Abstract
The neuropeptide alpha-melanocyte stimulating hormone (alpha-MSH) suppresses IFN-gamma production by DTH-mediating effector CD4+ T cells from mice. We discovered, however, that despite this significant supression of IFN-gamma production, alpha-MSH-treated effector T cells had the same level of IFN-gamma mRNA expression and intracellular IFN-gamma protein as untreated activated T cells. In order to explain why IFN-gamma production was suppressed in the face of unchanged mRNA and intracellular IFN-gamma levels, we looked for mechanisms that could increase the degradation of IFN-gamma within the alpha-MSH-treated T cells. Among the known pathways of post-translational intracellular protein modification, the ubiquitin-proteosome system was examined in alpha-MSH-treated T cells to see if a post-translational protein modification occurred to prevent IFN-gamma secretion from the cell. Immunoblots from alpha-MSH-treated T cells showed higher levels of protein ubiquitination when compared to untreated T cells. Resting T cells treated with alpha-MSH also demonstrated enhanced protein ubiquitination. We found that IFN-gamma is one of the ubiquitinated proteins in the alpha-MSH-treated activated T cells. Our results demonstrate that one of the mechanisms by which alpha-MSH regulates T cell activity is through mediating a change in the pattern of protein ubiquitination in T cells.
Keywords: T lymphocytes, Molecular Biology, Cytokines, Neuroimmunology, Rodent
INTRODUCTION
α-MSH is an evolutionarily conserved neuropeptide of 13 amino acids in length. It is produced by pituitary cells, macrophages, keratinocytes and centrally derived neurons expressing proopiomelanocortin hormone (8,16,17,26,28,34). α-MSH has been found to be an endogenous regulator of inflammation, limiting the inflammatory activity of macrophages and neutrophils induced by IL-1, tumor necrosis factor-α, IFN-γ, and endotoxin by inhibiting the intracellular activation of NF-κB (11,12,19). α-MSH also suppresses the generation of nitric oxide and reactive oxygen intermediates by neutrophils and macrophages, and their attraction to chemokines (5,6,20).
We previously demonstrated that α-MSH suppresses the activation of Th1 cells that mediate DTH and autoimmune disease (23,25,30). Treatment of activated, primed T cells with α-MSH suppresses their production of IFN-γ. In addition, there is enhanced production of TGF-β1 and subsequent generation of CD4+ T regulatory (Treg) cells that suppress other Th1 cells mediating DTH or autoimmune disease (23,30). α-MSH-induced Treg cells require antigen-specific activation, but suppress bystander T cells. α-MSH-induced Treg cells have been used to target specific tissue autoantigens and suppress autoimmune disease (23).
The ability of α-MSH to induce Treg cells depends on the expression of the MC5r, one of 5 known melanocortin receptors (30). MC1r, found on cells throughout the body, is expressed on macrophages, dendritic cells, B cells and CD8+ T cells, but not on CD4+ T cells (1,2,6,24). In comparison the MC5r, expressed on a limited set of lymphoid, muscle, and glandular cells, is expressed on macrophages, B cells and T cells (4,10,30). Both receptors are characterized as G-protein coupled receptors, but ligation activates more than an increase in cAMP production (19,29,33). The MC5r also signals the Jak/STAT pathways (4). Thus the effects of α-MSH induced MC5r signaling pathways are similar to other cytokine receptor signaling pathways that mediate T cell differentiation.
To analyze whether α-MSH has a role in T cell differentiation, we first wanted to determine how α-MSH suppressed IFN-γ production in activated primed T cells. Specifically, we asked what is the role of protein ubiquitination as a unique regulatory mechanism in α-MSH-mediated T cell differentiation. Since α-MSH-treated effector T cells showed similar IFN-γ levels for their mRNA and intracellular protein, we postulated that suppression of IFN-γ was by a post translational modification. One such mechanism suspected to be involved in this process of α-MSH-mediated suppression of T effector cell function was protein ubiquitination.
MATERIALS AND METHODS
Animals
The Institute’s Animal Care and Use Committee approved all animal use in our experiments. All mice used in the experiments were female BALB/c mice, 6 to 8 weeks old. They were obtained from Jackson Laboratory, Bar Harbor, Maine, USA.
Antigen-pulsed APC
Naïve spleen cells from whole spleens were suspended in RPMI 1640 (BioWhittaker, Walkersville MD, USA) with 5% fetal bovine serum (FBS: Hyclone Laboratories, Logan, UT, USA). The RPMI 1640 stock was supplemented with L-glutamine, 10Jg/ml gentamicin (Sigma-Aldrich St. Louis, MO, USA), 0.01M HEPES, 1X non-essential amino acids (NEAA) mixture, and 1mM sodium pyruvate (BioWhittaker). Each well of a 96 flat bottom culture plate was seeded with one million spleen cells and incubated for 90min at 37°C in 5% CO2. The wells were washed 4 times with Serum Free Media (SFM): RPMI 1640 supplemented as above without the FBS, but with a 1/500 dilution of ITS+ (Insulin/Transferrin/Selenium + linoleic acid) (Sigma-Aldrich) and 0.1% sterile BSA solution (Sigma-Aldrich). The cells were incubated for another 30min and washed 2 times with SFM. Desiccated Mycobacterium tuberculosis (MT) antigen (Difco, Detroit, MI, USA), suspended in SFM, was added at 0.3Jg/well to the cultures, and the cultures were incubated overnight at 37°C in 5% CO2.
