Abstract
The vitamin A metabolite retinoic acid (RA) regulates adaptive immunity in the intestines, with well-characterized effects on IgA responses, Treg induction and gut trafficking of T and B effector cells. It also controls the generation of cDC precursors in the bone marrow and regulates cDC subset representation, but its roles in the specialization of intestinal cDC subsets is understudied. Here we show that RA acts cell-intrinsically in developing gut-tropic pre-mucosal DC (pre-μDC) to effect the differentiation and drive the specialization of intestinal CD103+CD11b− (cDC1) and of CD103+CD11b+ (cDC2). Systemic deficiency or DC-restricted antagonism of RA signaling resulted in altered phenotypes of intestinal cDC1 and cDC2, and reduced numbers of cDC2. Effects of dietary deficiency were most apparent in the proximal small intestine, and were rapidly reversed by reintroducing vitamin A. In cultures of pre-μDC with Flt3L and GM-CSF, RA induced cDC with characteristic phenotypes of intestinal cDC1 and cDC2 by controlling subset-defining cell surface receptors, regulating subset-specific transcriptional programs, and suppressing proinflammatory NF-κB-dependent gene expression. Thus RA is required for transcriptional programming and maturation of intestinal cDC, and with GM-CSF and Flt3L provides a minimal environment for in vitro generation of intestinal cDC1- and cDC2-like cDC from specialized precursors.
Introduction
The intestines are continuously challenged by microbiota, digested food and invading pathogens. Intestinal dendritic cells (DC) play a key role in immune homeostasis by directing immune responses appropriate to each antigen and stimulus encountered. DC have the unique ability to sample and process antigens, and translate microenvironmental cues for T and B cells through presentation of cytokines and metabolites to initiate and regulate immune responses or to induce or maintain tolerance.
The two predominant cDC subsets in the small intestine (SI) are identified phenotypically as CD11c+MHCII+ cells that are either CD103+CD11b− (hereinafter called cDC1) or CD103+CD11b+ (cDC2). The subsets differ developmentally, requiring different transcription factors. Although both process local antigen, migrate to draining MLN, generate tolerogenic or immune stimulatory responses and imprint T cell trafficking to the gut, they also differ functionally: For example, cDC2 express TLR5 that recognizes flagellin and are better inducers of Th17 cells and of IgA synthesis; cDC1 express TLR3, a pattern receptor for double-stranded viral RNA, and display Clec9A and XCR1, receptors implicated in the specialized ability of cDC1 to cross-present cell-associated antigens to CD8 T cells in vivo1–9. Both subsets can derive from phenotypically diverse BM precursors10,11, including pre-mucosal DC (Pre-μDC), a gut-tropic precursor that can give rise to both cDC subsets as well as to plasmacytoid DC (pDC). Pre-μDC express the gut homing receptor α4β7, migrate preferentially to the intestinal lamina propria (LP), and efficiently repopulate intestinal cDC pools.
Vitamin A enters the body in the diet, and achieves its highest concentrations in the proximal intestines and the draining mesenteric LN. High concentrations are maintained by bile secretion of retinal from systemic reservoirs, even during short-term deficiency of dietary sources12. Vitamin A is metabolized by intestinal epithelial, stromal and dendritic cells to the active form, RA, which regulates transcription through its nuclear receptors 13. RA is intimately involved in all aspects of intestinal homeostasis, regulating epithelial differentiation and barrier function, as well as the development and function of cells of the innate and adaptive immune system with both immune stimulatory or tolerogenic effects depending on the context13 RARα-dependent RA signaling enhances IgA class switching14, promotes Treg induction15,16, and imprints activated B and T cells with gut trafficking programs by inducing expression of the α4β7 integrin receptor for the mucosal vascular addressin MAdCAM1, and the chemoattractant receptor CCR9 for the small intestinal chemokine CCL2514,17. RA can also affect DC: it regulates the generation and representation of notch-dependent CD4+ and CD4−CD8− splenic cDC 18,19, induces DC expression of aldh1a2, a retinaldehyde dehydrogenase (RALDH) that confers on intestinal CD103+ DC their characteristic ability to produce RA to present it directly to interacting lymphocytes20,21,22, and synergizes with TGFβ to induce a tolerogenic DC phenotype 23. Moreover, although RA precursors are most concentrated in the intestines and GALT, RA is also produced locally from circulating retinol in other sites including the bone marrow (BM) where it enhances production of gut tropic pre-μDC11.
Here we sought to determine whether RA also controls the further development of pre-μDC, and to examine its contribution to the specialization of intestinal cDC subsets. Our results show that cell intrinsic RARα signaling regulates pre-μDC generation of intestinal cDC, and plays a central role in transcriptional programming of both cDC1 and of cDC2. Moreover, we show that in combination with GM-CSF and Flt3L, RA directs efficient in vitro generation of cDC with unique characteristics of intestinal cDC1 and cDC2 from their gut tropic pre-uDC precursors.
Results
RA signaling in DC controls intestinal cDC development
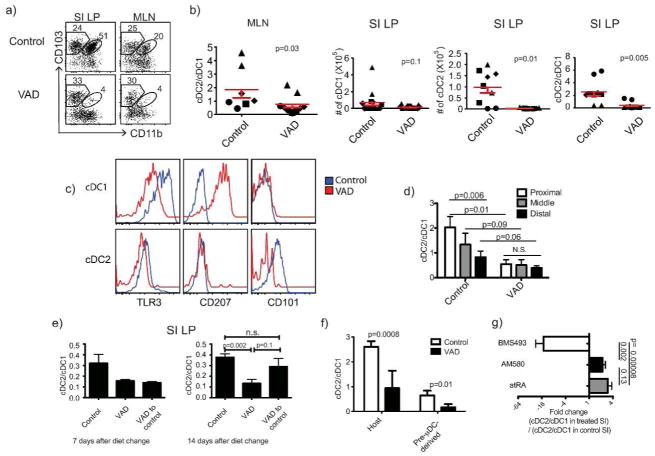
We initially evaluated cDC in mice rendered vitamin A deficient (VAD) by feeding a VAD diet for 12 weeks starting in utero17. VAD affected both the phenotypes and representation of intestinal cDC subsets. The frequency and number of cDC2 (CD103+CD11b+) was reduced in both SI LP and MLN of C57BL/6 mice, compared to mice on a control diet (Figures 1a,b). Similar results were obtained in BALB/c mice (Figure S1a) and in C57BL/6 mice fed a VAD diet beginning at weaning (Figure S1b). In contrast to the CD103+ cDC subsets, the mixed CD103-CD11b+ population, which consists of macrophages and minor CD103- intestinal cDC populations, although variable in numbers and frequency, showed no consistent difference between control and VAD mice in our studies (data not shown). Critical evaluation of the effect of RA on macrophage development will require studies with additional macrophage’s markers.
