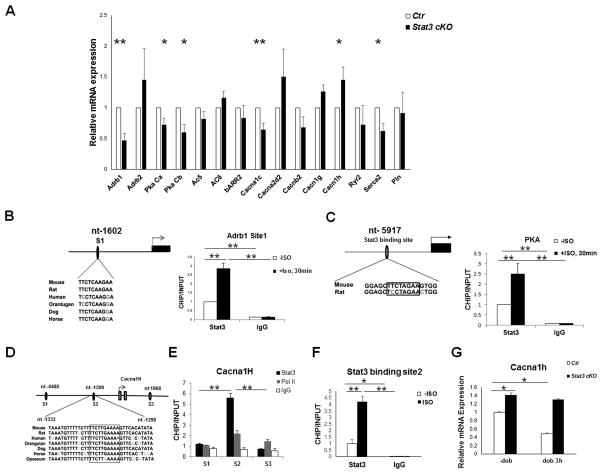
To further understand the molecular mechanisms by which STAT3 contributes to the functions of βAR, we investigated the effect of STAT3 deficiency on the transcription of signaling components downstream of βAR. Previously, using genome-wide chromatin immunoprecipitation (ChIP) and gene expression array analyses, the occupancy of STAT3 on different chromatin regions was examined in embryonic stem cells.30, 31 By exploring the published database in these studies, we found that the promoter regions of a number of key βAR signaling components (e.g., Adrb2, Ryr2, Serca2 and Cacna2d2, etc.) are occupied by STAT3 (Supplemental Table 1). To further investigate the transcriptional regulation in the heart, we surveyed the mRNA levels of major βAR signaling components in Stat3cKO hearts, which included β1AR (Adrb1), β2AR (Adrb2), PKA subunits, adenylyl cyclase (ACs), G protein-coupled receptor kinases (GRK2 and GRK5), and β-arrestin 2, and important effectors of βAR signaling in the hearts [e.g., cardiac troponin I (TnI), ryanodine receptor 2 (Ryr2) and phospholamban (Pln), voltage-gated Ca2+ channel subunits]. As shown in Figure 6A, Adrb1, PKA catalytic subunit α and β (Prkaca and Prkacb), L-type calcium channel subunits Cacnα1c, and Serca2 were significantly down-regulated in Stat3cKO hearts. These genes are known to be critical to the cardiac inotrophic and lusitropic response. Down-regulation of these genes explains the phenotype of decreased responses to acute βAR stimulation in Stat3cKO hearts. GRKs and β-arrestin 2 expression were not found altered. Interestingly, the pore forming α-subunit of voltage-gated T-type Ca2+ channel (Cacnα1h) was significantly up-regulated in Stat3cKO hearts when compared with littermate controls (Figure 6A). Cacnα1h up-regulation has been implicated to be a contributing factor in cardiac hypertrophy and heart failure.32–34 To understand whether these genes were direct targets of STAT3, we analyzed promoter regions of two critical signaling components, Adrb1 and Prkaca, and identified highly conserved STAT3 binding sites in the mouse Adrb1 and Prkaca promoter regions, located at nt-1602 and nt-5917 respectively (Figure 6B and C). ChIP assays demonstrated that STAT3 bound to both sites. Importantly, the binding activities increased dramatically in response to ISO treatment (Figure 6B and C). Additional experiments using luciferase assay further confirmed the function of both sites in response to STAT3 expression (Supplemental Figure 8).
Figure 6.
βAR signaling components as direct targets of STAT3. A, Quantitative RT-PCR analyses of βAR signaling components and calcium handling genes from hearts of 2-month old control and Stat3cKO mice (N = 3/each group). Ribosomal protein L7 mRNA was used for normalization. B, Positive regulation of Adrb1 by STAT3. Chromatin immunoprecipitation (ChIP) analysis of the STAT3 binding site in the promoter region of Adrb1 at nt-1602 relative to the transcription start site (TSS). C, Positive regulation of Prkaca by STAT3. ChIP analysis of the STAT3 binding site in the promoter region of Prkaca at nt-5917 relative to its TSS. D, Schematic diagram of putative STAT3 binding sites at the promoter region of Cacna1h. E–F, ChIP assay in combination with quantitative PCR demonstrates the active STAT3 binding site and its response to ISO stimulation. G, Cacna1h expression in 2-month old control and Stat3cKO hearts with or without dobutamine treatment (1 μg/g bodyweight), *P<0.05, **P<0.01.
Three potential STAT3 binding sites (S1, nt-4488; S2, nt-1309; S3, nt-1968) were initially identified in the promoter region of the mouse Cacnα1h gene by bioinformatics analysis (Figure 6D). ChIP analysis demonstrated that nt-1309 was the only binding site for STAT3 (Figure 6E). ISO-treatment enhanced the binding activity of STAT3 at nt-1309 site (Figure 6F). Unlike Adrb1 and Prkaca, ISO treatment suppressed Cacnα1h expression in control hearts, but not in Stat3cKO hearts (Figure 6G), further indicating that STAT3 is a negative transcriptional regulator of Cacnα1h.
