Abstract
Purpose
We have previously found that retinal pigment epithelial (RPE) cells suppressed endotoxin-stimulated macrophages; moreover, it induced expression of anti-inflammatory cytokines. We further assessed the possibility that the RPE is alternatively activating macrophages.
Methods
J774A.1 cells were stimulated with endotoxin and treated with the conditioned media (CM) of RPE, or neuroretinal eyecups from healthy mouse eyes. The supernatant was assayed for IL-1β, TNF-α, IL-6, IL-12(p70), and IL-10, and for nitric-oxide generation. The RPE conditioned media (RPE CM) was absorbed of known soluble factors to identify the factor that augments nitric-oxide generation.
Results
We found the RPE CM suppressed all cytokine production except IL-10, and augmented nitric-oxide generation. The augmented nitric-oxide levels were mediated by RPE derived alpha-melanocyte stimulating hormone (α-MSH).
Conclusions
Healthy RPE not only suppresses inflammatory activity, it promotes an alternative activation of macrophages that can further promote immune privilege.
Keywords: Immune privilege, Immunosuppression, Neuropeptides, Posterior eyecups
INTRODUCTION
The eye is an immune privileged microenvironment where there are evolutionary adapted mechanisms of immunoregulation and immunosuppression to protect the delicate, nonreplicative structure of the eye from the damaging and sight threatening consequences of inflammation.1 This adaptation involves the expression and release of immunomodulating proteins, cytokines, and neuropeptides by the parenchymal cells and neurons that makeup the ocular microenvironment.2 While much has been reported about the soluble immunosuppressive factors found in aqueous humor, the fluid filling the anterior chamber, more is being found about how the cells that line the microenvironment contribute through contact and soluble factors to ocular immune privilege.
Derived from the neural crest, retinal pigment epithelium (RPE) is a mononuclear layer of pigmented cells sandwiched between the choroid and the photoreceptors of the neuroretina. It performs a number of highly specialized functions which are important and necessary for visual processing and survival of the photoreceptor cells.3–8 The RPE tight junctions form the outer blood–retinal barrier to protect the delicate overlying neuroretina from direct contact with the systemic blood circulation of the choroid. This barrier together with the absence of lymphatics in the subretinal space (an area formed when the RPE is separated from the photoreceptors) partly account for the passive mechanisms of immune privilege in the RPE, neuroretina, and the subretinal space.9–12 Examples of active immunosuppressive activity in the posterior segment are the expression of contact and soluble immunomodulating molecules such as the expression of CD95L (Fas ligand) by RPE to induce apoptosis in effector T cells,13,14 the production of several immunosuppressive growth factors and neuropeptides by RPE that suppress T-cell activation,12,15–19 and that intact RPE monolayers can, in a contact dependent manner, induce regulatory activity in naive CD4+ T cells.20,21
The RPE also modulates the activity of macrophages and antigen presenting cells as demonstrated by the placement of foreign antigen into the subretinal space that induces systemic tolerance to the antigen in a phenomenologically similar manner as anterior chamber associated immune deviation (ACAID).16,22 This induction of ACAID-like tolerance is mediated by the constitutive production of transforming growth factor-b2 (TGF-β2) and the TGF-β activating molecule thrombospondin-1 by healthy RPE. In mice with disrupted RPE tight junctions, or thrombospondin-1 knocked-out do not support an ACAID-like tolerance to antigen placed into the subretinal space. The ACAID phenomena are mediated by F4/80 positive macrophages affected by TGF-β. These cells migrate to the spleen and set up a self-perpetuating network of cytokine, chemokines, and immune cells that results in the expansion of antigen-specific regulatory T cells.23 The resident macrophages/microglial cells, while few in number, they are seen in all layers of the neural retina, but usually not in the RPE layer unless the RPE layer or the photoreceptor layer is damaged. The macrophage/microglial cells turnover every 6 months.24–26 Microglial cells isolated from the retina paradoxically produce IL-10 in response to endotoxin and interferon-g stimulation.27 We reported that soluble RPE derived pigmented epithelial growth factor (PEGF) in the conditioned media (CM) of in situ RPE eyecups suppressed IL-12 production while promoting IL-10 production by endotoxin stimulated macrophages.15 These findings suggest that the functionality of retinal macrophage/microglial are tightly regulated in the immune privileged eye even when activated with proinflammatory stimuli.
Therefore, we further examined the potential for the RPE and the neuroretinal (NR) layers to regulate endotoxin-stimulated macrophage functionality, and see if the results can help classify the types of macrophages that could be functioning in the healthy immune privileged eye. We found that the RPE and the NR induced an alternative activation of the macrophages with the possibility that the RPE mediates activation of macrophages that can potentially suppress immunity.