Activation of primed T cells
Primed T cells were isolated from the popliteal lymph nodes of 6–8 week-old BALB/c mice that were subcutaneously immunized 7 days prior in one foot with 0.5mg of desiccated MT antigen suspension. T cell column-enriched (R&D Systems, Minneapolis, MN, USA) (99% CD3+, flow cytometry) CD3+ cells were incubated for 1 hour at 37°Cand 5% CO2 with or without 300pg/ml α-MSH. The cells were then washed in serum-free media, and 8x106 cells were added to each well already containing antigen-pulsed APC. The cultures were incubated for 24 (RPA), 48 (ELISA), or 72 (ubiquitin immunoblot) hours depending on the experiment. For the experiments examining unstimulated T cells, the APC were not pulsed with antigen.
Interferon-gamma ELISA
IFN-γ production was assayed by using a sandwich ELISA technique on the culture supernatant of stimulated T cells incubated for 48hrs as reported previously (23). Briefly, a 96 well microtiter plate was coated with rat anti-mouse antibody to IFN-γ (BD Pharmingen, San Diego, CA, USA) and incubated overnight at 4°C. After washing and blocking with PBS containing 1% BSA the wells were washed again and sample or standard were applied to the plate and incubated for 3hrs at room temperature. The plate was washed, and biotinylated-detecting antibody (BD Pharmingen, San Diego, CA USA) to IFN-γ was added for 1hr at room temperature and then washed out of the wells. Streptavidin-β-galactosidase (1: 1000) was added to the wells, and the plate was incubated at room temperature for 30min and washed. The substrate chlorophenyl-red-β-D-galactoside was added to the wells and the optical density of the color change was read on a standard ELISA plate reader at a wavelength of 570nm. Sample concentrations were calculated based on interpolated O.D. readings from a standard curve calculated from the O.D. of known IFN-γ concentrations run in parallel to the samples.
RNA analysis
RNA was analyzed by ribonuclease protection assay (RPA). For the RPA, non-adherent cells were collected 24hrs after the T cells were pretreated with α-MSH and antigen activated as described above. The mRNA was purified from the CD3+ T cells by TRI reagent LS (Molecular Research Center, Cincinnati, OH, USA) and DNase I (Ambion, Austin, TX, USA). The cell suspension was homogenized with a 23-gauge needle and left 5min at room temperature. Phase separation was achieved with 200Jl chloroform added to the suspension, vortexed 15s, and incubated for 15min at room temperature. The tubes were spun at 11000 x g for 15min at 4°C. The upper phase of the suspension was added to 500Jl 99% isopropanol and left for 15min at room temperature to precipitate the RNA. After spinning for 11000 x g for 15min at 4°C, the RNA pellet was resuspended with 1 ml of 75% ethanol and spun at 7500x g for 5min at 4°C. The RNA pellet was dissolved with 55Jl of ddH2O, and the RNA was quantified by spectrophotometry (OD 260/280). We used a RPA III kit (Ambion, Austin, TX, USA) with Biotin 16-UTP (Enzo, Germany) to create biotinylated probes. The RPA was performed by hybridizing the biotinylated probes with the isolated mRNA (5Jg) overnight at 56°C. Unhybridized RNA was digested with RNase, and the undigested RNA was denatured and loaded onto a 6% TBE/urea gel (Novex, San Diego, CA, USA) and run at 180V for 75min. The electrophoresed RNA was transferred onto a nylon membrane (Novex) and UV crosslinked. The membrane was blocked, incubated with Streptavidin-alkaline phosphatase, and a chemiluminescent substrate (BrightStar BioDetect, Ambion), and immediately exposed to X-ray film. The film was developed and the image was digitized. The intensity of the bands on the image were quantified by densitometry (NIH Image; National Institutes of Health, Bethesda, MD, USA) and normalized to the L32 band.
Intracellular Immunofluorescence Staining
Activated CD3+ T cells were collected from the cultures, spun down at 800 x g for 10min at room temperature. The cells (2 x 106 cells) were twice spun down and resuspended in 400Jl brefeldin solution (0.01M PBS, 1% BSA, 10Jg/ml brefeldin). After they were spun down for the second time, the cells were resuspended in 100Jl of the PBS/brefeldin solution and 100Jl of 4% paraformaldehyde for 20min at room temperature. The suspension was agitated gently, washed with 200Jl 0.01M PBS, and spun down at 800 x g for 10min. The pellet was resuspended in 50Jl permeabilization buffer (0.01M PBS, 1% BSA, 0.1% Na azide, 0.5% saponin, pH 7.4–7.6) for 10min at room temperature and either 5Jl of anti-IFN-γ (BD Pharmingen), or 2.5Jl of isoFITC (BD Pharmingen), was added. The suspension was incubated for 30min, washed twice with PBS/saponin, and washed once with staining buffer (0.01M PBS, 1% BSA, 0.1% Na azide, pH 7.4–7.6). For surface staining, the cells were resuspended in 50Jl of staining buffer into which was added 2.5Jl of anti-CD4-PE antibody (BD Pharmingen). The cells were incubated for 30min at room temperature, spun down at 800 x g for 10min. The cells were resuspended in 1ml staining buffer and analyzed by multi-color flow cytometry.