Altered expression of voltage-gated T-type Ca2+ channel contributes to excessive cardiomyocyte death in Stat3cKO hearts
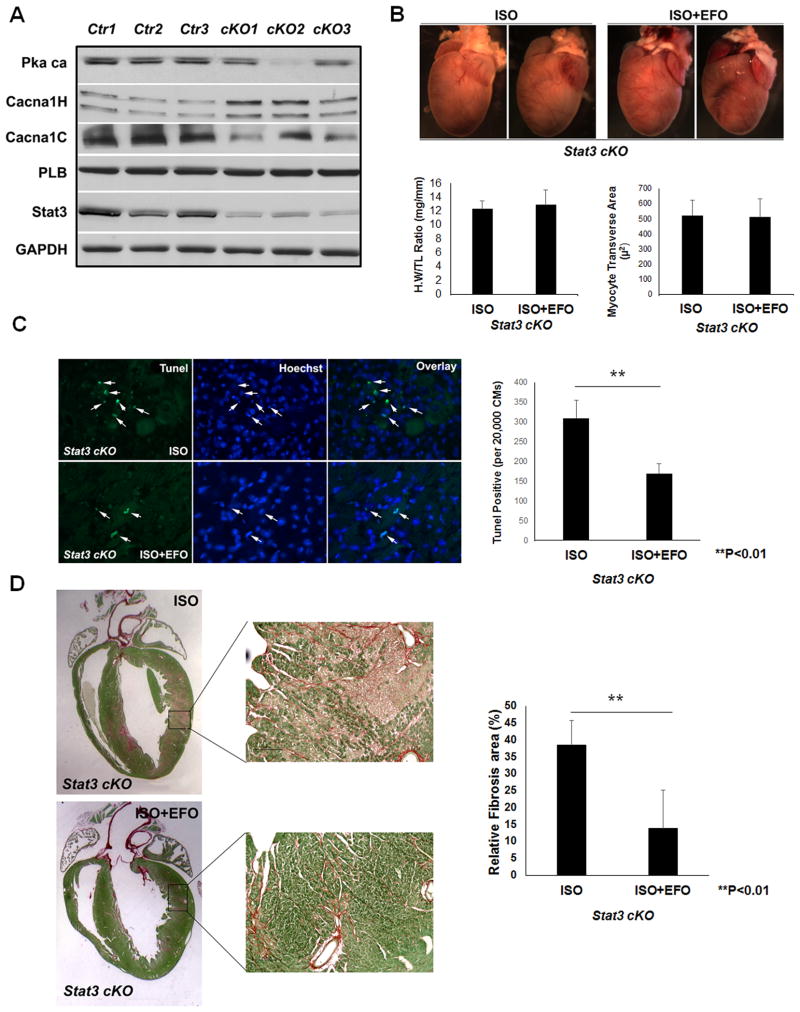
Given that calcium dynamics plays an important role in βAR mediated cardiac remodeling,5 we are particularly interested in the altered expression levels of Cacnα1h in Stat3cKO hearts. Consistent with quantitative RT-PCR data, Western blot confirmed that Cacnα1c was down-regulated and Cacnα1h was significantly up-regulated in Stat3cKO hearts (Figure 7A), which suggested a reduced L-type calcium current and increased t-type calcium current. As a number of recent studies demonstrated that the increase of T-type calcium currents is closely associated with the adverse cardiac remodeling,35, 36 we tested whether inhibition of T-type channel function will alleviate the cardiac hypertrophy and/or cardiomyocyte death and cardiac fibrosis in Stat3cKO hearts under chronic ISO stimulation. A T-type Ca2+ current suppressing does of efonidipine (25mg/kg bodyweight)33 was given to Stat3cKO mice (2-month old) under 7-day isoproterenol administration. We found that efonidipine treatment did not suppress chronic β-adrenergic stimulation induced cardiomyocyte hypertrophy; the heart weight-to-tibia length ratio and cardiomyocyte transverse area (μ2) were comparable between the mice treated with either isoproterenol alone or co-treated with efonidipine (Figure 7B). However, there was a significant reduction of cardiomyocyte death and cardiac fibrosis induced by β-adrenergic stimulation when efonidipine was co-administrated in Stat3cKO mice (Figure 7C and D). This data revealed that the elevated level of T-type calcium current is likely one of the major contributing factors to β-adrenergic-mediated cardiomyocyte death.
Figure 7.