Figure 1. Vitamin A deficiency decreases cDC2 in GALT.
a) FACS plots show subset composition of CD11c+MHCII+ cDC in SI LP and MLN from control and long-term AD mice. The number in each gate indicates percent of cells. b) Ratio of cDC2 to cDC1 in MLN and SI LP, numbers of cDC1 and cDC2 recovered from SI LP of control and VAD mice. Paired 2-tailed t test. Error bars show SEM. c) TLR3, CD207 and CD101 expression on SI cDC1 and cDC2 from control and VAD mice. d) Effects of VAD on cDC subsets in different segments of the SI. SI from control and VAD animals were divided into three equal segments. N=4 independent experiments, 3 mice each. Paired 1-tailed t test. e) Long-term VAD BALB/c mice were put on vitamin A sufficient diet for 7 or 14 days. Results for 7 days were from 2 animals in each group from 1 experiment; results for 14 days were pooled from 3 independent experiments with 2–3 animals per condition in each experiment. f) 0.5–1 millionsorted pre-μDC were adoptively transferred intravenously into control or VAD recipients and mice were sacrificed 6 or 7 days later. Histograms show ratios of host or pre-μDC derived SI cDC2 to cDC1..Unpaired 1-tailed t test. g) Pharmacological agonists of RAR, atRA and AM580, increased and antagonist BMS493 decreased the cDC2/cDC1 ratio in the SI of BALB/c mice. Fold change (FC) of the ratio of cDC2 to cDC1 in SI LP from treated vs. control animals. Results for each condition are pooled from 2 independent experiments with 3 animals each. Unpaired 1-tailed t-test.
Moreover, the phenotype of cDC was abnormal in VAD mice (Figure 1c): cDC1 in VAD SI expressed only low levels of the cDC1-specific pathogen recognition receptor TLR3, but as reported24 displayed upregulated CD207 (Langerin), a lectin receptor expressed by skin associated DC such as Langerhans cells and dermal DC25 but normally absent on intestinal DC. In contrast cDC2 in VAD mice lacked CD101, an immunoregulatory receptor characteristic of cDC2 in normal (Vitamin A sufficient) mice. RA and precursor concentrations are highest in the proximal small intestines20,26,27, where cDC2 predominate 28. Consistent with the role of RA in generating cDC2, vitamin A deficiency largely abolished the proximal to distal gradient in relative representation of the cDC2 and cDC1 populations (Figure 1d). Moreover, the effects of VAD were reversible: 1 week of dietary vitamin A had little impact, but after 2 weeks the phenotype (e.g. expression of CD101) and relative representation of cDC2 (ratio to cDC1) in previously long term VAD mice had returned to that of control animals (Figure 1e and data not shown). The time required for reconstitution of intestinal cDC2 is consistent with their reported half-life of 1–2 weeks29, suggesting that RA might affect subset development from precursors. Indeed, VAD decreased the generation of cDC2 from transferred pre-uDC precursors, as indicated by the decreased ratio of cDC2 to cDC1 among the recovered progeny in adoptive recipients (Figure 1f). These results suggest that RA acts locally on recruited precursors to influence their differentiation into one subset or the other, although an effect on pre-μDC homing or on differential survival or retention of progeny cDC subsets was not excluded. Mice treated with the selective RARα agonist AM580 displayed an increased ratio of cDC2 to cDC1 in the SI LP, mirroring the effects of atRA supplementation (Figure 1g) and suggesting that RA affects cDC differentiation via RARα signaling. In contrast, the pan-RAR antagonist BMS493 decreased the ratio of cDC2 to cDC1 (Figure 1g).
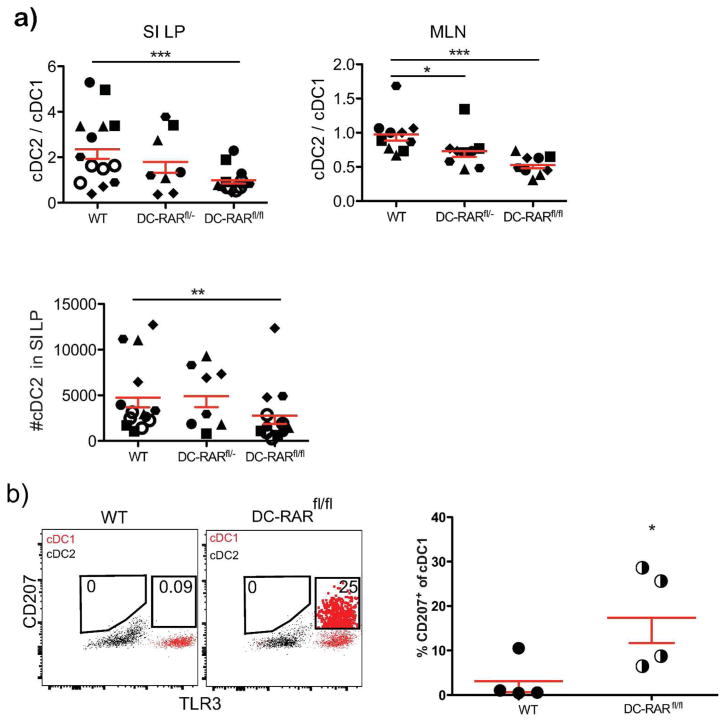
RA affects multiple cell types in the gut, including stromal cells and the epithelium, and could affect cDC indirectly. To determine whether DC-intrinsic RAR signaling was involved, we engineered mice to express a dominant negative RARα, RAR403, under the control of the CD11c promoter by crossing CD11c-Cre mice to mice carrying RAR403 downstream of a loxP-flanked STOP cassette (dnRaralsl/lsl) 30. These “DC-RAR403 mice” had a significant reduction in both absolute cDC2 numbers and in the cDC2/cDC1 ratio in the SI LP and MLN compared to their WT littermates (Figure 2a), and these effects were more prominent in mice that had two copies of the RAR403 gene (DC-RAR403fl/fl−) compared to mice with one copy (DC-RAR403fl/−). As in VAD mice, a subpopulation of intestinal cDC1 cells in DC-RAR403 mice expressed CD207 (Langerin) (Figure 2b). The results suggest that RA acts cell-intrinsically in intestinal cDC1 and cDC2 to influence their phenotype and development. However, subsets of intestinal macrophages are reported to exhibit cre recombination in CD11c-cre mice31 and may express RAR403 in our model ; thus indirect effects of macrophages on cDC phenotypes cannot be excluded.
Figure 2. Effects of DC-restricted deficiency in RA signaling on SI DC.
a) Ratio of cDC2 to cDC1 in SI LP and MLN and number of cDC2 in SI LP from DC-RAR403−/− (WT), DC-RAR403fl/− (Heterozygous) and DC-RAR403fl/fl (homozygous) mice. b) Aberrant expression of CD207 on SI cDC1 from DC-RAR403 mice. Shown are representative plots illustrating CD207 expression cells by SI cDC1 from DC-RARfl/fl mice (cells inside CD207+ gates are displayed as large dots for illustration). Scatter plot shows the percentage of cDC1 in SI that express CD207. Unpaired 1-tailed t-test.
Splenic cDC were also affected in DC-RAR403 mice (Figure S2). As in the SI of DC-RAR403 mice, a subset of splenic CD8α+ cDC1 in DC-RAR403 mice but not in WT controls expressed Langerin (CD207) (Figure S2a). Both CD11b+ and CD8α+ cDC numbers were diminished in the spleen of DC-RAR403 mice, but the ratio of CD11b+ cDC to CD8a+cDC was unchanged compared to WT littermates (Figure S2b and data not shown). However, cDC expressing Clec4a4 (DCIR2, recognized by Mab 33D1, a classical cDC2 marker) were significantly reduced, resulting in a reduced Clec4a4-defined cDC2 to cDC8α+ cDC1 ratio (Figure S2c). The Clec4a4+ subset that is strongly reduced in numbers in DC-RAR403 mice also expressed Esam in WT mice (Figure S2d). Thus cell-intrinsic RAR signaling influenced both cDC1 and cDC2 in the spleen as well as the SI.