Immunoprecipitation and Immunoblot
Protein lysates were collected from T cells treated with α-MSH, activated and incubated for 72hrs as described above. For each group, 30 to 40 x 106 cells were lysed in 200Jl RIPA buffer (1x PBS, 1%NP-40, 0.5% Na deoxycholate, 0.1% SDS containing aprotinin and PMSF). Cell lysates remained in the lysate buffer for 30min at 4°C and then were homogenized with a 21-gauge needle. Additional PMSF was added and the tubes were incubated for another 30min at 4°C. Cell lysates were spun at 10,000 x g for 10min at 4°C. The supernatant was collected and kept on ice. Protein concentration was determined from the supernatant using a Biorad assay kit (Hercules, CA, USA), and equal quantities of protein from each group was individually mixed with 10Jg of primary anti-ubiquitin antibody agarose conjugate (Santa Cruz Biotechnologies, Santa Cruz, CA, USA). The tubes were mixed end-over-end at 4°C overnight.
The beads were washed 4-times with 1x PBS and spun at 1000x g. The beads were suspended in NuPAGE Bis Tris sample buffer (Novex), heated to 70°C for 10min, and applied to a NuPAGE gel (4–12 % Bis Tris) under reducing conditions. The electrophoresed proteins were transferred onto a nitrocellulose membrane. The membrane was washed 2 times with 20ml ddH2O for 5min then blocked for 30min with 20ml of blocking solution (Invitrogen Western Breeze). After blocking, the membrane was washed 2 times with 20ml ddH2O for 5min, and incubated with either monoclonal mouse anti-ubiquitin antibody, FK2 (Affiniti, UK) at1: 1000 or a rat anti-mouse anti-IFN-γ (BD Pharmingen) at 1: 500. The membrane was incubated overnight on a rocking platform at room temperature. Bound primary antibody was detected using a Western Breeze mouse IgG kit (Invitrogen, Carlsbad, CA, USA) for the FK2 antibody or a goat anti-rat IgG-HRP antibody (Sigma) for the secondary antibody in the IFN-γ blot. An ECL chemiluminescent detection kit (Amersham, UK) was used to visualize the bands on radiographic film exposed to the membrane. The bands detected on the digitized image of the X-ray film were quantified by densitometry (NIH Image; National Institutes of Health, Bethesda, MD).
RESULTS
The effects of α-MSH on the production of IFN-γ
Previously we reported that α-MSH suppressed Th1 cell activity via suppression of their IFN-γ production (23,30). To examine the effects of α-MSH on enriched T cells we modified our previous procedure by pre-treating the T cells with α-MSH at 300pg/ml before stimulating the T cells with antigen-pulsed APC. The culture supernatants were collected at 48hrs and assayed for IFN-γ by ELISA. Effector T cells treated with α-MSH released 40–60% less IFN-γ into the culture supernatant than untreated T cells (Fig. 1A). mRNA was analyzed by ribonuclease protection assay for IL-4, IL10, IFN-γ, TGF-β1 and L32 (Fig. 1,B and C). α-MSH treatment did not induce a change in the message for IFN-γ, nor was there a change in message for TGF-β1, a cytokine we previously reported to be induced in T cells by α-MSH (25). The α-MSH induced the conversion of TGF-β from latent to active form rather than an increase in TGF-β mRNA as a mechanism of increasing the production and secretion of active TGF-β. To test for IFN-γ production in CD4+ cells, we also stained the T cells for intracellular IFN-γ. The α-MSH treated and untreated cells were collected 24hrs after activation and subsequently stained for intracellular IFN-γ and surface expression of CD4. Both treated and untreated cells had significantly higher IFN-γ staining than the unactivated CD4+ T cells, but the α-MSH-treated CD4+ T cells had an insignificant reduction intracellular IFN-γ expression in comparison to the untreated activated CD4+ T cells (Fig. 2). The CD4− cells did not stain for IFN-γ (data not shown). These findings further support the postulate that suppression of IFN-γ by α-MSH was through a mechanism that was post translational and interfered with the secretion of IFN-γ.
Fig. 1.