Altered expression of Ca2+ channel protein contributes to excessive cardiomyocyte death in Stat3cKO hearts. A, Representative Western blot analysis of Pkaca, Cacna1H, Cacna1C, and PLB protein expression in control and Stat3cKO mouse heart. B, Administration of Efonidipine (EFO) did not affect the cardiomyocyte hypertrophy induced by chronic isoproterenol stimulation in Stat3cKO mouse hearts. Representative images of the hearts from Stat3cKO mice under chronic ISO stimulation alone (Stat3cKO, ISO) or in combination with oral administration of efonidipine (Stat3cKO, ISO+EFO). Heart weight vs tibia length ratios is shown in left lower panel (Stat3cKO, ISO: N = 9; Stat3cKO, ISO+EFO: N = 6). Cardiomyocyte transverse area (μ2) is shown in the right lower panel (Stat3cKO, ISO: N = 3; Stat3cKO, ISO+EFO: N = 3). C, Efonidipine administration alleviated cardiomyocytes death and cardiac fibrosis induced by chronic isoproterenol stimulation in Stat3cKO hearts. Representative images of TUNEL staining (green) of Stat3cKO hearts treated with ISO for 7 days alone or co-administrated with Efonidipine (400x). Right panel shows the quantitation of TUNEL positive myocytes in Stat3cKO ISO vs Stat3cKO ISO+EFO mouse heart (N = 3/each group, **P<0.01). D, Sirius red/fast green staining showing the collagen deposition (red signal) is markedly decreased in Stat3cKO heart with co-administration of Efonidipine in comparison with Stat3cKO heart treated with ISO alone for 7 days (N =3 in each group, **P<0.01).
DISCUSSION
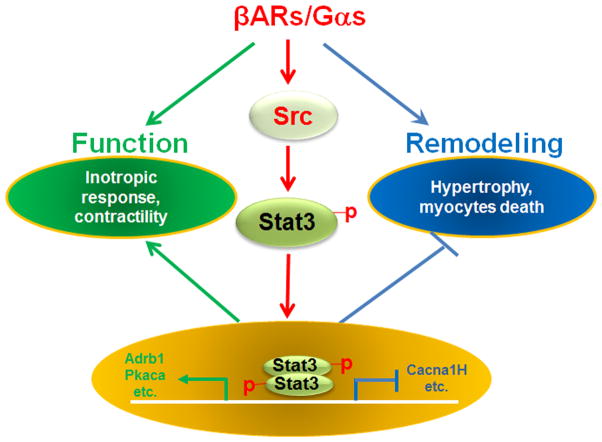
βAR signaling is essential for cardiac function under both physiological and pathophysiological conditions. As one of the most important targets of cardiovascular therapeutic drugs, understanding the mechanism by which the βAR signaling pathway is regulated has been a major effort in biomedical research in the past two decades. However, due to the complexity of its associated pathways, one key question- how βAR signaling elements are transcriptionally regulated- remains largely unanswered. Our current study demonstrates, for the first time, that STAT3 acts as a converging transcriptional regulator of several critical signaling components to maintain the normal homeostasis of βAR signaling. This function of STAT3 is essential for normal βAR-mediated cardiac contractile physiology and suppressing adverse myocardial remodeling (Figure 8); the latter is highly relevant to the pathogenesis of heart failure.
Figure 8.
Regulatory functions of STAT3 in cardiac β-adrenergic functions. Activation of STAT3 via the βAR-Gαs/Src pathway has an important regulatory role in maintaining normal cardiac function and in minimizing adverse cardiac remodeling in response to β-adrenergic stimulation.
Our study demonstrates that STAT3 function is one of the non-second messenger-generating signaling pathways activated by βARs in cardiomyocytes. The notion that βARs could trigger STST3 activation had been previously noted in non-cardiac cell types (e.g., myofibroblast cells). However, this was attributed to a secondary non-cell autonomous event of induced expression of cytokine IL-6.37, 38 Here we have demonstrated that βAR-mediated STAT3 activation is via a direct mechanism, and depends upon β1AR and β2AR, Gαs and Src-family kinases. Furthermore, while Src-family kinases are involved in both STAT3 and ERK activation, βAR-mediated trans-activation of EGFR is only required for ERK, but not STAT3 activation. In addition, deletion of Gαs in MEF cells completely abolishes STAT3 activation by βAR even at higher dose of ISO treatment (> 1 μM), which is different from βAR-mediated ERK activation that only requires Gαs at lower dose (<0.1μM).28 These data suggest that there exist distinct signaling transduction cascades for the activation of different non-second messenger-generating signaling pathways. A growing body of evidence suggests that βARs activate additional signaling factors in parallel to second messenger-generating mechanisms to exert their biological functions.8–10 Rockman and his colleagues have demonstrated that βAR-mediated ERK activation, via an EGFR and β–arrestin dependent mechanism, promotes the cardiac protective program and counteracts the effect of catecholamine toxicity.11, 12 ERK activation in Stat3cKO mice is normal in both basal and β-AR agonist stimulated states (Figure 5), suggesting that STAT3 does not exert its cardiac protective activity via ERK.
