Abstract
Background
Evidence is lacking to recommend one diet over another when treating polycystic ovary syndrome (PCOS).
Objectives
To obtain preliminary data, comparing the impact of a low-glycemic load (LGL) vs. low-fat (LF) diet on biochemical hyperandrogenism in overweight and obese adolescents with PCOS. To ascertain feasibility of recruiting study participants, in partnership with an adolescent clinic, and implementing dietary interventions.
Methods
Randomized controlled trial of 19 overweight and obese adolescents with PCOS and not using hormonal contraceptives (HCs). Interventions comprised nutrition education, dietary counseling, and cooking workshops to foster adherence to a LGL (45% carbohydrate, 35% fat, 20% protein) or LF (55% carbohydrate, 25% fat, 20% protein) diet over 6 months. Serum bioavailable testosterone was the primary outcome.
Results
Sixteen (LGL, n=7; LF, n=9) participants completed the study. Body fat percentage decreased (P<0.05) in response to the interventions, with no difference between the LGL and LF groups (−1.2% vs. −2.2%; P=0.16). Bioavailable testosterone did not change for either group (−0.4 vs. −1.8 ng/dL; P=0.35). Regarding feasibility, recruiting adolescents posed a challenge, and use of HCs was a main reason for ineligibility. Participants attended 5.9 of 6 in-person visits and 2.6 of 3 cooking workshops, completed 4.9 of 6 telephone counseling calls, and reported high satisfaction with the diets and cooking workshops (≥8 on a 10-point scale).
Conclusions
Dietary interventions were beneficial for weight control but did not attenuate biochemical hyperandrogenism. Innovative strategies are needed to recruit adolescents for studies aimed at assessing independent effects of diet on features of PCOS.
Keywords: polycystic ovary syndrome, bioavailable testosterone, dietary intervention, body composition, electronic medical record
INTRODUCTION
Polycystic ovary syndrome (PCOS) is characterized by hyperandrogenism and subsequent metabolic consequences including ovulatory and menstrual dysfunction, hirsutism, and acne (1). The syndrome often presents in susceptible girls during the peri-menarcheal period (2). In addition to insulin resistance and compensatory hyperinsulinemia that appear to play a role in the pathophysiology of elevated androgens (3, 4), adolescents with PCOS also may have glucose intolerance (5), metabolic syndrome (6), and dyslipidemia (3), increasing risk for type 2 diabetes and cardiovascular disease later in life (7). Obesity is prevalent among girls with PCOS and further exacerbates metabolic presentation of the syndrome, possibly mediated by insulin resistance (6, 8, 9). Moreover, obesity compromises health-related quality of life (HRQL) among adolescents with PCOS relative to their healthy counterparts (10).
Treatment guidelines specify lifestyle modification, including weight-loss diets, as the first line of therapy for PCOS among overweight and obese girls (11, 12). However, data are limited regarding effects of diets varying in composition on metabolic, reproductive, and psychological features of PCOS, particularly among adolescents. The overall aim of this pilot study was to obtain preliminary data, comparing impact of a low-glycemic load (LGL) vs. low-fat (LF) diet on biochemical hyperandrogenism and cardiometabolic risk factors. We hypothesized that a LGL diet, designed to attenuate postprandial glycemia and insulinemia, would be more efficacious than a LF diet for treating overweight and obese adolescent girls with PCOS. This pilot study also provided an opportunity to ascertain feasibility of recruiting study participants in partnership with an adolescent clinic, implementing dietary interventions, and assessing outcomes.
METHODS
Study Design and Setting
Participants were randomly assigned to receive either a LGL or LF dietary prescription. Outcomes were assessed at baseline, prior to random assignment, and at the end of a 6-month intervention period. The primary outcome was bioavailable testosterone. Eligible participants diagnosed with PCOS were recruited primarily through the Adolescent and Young Adult Medicine Clinic at Boston Children’s Hospital (BCH), and also through primary care practices and newspaper advertisements. A partnership between the clinic and research team was developed to identify potentially eligible participants using a flagging protocol of key eligibility criteria in an electronic medical record (EMR). When appropriate, providers presented the trial as a first-line non-pharmacologic treatment option. Interested participants underwent a multi-step screening and enrollment process to confirm eligibility. The institutional review board at BCH approved the protocol. Participants provided written informed consent or assent. For participants less than 18 years of age, a parent also provided written informed consent. Participants who completed the study received $100 as compensation for their time and effort. The study was conducted between July 2010 and November 2013.
Participants
Each participant had a diagnosis of PCOS from her treating physician with confirmed biochemical hyperandrogenism (elevated serum free testosterone within the last 6 months) and ovarian dysfunction (oligo-anovulation and/or polycystic ovaries on ultrasound), consistent with criteria established by the Androgen Excess Society (13). Other inclusion criteria were age between 13 and 21 years, body mass index (BMI) ≥85th percentile (14), and medical clearance from a treating physician. Exclusion criteria were type 2 diabetes (fasting plasma glucose ≥126 mg/dL), diagnosis of an eating disorder or any other major medical illness, abnormal screening laboratory measures indicating other causes of hyperandrogenism or obesity; and smoking (>1 cigarette per week). Use of medications (hormone contraceptives [HCs] within the past 3 months, insulin-sensitizing agents within the past month) also was exclusionary.
Interventions
We randomly assigned participants to the LGL or LF diet group. Both interventions comprised 12 sessions with a registered dietitian (6 monthly in-person visits for nutrition education, 6 monthly telephone counseling calls) and 3 cooking workshops with an executive chef (Table S1). Participants were accompanied by a parent (if <18 years of age) at the in-person visits and cooking workshops. At each in-person visit, take-home snack items consistent with random group assignments were provided to participants. At each cooking workshop, key ingredients were provided to participants to encourage repeat preparation of dishes at home. The telephone calls were conducted with the participants (no parental involvement) using a patient-centered counseling model (15) to encourage adherence to the diets.
Low-Glycemic Load Diet
Target macronutrient composition for the LGL diet was 45% of energy from carbohydrate, 35% from fat, and 20% from protein. The dietitian counseled participants to consume low-glycemic index sources of carbohydrate (including non-starchy vegetables, legumes, and fruits) and to limit intake of moderate or high glycemic index sources (including refined grains, starchy vegetables, and sweets). Attention also was directed towards consuming sources of healthful fat (including nuts, seeds, and oils). Take-home snack items included SoLoGI® energy bars and mixed nuts. Each participant was given a Corelle® plate with divisions to convey reasonable portion sizes, facilitate meal assembly, and thereby translate knowledge to behavior. The LGL plate was delineated as one-half non-starchy vegetables with oils, nuts, or other healthful sources of fat; one-quarter moderate glycemic load foods and/or legumes; and one-quarter lean protein.