Together the results suggest that DC-intrinsic RAR signaling regulates the development and phenotypic specialization of cDC subsets in both intestinal and extraintestinal sites, but support a particularly important role for RA in determining intestinal cDC phenotypes and regional specialization in the proximal small intestines and draining MLN where RA concentrations are high.
RA directs generation of intestine-like cDC1 and cDC2 subsets in vitro
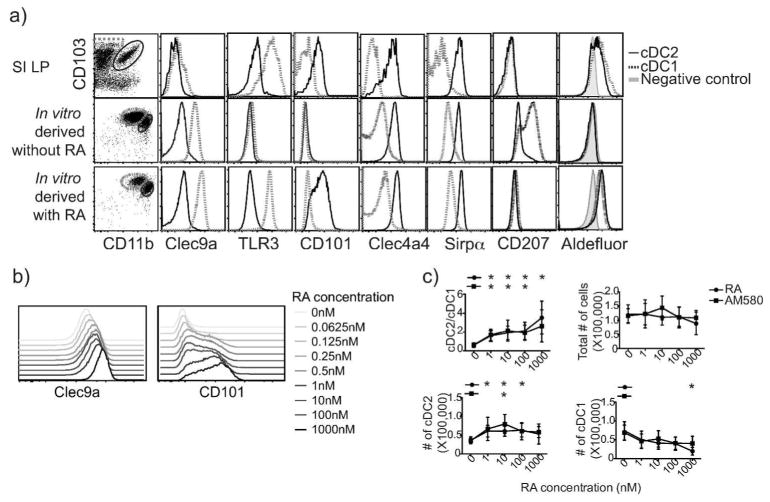
Although a variety of culture conditions have been used to generate DC from BM or monocyte precursors, classical culture conditions lead to DC whose phenotypes correspond poorly to those of intestinal cDC subsets observed in vivo32–34. We evaluated the phenotype of pre-μDC progeny generated in vitro. GM-CSF and Flt3L have been implicated in intestinal DC development: Flt3 KO mice have a global reduction in CD103+ cDC in the SI, while csf2r deficiency (GM-CSF receptor) selectively affects cDC2 numbers35 and leads to poor DC expression of RALDH activity 22. However, pre-μDC cultured with Flt3L alone developed into CD103+CD11b− and CD103−CD11b+ cells, but not CD103+CD11b+ cDC11. Culture of pre-μDC with Flt3L and GM-CSF gave rise to CD103+CD11b− and CD103+CD11b+ DCs (Figure 3a), but these DC lacked or displayed inappropriate expression of functionally important markers that define normal intestinal cDC1 and cDC2 in vivo. For instance, without RA in vitro derived cDC1 lacked TLR3 and had reduced Clec9a, while cDC2 lacked CD101 expression (Figure 3a). cDC1 derived in vitro without RA also aberrantly expressed CD207. Interestingly, these features (reduced TLR3 and expression of CD207 on cDC1, and absence of CD101 by cDC2 generated without RA) parallel the phenotypic abnormalities observed in VAD mouse intestinal cDC (Figure 1c). Moreover, cDC1 and cDC2 generated without RA lacked RALDH expression as indicated by lack of staining in the Aldefuor™ assay (Figure 3a).
Figure 3. RA directs generation of intestinal cDC-like DC subsets in vitro.
a–c) Pre-μDC were sorted from bone marrow of Flt3L-injected mice and cultured in complete IMDM media with 10% delipidated serum supplemented with Flt3L and GM-CSF without or with 100 nM of RA (or AM580 in c) unless otherwise specified. Cultures were harvested on day 4 and analyzed by flow cytometry. a) Surface markers and RALDH activity indicated by AldeFluor staining on in vivo intestinal and in vitro-derived cDC1 (dotted line) and cDC2 (solid line) are shown. Representative of at least 3 independent experiments. b) Expression of Clec9a on in vitro derived cDC1 and of CD101 on cDC2 with varying RA concentration. Representative of 2 independent experiments. c) Ratio of cDC2 to cDC1, numbers of total progeny cells, and numbers of cDC1 and cDC2 in cultures treated with indicated concentrations of RA or AM580 are shown. Data are pooled from 3 independent experiments.
Addition of RA, however, led to the generation of cDC1 and cDC2 with key phenotypic features of the two intestinal cDC subsets, including TLR3 on cDC1 and CD101 on cDC2 as well as RALDH expression by both subsets (Figure 3a). These results suggest that GM-CSF, Flt3L and RA act cooperatively to provide critical signals required for differentiation of intestinal cDCs from BM precursors. RA regulation of Clec9a on cDC1 and CD101 expression on the cDC2 subset was dose dependent (Figures 3b).
RA effected an increase in cDC2 in vitro, similar to its effect in vivo. When pre-μDC were cultured with varying concentrations of RA or the RARα agonist AM580 for 4 days, the presence of RA or AM580 increased the number of cDC2 generated while decreasing cDC1 numbers, effectively increasing the cDC2/cDC1 ratio. The effect of RA was dose dependent, with the cDC2/cDC1 ratio increasing from 0–1nM RA and plateauing as the RA concentration reached levels reported for the small intestine26 (~10nM). As the total number of progeny cells was not affected by RA or AM580 (Figure 3c), the results are consistent with direct action of RA on RARα in DC precursors to influence their fate.
Together, the results suggest a critical cell-intrinsic role for RA in intestinal cDC specialization, and define culture conditions for modeling of intestinal cDC development.