The activation of STAT3 provides another cardiac protective mechanism. The analyses of Adrb1 and Pkacb expression, and STAT3 binding to their promoter regions demonstrate that STAT3 is a direct positive transcriptional regulator of Adrb1 and Pkacb. Previously, it was shown that STAT3 occupied the promoter region of a number of key βAR signaling components (Supplemental Table 1 for a list of genes).30, 31 All these genes are known to be critical components in βAR signaling. Thus, STAT3 is primarily responsible for maintaining the homeostasis of βAR signaling. In the absence of STAT3, hearts become insensitive to acute β-adrenergic stimulation due to the down-regulation of β1AR, PKA, and several other downstream effectors (Figure 8).
In addition to the reduced sensitivity to acute β-adrenergic stimulation in Stat3cKO hearts, we observed increased cell death and cardiac fibrosis in response to chronic β-adrenergic stimulation. We attribute this phenomenon partially to the fact that STAT3 is a negative regulator of T-type Ca2+ channels (Cacnα1h) (Figure 7 and Figure 8) and a positive regulator of pro-survival factor Bcl-xl.39 The T-type calcium channel subunit, Cacnα1h, is dramatically increased in Stat3cKO hearts when compared to controls (Figure 7). Normally, Cacnα1h is abundantly expressed in embryonic hearts and subsequently down-regulated in the adult ventricular cardiomyocyte, while remaining high in atrial cardiomyocytes and cardiac conductive cells.36 It has been shown that the recurrence of inward Ca2+ T-currents (ICa, T) in adult ventricular cardiomyocytes is a contributing factor in the progression of heart failure.40 Administration of Ca2+ channel blocker, efonidipine, significantly blocked cell death and heart fibrosis induced by βAR-agonist in Stat3cKO mouse. Considering that there is a decrease of L-type calcium channel subunit Cacnα1c protein expression in Stat3cKO hearts, suggesting a reduced L-type calcium channel function, the reduction of cardiommyocytes death mediated by efonidipine administration under chronic ISO stimulation is most likely due to the inhibition of elevated T-type calcium channel function. This finding further suggested the contribution of elevated ICa, T to the heart failure phenotype in Stat3cKO mice. However, as efonidipine can also inhibit L-type Ca2+ channel at higher dose,41 we cannot fully exclude partial contribution from its inhibitory activity to L-type channel. Therefore, the future study using mice with genetically modified T-type and L-type Ca2+ channel subunits are necessary to further validate the role of both types of Ca2+ channels in mediating the cardiomyocytes intolerance to catecholamine stress in Stat3cKO mice. One possibility is that the elevated ICa, T exacerbates heart failure via the activation of NFAT,35 as conditional ablation of Cacnα1h leads to diminished NFAT activation in response to transverse aortic constriction (TAC). In Stat3cKO hearts, there is a significant increase of nuclear NFATc1 in cardiomyocytes (Supplemental Figure 9). Therefore, another potentially important function for STAT3 is to prevent over-activation of Cacnα1h in the heart. Although the genetic model used in this study specifically ablates STAT3 in cardiomyocytes, it leads to a great enhancement of STAT3 activation in vascular and interstitial myofibroblasts (Figure 5D and E) under prolonged ISO stimulation. This phenomenon may suggest a critical role of STAT3 in the cellular cross-talk between cardiomyocytes and other cell types in the heart, possibly via a paracrine pathway during inflammation or in response to oxidative stress in injured myocardium.16 The hyperactivation of STAT3 noted in myofibroblasts also reflects the complexity of STAT3 in different cardiac cell types, which could contribute directly at different pathogenic phases in diseased and failing hearts to develop excessive reparative and reactive fibrosis. Interestingly, a previous report demonstrated that hyperactivation of STAT3 led to desensitize TGFβ signaling.42 Consistent with that, we found that prolonged ISO treatment leads to elevated TGFβ-signaling (i.e., an increased level of pSmad2) in Stat3cKO hearts. Although the exacerbated oxidative stress induced by STAT3 deletion could be a major factor,43 the exact underlying mechanisms for the elevated TGFβ signaling in Stat3cKO hearts is still elusive and worth to be further studied.
One of the hallmarks of heart failure is the reduction of β1AR and cardiac insensitivity to β-adrenergic stimulation.44, 45 Given our finding that STAT3 directly regulates β1AR expression, this might provide a molecular basis for the observed heart failure in older Stat3cKO mice. It is known that STAT3 activation is altered in various human heart failure patients.17, 46 Based on our study, we propose a pivotal role for STAT3 in preventing the pathogenesis of heart failure in humans. Furthermore, in a mouse model carrying double mutations in cardiac Troponin I and α-myosin heavy chain (tni-203/mhc-403), which mimics a familial hypertrophic cardiomyopathy in humans,47 STAT3 has been found to be activated in cardiomyocytes during the course of heart failure.18 It is reasonable to propose that, based on our data, this activation of STAT3 provides a cardiac protective mechanism for the diseased heart. It would be interesting to generate compound mutant mice with a reduced level of STAT3 in tni-203/mhc-403, and analyze the impact of STAT3 in the development of hypertrophic cardiomyopathy and heart failure in future experiments.