Low-Fat Diet
Target macronutrient composition for the LF diet was 55% energy from carbohydrate, 25% from fat, and 20% from protein. The dietitian counseled participants to consume low-fat sources of whole grains, vegetables, and fruits and to limit intake of added fats, sweets, and high-fat snacks. Take-home snack items included Odwalla® bars and whole wheat pretzels.Each participant was given a Corelle® plate with divisions to convey reasonable portion sizes, facilitate meal assembly, and thereby translate knowledge to behavior. The LF plate was delineated as one-half low-fat vegetables and fruits, one-quarter low-fat grains (with an emphasis on whole grains), and one-quarter lean protein.
Treatment Fidelity
Procedures to promote treatment fidelity included scripts for presentation of topics during in-person visits, with well-defined nutrition messages for each diet; guides for telephone calls that provided both structure and flexibility, with prompts for adhering to a patient-centered counseling model (15); protocols for documenting each participant-dietitian interaction; and regular study team meetings to discuss strategies for promoting adherence without compromising differentiation between diets. We digitally recorded all telephone counseling calls, and two members of the research team reviewed a 10% random sample of the calls to ensure quality control.
Process Evaluation
Implementation of the dietary interventions was evaluated based on attendance at in-person visits and cooking workshops and completion of telephone counseling calls. We also evaluated participant adherence and satisfaction by interviews and questionnaire, respectively.
Three unannounced telephone interviews (2 weekdays, 1 weekend day) were conducted at baseline and again at 6 months to assess dietary intake and physical activity during the 24 hours preceding each call. The interviewer was masked to group assignment. Dietary intake was collected by a multiple-pass method using the Nutrition Data System for Research Software versions 2010–2012, and final calculations were completed with version 2013 (Nutrition Coordinating Center, University of Minnesota, Minneapolis). Participants also completed a satisfaction questionnaire at the end of the study, responding to questions using 10-cm visual analog scales with appropriate verbal anchors.
Outcomes
Study outcomes were assessed after a 12-hour overnight fast at baseline and the end of the 6-month intervention. Outcome assessors were masked to random assignment. Study data were managed using REDCap (Research Electronic Data Capture) hosted at BCH (16).
Biochemical analyses were carried out in CLIA-certified laboratories. The primary outcome was serum bioavailable testosterone (free and weakly bound). Other biochemical outcomes included blood levels of total testosterone, free testosterone, sex hormone binding globulin (SHBG), dehydroepiandrosterone sulfate (DHEAS), total cholesterol, LDL-cholesterol (direct determination by enzymatic spectrophotometric assay), HDL-cholesterol, triglycerides, high sensitivity C-reactive protein (hs-CRP), and hemoglobin (Hb) A1c. If serum progesterone was ≥4.0 mg/mL (indicating recent ovulation), measures of bioavailable testosterone, total testosterone, free testosterone, SHBG, DHEAS, lipids, and progesterone were repeated one week after the initial blood draw at baseline or 6 months.
A frequently-sampled oral glucose tolerance test (FS-OGTT), using a 75-gram dose of dextrose, was conducted with blood sampling at −10, −5, 0, 10, 20, 30, 60, 90, and 120 minutes relative to the dose. We used data obtained at −10, −5, and 0 minutes to calculate mean fasting plasma glucose and serum insulin. Incremental area under respective 2-hour glucose and insulin curves (iAUC), in excess of mean fasting levels, was calculated by the trapezoidal rule.
Body weight and height were measured using a calibrated electronic scale and wall-mounted stadiometer, respectively, to calculate BMIand determine BMI percentile (14). Blood pressure was measured by auscultation, with the participant sitting quietly. Body composition was assessed by dual-energy x-ray absorptiometry (DXA, Discovery A, Hologic, Inc., Bedford, MA).
Self-reported HRQL was assessed using the Child Health Questionnaire (CHQ-CF87, HealthActCHQ, Boston, MA).
Statistical Analyses
According to a priori power calculations, a sample size of 40 participants would provide 80% power to detect a group differential approximating 20% when testing our primary hypothesis, and we proposed to recruit 50 participants to account for attrition. In light of recruitment challenges, we pooled available data from both dietary intervention groups to construct conditional power curves in July 2013. From these curves, we concluded that enrollment of additional participants would not substantially enhance power to detect a group effect for change in bioavailable testosterone. Thus, we stopped recruitment in July 2013, and this report is based on data from 16 of 19 (7/9 LGL, 9/10 LF) randomly assigned participants who completed the study.
Baseline characteristics were compared between the diet groups using the Fisher exact test for categorical variables and t-test for continuous variables. The primary outcome was the comparison of 6-month changes in bioavailable testosterone in the two diet groups, using Student’s independent t-test with two-sided P<0.05 as critical value. Secondary outcomes were analyzed similarly. Relationships between bioavailable testosterone and other outcomes at baseline and for changes over 6 months were calculated using Pearson correlations. SAS software (SAS Institute Inc., Cary, NC) was used for all computations. Data are presented as mean and standard deviation (SD) or standard error (SE).