RA drives the transcriptomes of IN VITRO derived cDC1 and cDC2 towards physiologic patterns of gene expression
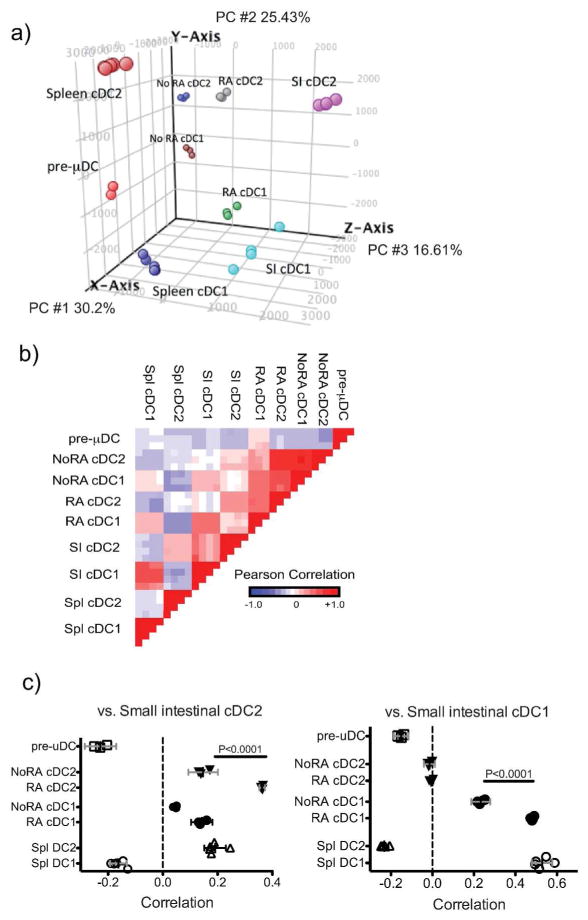
To assess the effects of RA on transcriptional programming of differentiating cDC, we carried out whole genome expression analyses on sorted pre-μDC, and on cDC1 and cDC2 generated from pre-μDC during 5 d culture with Flt3L and GM-CSF, with or without 100nM RA. Expression profiles of SI and spleen cDC1 and cDC236 (from http://immgen.org, GEO accession number GSE15907) were included for comparison. Principal component analysis (PCA) using all expressed genes (raw expression values (EV >120, and a coefficient variance of <50% in at least half of the samples) showed clustering of samples by cell type, indicating low technical and biological variation (Figure S3). To assess effects of RA on expression of genes associated with cDC1 vs. cDC2 specialization, we focused on a set of 1200 genes with 2 fold different expression between SI cDC1 and cDC2, and/or between spleen cDC2(CD8α−CD4+CD11b+ cDC) and cDC1 (CD8α+CD4−CD11b−cDC). PCA using these cDC1- and cDC2-associated gene sets revealed that cDC generated without RA remained distant from their in vivo counterparts (Figure 4a). In contrast, when generated in the presence of RA, cDC1 and to some extent cDC2 clustered more closely to their in vivo counterparts. In direct comparison of correlation of gene expression with SI cDC, in vitro-derived cDC1 aligned with SI cDC1, and their gene expression profile displayed a significantly higher correlation with that of SI cDC1 in vivo when RA was present during their development (Figure 4b,c). Similarly in vitro-derived cDC2 displayed significant correlation in gene expression with SI cDC2, and the presence of RA again resulted in a higher correlation with in vivo gene profiles (Figure 4b,c). Both populations derived in vitro with RA aligned more closely with their SI cDC counterparts than with spleen cDC (Figure 4b,c).
Figure 4. RA drives the transcriptomes of in vitro derived cDC1 and cDC2 towards patterns of physiologic gene expression.
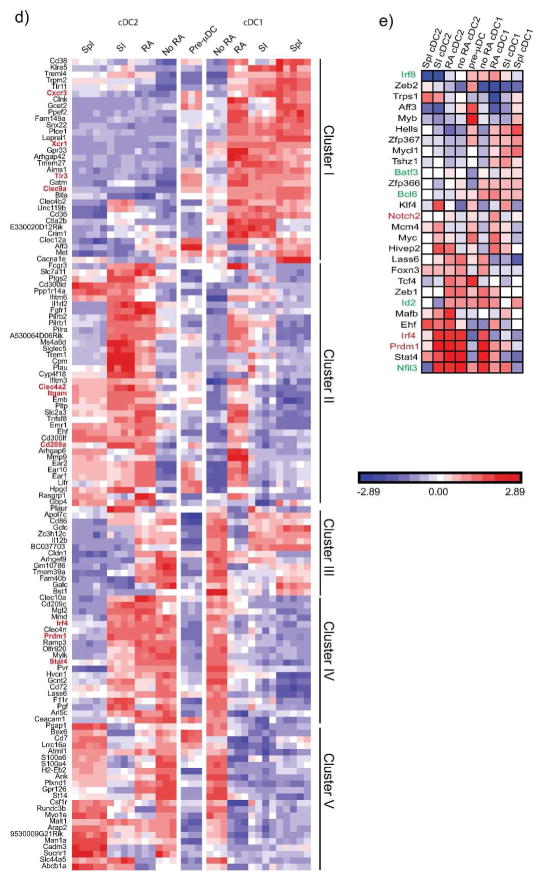
a) PCA of pre-μDC derived cDC1 and cDC2 generated in vitro in the presence or absence of RA, and spleen and SI cDC using 1200 genes with EV>120 that differed at least 2-fold between SI and/or spleen cDC1 and cDC2. b) Pair-wise comparison of the indicated cDC subsets and BM pre-μDC using the same genes as in a). c) Pairwise correlation of gene expression by in vitro derived cDC1 and cDC2 or spleen cDC1 and cDC2 replicates with the mean gene-expression profiles of SI cDC1 or cDC2, using the same genes as in a). Error bars are 95% CL. d) Heatmap of genes that are differentially expressed (>3X FC) between SI or spleen cDC1 and cDC2 and regulated by RA (>3X FC between in vitro cDC1 derived with and without RA OR cDC2 with and without RA). Clustering of genes is based on correlation. e) Transcription factor expression. Shown is expression of TF implicated previously in DC development, or regulated (2 FC) by RA in vitro and also differently and coordinately expressed by spleen and SI cDC1 vs. cDC2 (at least 1.5FC in both SI and spleen). TF implicated in cDC1 or cDC2 development are highlighted in green or red respectively.
To evaluate patterns of gene regulation, we selected 127 genes regulated in vitro by RA (>3 fold different expression by in vitro derived cDC1 with or without RA, or between cDC2 with or without RA) that also differed at least 3 fold between SI cDC1 vs. cDC2 and/or between spleen cDC1 vs. cDC2 (Table S1 and Figure 4d). These genes could be divided into 5 main clusters based on the effects of RA on their expression (Figure 4d). Cluster I consisted of genes that are higher in both SI and spleen cDC1 than in cDC2, and that were induced or maintained (from basal pre-μDC expression values (EV)) by RA in in vitro derived cDC1 but not cDC2, making the expression pattern of this group of genes in the in vitro-derived subsets more similar to their in vivo counterparts. Functionally important genes in this cluster included Clec9a, Xcr1, TLR3, and Cxcr3 encoding the chemokine receptor CXCR3. Genes in cluster II were more highly expressed in cDC2, especially in cDC2 from the SI, and were induced by RA in developing cDC2. However, RA also inappropriately induced the expression of some of these genes in cDC1. Examples of cluster 2 genes included CD209a, a c-type lectin receptor that has been implicated in pathogenic Th17 development in murine schistosomiasis; Itgam which encodes CD11b; and Clec4a2, a C-type lectin family regulatory receptor37. Cluster III genes were more highly expressed in cDC1 in vivo, but in most cases were actually suppressed in developing cDC1 by RA: these genes must be programmed by additional factors or conditions not replicated in our in vitro culture conditions. Cluster IV genes were more highly expressed in SI cDC2 than in cDC1 but were not differentially expressed between spleen cDC1 and cDC2. Interestingly, RA suppressed expression of most of these cDC2-specific genes in cDC1 without affecting their expression in cDC2. Genes in this category included genes for transcription factors important in intestinal cDC2 development (see below). Cluster V genes were more highly expressed in spleen cDC2 than cDC1 but were not as differentially expressed between the two SI cDC subsets. RA suppressed expression of these genes in cDC2 and more in cDC1. Together the results show that RA regulates gene expression in both cDC1 and cDC2 lineages, acting in a subset-specific manner to influence their specialization. For the majority of genes, regulation by RA leads to patterns of subset expression that better mimics expression by in vivo subsets, particularly cDC of the small intestines.
