Abstract
Background:
Although healthcare quality has considerably improved in many countries, pressure ulcer is still a major health challenge worldwide.
Objectives:
The current study aimed to evaluate the effects of TWOT on the healing of pressure ulcers.
Patients and Methods:
This study was a randomized controlled trial, and the convenient sample including 100 patients hospitalized in two university-affiliated medical-surgical intensive care units and one neurology unit located in Qazvin, Iran were studied. Patients with stage II-IV pressure ulcer on the sacral or ischial areas were randomly assigned to either the control or the experimental groups. The experimental group received a 12-day transdermal wound oxygen therapy. Wound status was assessed seven times before the intervention, as well as two, four, six, eight, ten, and twelve days after the intervention.
Results:
After 12 days of wound oxygen therapy, the number of patients with complete wound healing in the experimental group was significantly greater than that of the control group. Moreover, the total mean of wound area in the experimental group was significantly lower than that of the control group.
Conclusions:
Transdermal wound oxygen therapy can effectively promote wound healing in patients with pressure ulcers.
Keywords: Pressure Ulcer, Oxygen Therapy, Wounds
1. Background
Pressure ulcer is one of the major healthcare challenges (1). Although health care quality has considerably improved in many countries, pressure ulcer is still a common problem worldwide (2). The prevalence of pressure ulcers in the USA is about 25% (3). In Iran, the prevalence varies widely from 10% to 50% (4).
Pressure ulcers are usually developed secondary to insufficient blood supply, ischemia, and tissue necrosis over bony prominences. Tissue necrosis happens due to the compression of soft tissue between bony prominences and external pressure (5-7).
Pressure ulcers are associated with many serious complications such as infection and sepsis. Moreover, pressure ulcers also increase the length of hospital stay and the health care costs (8-11). Since 1993 to 2006, the prevalence of pressure ulcers in hospitalized adult patients has increased by 80%, imposing about US$11 billion on healthcare systems (12). The cost of treating a pressure ulcer in the UK varies from £1064 (stage I ulcer) to £10551 (stage IV ulcer) and the total cost is about £1.4 - 2.1 billion a year. It is also estimated that pressure ulcers can increase the cost of nursing cares up to 50% (13, 14).
One of the new interventions developed to treat pressure ulcers is transdermal or topical wound oxygen therapy (TWOT). Studies show that delivering oxygen directly to the wound site accelerates the angiogenesis, the collagen synthesis, the fibroblast growth processes, and suppresses the bacterial growth, which in turn facilitate tissue regeneration (3, 15, 16). This method has several advantages, including fewer complications and lower costs and more safety (17). Despite the potential effects of TWOT on wound healing, pressure ulcers are mainly managed pharmacologically. Therefore, there is a literature reporting the application of topical oxygen to heal wounds (15), and the results of researches declared that TWOT positively affected the pressure ulcers healing process (18-20) or had less significant effect on healing the pressure ulcers (21). Although there were not such researches in Iran; and well-designed clinical trials are needed to prove this notion.
2. Objectives
The current study aimed to evaluate the effects of TWOT on the healing of pressure ulcers.
3. Patients and Methods
It was a single-blind multi-center randomized controlled trial conducted in 2009.
3.1. Participants
A convenient sample of 100 adult patients hospitalized in two general state university-affiliated medical-surgical intensive care units and one neurology unit located in Qazvin, Iran were recruited. The Inclusion criteria were being at least 18 years old with a stage II-IV pressure ulcer, according to the University of Texas wound classification system (5), on the sacral or ischial areas. The exclusion criterion was having any kind of peripheral vascular diseases such as diabetes and mellitus-induced vascular complications. The coin-tossing method and block randomization technique (five blocks and ten samples in each block) were used to randomly assign the patients to either the control or the experimental groups.
The participants consisted of 100 patients with pressure sore enrolled in the study based on convenient sampling method. One patient from the intervention group and two patients from the control group were excluded. Patients or their families were blind for allocation; therefore, it is a single blind research.