RESULTS
Recruitment and Retention
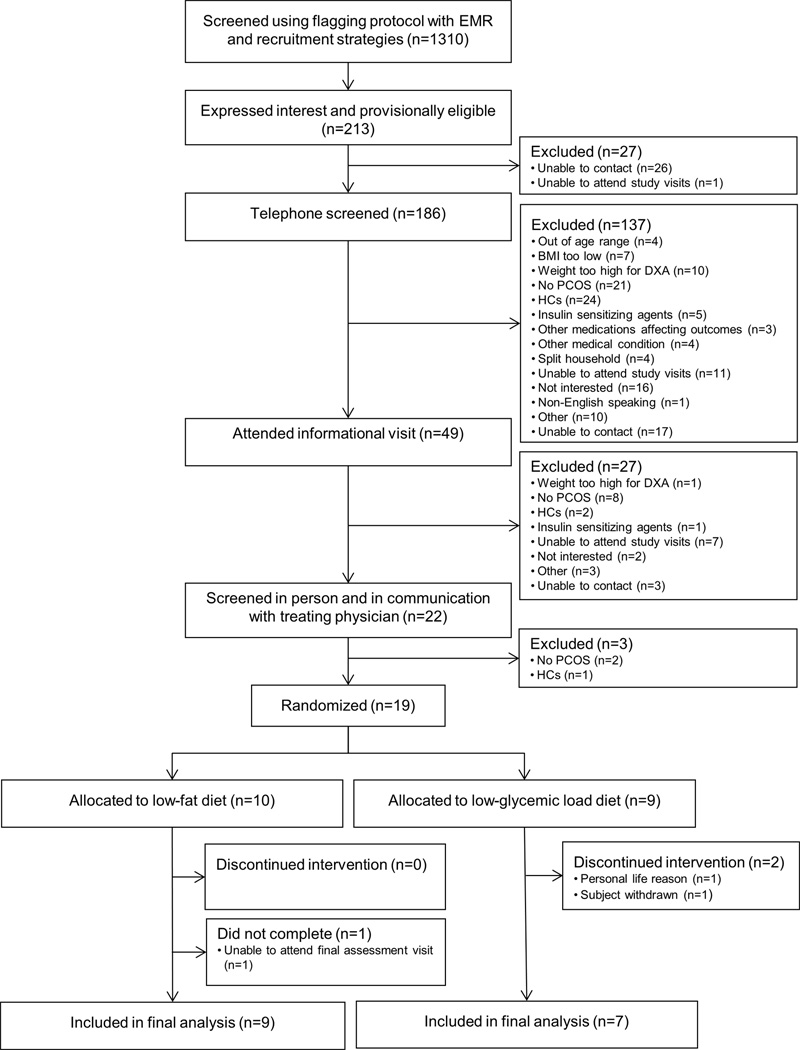
More than 33% of 442 girls flagged as having a diagnosis PCOS, based on information in the EMR, reported use of HCs (with no plans to discontinue) and thus were not screened for the study. As presented in Figure 1, 1310 adolescent girls were identified as potentially eligible, primarily using the flagging protocol. The main reasons for ineligibility upon initial screening included not meeting study criteria for PCOS (27%), BMI less than the 85th percentile (23%), and use of exclusionary medications (19%). Among those who were provisionally eligible (213/1310, 16%), 19 girls met all eligibility criteria upon further screening and were randomly assigned to a dietary intervention group. Baseline data are presented in Table 1.
Figure 1.
Flow of Participants Through the Trial
Table 1.
Baseline Characteristics *
| Variable | Unadjusted Data (Mean ± SD) | P value | |
|---|---|---|---|
| Low-GL (n=10) |
Low-Fat (n=9) |
||
| Race or Ethnic Group | |||
| Race | 1.00 | ||
| White | 5 | 5 | |
| Black | 1 | 1 | |
| Multiple or Other | 3 | 4 | |
| Ethnic Group | 1.00 | ||
| Hispanic | 4 | 5 | |
| Non Hispanic | 5 | 5 | |
| Annual Household Income | 0.57 | ||
| < $30,000 | 0 | 4 | |
| $30,000 – $59,999 | 1 | 2 | |
| $60,000 – $89,999 | 2 | 1 | |
| > $90,000 | 6 | 3 | |
| Age (yr) | 15.4 ± 1.3 | 16.3 ± 2.2 | 0.35 |
| Weight (kg) | 96.5 ± 10.0 | 90.2 ± 13.9 | 0.28 |
| Height (m) | 163.7 ± 4.5 | 163.1 ± 6.4 | 0.82 |
| BMI (kg/m2) | 36.2 ± 5.3 | 33.9 ± 4.7 | 0.32 |
| BMI Percentile | 98.0 ± 2.2 | 97.2 ± 2.1 | 0.43 |
| Body Fat (%) | 44.9 ± 4.5 | 44.1 ± 3.9 | 0.66 |
| Trunk Fat (kg) | 20.6 ± 4.0 | 19.6 ± 5.4 | 0.68 |
| Bioavailable Testosterone (ng/dL) | 14.9 ± 5.2 | 15.7 ± 8.7 | 0.80 |
| Bioavailable Testosterone (%) | 25.0 ± 7.3 | 27.3 ± 10.4 | 0.59 |
| Total Testosterone (ng/dL) | 64.2 ± 28.3 | 57.3 ± 16.7 | 0.52 |
| Free Testosterone (direct, pg/mL) | 1.9 ± 1.0 | 1.6 ± 0.8 | 0.40 |
| SHBG (nmol/L) | 21.6 ± 8.6 | 19.3 ± 11.3 | 0.63 |
| DHEAS (ug/dL) | 241.1 ± 120.9 | 265.0 ± 130.9 | 0.69 |
| Total Cholesterol (mg/dL) | 177.1 ± 14.0 | 167.0 ± 14.4 | 0.14 |
| LDL-C (direct) (mg/dL) | 120.8 ± 19.0 | 117.2 ± 13.5 | 0.64 |
| HDL-C (mg/dL) | 46.8 ± 12.6 | 42.7 ± 8.1 | 0.41 |
| Triglycerides (mg/dL) | 107.7 ± 47.1 | 91.5 ± 31.4 | 0.39 |
| Total Cholesterol / HDL-C ratio | 4.0 ± 1.0 | 4.0 ± 0.7 | 0.98 |
| hs-CRP (mg/L) | 4.1 ± 3.8 | 1.6 ± 1.4 | 0.06 |
| Fasting Glucose (mg/dL) | 80.5 ± 4.3 | 79.9 ± 6.6 | 0.84 |
| Fasting Insulin (uIU/mL) | 16.2 ± 6.9 | 15.0 ± 7.7 | 0.73 |
| HbA1c (%) | 5.7 ± 0.3 | 5.4 ± 0.3 | 0.04 |
| Systolic Blood Pressure (mmHg) | 100.9 ± 4.4 | 101.3 ± 6.6 | 0.89 |
| Diastolic Blood Pressure (mmHg) | 64.5 ± 5.3 | 63.1 ± 6.9 | 0.62 |
Abbreviations: BMI, body mass index; SHBG, sex hormone binding globulin; DHEAS, dehydroepiandrosterone sulfate; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; HbA1c, hemoglobin A1c; hs-CRP, high sensitivity C-reactive protein
SI Conversion Factors: To convert bioavailable and total testosterone to nmol/L, multiply by 0.0347; direct free testosterone to pmol/L, multiply by 3.47; DHEAS to umol/L, multiply by 0.027; total cholesterol, LDL-C, and HDL-C to mmol/L, multiply by 0.0259; triglycerides to mmolL, multiply by 0.0113; glucose to mmol/L, multiply by 0.0555; insulin to pmol/L, multiply by 6.945; hs-CRP to nmol/L, multiply by 9.524.