RA controls expression of transcription factors involved in cDC differentiation
We next evaluated expression of selected genes encoding regulators of DNA-templated transcription (Gene ontology term 0006355) that have either been implicated previously in DC development, or that were regulated (2-fold different expression) by RA in vitro and are also differently and coordinately expressed by spleen and SI cDC1 vs. cDC2 (at least 1.5FC in both SI and spleen). RA regulated several genes required for cDC2 development (highlighted in red in Figure 4e): it upregulated Notch2 in developing cDC1 and in cDC2 (compared to pre-μDC progeny in cultures without RA), and downregulated Prdm1 and Irf4 in cDC1. RA also downregulated Foxn3 in cDC1. Foxn3, Prdm1 and Irf4 are three of only five TF’s whose expression was identified as coordinately higher in both human and mouse intestinal cDC2 compared to cDC1; the other two being Zeb2 and Hivep238. Zeb2, highest in pre-μDC, was maintained or upregulated (compared to pre-μDC progeny in cultures without RA) by RA selectively in cDC2, correlating with high expression in cDC2 in vivo as well. Hivep2 was upregulated by RA preferentially in cDC2, although RA also enhanced Hivep2 expression in developing cDC1 in vitro. Among genes required for cDC1 development, RA upregulated Bcl6 in cDC1 and repressed it in cDC2; but it had no significant effect on expression of Id2, Irf8, or Batf3. The results suggest a significant role for RA in the regulation of subset-associated TF expression in developing cDC.
Overrepresentation of NF-κB/REL binding sites in promoters of RA suppressed genes
RA commonly activates gene expression, yet RA treatment suppressed the expression of many genes otherwise induced or expressed in our culture model. We used Enrichr39 to identify overrepresented transcription factor binding motifs in the promoter regions of genes whose expression was twofold lower in RA-treated vs. control cDC1 cells: the highest scoring were NF-κB and REL TFBS (TRANSFAC and JASPAR pwm module, P≤10−20 and <10−12 respectively (see below).). Genes downregulated by RA in cDC1 were also significantly enriched in genes involved in NF-κB mediated pro-inflammatory signaling pathways (Wikipathways, P=0.00004). NF-κB motifs were also enriched in promoters of RA-suppressed (twofold or more) genes in differentiating cDC2 (TRANSFAC and JASPAR pwm module; P<10−9). Thus, in addition to its role in enhancing cDC2 generation, under in vitro conditions that support differentiation of cDC1 and cDC2, RA suppresses pro-inflammatory gene programs.
Discussion
We evaluated the role of RA and RAR signaling in the generation and transcriptional programming of intestinal specialized cDC subsets from gut-tropic BM precursors both in vivo, and in a novel in vitro model of the development of cDC1 and cDC2. Our results indicate that RA acts cell-intrinsically to exert global effects on gene expression in both cDC1 and cDC2 lineages, and drives transcriptional programs that control subset generation and expression of functional and phenotypic features that define intestinal cDC1 and cDC2 specialization in vivo.
The combination of Flt3L, GM-CSF and RA was sufficient to drive the in vitro development of gut-tropic pre-μDC into CD103+ cDC1 and cDC2 that were similar in cell surface receptor expression to the two subsets found in the gut wall. Mayer CT et al showed that Flt3L and GM-CSF together support development of IRF8- and Batf3-dependent CD103+ cDC33, but we found pre-μDC -derived cDC1 generated in the absence of RA had an aberrant cell surface phenotype and were less aligned with intestinal cDC1 in gene expression than cDC1 generated with all three factors. Moreover, culture with RA allows generation of both intestinal DC-like populations. Consistent with our observations in VAD mice, increasing RA concentrations to within the range reported in the proximal SI (~10nM), increased the ratio of cDC2 to cDC1 among pre-μDC progeny in vitro. Higher concentrations further enhanced phenotypic specialization as indicated, for example, by progressive upregulation of CD101 and Clec9a. These observations support a critical role for RA in the normal development of cDC, and are consistent with a particularly important role for RA in cDC specialization in the intestines and GALT where vitamin A concentrations are highest. The culture model described here should facilitate future studies of the mechanisms involved.
RA regulated expression of many cDC-subset specific receptors and differentiation antigens during pre-μDC development into cDC1 and cDC2. Clec9a, an uptake receptor for apoptotic cells4,5,8, and TLR3, a receptor for double-stranded RNA implicated in responses to viruses, are both markers of cDC138. During in vitro development of cDC1 from pre-μDC, RA enhanced Clec9a and was required for expression of TLR3. Similarly, cDC1 from VAD mice have lower TLR3 expression compared to control mice. Defective TLR3 or Clec9a expression on cDC1 might thus lead to impaired immune responses to RNA viruses or apoptotic cells in Vitamin A deficient states. Our data show that, as in the human, CD101 is a specific marker of intestinal cDC2, and that RA upregulates it selectively in developing cDC2 in vitro. Consistent with an RA requirement, intestinal cDC2 from VAD mice expressed little if any CD101. Genes for TLR11, Cd300ld, leukocyte inhibitory factor receptor Lifr, triggering receptor expressed on myeloid cells Trem1, and for the lectins Siglec5 and Mgl2, which are selectively expressed by intestinal cDC2 in vivo, were highly upregulated by RA in developing cDC2 as well. Although the biological function of many of these RA regulated receptors remains to be fully clarified, they are likely to control cell-cell interactions, and pathogen and apoptotic cell recognition and signaling activities important to local immune homoestasis and function. CD101 is an immunoregulatory Ig family member implicated in moderation of NF-κB signaling in T cells40 and in IL10 production and inhibition of T cell proliferation by by cutaneous dendritic cells41 The C-type lectins Clec4a4 (DCIR2, the 33D1 antigen), Siglec5 and Mgl2 are potential cell-cell interaction or pattern recognition receptors. ESAM mediates cell adhesion. Cd300ld and TREM1 are likely DC activating receptors42,43. Lifr, the leukocyte inhibitory factor receptor, is a member of the type 1 cytokine receptor family with roles in cellular differentiation and regulation of immune function44 Thus RA controls diverse immune regulatory genes in developing cDC, effects that are likely to contribute to defective and dysregulated immunity in Vitamin A deficiency.
Our transcriptomic analyses confirmed that the progeny of pre-μDC cultured with RA align more closely in gene expression to in vivo intestinal cDC1 and cDC2 than their counterparts cultured with GM-CSF and Flt3L alone. This was particularly apparent when comparing subsets based on expression of genes differentially expressed by cDC1 vs. cDC2 in vivo. Interestingly, in vitro-derived cDC2 deviated more from their in vivo counterparts than cDC1, even when generated in the presence of RA, suggesting that they may be particularly sensitive to and dependent on local environmental influences. Consistent with this, previous studies of gene expression by cDC from intestines, skin, lymphoid tissues and blood showed that cDC2 from different tissue sites varied much more in gene expression than cDC138.