3.2. Sample Size
The total sample size was 50 for each intervention and control group. The sample size was calculated based on the prior studies considering 95% confidence level; 80% power, d = 1 and σ = 1.8. The sampling formula was:
| (1) |
3.3. Intervention
Patients in the control group received only the routine care of the study setting. Patients in the experimental group received the routine care of the study setting in addition to TWOT. TWOT consisted of direct application of humidified high pressure ten liters per minute oxygen to the wound site for 20 minutes, three times a day for 12 days. A disposable catheter was used to deliver oxygen to the wound site. Thereafter, wound dressing was applied by using saline-soaked gauzes. Dressing was changed once in every working shift. This type of dressing was used to minimize wound site irritation and to promote patients’ comfort. All the interventions were implemented by a nurse holding B. Sc. and experienced in wound care.
3.4. Data Collection
The primary outcome measure was the wound status. To assess the wound status the Pressure Ulcers Scale for Healing (PUSH) was employed (22, 23). PUSH was developed by the national pressure ulcer advisory panel in 1991. The validity and reliability of PUSH were confirmed in previous studies (17, 23). Wound area was measured in square millimeters by a standard sterile graded clipper. Wound status was assessed before and every 48 hours after the beginning of the intervention. Accordingly, wound status was assessed seven times, i.e., before the intervention (T1), as well as two days (T2), four days (T3), six days (T4), eight days (T5), ten days (T6), and twelve days (T7) after the intervention. According to the average patient stay in the units (12 days) and limited resources, the intervention was carried out for 12 days. And wound assessment was performed by two educated nurses. The inter-rater reliability coefficient was 0.97.
3.5. Data Analysis
The Statistical Package for Social Sciences version 17.0 (SPSS v.17.0; SPSS Inc., Chicago, IL, USA) was employed for data analysis. To analyze the study data, descriptive statistics measures such as percentage, mean, and standard deviation were used. First the Kolmogorov- Smirnov test was performed, which confirmed the normality of data. The study groups were compared in terms of the study variables by the independent-sample t-test, the binominal, and the Chi-square tests. Moreover, the repeated measures analysis of variance (ANOVA) test was used to compare changes in the wound status across the seven assessment time-points. P values less than 0.05 were considered as significant. The effect of other variables such as gender, age and other confounding factors were transferred into the model.
3.6. Ethical Consideration
Ethical consideration (including plagiarism, research misconduct, data fabrication or falsification, redundancy, double submission, etc.) was completely observed by the authors. The university-affiliated institutional review board and ethics committee approved the study (No: k 2635/20/28). Study participants were informed that participation in and withdrawal from the study were voluntary. It was ensured that the patients’ personal information will be analyzed and reported anonymously. Finally, they were asked to read and sign the informed consent form of the study. The ethical code number and further ethical consideration is in IRCT website (the registration number: IRCT138708051407N1).
4. Results
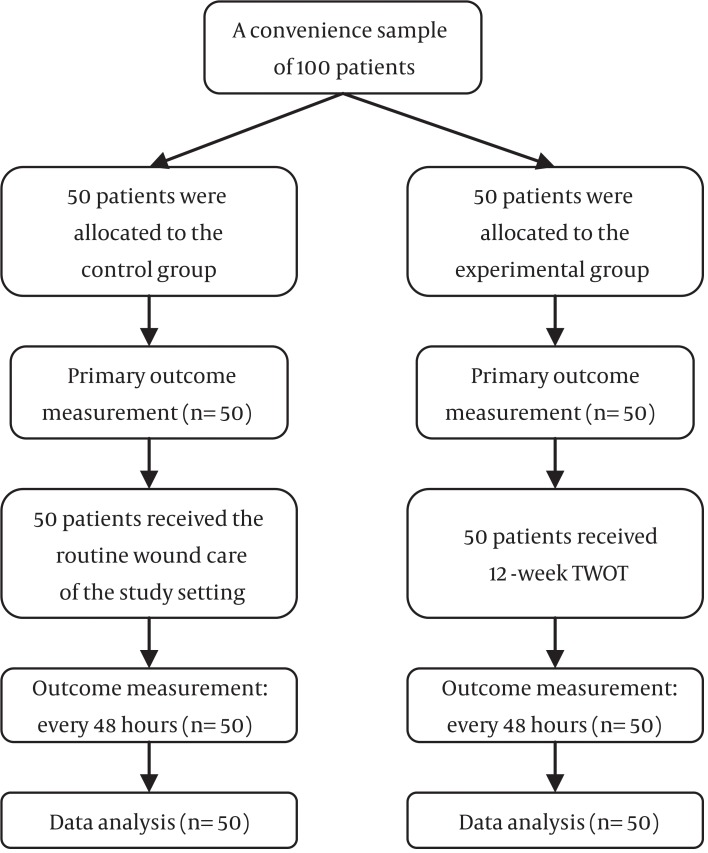
Totally, 100 patients, 50 patients (males 48% and females 52%) in the intervention group and 50 patients (males 52% and females 48%), participated in the control group in the study (Figure 1). The results of the independent-samples t-test and Chi-square test showed that the control and the experimental groups did not differ significantly in terms of age, gender, previous history of cerebrovascular accident, level of consciousness, mobility, and baseline wound stage and wound area (P value > 0.05; Table 1).