Differences in baseline characteristics by diet group assessed using the Fisher exact test for categorical variables and t-test for continuous variables. P-value tests the hypothesis of zero difference between diet groups.
Process Measures
Attendance at in-person visits and cooking workshops and completion of telephone counseling calls did not differ between dietary intervention groups. Participants attended a mean (SE) of 5.9 (0.1) of the 6 in-person sessions and 2.6 (0.2) of the 3 cooking workshops and completed 4.9 (0.3) of the 6 telephone calls.
Dietary intake, based on self-report, is presented in Table S2. Percentage of energy from carbohydrate (P=0.046), glycemic index (P=0.03), glycemic load (P=0.007), and available carbohydrate (per 1000 kcal) (P=0.01) decreased from baseline in the LGL group. The difference between groups for change in glycemic load from baseline approached significance (P=0.10). Dietary fat did not change in either group.
Satisfaction with the diet and cooking workshops was ≥8, on a 10-point scale, and did not differ between groups (Table S3).
Study Outcomes
Study outcomes are presented in Table 2. BMI percentile, body fat percentage, and trunk fat decreased in both groups (P<0.05), and the decrease in BMI percentile was greater for the LF compared to LGL group (P=0.03). Bioavailable testosterone, the primary outcome, and SHBG did not change in either group. Total/HDL-cholesterol ratio decreased in the LGL (P=0.02) but not in the LF group, such that the group effect approached significance (P=0.05). Other risk factors did not change in either group.
Table 2.
Study Outcomes for Participants who Completed the Study *
| Variable | Study Group | Unadjusted Data (Mean ± SD) |
Change from Baseline (Mean ± SE) |
||
|---|---|---|---|---|---|
| Baseline** | 6 months | 6 months | P value | ||
| Body Weight (kg) |
Low-GL | 97.4 ± 8.8 | 96.2 ± 9.9 | −1.2 ± 0.8 | 0.19 |
| Low-Fat | 87.4 ± 11.3 | 82.6 ± 12.2 | −4.8 ± 1.6 | 0.02 | |
| Low-Fat - Low-GL | −3.6 ± 1.9 | 0.08 | |||
| Height (cm) |
Low-GL | 163.5 ± 3.5 | 163.6 ± 3.5 | 0.1 ± 0.2 | 0.70 |
| Low-Fat | 163.2 ± 6.8 | 163.4 ± 6.9 | 0.2 ± 0.1 | 0.25 | |
| Low-Fat - Low-GL | 0.1 ± 0.2 | 0.64 | |||
| BMI (kg/m2) |
Low-GL | 36.5 ± 4.3 | 36.1 ± 4.7 | −0.5 ± 0.3 | 0.17 |
| Low-Fat | 32.8 ± 3.2 | 30.9 ± 3.7 | −1.9 ± 0.6 | 0.01 | |
| Low-Fat - Low-GL | −1.4 ± 0.7 | 0.08 | |||
| BMI Percentile | Low-GL | 98.6 ± 1.0 | 98.3 ± 1.3 | −0.4 ± 0.1 | 0.04 |
| Low-Fat | 96.9 ± 2.0 | 94.9 ± 3.3 | −2.0 ± 0.6 | 0.009 | |
| Low-Fat - Low-GL | −1.7 ± 0.7 | 0.03 | |||
| Body Fat (%) |
Low-GL | 46.3 ± 4.2 | 45.1 ± 4.6 | −1.2 ± 0.4 | 0.01 |
| Low-Fat | 43.2 ± 2.7 | 40.9 ± 3.5 | −2.2 ± 0.5 | 0.004 | |
| Low-Fat - Low-GL | −1.0 ± 0.7 | 0.16 | |||
| Trunk Fat (kg) |
Low-GL | 21.0 ± 4.4 | 20.1 ± 4.5 | −0.9 ± 0.3 | 0.04 |
| Low-Fat | 18.4 ± 4.0 | 16.3 ± 4.6 | −2.0 ± 0.6 | 0.01 | |
| Low-Fat - Low-GL | −1.1 ± 0.7 | 0.16 | |||
| Bioavailable Testosterone (ng/dL) |
Low-GL | 13.6 ± 4.8 | 14.0 ± 7.4 | 0.4 ± 1.5 | 0.81 |
| Low-Fat | 14.7 ± 8.5 | 12.9 ± 7.4 | −1.8 ± 1.6 | 0.29 | |
| Low-Fat - Low-GL | −2.2 ± 2.2 | 0.35 | |||
| Bioavailable Testosterone (%) |
Low-GL | 24.5 ± 8.4 | 24.5 ± 8.4 | −0.1 ± 1.7 | 0.97 |
| Low-Fat | 26.6 ± 10.8 | 25.2 ± 11.0 | −1.4 ± 1.3 | 0.30 | |
| Low-Fat - Low-GL | −1.4 ± 2.1 | 0.52 | |||
| Total Testosterone (ng/dL |
Low-GL | 61.3 ± 30.1 | 60.0 ± 24.8 | −1.3 ± 5.1 | 0.81 |
| Low-Fat | 55.2 ± 16.3 | 51.8 ± 19.7 | −3.4 ± 4.6 | 0.48 | |
| Low-Fat - Low-GL | −2.2 ± 7.0 | 0.76 | |||
| Free Testosterone (direct, pg/mL) |
Low-GL | 1.8 ± 1.0 | 1.7 ± 0.8 | −0.1 ± 0.2 | 0.48 |
| Low-Fat | 1.6 ± 0.9 | 1.2 ± 0.4 | −0.4 ± 0.3 | 0.13 | |
| Low-Fat - Low-GL | −0.3 ± 0.3 | 0.40 | |||
| SHBG (nmol/L) |
Low-GL | 22.9 ± 9.4 | 21.4 ± 10.5 | −1.5 ± 1.3 | 0.30 |
| Low-Fat | 19.6 ± 11.9 | 22.0 ± 14.3 | 2.4 ± 2.6 | 0.38 | |