In addition to controlling cDC1 and cDC2 transcriptional programs, RA influences the subset composition of intestinal cDC, and of pre-μDC derived progeny both in vivo and in vitro. These results confirm and extend previous findings showing a role for RA in peripheral cDC2 development19,23: VAD mice displayed a selective reduction in peripheral cDC2, and in splenic CD4−CD8− cDC19. This effect on endogenous cDC2 paralleled the enhancement of cDC2 generation by RA from pre-μDC both in vitro in culture, and in vivo in recipients of transferred pre-μDC. Moreover, as in VAD mice, mice expressing the dominant negative RAR403 selectively in DC had reduced cDC2 but not cDC1 numbers, and a decreased cDC2/cDC1 ratio in the SI LP. Although CD11c-RAR403 cDC1 showed aberrant CD207 expression as in VAD mice, changes in cDC ratios and phenotypic features were less dramatic in DC-RAR403 mice than in VAD or BMS493 -treated mice: this may reflect incomplete inhibition of RA signaling by the dominant negative receptor45. For example, TLR3, which was reduced in SI cDC1 in VAD mice and nearly absent in cDC1 generated in vitro without RA, was retained on cDC1 in RAR403fl/fl mice. Indeed, we observed a gene dose effect on SI cDC1/cDC2 ratios when comparing RAR403fl/fl, RAR403fl/− and WT mice, consistent with incomplete shutdown of RAR transcriptional activity by the dominant negative RAR403. Together our results demonstrate a cell-intrinsic role of RA in pre-μDC fate as well as DC subset phenotypic maturation.. However, they do not preclude an additional role for indirect effects.
Cell fate determination is controlled by transcription factors. Id2, IRF8, Baft3, Nfil3 and Bcl6 are essential for development of intestinal cDC138,46, whereas IRF4, Notch2 and Blimp1 drive development of intestinal cDC238,47,48. RA regulation of the subset-specific expression of some of these critical transcription factors during cDC development from pre-μDC may contribute to the RA effect on precursor fate, as well as to subset-specific transcriptional programs. RA suppressed upregulation of the cDC2-associted TF’s Irf4 and Prdm1 encoding Blimp1 in cDC1, which may prevent developing cDC1 from acquiring a cDC2-phenotype. RA also fine-tuned expression of Bcl6, a TF required for cDC1 development, by increasing its expression in cDC1 and decreasing it in cDC2. These effects result in patterns of expression of subset-determining TF that reflect more accurately those of in vivo cDC1 and cDC2. Genes for other transcription factors such as Rel, Stat4 and Zeb2 that are differently expressed by intestinal cDC1 vs. cDC2 are also regulated by RA. Whether and to what extent the cDC2-promoting property of RA and RA control of subset-specific gene expression are mediated by these effects on TF regulation remains to be determined. Analysis of cis-elements in promoters of RA-repressed genes revealed enrichment for NF-κB TFBS motifs, especially in cDC1; and pathway analysis showed RA suppression of genes involved in proinflammatory signaling, a finding consistent with the reported role of RA in inducing or maintaining a tolerogenic phenotype of intestinal DC 23. Thus an additional function of RA signaling may be to suppress activation of cDC during their initial development from gut-homing precursors. Hivep2, a transcription factor that binds NF-κB TFBS and inhibits transcription, was substantially upregulated by RA in both cDC1 and cDC2 in culture and could contribute to the broad suppression of NF-kB target genes.
Taken together, our findings suggest a scenario in which RA regulation of intestinal DC starts in the BM where it positively regulates development of the gut-tropic DC precursor, pre-μDC. Pre-μDC home to and give rise to both cDC1 and cDC2 in the SI. In the gut wall, RA acts directly on pre-μDC, likely through regulation of fate-determining transcription factors, to enhance the generation of cDC2 and to direct gene expression programs in both cDC1 and cDC2 that contribute to their functional specialization, and their ability to maintain immune homeostasis and coordinate the immune response to invading pathogens.
MATERIALS AND METHODS
Mice
C57BL/6.CD45.2 (B6.CD45.2), C57BL/6.CD45.1 (B6.CD45.1) and BALB/c mice were originally purchased from Jackson Laboratory and were maintained and bred in specific pathogen-free conditions in the animal facility in Veterans Affairs Palo Alto Health Care Systems (VAPAHCS). To generate B16/Flt3L-injected mice, 5 million B16/Flt3L cells were injected subcutaneously near the neck and animals were sacrificed 11–14 days later. All animals were used in accordance with guidelines set forth by the animal committee of VAPAHCS. Vitamin A deficient and control mice were generated as described17. Short-term VAD mice were generated by putting newly weaned mice (3–4 weeks) on VAD or control diet for 1–3 months. In some experiments, mice were i.p. injected with BMS493 (25mM in DMSO) at 1μl per gram of weight in 100 μl of olive oil. Control animals received the same amount of DMSO in olive oil. All trans-RA was made into suspension in olive oil at 25 mg/ml and injected i.p. at 125μg per gram of weight, control animals received olive oil. AM580 was dissolved in DMSO at a concentration of 20 mg/ml and injected i.p. at 1μl per gram of weight in 100 μl of olive oil; control animals received the same amount of DMSO in olive oil. Animals were injected every 24 hours for 5 days.
Flow cytometry
Samples (single-cell suspensions) were first blocked with FACS buffer (HBSS with 2% FCS) containing 100X dilution of antibody against mouse Fcγ III/II receptor (BD Bioscience) and rat serum to prevent non-specific binding of monoclonal antibodies. The following antibodies were used for staining: CD3-PECy7/CD3-Biotin (145–2C11), CD19-PECy7/CD19-biotin (ID3), NK1.1-PECy7/NK1.1-biotin (PK136), CD49b-Biotin(DX-5), Ly6G-Biotin (1A8), Ter-119-biotin(Ter-119), B220-PECy7/B220-Biotin/B220-PerCPCy5.5 (RA3-6B2), MHCII-AF700/MHCII-FITC(M5/114.15.2)/MHCII-Biotin (2G9), CD11c-PB (N418), α4β7-APC/α4β7-PE (DATK32), CCR9-APC/CCR9-PE/CCR9-FITC (242503), CD103-PE/CD103-APC(M290), CD11b-AF700 (M1/70), CD8α-PE (53–6.7), CD45.1-PerCPCy5.5/CD45.1-APC, CD45.2-FITC/CD45.2- PerCPCy5.5 (RA3-6B2), CD135-PE/CD135-PerCP-EfluorF710 (A2F10), CD4-AF700(RM4-5), Sirpα-FITC/Sirpα-PerCP-eFluor710 (P84), TLR3-PE (11F8), Clec4a4-PE/Clec4a4-Biotin (33D1), CD101-PE (Mousei101), Clec9a-PerCP-eFluor710 (44D2), Langerin-FITC. All experiments analyzing cDC subsets included pre-gating on live, CD3e−,CD19−, NK1.1−, MHCII+, CD11c+.
Cell isolation from tissues11
Cell sorting
Pre-μDC and pre-cDC were sorted from B16/Flt3L treated mice. BM cells were isolated as described above and enriched by magnetic activated cell sorting (MACS) using the pan-DC kits from Miltenyi. The cells were then sorted on Aria II or III (BD) for Lineage (CD3, CD19, NK1.1)-CD11cintB220+α4β7+CCR9− pre-μDC and Lineage (CD3, CD19, NK1.1, B220)−CD11cintMHCII−Sirpa+ pre-cDC.
Adoptive transfer
0.5–1×106 sorted pre-μDC (CD45.2 Or CD45.1) were injected retro-orbitally into congenic recipients (CD45.1 or CD45.2). Recipient animals were sacrificed 5–7 days after transfer.
in vitro culture
Pre-μDC were sorted from BM from Flt3L-treated mice and cultured 0.5 million cells/ml, 200 μl per well in flat-bottom 96-well plate or 2 ml per well in 6-well plate in complete IMDM media (10% delipidated FCS, 1X penicillin/streptomycin) supplemented with 100ng/ml recombinant Flt3L, 10 ng/ml recombinant GM-CSF and RA of indicated concentration. Media were changed on day 3.