In summary, we have shown that, in the myocardial-restricted STAT3 knockout mice, cardiac contractile function and calcium release properties by acute βAR stimulation are impaired. Furthermore, chronic βAR stimulation induces excessive hypertrophy, myocyte death and cardiac fibrosis in the absence of STAT3. Moreover, we have revealed the molecular mechanisms by which STAT3 contributes to βAR functions. βARs activate STAT3 through Gαs and Src kinases, and STAT3 directly regulates the gene expression of several key components in the adrenergic signaling pathways. Together, these data clearly demonstrate critical roles for STAT3 in β-adrenergic functions in the heart.
Supplementary Material
Clinical Perspectives.
βAR signaling plays a central role in cardiac stress adaption, pathological remodeling and heart failure. Diminished contractile responsiveness to βAR stimulation is often correlated with increase of myocyte apoptosis, compensatory hypertrophy and cardiac fibrosis in failing human heart. The STAT (signal transducer and activator of transcription) family of transcriptional regulators mediates a wide range of biological functions. Tyrosine phosphorylation in response to varies of cytokine and stress stimulation is required for STAT3 dimerization, nuclear translocation, and DNA binding. Previous studies demonstrated that STAT3 activation and expression was severely reduced in failing hearts. Here, we show that STAT3 plays a critical role in maintaining cardiac homeostasis via its transcriptional regulation of major βAR signaling components. Conditional loss of STAT3 in cardiomyocytes impairs the inotropic and lusitropic response to acute βAR stimulation, and augments chronic βAR stimulation-induced hypertrophic response, cardiomyocyte death, and cardiac fibrosis. These findings suggest a novel pharmacological intervention for the management for heart failure. In addition, pharmacologic blockage of STAT3 signaling has been explored for the treatment of several malignancies; the results presented here suggest that close monitoring of cardiac function in these patients during treatment is warranted.
Acknowledgments
We thank Mark H. Soonpaa for critical review of the manuscript.
Funding Sources: This work was supported, in whole or in part, by 863 project SS2012AA023503 (YT), National Institutes of Health Grants HL81092 (WS), HL91525 (XYH), HL090905 (LSS), and CA125568-05 (XYF), NSFC81470446 and 31401237 (XQ and WS), and by Indiana University School of Medicine Strategic Research Initiative (MR) and CECARE (WS).
Footnotes
Disclosures: None.
References
- 1.Lefkowitz RJ, Rockman HA, Koch WJ. Catecholamines, cardiac beta-adrenergic receptors, and heart failure. Circulation. 2000;101:1634–7. doi: 10.1161/01.cir.101.14.1634. [DOI] [PubMed] [Google Scholar]
- 2.Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–12. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- 3.Bristow MR, Ginsburg R, Umans V, Fowler M, Minobe W, Rasmussen R, Zera P, Menlove R, Shah P, Jamieson S. Beta 1- and beta 2-adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: coupling of both receptor subtypes to muscle contraction and selective beta 1-receptor down-regulation in heart failure. Circ Res. 1986;59:297–309. doi: 10.1161/01.res.59.3.297. [DOI] [PubMed] [Google Scholar]
- 4.Koch WJ, Lefkowitz RJ, Rockman HA. Functional consequences of altering myocardial adrenergic receptor signaling. Annu Rev Physiol. 2000;62:237–60. doi: 10.1146/annurev.physiol.62.1.237. [DOI] [PubMed] [Google Scholar]
- 5.Lohse MJ, Engelhardt S, Eschenhagen T. What is the role of beta-adrenergic signaling in heart failure? Circ Res. 2003;93:896–906. doi: 10.1161/01.RES.0000102042.83024.CA. [DOI] [PubMed] [Google Scholar]
- 6.Zhu WZ, Wang SQ, Chakir K, Yang D, Zhang T, Brown JH, Devic E, Kobilka BK, Cheng H, Xiao RP. Linkage of beta1-adrenergic stimulation to apoptotic heart cell death through protein kinase A-independent activation of Ca2+/calmodulin kinase II. J Clin Invest. 2003;111:617–25. doi: 10.1172/JCI16326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh K, Communal C, Sawyer DB, Colucci WS. Adrenergic regulation of myocardial apoptosis. Cardiovasc Res. 2000;45:713–9. doi: 10.1016/s0008-6363(99)00370-3. [DOI] [PubMed] [Google Scholar]