| Low-Fat - Low-GL | 3.9 ± 3.2 | 0.24 | |||
| DHEAS (ug/dL) |
Low-GL | 232.5 ± 129.3 | 261.4 ± 147.4 | 28.9 ± 19.7 | 0.19 |
| Low-Fat | 273.7 ± 135.7 | 268.4 ± 126.4 | −5.3 ± 22.8 | 0.82 | |
| Low-Fat - Low-GL | −34.2 ± 31.2 | 0.29 | |||
| Total Cholesterol (mg/dL) |
Low-GL | 177.0 ± 16.0 | 169.4 ± 13.4 | −7.6 ± 18.7 | 0.32 |
| Low-Fat | 168.2 ± 14.7 | 161.3 ± 15.7 | −6.9 ± 12.0 | 0.12 | |
| Low-Fat - Low-GL | 0.7 ± 7.7 | 0.93 | |||
| LDL-C (direct) (mg/dL) |
Low-GL | 120.6 ± 21.7 | 112.0 ± 17.2 | −8.6 ± 6.0 | 0.20 |
| Low-Fat | 118.0 ± 14.0 | 111.6 ± 11.1 | −6.4 ± 3.4 | 0.10 | |
| Low-Fat - Low-GL | 2.1 ± 6.5 | 0.75 | |||
| HDL-C (mg/dL) |
Low-GL | 49.3 ± 13.3 | 52.6 ± 12.4 | 3.3 ± 2.2 | 0.18 |
| Low-Fat | 43.3 ± 8.3 | 42.1 ± 6.1 | −1.2 ± 1.7 | 0.49 | |
| Low-Fat - Low-GL | −4.5 ± 2.7 | 0.12 | |||
| Triglycerides (mg/dL) |
Low-GL | 93.9 ± 44.0 | 77.0 ± 30.5 | −16.9 ± 12.5 | 0.23 |
| Low-Fat | 92.7 ± 33.1 | 88.7 ± 37.0 | −4.0 ± 10.7 | 0.72 | |
| Low-Fat - Low-GL | 12.9 ± 16.4 | 0.45 | |||
| Total Cholesterol:HDL-C Ratio |
Low-GL | 3.8 ± 1.1 | 3.4 ± 0.9 | −0.4 ± 0.1 | 0.02 |
| Low-Fat | 4.0 ± 0.8 | 3.9 ± 0.6 | −0.1 ± 0.1 | 0.28 | |
| Low-Fat - Low-GL | 0.3 ± 0.2 | 0.05 | |||
| hs-CRP (mg/L) | Low-GL | 4.0 ± 4.1 | 3.3 ± 3.7 | −0.7 ± 1.4 | 0.62 |
| Low-Fat | 1.6 ± 1.5 | 2.1 ± 2.2 | 0.5 ± 0.6 | 0.46 | |
| Low-Fat - Low-GL | 1.2 ± 1.4 | 0.40 | |||
| Fasting Glucose (mg/dL) |
Low-GL | 80.9 ± 4.0 | 81.0 ± 4.0 | 0.1 ± 2.2 | 0.97 |
| Low-Fat | 80.3 ± 6.9 | 78.7 ± 7.4 | −1.6 ± 1.5 | 0.31 | |
| Low-Fat - Low-GL | −1.7 ± 2.6 | 0.52 | |||
| 120-min Glucose† (mg/dL) |
Low-GL | 129.3 ± 8.6 | 122.7 ± 11.9 | −5.7± 3.8 | 0.19 |
| Low-Fat | 124.7 ± 23.0 | 122.8 ± 28.9 | −1.9 ± 6.3 | 0.77 | |
| Low-Fat - Low-GL | 3.8 ± 8.3 | 0.66 | |||
| Fasting Insulin (uIU/mL) |
Low-GL | 16.4 ± 7.5 | 18.8 ± 15.6 | 2.4 ± 5.2 | 0.66 |
| Low-Fat | 13.2 ± 5.7 | 10.3 ± 7.0 | −2.9 ± 1.4 | 0.08 | |
| Low-Fat - Low-GL | 27.2 ± 39.9 | 0.51 | |||
| 120-min Insulin‡ (uIU/mL) |
Low-GL | 131.8 ± 98.4 | 115.7 ± 68.0 | −21.7 ± 29.1 | 0.49 |
| Low-Fat | 98.2 ± 72.2 | 96.9 ± 84.5 | 5.5 ± 26.8 | 0.84 | |
| Low-Fat - Low-GL | −5.3 ± 4.8 | 0.29 | |||
| Glucose iAUC | Low-GL | 97.9 ± 29.5 | 109.5 ± 20.3 | 11.6 ± 11.5 | 0.35 |
| Low-Fat | 100.7 ± 31.4 | 90.5 ± 22.0 | −10.2 ± 8.5 | 0.27 | |
| Low-Fat - Low-GL | −21.8 ± 14.0 | 0.14 | |||
| Insulin iAUC | Low-GL | 281.6 ± 242.8 | 230.0 ± 127.7 | −51.6 ± 53.8 | 0.37 |
| Low-Fat | 175.5 ± 72.0 | 143.8 ± 82.3 | −31.7 ± 17.7 | 0.11 | |
| Low-Fat - Low-GL | 20.0 ± 51.1 | 0.70 | |||
| HbA1c (%) |
Low-GL | 5.7 ± 0.3 | 5.7 ± 0.2 | −0.0 ± 0.1 | 0.75 |
| Low-Fat | 5.5 ± 0.3 | 5.3 ± 0.3 | −0.1 ± 0.1 | 0.15 | |
| Low-Fat - Low-GL | −0.1 ± 0.1 | 0.55 | |||
| Systolic Blood Pressure (mmHg) |
Low-GL | 101.0 ± 4.9 | 102.8 ± 5.9 | 1.8 ± 2.5 | 0.49 |
| Low-Fat | 101.6 ± 6.9 | 102.8 ± 5.2 | 1.1 ± 2.3 | 0.64 | |
| Low-Fat - Low-GL | −0.6 ± 3.4 | 0.85 | |||
| Diastolic Blood Pressure (mmHg) |
Low-GL | 64.8 ± 4.6 | 63.4 ± 3.5 | −1.4 ± 1.3 | 0.32 |
| Low-Fat | 62.6 ± 7.1 | 62.1 ± 5.6 | −0.4 ± 2.4 | 0.86 | |
| Low-Fat - Low-GL | 1.0 ± 2.9 | 0.74 | |||
Abbreviations: BMI, body mass index; SHBG, sex hormone binding globulin; DHEAS, dehydroepiandrosterone sulfate; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; iAUC, incremental area under the 2-hr FS-OGTT curve, in excess of the mean fasting level, calculated by the trapezoidal rule; HbA1c, hemoglobin A1c; hs-CRP, high sensitivity C-reactive protein
SI Conversion Factors: To convert bioavailable and total testosterone to nmol/L, multiply by 0.0347; direct free testosterone to pmol/L, multiply by 3.47; DHEAS to umol/L, multiply by 0.027; total cholesterol, LDL-C, and HDL-C to mmol/L, multiply by 0.0259; triglycerides to mmolL, multiply by 0.0113; glucose to mmol/L, multiply by 0.0555; insulin to pmol/L, multiply by 6.945; hs-CRP to nmol/L, multiply by 9.524.