Analysis of ALDH activity was as described11.
Microarray
Total RNA was isolated from in vitro-derived cDC1 and cDC2 in the presence or absence of 100 nM RA using phenol-chloroform extraction method provided by Immgen (immgen.org). RNA integrity was determined using an Agilent Bioanalyzer (Stanford PAN Facility). Intact total RNA from each sample was used for amplification, labeling and hybridization by Expression Analysis. Samples were hybridized on a GeneChip mouse Gene 1.0 ST Array (Affymetrix). GeneSpring GX 12.6 software and the Partek Genomic Suite (version 6.6) were used to process and analyze the microarray data.
Statistical Analysis
The statistical significance of differences between two sets of data was assessed by Student’s t-test unless stated otherwise.
Supplementary Material
Acknowledgments
Funding
R.Z. is a recipient of the National Science Scholarship awarded by the Agency for Science, Technology And Research, Singapore; M.B. is funded by the German Research Foundation (DFG) grant BS56/1-1; KL was supported by fellowships from the German Research Foundation (DFG), the Crohn’s and Colitis Foundation of America, and an ITI Young Investigator Award from Stanford; M.L. is a recipient of a senior fellowship from the Crohn’s and Colitis Foundation, and was a fellow under the NIH Training Grant AI07290; The work was supported in part by NIH grants R01 AI093981 and R37 AI047822 to E.C.B.
We thank L. Rott for assistance with flow cytometry and cell sorting. The B16 melanoma cell line stably transfected with murine Flt3L was a kind gift from G. Dranoff, Dana-Farber Cancer Institute, Boston, MA. RAR403fl/fl mice are a generous gift from Dr. Shanthini Sockanathan (Johns Hopkins School of Medicine).
Footnotes
Conflicts of interest
The authors disclose no conflicts.
Author Contributions
R.Z. designed and performed experiments, analyzed and interpreted data and wrote the manuscript; M.B. performed experiments, analyzed and interpreted data and edited the manuscript; K.L and M.L performed experiments; E.C.B directed the study and wrote the manuscript. All authors reviewed and commented on the manuscript.
Transcript Profiling
Accession number of repository for expression microarray data: Pending
References
- 1.Gottschalk C, Damuzzo V, Gotot J, et al. Batf3-dependent dendritic cells in the renal lymph node induce tolerance against circulating antigens. J Am Soc Nephrol. 2013 Mar;24(4):543–549. doi: 10.1681/ASN.2012101022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crozat K, Tamoutounour S, Vu Manh TP, et al. Cutting edge: expression of XCR1 defines mouse lymphoid-tissue resident and migratory dendritic cells of the CD8alpha+ type. J Immunol. 2011 Nov 1;187(9):4411–4415. doi: 10.4049/jimmunol.1101717. [DOI] [PubMed] [Google Scholar]
- 3.Bachem A, Hartung E, Guttler S, et al. Expression of XCR1 Characterizes the Batf3- Dependent Lineage of Dendritic Cells Capable of Antigen Cross-Presentation. Front Immunol. 2012;3:214. doi: 10.3389/fimmu.2012.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang JG, Czabotar PE, Policheni AN, et al. The dendritic cell receptor Clec9A binds damaged cells via exposed actin filaments. Immunity. 2012 Apr 20;36(4):646–657. doi: 10.1016/j.immuni.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Zelenay S, Keller AM, Whitney PG, et al. The dendritic cell receptor DNGR-1 controls endocytic handling of necrotic cell antigens to favor cross-priming of CTLs in virusinfected mice. J Clin Invest. 2012 May 1;122(5):1615–1627. doi: 10.1172/JCI60644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sancho D, Joffre OP, Keller AM, et al. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature. 2009 Apr 16;458(7240):899–903. doi: 10.1038/nature07750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poulin LF, Reyal Y, Uronen-Hansson H, et al. DNGR-1 is a specific and universal marker of mouse and human Batf3-dependent dendritic cells in lymphoid and nonlymphoid tissues. Blood. 2012 Jun 21;119(25):6052–6062. doi: 10.1182/blood-2012-01-406967. [DOI] [PubMed] [Google Scholar]
- 8.Iborra S, Izquierdo HM, Martinez-Lopez M, Blanco-Menendez N, Reis e Sousa C, Sancho D. The DC receptor DNGR-1 mediates cross-priming of CTLs during vaccinia virus infection in mice. J Clin Invest. 2012 May 1;122(5):1628–1643. doi: 10.1172/JCI60660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caminschi I, Vremec D, Ahmet F, et al. Antibody responses initiated by Clec9Abearing dendritic cells in normal and Batf3(-/-) mice. Mol Immunol. 2012 Feb;50(1–2):9–17. doi: 10.1016/j.molimm.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Liu K, Victora GD, Schwickert TA, et al. In vivo analysis of dendritic cell development and homeostasis. Science. 2009 Apr 17;324(5925):392–397. doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeng R, Oderup C, Yuan R, et al. Retinoic acid regulates the development of a guthoming precursor for intestinal dendritic cells. Mucosal Immunol. 2013 Jul;6(4):847–856. doi: 10.1038/mi.2012.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaensson-Gyllenback E, Kotarsky K, Zapata F, et al. Bile retinoids imprint intestinal CD103+ dendritic cells with the ability to generate gut-tropic T cells. Mucosal Immunol. 2011 Jul;4(4):438–447. doi: 10.1038/mi.2010.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall JA, Grainger JR, Spencer SP, Belkaid Y. The role of retinoic acid in tolerance and immunity. Immunity. 2011 Jul 22;35(1):13–22. doi: 10.1016/j.immuni.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mora JR, Iwata M, Eksteen B, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006 Nov 17;314(5802):1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 15.Sun CM, Hall JA, Blank RB, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007 Aug 6;204(8):1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hill JA, Hall JA, Sun CM, et al. Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi Cells. Immunity. 2008 Nov 14;29(5):758–770. doi: 10.1016/j.immuni.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004 Oct;21(4):527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Beijer MR, Molenaar R, Goverse G, Mebius RE, Kraal G, den Haan JM. A crucial role for retinoic acid in the development of Notch-dependent murine splenic CD8- CD4- and CD4+ dendritic cells. European journal of immunology. 2013 Jun;43(6):1608–1616. doi: 10.1002/eji.201343325. [DOI] [PubMed] [Google Scholar]
- 19.Klebanoff CA, Spencer SP, Torabi-Parizi P, et al. Retinoic acid controls the homeostasis of pre-cDC-derived splenic and intestinal dendritic cells. J Exp Med. 2013 Sep 23;210(10):1961–1976. doi: 10.1084/jem.20122508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaensson-Gyllenback E, Kotarsky K, Zapata F, et al. Bile retinoids imprint intestinal CD103+ dendritic cells with the ability to generate gut-tropic T cells. Mucosal Immunol. 2011 Jul;4(4):438–447. doi: 10.1038/mi.2010.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molenaar R, Knippenberg M, Goverse G, et al. Expression of retinaldehyde dehydrogenase enzymes in mucosal dendritic cells and gut-draining lymph node stromal cells is controlled by dietary vitamin A. J Immunol. 2011 Feb 15;186(4):1934–1942. doi: 10.4049/jimmunol.1001672. [DOI] [PubMed] [Google Scholar]