Figure 1. The Flow Diagram of the Study.
Table 1. Patients’ Demographic Data and Wound Characteristics.
| Variable | Control Group | Intervention Group | P Value |
|---|---|---|---|
| Age, y a | 69.56 ± 12.26 | 70.48 ± 12.33 | 0.17 b |
| Gender male/female, n | 26/24 | 26/24 | 1.00 c |
| History of CVA (yes/no), n | 38/12 | 36/14 | 0.08 c |
| Loss of consciousness (coma), n | 34 | 32 | 0.09 c |
| Immobility, n | 50 | 50 | 1.00 c |
| Wound area, cm 2 | 28.74 ± 15.88 | 31.81 ± 12.94 | 0.153 b |
| Wound stage d | |||
| Class II | 29 (58) | 27 (54) | 0.09 b |
| Class III | 16 (32) | 18 (36) | 0.08 b |
| Class IV | 5 (10) | 5 (10) | 1.00 b |
aData are presented as mean ± SD.
bThe result of the independent samples t-test.
cThe result of Chi-square test.
dData are presented as No. (%).
On the other hand, after 12 days of TWOT, the number of patients with complete wound healing, defined as complete epithelialization of the wound with the absence of drainage, in the experimental group (sixteen patients) was greater than that of the control group (only one patient). The results of the binominal test revealed that this difference was statistically significant (P value < 0.001).
Moreover, the results of the repeated measure ANOVA test for the within subjects factor of time showed that the wound area differed significantly across the seven assessment time-points in the experimental group (P < 0.001; Table 2). However, the results of this test in the control group indicated that, the wound area did not differ significantly across the assessment time-points (P value = 0.16; Table 2).
Table 2. Wound Area in the Study Groupsa.
| Groups | Baseline | Day Two | Day Four | Day Six | Day Eight | Day Ten | Day Twelve | Mean | P Value b |
|---|---|---|---|---|---|---|---|---|---|
| Intervention | 28.74 ± 5.88 | 25.31 ± 3.73 | 20.35 ± 3.02 | 18.89 ± 2.63 | 17.59 ± 6.79 | 21.02 ± 8.60 | 8.52 ± 6.36 | 13.36 ± 7.07 | 0.001 |
| Control | 31.81 ± 3.94 | 31.91 ± 3.74 | 31.79 ± 3.10 | 30.39 ± 3.56 | 29.26 ± 3.33 | 33.18 ± 4.82 | 35.68 ± 7.06 | 31.81 ± 3.94 | 0.16 |
| P Value c | 0.153 | 0.825 | 0.928 | 0.003 | 0.0011 | 0.116 | 0.0011 | 0.0011 |
aData are presented as mean ± SD.
bThe result of the repeated measure test.
cThe result of the independent samples t-test.
On the other hand, the results of the independent-samples t-test showed that the study groups significantly differed in terms of wound area on sixth, eighth, and twelfth days (P value< 0.01; Table 2). Moreover, the results of this test revealed that the total mean of wound area in the experimental group was significantly lower than that of the control group (P value < 0.001; Table 2).
5. Discussion
The current study aimed to evaluate the effects of TWOT on the healing of pressure ulcers. The study findings revealed that TWOT positively affected the pressure ulcers healing process. This finding is in line with the findings of the previous studies (3, 4, 24-26).
It was also found that the number of patients with complete wound healing was greater in the experimental group than the control group. Tawfick and Sultan also evaluated the effects of TWOT on the healing of venous ulcers and reported that 80% of the patients in the experimental group and only 35% of patients in the control group experienced complete wound healing (20). Robson et al. evaluated the cost-effectiveness of transdermal oxygen therapy in the treatment of necrotic ulcers and reported that in the experimental group, the wound healing rate was significantly higher than that of the control group (19).