N=16 (9 in Low-Fat Group, 7 in Low-GL Group).
Levels (mean ± SD) by study group at baseline and 6 months. Change (mean ± SE) from baseline assessed using t-test. P-value for each diet group tests the hypothesis of zero mean change from baseline within group. P-value for “Low-Fat – Low-GL” tests the hypothesis of zero difference between diet groups.
There were no differences between diet groups at baseline (P≥0.05).
At baseline, data were available for 15 participants (9 in Low-Fat Group, 6 in Low-GL Group).
At baseline, data were available for 15 participants (9 in Low-Fat Group, 6 in Low-GL Group). At 6-months, data were available for 15 participants (8 in Low-Fat Group, 7 in Low-GL Group).
Concerning results from the CHQ-CF87 (Table S4), scores on the “global health” subscale improved in the LGL group (P=0.03), and scores on the “change in health in the last year” subscale improved in both groups (P<0.01). Changes from baseline did not differ between groups for any of the subscales.
Correlations
Correlations of bioavailable testosterone with other outcomes are presented in Table S5. Change in bioavailable testosterone was positively correlated with change in fasting insulin (r=0.64, P=0.01). In a sensitivity analysis with removal of an influential outlier, the correlation remained near-significant (r=0.49, P=0.06).
DISCUSSION
We conducted a 6-month dietary intervention study in overweight and obese adolescents with PCOS. The retention rate of 84% in this study compares favorably to rates reported in other studies of adolescents with PCOS (17–19). Participants attended 98% of the in-person visits and 87% of the cooking workshops, received 82% of the telephone counseling calls, and reported high satisfaction with the diets and perceived usefulness of the cooking workshops. We carefully monitored treatment fidelity and assessed all study outcomes with minimal missing data. As such, we demonstrated feasibility of implementing intensive dietary interventions and assessing outcomes in adolescents with PCOS. However, with respect to our primary hypothesis, we found no indication that a LGL diet was better than a LF diet for attenuating hyperandrogenism. Bioavailable testosterone did not change for either group, despite significant reductions in body fat. Our findings may be attributed to true null effects of weight-loss diets on bioavailable testosterone in adolescents, insufficient weight loss to achieve hormonal and metabolic benefits, limited statistical power due to challenges with recruiting participants, insufficient adherence of some participants to dietary prescriptions, and/or disparate responses to diet associated with different phenotypes and metabolic profiles among adolescents with PCOS.
Regarding recruitment challenges, we were unable to achieve our proposed sample size of 50 subjects, even with extensive efforts to develop a clinic partnership and implement an EMR flagging protocol. Many patients who were flagged as potentially eligible for the study were not interested in participating. Based on communication with clinic providers, adolescents with PCOS who are referred to a tertiary care center by their primary care providers typically are seeking pharmacological treatment, such as HCs, and do not want to delay or discontinue such treatment. The attained sample size provided only 15% power to detect a difference of the observed magnitude (−2.2 ng/dL in bioavailable testosterone).
Variable adherence is a common challenge in dietary intervention studies, particularly among adolescents (19). Recognizing that adolescents desire increasing autonomy but often are ambivalent about behavior change, we implemented an intensive intervention comprising nutrition education and behavioral counseling to promote adherence to dietary prescriptions. Moreover, the participants were a self-selected group of patients who expressed an interest in dietary intervention, rather than pharmacological therapy, for managing PCOS and displayed motivation to take part in the study. We achieved some success in promoting adherence given that self-reported dietary intake shifted as intended, and the total/HDL-cholesterol ratio decreased more with the LGI vs. LF diet, as expected based on usual changes in blood lipids observed in previous studies of dietary composition (20, 21). However, differences in macronutrient intakes between groups were not statistically significant, suggesting that overall adherence may have been inadequate to elicit differential changes in metabolism and study outcomes, despite an intensive intervention.
Adolescents with PCOS represent a heterogeneous population, and criteria for the syndrome have evoked debate (12, 22). Due to heterogeneity, responses to dietary interventions may vary widely among individuals (23). Although careful evaluation of effect modification by covariates is beyond the scope of this pilot study, we used data pooled across groups in correlational analyses to evaluate relationships between bioavailable testosterone and other outcome variables. We found that change in fasting serum insulin was related to change in bioavailable testosterone, consistent with results from a study of adults (24) and suggesting that dietary intervention may have beneficial effects for treating hyperandrogenism via effects on insulin levels in some adolescents with PCOS.
Data are limited regarding the effects of dietary interventions, independent of pharmacological therapy, for managing PCOS in adolescents. Hoeger et al. (17) found that free androgen index (FAI) and SHBG improved from baseline, but total testosterone did not change, among 11 overweight and obese girls with PCOS who were assigned to a 24-week lifestyle management program with training in diet, exercise, and behavioral modification skills. Lass et al. (25) conducted a one-year lifestyle intervention with 59 obese girls who had PCOS, noting improvements in serum total testosterone, FAI, SHBG, menstrual function, and various cardiovascular disease risk factors among 26 girls who lost weight. Ornstein et al. (19) compared the effects of a very low-carbohydrate vs. hypocaloric low-fat dietary prescription in a pilot study of 16 girls with a BMI greater than the 85th percentile for age. They noted weight loss and improved menstrual function in analyses of pooled data, but no differences between dietary intervention groups. Regarding the effects of dietary interventions on biochemical hyperandrogenism, most studies have focused on adults with PCOS. While some studies indicate benefit but no significant effect of macronutrient composition (24, 26, 27), others indicate no effect (28, 29).