- 22.Yokota A, Takeuchi H, Maeda N, et al. GM-CSF and IL-4 synergistically trigger dendritic cells to acquire retinoic acid-producing capacity. International immunology. 2009 Apr;21(4):361–377. doi: 10.1093/intimm/dxp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iliev ID, Mileti E, Matteoli G, Chieppa M, Rescigno M. Intestinal epithelial cells promote colitis-protective regulatory T-cell differentiation through dendritic cell conditioning. Mucosal Immunol. 2009 Jul;2(4):340–350. doi: 10.1038/mi.2009.13. [DOI] [PubMed] [Google Scholar]
- 24.Chang SY, Cha HR, Chang JH, et al. Lack of retinoic acid leads to increased langerinexpressing dendritic cells in gut-associated lymphoid tissues. Gastroenterology. 2010 Apr;138(4):1468–1478. 1478 e1461–1466. doi: 10.1053/j.gastro.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Hashimoto D, Miller J, Merad M. Dendritic cell and macrophage heterogeneity in vivo. Immunity. 2011 Sep 23;35(3):323–335. doi: 10.1016/j.immuni.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Villablanca EJ, Wang S, de Calisto J, et al. MyD88 and retinoic acid signaling pathways interact to modulate gastrointestinal activities of dendritic cells. Gastroenterology. 2011 Jul;141(1):176–185. doi: 10.1053/j.gastro.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mowat AM, Agace WW. Regional specialization within the intestinal immune system. Nat Rev Immunol. 2014 Oct;14(10):667–685. doi: 10.1038/nri3738. [DOI] [PubMed] [Google Scholar]
- 28.Denning TL, Norris BA, Medina-Contreras O, et al. Functional specializations of intestinal dendritic cell and macrophage subsets that control Th17 and regulatory T cell responses are dependent on the T cell/APC ratio, source of mouse strain, and regional localization. J Immunol. 2011 Jul 15;187(2):733–747. doi: 10.4049/jimmunol.1002701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Helft J, Ginhoux F, Bogunovic M, Merad M. Origin and functional heterogeneity of non-lymphoid tissue dendritic cells in mice. Immunol Rev. 2010 Mar;234(1):55–75. doi: 10.1111/j.0105-2896.2009.00885.x. [DOI] [PubMed] [Google Scholar]
- 30.Rajaii F, Bitzer ZT, Xu Q, Sockanathan S. Expression of the dominant negative retinoid receptor, RAR403, alters telencephalic progenitor proliferation, survival, and cell fate specification. Dev Biol. 2008 Apr 15;316(2):371–382. doi: 10.1016/j.ydbio.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 31.Murphy KM. Comment on “Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine”. Science. 2011 Jul 22;333(6041):405. doi: 10.1126/science.1198277. author reply 405. [DOI] [PubMed] [Google Scholar]
- 32.Inaba K, Inaba M, Romani N, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992 Dec 1;176(6):1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mayer CT, Ghorbani P, Nandan A, et al. Selective and efficient generation of functional Batf3-dependent CD103+ dendritic cells from mouse bone marrow. Blood. 2014 Nov 13;124(20):3081–3091. doi: 10.1182/blood-2013-12-545772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naik SH, Proietto AI, Wilson NS, et al. Cutting edge: generation of splenic CD8+ and CD8- dendritic cell equivalents in Fms-like tyrosine kinase 3 ligand bone marrow cultures. J Immunol. 2005 Jun 1;174(11):6592–6597. doi: 10.4049/jimmunol.174.11.6592. [DOI] [PubMed] [Google Scholar]
- 35.Bogunovic M, Ginhoux F, Helft J, et al. Origin of the lamina propria dendritic cell network. Immunity. 2009 Sep 18;31(3):513–525. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heng TS, Painter MW. The Immunological Genome Project: networks of gene expression in immune cells. Nat Immunol. 2008 Oct;9(10):1091–1094. doi: 10.1038/ni1008-1091. [DOI] [PubMed] [Google Scholar]
- 37.Fujikado N, Saijo S, Yonezawa T, et al. Dcir deficiency causes development of autoimmune diseases in mice due to excess expansion of dendritic cells. Nat Med. 2008 Feb;14(2):176–180. doi: 10.1038/nm1697. [DOI] [PubMed] [Google Scholar]
- 38.Watchmaker PB, Lahl K, Lee M, et al. Comparative transcriptional and functional profiling defines conserved programs of intestinal DC differentiation in humans and mice. Nat Immunol. 2014 Jan;15(1):98–108. doi: 10.1038/ni.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen EY, Tan CM, Kou Y, et al. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics. 2013;14:128. doi: 10.1186/1471-2105-14-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soares LR, Tsavaler L, Rivas A, Engleman EG. V7 (CD101) ligation inhibits TCR/CD3- induced IL-2 production by blocking Ca2+ flux and nuclear factor of activated T cell nuclear translocation. J Immunol. 1998 Jul 1;161(1):209–217. [PubMed] [Google Scholar]
- 41.Bouloc A, Bagot M, Delaire S, Bensussan A, Boumsell L. Triggering CD101 molecule on human cutaneous dendritic cells inhibits T cell proliferation via IL-10 production. European journal of immunology. 2000 Nov;30(11):3132–3139. doi: 10.1002/1521-4141(200011)30:11<3132::AID-IMMU3132>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 42.Bouchon A, Facchetti F, Weigand MA, Colonna M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature. 2001 Apr 26;410(6832):1103–1107. doi: 10.1038/35074114. [DOI] [PubMed] [Google Scholar]
- 43.Enomoto Y, Yamanishi Y, Izawa K, et al. Characterization of leukocyte monoimmunoglobulin- like receptor 7 (LMIR7)/CLM-3 as an activating receptor: its similarities to and differences from LMIR4/CLM-5. The Journal of biological chemistry. 2010 Nov 12;285(46):35274–35283. doi: 10.1074/jbc.M110.137166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weber MA, Schnyder-Candrian S, Schnyder B, et al. Endogenous leukemia inhibitory factor attenuates endotoxin response. Laboratory investigation; a journal of technical methods and pathology. 2005 Feb;85(2):276–284. doi: 10.1038/labinvest.3700216. [DOI] [PubMed] [Google Scholar]
- 45.Caton ML, Smith-Raska MR, Reizis B. Notch-RBP-J signaling controls the homeostasis of CD8- dendritic cells in the spleen. J Exp Med. 2007 Jul 9;204(7):1653–1664. doi: 10.1084/jem.20062648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Edelson BT, Kc W, Juang R, et al. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8alpha+ conventional dendritic cells. J Exp Med. 2010 Apr 12;207(4):823–836. doi: 10.1084/jem.20091627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Persson EK, Uronen-Hansson H, Semmrich M, et al. IRF4 transcription-factordependent CD103(+)CD11b(+) dendritic cells drive mucosal T helper 17 cell differentiation. Immunity. 2013 May 23;38(5):958–969. doi: 10.1016/j.immuni.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 48.Schlitzer A, McGovern N, Teo P, et al. IRF4 transcription factor-dependent CD11b+ dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity. 2013 May 23;38(5):970–983. doi: 10.1016/j.immuni.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.