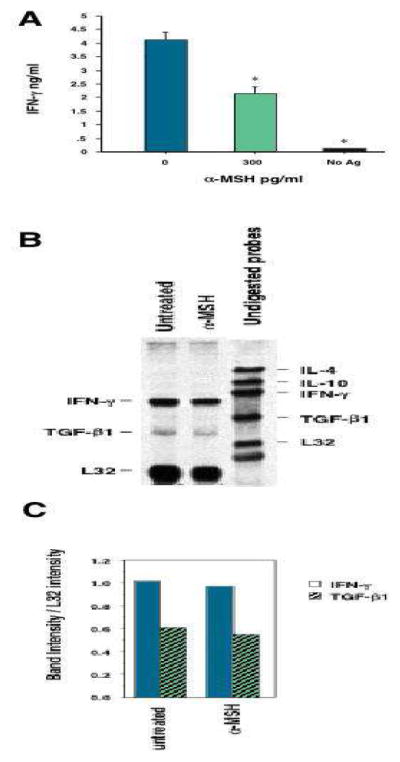
The effects of α-MSH on IFN-γ production by activated primed T cells. (A), Primed T cells (8 x 106 cells/well) were enriched from draining lymph nodes of mice immunized with M. tuberculosis. The T cells were pre-treated with α-MSH (300pg/ml) or without α-MSH (0pg/ml) and were subsequently added to cultures of M. tuberculosis antigen-pulsed APC (1 x 105 cells/well). One culture was primed T cells added to APC not pulsed with antigen (No Ag). The cultures were incubated for 48hrs, and their supernatants were assayed for IFN-γ by ELISA. The results are the average of 4 independent experiments presented as ng/ml ± standard error of the mean. *The production of IFN-γ is significantly (P<0.05) less than the IFN-γ produced by untreated cultures. (B) α-MSH treated or untreated primed T cells were activated by antigen-pulsed APC and cultured for 24hrs. The mRNA of the T cells was isolated and assayed in a chemiluminescent ribonuclease protection assay. The results presented are the digital images of the chemiluminescent bands visualized on x-ray film. Also, the relative intensities of the bands in each lane to L32 were calculated and presented in (C). These results are representative of 3 independent experiments.
Fig. 2.
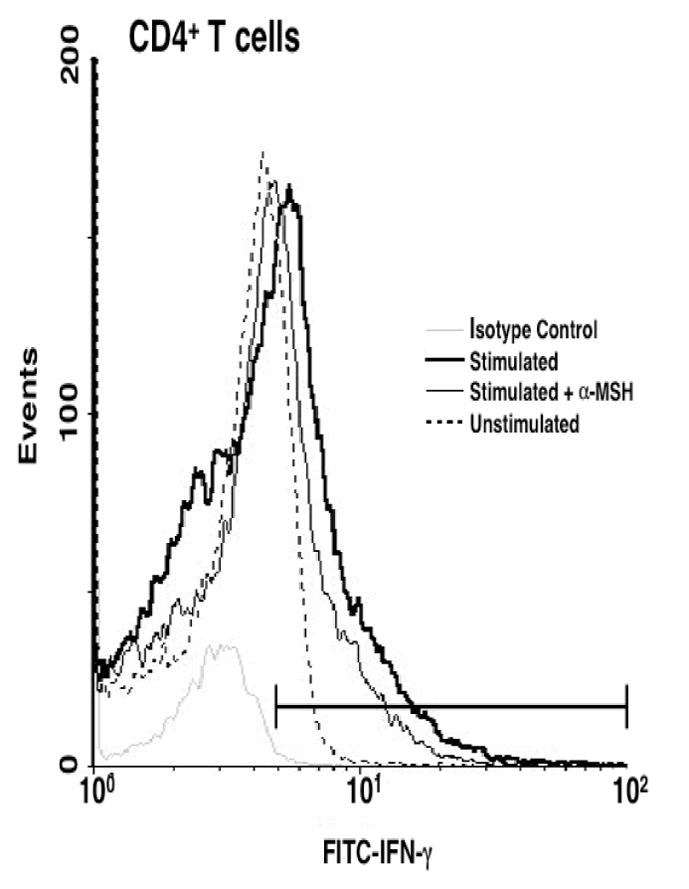
Flow cytometry analysis of intracellular IFN-γ. Primed T cells treated with α-MSH (300pg/ml) or without α-MSH (0pg/ml) were activated for 24hrs prior to staining for intracellular IFN-γ and surface CD4 molecules. The histogram presents the intracellular IFN-γ staining of gated CD4+ T cells. The number of events counted is represented on the ordinate. The abscissa axis in the histogram defines the signals differentiated from the isotype control (stippled line). The percent positive was 5% for unstimulated (dashed line), 29% for untreated (thick line), and 21% for the α-MSH treated (thin line) primed T cells. This figure is representative of 3 independent experiments.
The effect of α-MSH on IFN-γ ubiquitination in activated T cells
To investigate the role of ubiquitination in α-MSH-treated T cells, we lysed the α-MSH-treated T cells and immunoprecipitated the proteins using an anti-ubiquitin antibody. The immunoprecipitated proteins were electrophoresed, transferred to a nitrocellulose membrane, and immunoblotted for ubiquitin. T cells treated with α-MSH had a 2- to 3-fold increase in ubiquitinated proteins between 65 and 206kDa when compared to the levels of ubiquitination in untreated, activated T cells (Fig. 3). This effect is enhanced by the addition of the proteasome inhibitor N-acetyl-L-leucinyl-L-leucinal-L-norleucinal (LLnL) (Sigma) (data not shown). Also, α-MSH-treated unstimulated T cells had upregulated protein ubiquitination (Fig. 3); the proteins ubiquitinated are found to have lower molecular weights (50–80kDa) suggesting these ubiquitinated proteins may either have fewer ubiquitin moieties attacheds at the time they are examined, or alternatively α-MSH induces a different set of ubiquitinated proteins in unstimulated T cells. Therefore α-MSH induces ubiquitination in T cells.