Transdermal wound oxygen therapy can effectively promote wound healing in patients with pressure ulcers. Compared to the expensive wound management approaches, using a simple catheter to deliver oxygen to the wound site bears no considerable cost. Therefore, transdermal wound oxygen therapy is a cost-effective method to treat pressure ulcers. This simple method is recommended as an alternative strategy to treat pressure ulcers. However, further studies are still needed to provide more evidence on the effectiveness of transdermal wound oxygen therapy.
Patients with diabetic foot ulcers were not included in the current study. Therefore, conducting studies to assess the effect of TWOT on diabetic foot ulcers is recommended. Moreover, the current study only assessed the effectiveness of TWOT in the healing of pressure ulcers located on the sacral and ischial areas. Consequently, further studies are recommended to examine the effectiveness of TWOT to treat pressure ulcers located on the other parts of the body. Finally, according to the relatively small sample size of the current study, studies with larger sample sizes are recommended.
Acknowledgments
Authors wish to acknowledge their sincere gratitude to the research administration of Qazvin university of medical sciences for financial supporting of the current study. Authors also would like to gratefully thank the managers of Boali and Kowsar hospitals who strongly supported the researchers throughout the research process.
Footnotes
Authors’ Contributions:Study concept and design: Jalil Azimian; data guttering: Jalil Azimian, Enis Pourkhaleghi and Monireh Ansari; analysis and interpretation of data: Jalil Azimian; drafting of the manuscript: Jalil Azimian; revision of the manuscript for important intellectual content: Jalil Azimian, Nahid Dehghan Nayeri; Statistical analysis: Jalil Azimian.
Financial Disclosure:This work was sponsored by Qazvin University of medical sciences, IR Iran.
Funding Support:This study was supported by the grant of Qazvin University of medical sciences, IR Iran.
References
- 1.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453(7193):314–21. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 2.Dib M. Pressure ulcers: prevention and management. Lebanese Med J. 2007;56(2):112–7. [PubMed] [Google Scholar]
- 3.Hunter SM, Cathcart-Silberberg T, Langemo DK, Olson B, Hanson D, Burd C, et al. Pressure Ulcer Prevalence and Incidence in a Rehabilitation Hospital. Rehabil Nurs. 1992;17(5):239–43. doi: 10.1002/j.2048-7940.1992.tb01557.x. [DOI] [PubMed] [Google Scholar]
- 4.Fatemi E. Comparing the Effect of lazer therapy ,ultrasound and routine wound care in bed sore healing. J Kosar. 2000;4(3):33–6. [Google Scholar]
- 5.Grey JE, Harding KG, Enoch S. Venous and arterial leg ulcers. BMJ. 2006;332(7537):347–50. doi: 10.1136/bmj.332.7537.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pancorbo-Hidalgo PL, Garcia-Fernandez FP, Lopez-Medina IM, Alvarez-Nieto C. Risk assessment scales for pressure ulcer prevention: a systematic review. J Adv Nurs. 2006;54(1):94–110. doi: 10.1111/j.1365-2648.2006.03794.x. [DOI] [PubMed] [Google Scholar]
- 7.Stechmiller JK, Cowan L, Whitney JD, Phillips L, Aslam R, Barbul A, et al. Guidelines for the prevention of pressure ulcers. Wound Repair Regen. 2008;16(2):151–68. doi: 10.1111/j.1524-475X.2008.00356.x. [DOI] [PubMed] [Google Scholar]
- 8.Graves N, Birrell F, Whitby M. Effect of pressure ulcers on length of hospital stay. Infect Control Hosp Epidemiol. 2005;26(3):293–7. doi: 10.1086/502542. [DOI] [PubMed] [Google Scholar]
- 9.Hopkins A, Dealey C, Bale S, Defloor T, Worboys F. Patient stories of living with a pressure ulcer. J Adv Nurs. 2006;56(4):345–53. doi: 10.1111/j.1365-2648.2006.04007.x. [DOI] [PubMed] [Google Scholar]