- 8.Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, Lin F, Kawakatsu H, Owada K, Luttrell DK, Caron MG, Lefkowitz RJ. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283:655–61. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- 9.Lavoie C, Mercier JF, Salahpour A, Umapathy D, Breit A, Villeneuve LR, Zhu WZ, Xiao RP, Lakatta EG, Bouvier M, Hebert TE. Beta 1/beta 2-adrenergic receptor heterodimerization regulates beta 2-adrenergic receptor internalization and ERK signaling efficacy. J Biol Chem. 2002;277:35402–10. doi: 10.1074/jbc.M204163200. [DOI] [PubMed] [Google Scholar]
- 10.Ma YC, Huang J, Ali S, Lowry W, Huang XY. Src tyrosine kinase is a novel direct effector of G proteins. Cell. 2000;102:635–46. doi: 10.1016/s0092-8674(00)00086-6. [DOI] [PubMed] [Google Scholar]
- 11.Noma T, Lemaire A, Naga Prasad SV, Barki-Harrington L, Tilley DG, Chen J, Le Corvoisier P, Violin JD, Wei H, Lefkowitz RJ, Rockman HA. Beta-arrestin-mediated beta1-adrenergic receptor transactivation of the EGFR confers cardioprotection. J Clin Invest. 2007;117:2445–58. doi: 10.1172/JCI31901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim IM, Tilley DG, Chen J, Salazar NC, Whalen EJ, Violin JD, Rockman HA. Beta-blockers alprenolol and carvedilol stimulate beta-arrestin-mediated EGFR transactivation. Proc Natl Acad Sci U S A. 2008;105:14555–60. doi: 10.1073/pnas.0804745105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bromberg JF, Darnell JE., Jr Potential roles of Stat1 and Stat3 in cellular transformation. Cold Spring Harb Symp Quant Biol. 1999;64:425–8. doi: 10.1101/sqb.1999.64.425. [DOI] [PubMed] [Google Scholar]
- 14.Levy DE, Lee CK. What does Stat3 do? J Clin Invest. 2002;109:1143–8. doi: 10.1172/JCI15650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stark GR, Darnell JE., Jr The JAK-STAT pathway at twenty. Immunity. 2012;36:503–14. doi: 10.1016/j.immuni.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischer P, Hilfiker-Kleiner D. Survival pathways in hypertrophy and heart failure: the gp130-STAT3 axis. Basic Res Cardiol. 2007;102:279–97. doi: 10.1007/s00395-007-0658-z. [DOI] [PubMed] [Google Scholar]
- 17.Ng DC, Court NW, dos Remedios CG, Bogoyevitch MA. Activation of signal transducer and activator of transcription (STAT) pathways in failing human hearts. Cardiovasc Res. 2003;57:333–46. doi: 10.1016/s0008-6363(02)00664-8. [DOI] [PubMed] [Google Scholar]
- 18.Tsoutsman T, Kelly M, Ng DC, Tan JE, Tu E, Lam L, Bogoyevitch MA, Seidman CE, Seidman JG, Semsarian C. Severe heart failure and early mortality in a double-mutation mouse model of familial hypertrophic cardiomyopathy. Circulation. 2008;117:1820–31. doi: 10.1161/CIRCULATIONAHA.107.755777. [DOI] [PubMed] [Google Scholar]
- 19.Jacoby JJ, Kalinowski A, Liu MG, Zhang SS, Gao Q, Chai GX, Ji L, Iwamoto Y, Li E, Schneider M, Russell KS, Fu XY. Cardiomyocyte-restricted knockout of STAT3 results in higher sensitivity to inflammation, cardiac fibrosis, and heart failure with advanced age. Proc Natl Acad Sci U S A. 2003;100:12929–34. doi: 10.1073/pnas.2134694100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hilfiker-Kleiner D, Hilfiker A, Fuchs M, Kaminski K, Schaefer A, Schieffer B, Hillmer A, Schmiedl A, Ding Z, Podewski E, Podewski E, Poli V, Schneider MD, Schulz R, Park JK, Wollert KC, Drexler H. Signal transducer and activator of transcription 3 is required for myocardial capillary growth, control of interstitial matrix deposition, and heart protection from ischemic injury. Circ Res. 2004;95:187–95. doi: 10.1161/01.RES.0000134921.50377.61. [DOI] [PubMed] [Google Scholar]