Fig. 3.
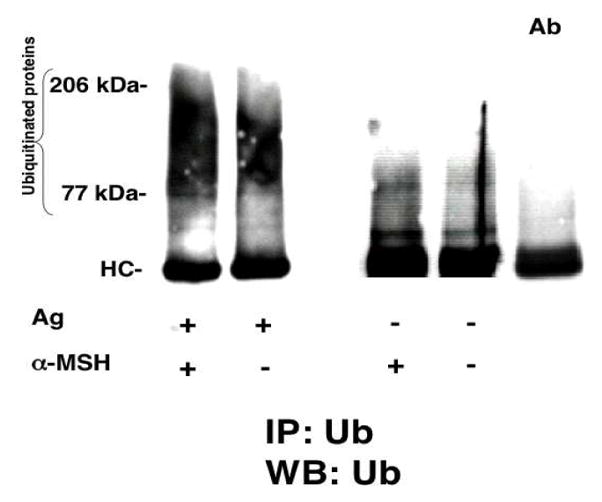
Immunoblot of the effects of α-MSH on protein ubiquitination in primed T cells. Primed CD3+ T cells enriched from draining lymph nodes of immunized mice were pre-treated with or without α-MSH (300pg/ml), washed, and added to in vitro cultures of either antigen-pulsed APC or unactivated APC for 72hrs. The T cells were collected and lysates of the T cells were immunoprecipitated with anti-ubiquitin antibody (FK2, Affiniti, UK), and the proteins were separated on a Nu-PAGE 4–20% gradient gel and transferred to nitrocellulose membranes. The membranes were immunoblotted with FK2 and chemiluminescent bands were visualized on x-ray film. The results presented are the digital image of the x-ray film and are representative of 3 independent experiments. The last column (Ab) contains only FK2 antibody as immunoprecipitate and immunoblot control.
To determine if one of the ubiquitinated proteins in the activated T cell population was IFN-γ, we immunoblotted the ubiquitin immunoprecipitates for mouse IFN-γ protein (Fig. 4A). We found a 3- to 4-fold increase in the ubiquitinated-IFN-γ (77kDa band) in α-MSH-treated T cells when contrasted to untreated T cells (Fig. 4A). As a control we could not detect the 77kDa band in immunoblotted ubiquitinated proteins from α-MSH-treated T cells from IFN-γ knock-out mice (Fig. 4B).
Fig. 4.
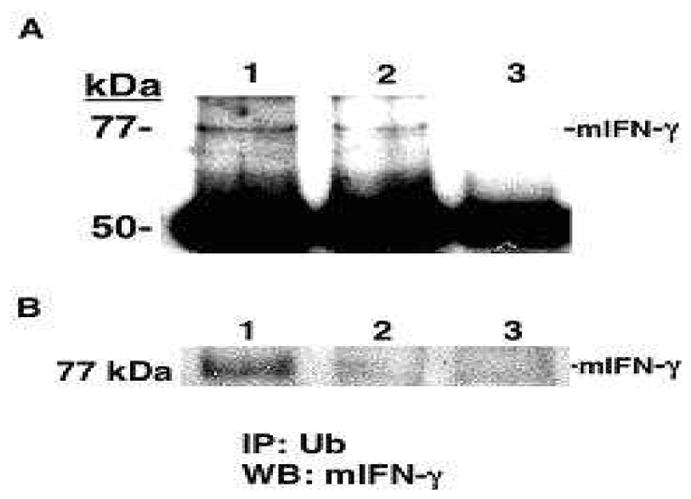
Immunoblot of the effects of α-MSH on IFN-γ ubiquitination in activated primed T cells. (A), The lysates from α-MSH treated (Lane 1) or untreated (Lane 2) activated primed T cells were immunoprecipitated with FK2 and immunoblotted with anti-mouse IFN-γ. Lane 3 is the antibody control for the immunoprecipitation. (B), the lysates from α-MSH treated (Lane 1) or untreated (Lane 2) activated primed T cells from wild type mice were immunoprecipitated with FK2 and immunoblotted with anti-mouse IFN-γ. Lane 3 is the lysate from IFN-γ knock out mice. Shown are the 77kDa bands for mouse IFN-γ. This figure is representative of 3 independent experiments of the same result.
DISCUSSION
We examined the effects of the immunosuppressive neuropeptide α-MSH on the expression of IFN-γ by effector T cells. We found that α-MSH activity did not mediate a specific decrease in IFN-γ synthesis, but rather facilitated a post-translational ubiquitination of IFN-γ. The message levels of IFN-γ were unchanged in activated T cells treated with α-MSH, and intracellular staining demonstrated the presence of intracellular IFN-γ in the treated T cells. Examination of immunoprecipitated ubiquitinated proteins from cellular lysates of α-MSH treated T cells clearly showed a significant increase in protein ubiquitination when compared to untreated activated T cells. The 2- to 3-fold increase of IFN-γ protein from ubiquitin immunoprecipitates of α-MSH-treated T cells suggests that the IFN-γ is targeted for degradation within the cell. Therefore, α-MSH works uniquely through the ubiquitin-proteosome pathway to suppress IFN-γ release by effector T cells.