Several issues pertaining to study design warrant comment. Strengths include a randomized design with close monitoring of treatment fidelity, a primary outcome (bioavailable testosterone) with direct relevance to the pathophysiology of PCOS, assessment of dietary process measures, and evaluation of feasibility in the context of a pilot study. The primary limitations include small sample size, challenges in promoting adherence (even with intensive intervention), reliance on self-report to assess diet, and relatively short intervention duration.
In conclusion, we successfully implemented in-person visits, cooking workshops, and telephone counseling calls and assessed outcomes. Despite challenges with recruitment and adherence, we noted that LGL and LF dietary prescriptions were efficacious for promoting weight loss and reductions in body fat but did not attenuate biochemical hyperandrogenism among overweight and obese adolescents with PCOS. Clinical trials with adequate statistical power are needed to fully elucidate effects of diet on metabolic profiles of adolescents with PCOS, including biochemical hyperandrogenism and cardiometabolic risk factors. Success of trials aimed at assessing independent effects of diet on features of PCOS will depend on innovative recruitment strategies and novel approaches for promoting adherence to dietary interventions.
Supplementary Material
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Treatment guidelines for PCOS among overweight and obese adolescents specify lifestyle modification to achieve weight loss and thereby lower testosterone levels in the blood.
Data are limited regarding effects of different diets on metabolic features of PCOS, particularly among adolescents.
WHAT THIS ADDS
When comparing the impact of LGL and LF diet, we held in-person visits, cooking workshops, and telephone counseling calls to encourage adherence.
These intensive dietary interventions promoted weight loss but did not lower testosterone levels.
ACKNOWLEDGMENTS
We thank Margaret Apura for assistance in developing the dietary interventions; Joshua Riazi for conducting the cooking workshops; Jillian Aronovitz and Meghan Leary for clinic recruitment, organization of study visits, data collection, and data entry; Linda Seger-Shippee for conducting the 24-hour dietary and physical activity recall interviews; Sarah Steltz for quality control protocols and development and maintenance of the REDCap database; Catherine Matero and Veronica Gragnano for administrative support; Saul Katz for donating SoLo GI® Energy Bars (New Era Nutrition, Inc., Kelowna, British Columbia, Canada); clinic providers at Boston Children’s Hospital in the Division of Adolescent and Young Adult Medicine, Division of Gynecology, Division of Endocrinology, Optimal Weight for Life (OWL) Clinic, and Martha Elliot Health Center.
Funding/Support
This work was conducted with grants from the Thrasher Research Fund and New Balance Foundation, support from the Harvard Catalyst (Harvard Clinical and Translational Science Center, National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102), and financial contributions from Harvard University and its affiliated academic healthcare centers.
Dr. Ludwig was supported by a mid-career mentoring award from the National Institute of Diabetes and Digestive and Kidney Diseases (K24 DK082730). Dr. Wong was a recipient of a Canadian Institutes of Health Research (CIHR) Fellowship Award in the area of Clinical Research and a CIHR Randomized Controlled Trials—Mentoring Program Training Grant.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the Thrasher Research Fund, New Balance Foundation, Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
Role of Funder/Sponsor
The funding organizations played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Abbreviations
- PCOS
polycystic ovary syndrome
- LGL
low-glycemic load
- LF
low-fat
- BCH
Boston Children’s Hospital
- EMR
electronic medical record
- BMI
body mass index
- HC
hormonal contraceptive
- REDCap
Research Electronic Data Capture
- CLIA
Clinical Laboratory Improvement Amendments
- SHBG
sex hormone binding globulin
- DHEAS
dehydroepiandrosterone sulfate
- LDL
low-density lipoprotein
- HDL
high-density lipoprotein
- hs-CRP
high sensitivity C-reactive protein
- Hb
hemoglobin
- FS-OGTT
frequently-sampled oral glucose tolerance test
- iAUC
incremental area under the curve
- DXA
dual-energy x-ray absorptiometry
- HRQL
health-related quality of life
- CHQ
Child Health Questionnaire
Footnotes
Trial Registration: ClinicalTrials.gov, NCT01079845, http://clinicaltrials.gov/ct2/show/NCT01079845
CONFLICTS OF INTEREST STATEMENT
Dr. Ludwig received royalties for books on obesity and nutrition. Other authors declare no conflicts of interest relevant to this manuscript.
Author Contributions
HF, CG, DL, and CE conceptualized and designed the study and obtained the funding. JW, MG, HG, HF, and CE acquired, analyzed and interpreted the data. JW, HF, and CE drafted the manuscript. JW, MG, HG, HF, CG, DL, and CE provided critical revision of the manuscript for important intellectual content. JW and HF conducted the statistical analysis. JW and CE provided study supervision.
All authors have reviewed the manuscript and take responsibility of the submitted and published versions.