- 21.Wu B, Zhou B, Wang Y, Cheng HL, Hang CT, Pu WT, Chang CP, Zhou B. Inducible cardiomyocyte-specific gene disruption directed by the rat Tnnt2 promoter in the mouse. Genesis. 2010;48:63–72. doi: 10.1002/dvg.20573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hang CT, Yang J, Han P, Cheng HL, Shang C, Ashley E, Zhou B, Chang CP. Chromatin regulation by Brg1 underlies heart muscle development and disease. Nature. 2010;466:62–7. doi: 10.1038/nature09130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim KK, Soonpaa MH, Daud AI, Koh GY, Kim JS, Field LJ. Tumor suppressor gene expression during normal and pathologic myocardial growth. J Biol Chem. 1994;269:22607–13. [PubMed] [Google Scholar]
- 24.Chen H, Yong W, Ren S, Shen W, He Y, Cox KA, Zhu W, Li W, Soonpaa M, Payne RM, Franco D, Field LJ, Rosen V, Wang Y, Shou W. Overexpression of bone morphogenetic protein 10 in myocardium disrupts cardiac postnatal hypertrophic growth. J Biol Chem. 2006;281:27481–91. doi: 10.1074/jbc.M604818200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang C, Gu H, Zhang W, Manukyan MC, Shou W, Wang M. SDF-1/CXCR4 mediates acute protection of cardiac function through myocardial STAT3 signaling following global ischemia/reperfusion injury. Am J Physiol Heart Circ Physiol. 2011;301:H1496–505. doi: 10.1152/ajpheart.00365.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang M, Zhang W, Crisostomo P, Markel T, Meldrum KK, Fu XY, Meldrum DR. Endothelial STAT3 plays a critical role in generalized myocardial proinflammatory and proapoptotic signaling. Am J Physiol Heart Circ Physiol. 2007;293:H2101–8. doi: 10.1152/ajpheart.00125.2007. [DOI] [PubMed] [Google Scholar]
- 27.Bastepe M, Gunes Y, Perez-Villamil B, Hunzelman J, Weinstein LS, Juppner H. Receptor-mediated adenylyl cyclase activation through XLalpha(s), the extra-large variant of the stimulatory G protein alpha-subunit. Mol Endocrinol. 2002;16:1912–9. doi: 10.1210/me.2002-0054. [DOI] [PubMed] [Google Scholar]
- 28.Sun Y, Huang J, Xiang Y, Bastepe M, Juppner H, Kobilka BK, Zhang JJ, Huang XY. Dosage-dependent switch from G protein-coupled to G protein-independent signaling by a GPCR. EMBO J. 2007;26:53–64. doi: 10.1038/sj.emboj.7601502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sulakhe PV, Vo XT. Regulation of phospholamban and troponin-I phosphorylation in the intact rat cardiomyocytes by adrenergic and cholinergic stimuli: roles of cyclic nucleotides, calcium, protein kinases and phosphatases and depolarization. Mol Cell Biochem. 1995;149–150:103–26. doi: 10.1007/BF01076569. [DOI] [PubMed] [Google Scholar]
- 30.Chen X, Xu H, Yuan P, Fang F, Huss M, Vega VB, Wong E, Orlov YL, Zhang W, Jiang J, Loh YH, Yeo HC, Yeo ZX, Narang V, Govindarajan KR, Leong B, Shahab A, Ruan Y, Bourque G, Sung WK, Clarke ND, Wei CL, Ng HH. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell. 2008;133:1106–17. doi: 10.1016/j.cell.2008.04.043. [DOI] [PubMed] [Google Scholar]
- 31.Kidder BL, Yang J, Palmer S. Stat3 and c-Myc genome-wide promoter occupancy in embryonic stem cells. PLoS One. 2008;3:e3932. doi: 10.1371/journal.pone.0003932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinez ML, Heredia MP, Delgado C. Expression of T-type Ca(2+) channels in ventricular cells from hypertrophied rat hearts. J Mol Cell Cardiol. 1999;31:1617–25. doi: 10.1006/jmcc.1999.0998. [DOI] [PubMed] [Google Scholar]
- 33.Kinoshita H, Kuwahara K, Takano M, Arai Y, Kuwabara Y, Yasuno S, Nakagawa Y, Nakanishi M, Harada M, Fujiwara M, Murakami M, Ueshima K, Nakao K. T-type Ca2+ channel blockade prevents sudden death in mice with heart failure. Circulation. 2009;120:743–52. doi: 10.1161/CIRCULATIONAHA.109.857011. [DOI] [PubMed] [Google Scholar]
- 34.Montgomery RL, Davis CA, Potthoff MJ, Haberland M, Fielitz J, Qi X, Hill JA, Richardson JA, Olson EN. Histone deacetylases 1 and 2 redundantly regulate cardiac morphogenesis, growth, and contractility. Genes Dev. 2007;21:1790–802. doi: 10.1101/gad.1563807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiang CS, Huang CH, Chieng H, Chang YT, Chang D, Chen JJ, Chen YC, Chen YH, Shin HS, Campbell KP, Chen CC. The Ca(v)3.2 T-type Ca(2+) channel is required for pressure overload-induced cardiac hypertrophy in mice. Circ Res. 2009;104:522–30. doi: 10.1161/CIRCRESAHA.108.184051. [DOI] [PubMed] [Google Scholar]