Based on the increased level of total ubiquitinated proteins observed in α-MSH-treated activated T cells, a number of other proteins must be altered by α-MSH-mediated upregulation of ubiquitination. The other ubiquitinated proteins may also be proinflammatory cytokines, receptors, or responsive elements associated with Th1 responses. In unstimulated T cells, α-MSH upregulates protein ubiquitination suggesting that the effects of α-MSH on T cells is independent of T cell activation pathways. Ubiquitination of inflammatory proteins may be an important regulatory mechanism for immune privileged sites such as the eye where α-MSH is produced constitutively (31). In this scenario, a T cell within an immune privileged site, exposed to α-MSH, should be less conducive to activation and production of IFN-γ.
While this is the first report of an immunomodulatory factor (α-MSH) can induce selective ubiquitination of an inflammatory molecule (IFN-γ) to suppress effector T cell activity, post translational modification is a well known mechanism of intrinsic regulation of an immune response (3,7,9,13,14,18,22,32,36). One example of this involves proteolytic processing that modifies the T cell receptor (TCR) (7,13). Activation-induced post-translational modification of the tails of CD3 chains results in downmodulation of the TCR. This change is characterized by increased retention or degradation of the internalized TCR by ligand stimulation. Cbl proteins, a family of intracellular tyrosine kinase binding proteins are involved in ligand-induced TCR protein ubiquitination and regulate the clearance of activated TCR complexes from the cell surface (18, 32). Inhibitors of tyrosine kinase activation or mutation of the cytoplasmic tails of the CD3 chains are known to counter the clearance of ligand-bound TCR. Protein ubiquitination is also associated with regulating Cbl itself (3,9,36). The costimulation of TCR and CD28, but not TCR stimulation alone, enhances ubiquitin-mediated Cbl degradation in T cell activation. In patients with systemic lupus erythematosus, there is an upregulation of TCR tyrosine kinase ubiquitination in T cells. (14).
Ubiquitin-associated protein degradation also alters the T cells expression of cytokine receptors (15,21,27,35). Receptors with tyrosine kinase domains such as PDGF, have chains that are internalized and degraded following polyubiquitination (21). Ubiquitination is also required for the sorting of the non-tyrosine kinase IL-2 receptor β–chain toward lysosomal degradation (27). Other related non-tyrosine kinase receptors IL-9 and erythropoietin have components that are also ubiquitinated (35). In another example, an F-box protein has been found to be integral to IFN-α dependent ubiquitination of the IFN-α receptor 1 (15). This interaction has downstream effects on the extent of STAT 1 phosphorylation, STAT-mediated transcriptional activities, and the magnitude of the antiproliferative effects of type I interferons.
The regulation of immunity by the ubiquitin-proteosome pathway suggests an important mechanism by which immune cell activity can be rapidly altered before gene expression is affected. The novel finding in this report is that the immunomodulatory factor α-MSH prevalent in the eye may in part regulate immunity, mediate homeostasis, and promote immune privilege through the ubiquitin-proteosome pathway.
Acknowledgments
We thank David Yee and Randy Huang for their technical assistance with the flow cytometric analysis by the Schepens flow cytometry core facility.
This work is supported in part by National Institutes of Health Grants EY13913 (to D.J.B.) and EY10752 (to A.W.T.).
Abbreviations
- α-MSH
Alpha-Melanocyte Stimulating Hormone
- Jak/STAT
Janus kinase/signal transducer and activators of transcription
- MC1r
The melanocortin-1 receptor
- MC5r
melanocortin-5 receptor
- TGF-β1
transforming growth factor beta-1
- Treg
T regulatory
References
- 1.Adachi S, Morii E, Kim D, Ogihara H, Jippo T, Ito A, Lee YM, Kitamura Y. Involvement of mi-transcription factor in expression of alpha-melanocyte-stimulating hormone receptor in cultured mast cells of mice. J Immunol. 2000;164:855–860. doi: 10.4049/jimmunol.164.2.855. [DOI] [PubMed] [Google Scholar]
- 2.Artuc M, Grutzkau A, Luger T, Henz BM. Expression of MC1- and MC5-receptors on the human mast cell line HMC-1. Ann N Y Acad Sci. 1999;885:364–367. doi: 10.1111/j.1749-6632.1999.tb08691.x. [DOI] [PubMed] [Google Scholar]