Additional Contributions
References
- 1.Azziz R, Carmina E, Dewailly D, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456–488. doi: 10.1016/j.fertnstert.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 2.Franks S. Adult polycystic ovary syndrome begins in childhood. Best Pract Res Clin Endocrinol Metab. 2002;16:263–272. doi: 10.1053/beem.2002.0203. [DOI] [PubMed] [Google Scholar]
- 3.Fruzzetti F, Perini D, Lazzarini V, Parrini D, Genazzani AR. Adolescent girls with polycystic ovary syndrome showing different phenotypes have a different metabolic profile associated with increasing androgen levels. Fertil Steril. 2009;92:626–634. doi: 10.1016/j.fertnstert.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Lewy VD, Danadian K, Witchel SF, Arslanian S. Early metabolic abnormalities in adolescent girls with polycystic ovarian syndrome. J Pediatr. 2001;138:38–44. doi: 10.1067/mpd.2001.109603. [DOI] [PubMed] [Google Scholar]
- 5.Palmert MR, Gordon CM, Kartashov AI, Legro RS, Emans SJ, Dunaif A. Screening for abnormal glucose tolerance in adolescents with polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87:1017–1023. doi: 10.1210/jcem.87.3.8305. [DOI] [PubMed] [Google Scholar]
- 6.Coviello AD, Legro RS, Dunaif A. Adolescent girls with polycystic ovary syndrome have an increased risk of the metabolic syndrome associated with increasing androgen levels independent of obesity and insulin resistance. J Clin Endocrinol Metab. 2006;91:492–497. doi: 10.1210/jc.2005-1666. [DOI] [PubMed] [Google Scholar]
- 7.Steinberger J, Daniels SR American Heart Association Atherosclerosis H, Obesity in the Young C, American Heart Association Diabetes C. Obesity, insulin resistance, diabetes, and cardiovascular risk in children: an American Heart Association scientific statement from the Atherosclerosis, Hypertension, and Obesity in the Young Committee (Council on Cardiovascular Disease in the Young) and the Diabetes Committee (Council on Nutrition, Physical Activity, and Metabolism) Circulation. 2003;107:1448–1453. doi: 10.1161/01.cir.0000060923.07573.f2. [DOI] [PubMed] [Google Scholar]
- 8.Silfen ME, Denburg MR, Manibo AM, et al. Early endocrine, metabolic, and sonographic characteristics of polycystic ovary syndrome (PCOS): comparison between nonobese and obese adolescents. J Clin Endocrinol Metab. 2003;88:4682–4688. doi: 10.1210/jc.2003-030617. [DOI] [PubMed] [Google Scholar]
- 9.Glueck CJ, Morrison JA, Friedman LA, Goldenberg N, Stroop DM, Wang P. Obesity, free testosterone, and cardiovascular risk factors in adolescents with polycystic ovary syndrome and regularly cycling adolescents. Metabolism. 2006;55:508–514. doi: 10.1016/j.metabol.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Trent M, Austin SB, Rich M, Gordon CM. Overweight status of adolescent girls with polycystic ovary syndrome: body mass index as mediator of quality of life. Ambul Pediatr. 2005;5:107–111. doi: 10.1367/A04-130R.1. [DOI] [PubMed] [Google Scholar]
- 11.Moran LJ, Pasquali R, Teede HJ, Hoeger KM, Norman RJ. Treatment of obesity in polycystic ovary syndrome: a position statement of the Androgen Excess and Polycystic Ovary Syndrome Society. Fertil Steril. 2009;92:1966–1982. doi: 10.1016/j.fertnstert.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 12.Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565–4592. doi: 10.1210/jc.2013-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azziz R, Carmina E, Dewailly D, et al. Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metab. 2006;91:4237–4245. doi: 10.1210/jc.2006-0178. [DOI] [PubMed] [Google Scholar]
- 14.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000:1–27. [PubMed] [Google Scholar]
- 15.Rosal MC, Ebbeling CB, Lofgren I, Ockene JK, Ockene IS, Hebert JR. Facilitating dietary change: the patient-centered counseling model. J Am Diet Assoc. 2001;101:332–341. doi: 10.1016/S0002-8223(01)00086-4. [DOI] [PubMed] [Google Scholar]
- 16.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoeger K, Davidson K, Kochman L, Cherry T, Kopin L, Guzick DS. The impact of metformin, oral contraceptives, and lifestyle modification on polycystic ovary syndrome in obese adolescent women in two randomized, placebo-controlled clinical trials. J Clin Endocrinol Metab. 2008;93:4299–4306. doi: 10.1210/jc.2008-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bridger T, MacDonald S, Baltzer F, Rodd C. Randomized placebo-controlled trial of metformin for adolescents with polycystic ovary syndrome. Arch Pediatr Adolesc Med. 2006;160:241–246. doi: 10.1001/archpedi.160.3.241. [DOI] [PubMed] [Google Scholar]
- 19.Ornstein RM, Copperman NM, Jacobson MS. Effect of weight loss on menstrual function in adolescents with polycystic ovary syndrome. J Pediatr Adolesc Gynecol. 2011;24:161–165. doi: 10.1016/j.jpag.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Sacks FM, Bray GA, Carey VJ, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360:859–873. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77:1146–1155. doi: 10.1093/ajcn/77.5.1146. [DOI] [PubMed] [Google Scholar]
- 22.Roe AH, Dokras A. The diagnosis of polycystic ovary syndrome in adolescents. Rev Obstet Gynecol. 2011;4:45–51. [PMC free article] [PubMed] [Google Scholar]
- 23.Pasquali R, Gambineri A, Cavazza C, et al. Heterogeneity in the responsiveness to long-term lifestyle intervention and predictability in obese women with polycystic ovary syndrome. Eur J Endocrinol. 2011;164:53–60. doi: 10.1530/EJE-10-0692. [DOI] [PubMed] [Google Scholar]
- 24.Gower BA, Chandler-Laney PC, Ovalle F, et al. Favourable metabolic effects of a eucaloric lower-carbohydrate diet in women with PCOS. Clin Endocrinol (Oxf) 2013;79:550–557. doi: 10.1111/cen.12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lass N, Kleber M, Winkel K, Wunsch R, Reinehr T. Effect of lifestyle intervention on features of polycystic ovarian syndrome, metabolic syndrome, and intima-media thickness in obese adolescent girls. J Clin Endocrinol Metab. 2011;96:3533–3540. doi: 10.1210/jc.2011-1609. [DOI] [PubMed] [Google Scholar]
- 26.Moran LJ, Noakes M, Clifton PM, Tomlinson L, Galletly C, Norman RJ. Dietary composition in restoring reproductive and metabolic physiology in overweight women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:812–819. doi: 10.1210/jc.2002-020815. [DOI] [PubMed] [Google Scholar]
- 27.Moran LJ, Noakes M, Clifton PM, Wittert GA, Williams G, Norman RJ. Short-term meal replacements followed by dietary macronutrient restriction enhance weight loss in polycystic ovary syndrome. Am J Clin Nutr. 2006;84:77–87. doi: 10.1093/ajcn/84.1.77. [DOI] [PubMed] [Google Scholar]
- 28.Douglas CC, Gower BA, Darnell BE, Ovalle F, Oster RA, Azziz R. Role of diet in the treatment of polycystic ovary syndrome. Fertil Steril. 2006;85:679–688. doi: 10.1016/j.fertnstert.2005.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marsh KA, Steinbeck KS, Atkinson FS, Petocz P, Brand-Miller JC. Effect of a low glycemic index compared with a conventional healthy diet on polycystic ovary syndrome. Am J Clin Nutr. 2010;92:83–92. doi: 10.3945/ajcn.2010.29261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.