- 36.Nuss HB, Houser SR. T-type Ca2+ current is expressed in hypertrophied adult feline left ventricular myocytes. Circ Res. 1993;73:777–82. doi: 10.1161/01.res.73.4.777. [DOI] [PubMed] [Google Scholar]
- 37.Yin F, Li P, Zheng M, Chen L, Xu Q, Chen K, Wang YY, Zhang YY, Han C. Interleukin-6 family of cytokines mediates isoproterenol-induced delayed STAT3 activation in mouse heart. J Biol Chem. 2003;278:21070–5. doi: 10.1074/jbc.M211028200. [DOI] [PubMed] [Google Scholar]
- 38.Szabo-Fresnais N, Lefebvre F, Germain A, Fischmeister R, Pomerance M. A new regulation of IL-6 production in adult cardiomyocytes by beta-adrenergic and IL-1 beta receptors and induction of cellular hypertrophy by IL-6 trans-signalling. Cell Signal. 2010;22:1143–52. doi: 10.1016/j.cellsig.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 39.Cambi GE, Lucchese G, Djeokeng MM, Modesti A, Fiaschi T, Faggian G, Sani G, Modesti PA. Impaired JAK2-induced activation of STAT3 in failing human myocytes. Mol Biosyst. 2012;8:2351–9. doi: 10.1039/c2mb25120e. [DOI] [PubMed] [Google Scholar]
- 40.Kuwahara K, Saito Y, Takano M, Arai Y, Yasuno S, Nakagawa Y, Takahashi N, Adachi Y, Takemura G, Horie M, Miyamoto Y, Morisaki T, Kuratomi S, Noma A, Fujiwara H, Yoshimasa Y, Kinoshita H, Kawakami R, Kishimoto I, Nakanishi M, Usami S, Saito Y, Harada M, Nakao K. NRSF regulates the fetal cardiac gene program and maintains normal cardiac structure and function. EMBO J. 2003;22:6310–21. doi: 10.1093/emboj/cdg601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanaka H, Shigenobu K. Efonidipine hydrochloride: a dual blocker of L- and T-type ca(2+) channels. Cardiovasc Drug Rev. 2002;20:81–92. doi: 10.1111/j.1527-3466.2002.tb00084.x. [DOI] [PubMed] [Google Scholar]
- 42.Jenkins BJ, Grail D, Nheu T, Najdovska M, Wang B, Waring P, Inglese M, McLoughlin RM, Jones SA, Topley N, Baumann H, Judd LM, Giraud AS, Boussioutas A, Zhu HJ, Ernst M. Hyperactivation of Stat3 in gp130 mutant mice promotes gastric hyperproliferation and desensitizes TGF-beta signaling. Nat Med. 2005;11:845–52. doi: 10.1038/nm1282. [DOI] [PubMed] [Google Scholar]
- 43.Hilfiker-Kleiner D, Kaminski K, Podewski E, Bonda T, Schaefer A, Sliwa K, Forster O, Quint A, Landmesser U, Doerries C, Luchtefeld M, Poli V, Schneider MD, Balligand JL, Desjardins F, Ansari A, Struman I, Nguyen NQ, Zschemisch NH, Klein G, Heusch G, Schulz R, Hilfiker A, Drexler H. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell. 2007;128:589–600. doi: 10.1016/j.cell.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 44.Bristow MR, Ginsburg R, Minobe W, Cubicciotti RS, Sageman WS, Lurie K, Billingham ME, Harrison DC, Stinson EB. Decreased catecholamine sensitivity and beta-adrenergic-receptor density in failing human hearts. N Engl J Med. 1982;307:205–11. doi: 10.1056/NEJM198207223070401. [DOI] [PubMed] [Google Scholar]
- 45.Brodde OE, Vogelsang M, Broede A, Michel-Reher M, Beisenbusch-Schafer E, Hakim K, Zerkowski HR. Diminished responsiveness of Gs-coupled receptors in severely failing human hearts: no difference in dilated versus ischemic cardiomyopathy. J Cardiovasc Pharmacol. 1998;31:585–94. doi: 10.1097/00005344-199804000-00018. [DOI] [PubMed] [Google Scholar]
- 46.Podewski EK, Hilfiker-Kleiner D, Hilfiker A, Morawietz H, Lichtenberg A, Wollert KC, Drexler H. Alterations in Janus kinase (JAK)-signal transducers and activators of transcription (STAT) signaling in patients with end-stage dilated cardiomyopathy. Circulation. 2003;107:798–802. doi: 10.1161/01.cir.0000057545.82749.ff. [DOI] [PubMed] [Google Scholar]
- 47.Tsoutsman T, Bagnall RD, Semsarian C. Impact of multiple gene mutations in determining the severity of cardiomyopathy and heart failure. Clin Exp Pharmacol Physiol. 2008;35:1349–57. doi: 10.1111/j.1440-1681.2008.05037.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.