- 3.Bachmaier K, Krawczyk C, Kozieradzki I, Kong YY, Sasaki T, Oliveira-dos-Santos A, Mariathasan S, Bouchard D, Wakeham A, Itie A, Le J, Ohashi PS, Sarosi I, Nishina H, Lipkowitz S, Penninger JM. Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature. 2000;403:211–216. doi: 10.1038/35003228. [DOI] [PubMed] [Google Scholar]
- 4.Buggy JJ. Binding of alpha-melanocyte-stimulating hormone to its G-protein-coupled receptor on B-lymphocytes activates the Jak/STAT pathway. Biochem J. 1998;331(Pt 1):211–216. doi: 10.1042/bj3310211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cannon JG, Tatro JB, Reichlin S, Dinarello CA. α Melanocyte stimulating hormone inhibits immunostimulatory and inflammatory actions of interleukin 1. J Immunol. 1986;137:2232–2236. [PubMed] [Google Scholar]
- 6.Catania A, Rajora N, Capsoni F, Minonzio F, Star RA, Lipton JM. The neuropeptide alpha-MSH has specific receptors on neutrophils and reduces chemotaxis in vitro. Peptides. 1996;17:675–679. doi: 10.1016/0196-9781(96)00037-x. [DOI] [PubMed] [Google Scholar]
- 7.Cenciarelli C, Hou D, Hsu KC, Rellahan BL, Wiest DL, Smith HT, Fried VA, Weissman AM. Activation-induced ubiquitination of the T cell antigen receptor. Science. 1992;257:795–797. doi: 10.1126/science.1323144. [DOI] [PubMed] [Google Scholar]
- 8.Chakraborty AK, Funasaka Y, Slominski A, Ermak G, Hwang J, Pawelek JM, Ichihashi M. Production and release of proopiomelanocortin (POMC) derived peptides by human melanocytes and keratinocytes in culture: regulation by ultraviolet B. Biochimica et Biophysica Acta. 1996;1313:130–138. doi: 10.1016/0167-4889(96)00063-8. [DOI] [PubMed] [Google Scholar]
- 9.Chiang YJ, Kole HK, Brown K, Naramura M, Fukuhara S, Hu RJ, Jang IK, Gutkind JS, Shevach E, Gu H. Cbl-b regulates the CD28 dependence of T-cell activation. Nature. 2000;403:216–220. doi: 10.1038/35003235. [DOI] [PubMed] [Google Scholar]
- 10.Clarke BL. Binding and processing of (125) I-ACTH by isolated rat splenic lymphocytes. Biochem Biophys Res Commun. 1999;266:542–546. doi: 10.1006/bbrc.1999.1848. [DOI] [PubMed] [Google Scholar]
- 11.Ichiyama T, Sakai T, Catania A, Barsh GS, Furukawa S, Lipton JM. Systemically administered alpha-melanocyte-stimulating peptides inhibit NF-kappa B activation in experimental brain inflammation. Brain Res. 1999;836:31–37. doi: 10.1016/s0006-8993(99)01584-x. [DOI] [PubMed] [Google Scholar]
- 12.Ichiyama T, Sakai T, Catania A, Barsh GS, Furukawa S, Lipton JM. Inhibition of peripheral NF-kappaB activation by central action of alpha-melanocyte-stimulating hormone. J Neuroimmunol. 1999;99:211–217. doi: 10.1016/s0165-5728(99)00122-8. [DOI] [PubMed] [Google Scholar]
- 13.Jang IK, Gu H. Negative regulation of TCR signaling and T-cell activation by selective protein degradation. Curr Opin Immunol. 2003;15:315–320. doi: 10.1016/s0952-7915(03)00048-7. [DOI] [PubMed] [Google Scholar]
- 14.Jury EC, Kabouridis PS, Abba A, Mageed RA, Isenberg DA. Increased ubiquitination and reduced expression of LCK in T lymphocytes from patients with systemic lupus erythematosus. Arthritis Rheum. 2003;48:1343–1354. doi: 10.1002/art.10978. [DOI] [PubMed] [Google Scholar]
- 15.Kumar KG, Tang W, Ravindranath AK, Clark WA, Croze E, Fuchs SY. SCF(HOS) ubiquitin ligase mediates the ligand-induced down-regulation of the interferon-alpha receptor. Embo J. 2003;22:5480–5490. doi: 10.1093/emboj/cdg524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee TH, Lerner AB, Buettner-Janusch V. The isolation and structure of α- and β-melanocyte-stimulating hormones from monkey pituitary glands. J Biol Chem. 1961;236:1390–1394. [PubMed] [Google Scholar]
- 17.Lipton JM, Catania A. Anti-inflammatory actions of the neuroimmunomodulator α-MSH. Immunol Today. 1997;18:140–145. doi: 10.1016/s0167-5699(97)01009-8. [DOI] [PubMed] [Google Scholar]
- 18.Lupher ML, Jr, Rao N, Eck MJ, Band H. The Cbl protooncoprotein: a negative regulator of immune receptor signal transduction. Immunol Today. 1999;20:375–382. doi: 10.1016/s0167-5699(99)01484-x. [DOI] [PubMed] [Google Scholar]
- 19.Manna SK, Aggarwal BB. α-Melanocyte stimulating hormone inhibits the nuclear transcription factor NF-κB activation induced by various inflammatory agents. J Immunol. 1998;161:2873–2880. [PubMed] [Google Scholar]