- 10.Lyder CH. Pressure ulcer prevention and management. JAMA. 2003;289(2):223–6. doi: 10.1001/jama.289.2.223. [DOI] [PubMed] [Google Scholar]
- 11.Gorecki C, Brown JM, Nelson EA, Briggs M, Schoonhoven L, Dealey C, et al. Impact of pressure ulcers on quality of life in older patients: a systematic review. J Am Geriatr Soc. 2009;57(7):1175–83. doi: 10.1111/j.1532-5415.2009.02307.x. [DOI] [PubMed] [Google Scholar]
- 12.Cox J. Predictors of pressure ulcers in adult critical care patients. Am J Crit Care. 2011;20(5):364–75. doi: 10.4037/ajcc2011934. [DOI] [PubMed] [Google Scholar]
- 13.Kuhn BA, Coulter SJ. Balancing the pressure ulcer cost and quality equation. Nurs Econ. 1992;10(5):353–9. [PubMed] [Google Scholar]
- 14.Moore ZE, Webster J, Samuriwo R. Wound-care teams for preventing and treating pressure ulcers. Cochrane Database Syst Rev. 2015;9:CD011011. doi: 10.1002/14651858.CD011011.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feldmeier JJ, Hopf HW, Warriner R3, Fife CE, Gesell LB, Bennett M. UHMS position statement: topical oxygen for chronic wounds. Undersea Hyperb Med. 2005;32(3):157–68. [PubMed] [Google Scholar]
- 16.Roe DF, Gibbins BL, Ladizinsky DA. Topical dissolved oxygen penetrates skin: model and method. J Surg Res. 2010;159(1):e29–36. doi: 10.1016/j.jss.2009.10.039. [DOI] [PubMed] [Google Scholar]
- 17.Roeckl-Wiedmann I, Bennett M, Kranke P. Systematic review of hyperbaric oxygen in the management of chronic wounds. Br J Surg. 2005;92(1):24–32. doi: 10.1002/bjs.4863. [DOI] [PubMed] [Google Scholar]
- 18.Kranke P, Bennett MH, Martyn-St James M, Schnabel A, Debus SE, Kranke P. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev. 2012;6:CD004123. doi: 10.1002/14651858.CD004123.pub3. [DOI] [PubMed] [Google Scholar]
- 19.Robson MC, Hill DP, Woodske ME, Steed DL. Wound healing trajectories as predictors of effectiveness of therapeutic agents. Arch Surg. 2000;135(7):773–7. doi: 10.1001/archsurg.135.7.773. [DOI] [PubMed] [Google Scholar]
- 20.Tawfick W, Sultan S. Does topical wound oxygen (TWO2) offer an improved outcome over conventional compression dressings (CCD) in the management of refractory venous ulcers (RVU)? A parallel observational comparative study. Eur J Vasc Endovasc Surg. 2009;38(1):125–32. doi: 10.1016/j.ejvs.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 21.Whitney J, Phillips L, Aslam R, Barbul A, Gottrup F, Gould L, et al. Guidelines for the treatment of pressure ulcers. Wound Repair Regen. 2006;14(6):663–79. doi: 10.1111/j.1524-475X.2006.00175.x. [DOI] [PubMed] [Google Scholar]
- 22.Stotts NA, Rodeheaver GT, Thomas DR, Frantz RA, Bartolucci AA, Sussman C, et al. An Instrument to Measure Healing in Pressure Ulcers: Development and Validation of the Pressure Ulcer Scale for Healing (PUSH). J Gerontol Series A Biol Sci Med Sci. 2001;56(12):M795–9. doi: 10.1093/gerona/56.12.M795. [DOI] [PubMed] [Google Scholar]
- 23.Thomas DR. Prevention and treatment of pressure ulcers: what works? what doesn't? Cleve Clin J Med. 2001;68(8):704-7–717-22. doi: 10.3949/ccjm.68.8.704. [DOI] [PubMed] [Google Scholar]
- 24.Kalliainen LK, Gordillo GM, Schlanger R, Sen CK. Topical oxygen as an adjunct to wound healing: a clinical case series. Pathophysiology. 2003;9(2):81–7. doi: 10.1016/s0928-4680(02)00079-2. [DOI] [PubMed] [Google Scholar]
- 25.Tandara AA, Mustoe TA. Oxygen in wound healing--more than a nutrient. World J Surg. 2004;28(3):294–300. doi: 10.1007/s00268-003-7400-2. [DOI] [PubMed] [Google Scholar]
- 26.Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89(3):219–29. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]