- 20.Mason MJ, Van Epps D. Modulation of IL-1, Tumor necrosis factor, and C5A-mediated murine neutrophil migration by α-melanocyte-stimulating hormone. J Immunol. 1989;142:1646–1651. [PubMed] [Google Scholar]
- 21.Mori S, Heldin CH, Claesson-Welsh L. Ligand-induced polyubiquitination of the platelet-derived growth factor beta-receptor. J Biol Chem. 1992;267:6429–6434. [PubMed] [Google Scholar]
- 22.Nakajima H, Suzuki K, Iwamoto I. Lineage-specific negative regulation of STAT-mediated signaling by proteolytic processing. Cytokine Growth Factor Rev. 2003;14:375–380. doi: 10.1016/s1359-6101(03)00048-0. [DOI] [PubMed] [Google Scholar]
- 23.Namba K, Kitaichi N, Nishida T, Taylor AW. Induction of regulatory T cells by the immunomodulating cytokines alpha-melanocyte-stimulating hormone and transforming growth factor-beta2. J Leukoc Biol. 2002;72:946–952. [PubMed] [Google Scholar]
- 24.Neumann Andersen G, Nagaeva O, Mandrika I, Petrovska R, Muceniece R, Mincheva-Nilsson L, Wikberg JE. MC(1) receptors are constitutively expressed on leucocyte subpopulations with antigen presenting and cytotoxic functions. Clin Exp Immunol. 2001;126:441–446. doi: 10.1046/j.1365-2249.2001.01604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishida T, Taylor AW. Specific aqueous humor factors induce activation of regulatory T cells. Invest Ophthal Vis Sci. 1999;40:2268–2274. [PubMed] [Google Scholar]
- 26.Rajora N, Ceriani G, Catania A, Star RA, Murphy MT, Lipton JM. α-MSH production, receptors, and influence on neopterin in a human monocyte/macrophage cell line. J Leukoc Biol. 1996;59:248–253. doi: 10.1002/jlb.59.2.248. [DOI] [PubMed] [Google Scholar]
- 27.Rocca A, Lamaze C, Subtil A, Dautry-Varsat A. Involvement of the ubiquitin/proteasome system in sorting of the interleukin 2 receptor beta chain to late endocytic compartments. Mol Biol Cell. 2001;12:1293–1301. doi: 10.1091/mbc.12.5.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Star RA, Rajora N, Huang J, Chavez R, Catania A, Lipton JM. Evidence of autocrine modulation of macrophage nitric oxide synthase by α-MSH. Proc Nat Acad Sci. 1995;90:8856–8860. doi: 10.1073/pnas.92.17.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sukhanov VA, Voronkova IM, Shvets SV, Dyakov VL, Morozova LF. Melanocyte-stimulating hormone (alpha-MSH) inhibits the growth of human malignant melanoma cells with the induction of phosphatidyl inositol and myo-inositol phosphate levels. Biochem Int. 1991;24:625–632. [PubMed] [Google Scholar]
- 30.Taylor A, Namba K. in vitro induction of CD25+ CD4+ regulatory T cells by the neuropeptide alpha-melanocyte stimulating hormone (alpha-MSH) Immunol Cell Biol. 2001;79:358–367. doi: 10.1046/j.1440-1711.2001.01022.x. [DOI] [PubMed] [Google Scholar]
- 31.Taylor AW, Streilein JW, Cousins SW. Identification of alpha-melanocyte stimulating hormone as a potential immunosuppressive factor in aqueous humor. Curr Eye Res. 1992;11:1199–1206. doi: 10.3109/02713689208999545. [DOI] [PubMed] [Google Scholar]
- 32.Thien CB, Scaife RM, Papadimitriou JM, Murphy MA, Bowtell DD, Langdon WY. A mouse with a loss-of-function mutation in the c-Cbl TKB domain shows perturbed thymocyte signaling without enhancing the activity of the ZAP-70 tyrosine kinase. J Exp Med. 2003;197:503–513. doi: 10.1084/jem.20021498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weatherhead B, Logan A. Interaction of alpha-melanocyte-stimulating hormone, melatonin, cyclic AMP and cyclic GMP in the control of melanogenesis in hair follicle melanocytes in vitro. J Endocrinol. 1981;90:89–96. doi: 10.1677/joe.0.0900089. [DOI] [PubMed] [Google Scholar]
- 34.Wintzen M, Gilchrest BA. Proopiomelanocortin, its derived peptides, and the skin. J Invest Dermatol. 1996;106:3–10. doi: 10.1111/1523-1747.ep12326950. [DOI] [PubMed] [Google Scholar]
- 35.Yen CH, Yang YC, Ruscetti SK, Kirken RA, Dai RM, Li CC. Involvement of the ubiquitin-proteasome pathway in the degradation of nontyrosine kinase-type cytokine receptors of IL-9, IL-2, and erythropoietin. J Immunol. 2000;165:6372–6380. doi: 10.4049/jimmunol.165.11.6372. [DOI] [PubMed] [Google Scholar]
- 36.Zhang J, Bardos T, Li D, Gal I, Vermes C, Xu J, Mikecz K, Finnegan A, Lipkowitz S, Glant TT. Cutting edge: regulation of T cell activation threshold by CD28 costimulation through targeting Cbl-b for ubiquitination. J Immunol. 2002;169:2236–2240. doi: 10.4049/jimmunol.169.5.2236. [DOI] [PubMed] [Google Scholar]