MATERIAL AND METHODS
Reagents, cell lines, and mice
Phenol-red-free (to avoid interference with spectrophotometry) culture medium was either DMEM (Dulbecco’s modified Eagle’s medium, serum-free) or EMEM (Eagle’s minimal essential medium with 0.5% FBS) with both supplemented with 0.01 M HEPES, 1 mM sodium pyruvate, 2 mM L-glutamine, 1% nonessential amino acid (NEAA) (all from Cambrex, Walkersville, MD), 50 μg/mL gentamicin, ITS + 1 liquid media supplement (Sigma, St Louis, MO), and 0.1% bovine serum solution (Sigma). The mouse macrophage cell line was the monocytic leukemic cell line J774A.1 (ATCC, Rockville, MD). Ocular tissues and primary immune cells were from 7 weeks old female C57BL/6J mice from Jackson Laboratories (Bar Harbor, ME). All animal use was approved by the Schepens Institutional Animal Care and Use Committee and adhered to the ARVO Statement for the Use of Animals for Ophthalmic and Vision Research. The antibodies used were anti-pigmented epithelium derived factor (PEDF) (USBiological, Swampscott, MA), anti-TGF-β (R&D Systems, Minneapolis, MN), and from Bachem (Torrance, CA) anti-neuropeptide Y, anti-α-MSH, anti-somatostatin (SOM), and anti-calcitonin gene related peptide (CGRP). The control unimmunized rabbit isotype IgG was from Jackson Immunoresearch (West Grove, PA).
Preparation of RPE eyecup
RPE eyecups were prepared as previously described.15,16,28 Eyes were enucleated from euthanized mice and the eyes were immediately immersed in Ca++/Mg++-free HBSS for 30 min on ice. In serum-free and phenol-red-free DMEM under a dissecting microscope the optic nerve, extraocular muscles, connective tissues, and conjunctiva were excised from each eye. Using a 25-G needle, a perpendicular entry was made posterior to the anterior boundary of the retina and extended circumferentially for 360°. The anterior segment (cornea, iris, and anterior part of sclera together with the lens) were separated and removed from the posterior segment (eyecup). The neuroretina layer was gently peeled away from the underlying RPE monolayer, and individually placed into serum-free and phenol-red-free DMEM in the wells of a 96-well tissue culture plate (Corning, NY). The remaining RPE eyecups (RPE monolayer attached to choroid and sclera) were individually cultured in the wells of a 96-well tissue culture plate in serum-free and phenol-red-free DMEM. In some cases we did not extract the neuoretina, but incubated the whole posterior eyecup segment. For a control the neuroretina was removed as described and the RPE was also removed using surgical microsponges to wipe the RPE cells off Bruch’s membrane leaving only the choriod. Both the eyecups were incubated for 48 h at 37°C and 5% CO2. The CM was collected, assayed, and diluted 1:5 to treat the macrophages.
Quantification of TGF-β produced by the RPE and NR eyecups
To measure TGF-β in the RPE and NR eyecup CM, we assayed the CM using the standard mink lung epithelial cell bioassay we have previously described.16 We assayed for total TGF-β and had to dilute the CM 1:50 for the assay. Specificity of the assay was monitored by treating the CM with neutralizing anti-pan-TGF-β antibody (R&D Systems, Minneapolis, MN).
Multiplex assay for cytokines production by J774 macrophage cells
To assay the production of cytokines by LPS-stimulated J774 macrophage cells treated 48 h with RPE or NR CM we used the BioRad (Hercules, CA) Multiplex Assay system that uses Luminex technology (Austin, TX). We used a prepared array of 5 cytokines associated with an inflammatory response reflecting products of parenchymal cells and activated macrophages. They were IL-1b, TNF-a, IL-6, IL-10, IL-12p70. The J774 macrophages were added to the wells of a flat-bottomed 96-well tissue culture plate at 1.5 × 105 cells per well in serum-free, phenol-red-free DMEM. After 2 h at 5% CO2 and 37°C, the culture medium was replaced with fresh media containing 1 μg/mL of LPS, and diluted (1:5) RPE or NR CM. The macrophage cultures were incubated for another 48 h at 5% CO2 and 37°C, and the culture supernatant was assayed following the instructions provided by BioRad for the multiplex assay. To duplicate wells of 50 μL samples or standards, fluorescent beads covalently coupled with antibodies specific for capturing the cytokines were added and mixed. The beads were washed, and biotinylated detection antibodies were added to form a sandwich of antibodies around their specific cytokine. After incubation, and wash, streptavidin–phycoerythrin (streptavidin-PE) was added. The beads in the individual wells were passed through the Luminex detector. The fluorescent intensity for each individual cytokine bead group was measured. The concentration of each individual cytokine in the sample was determined by comparing the individual cytokine fluorescent intensities of the samples to a standard curve derived from standards containing known amount of the individual cytokines.
Assay for nitric oxide production and neuropeptide neutralization
The production of nitric oxide (NO) by LPS-stimulated J774 macrophages was indirectly assayed in the macrophage culture supernatant using a standard Griess reagent assay. The J774 macrophages were added to the wells of a flat-bottomed 96-well plate at 1.5 × 105 cells per well in serum-free and phenol-red-free DMEM. After 2 h at 5% CO2 and 37°C, the media was replaced with fresh media containing LPS (1 μg/mL), and diluted (1:5) RPE or NR CM. After 24 h incubation at 37°C, 5% CO2, the supernatants were assayed for nitrite by mixing 50 μL of the supernatants with 50 μL of Griess reagent (1% sulfanilamide–0.1% naphthylethylene diamine dihydrochloride in 2% H3PO4, Molecular Probes, Oregon) in wells of a 96-well plate. After 15 min of incubation at room temperature, the plate was read by a spectrophotometric plate reader at a wavelength of 548 nm. The concentration of nitrite in the culture supernatant was then determined by comparison of the absorbance readings to a standard curve of known sodium nitrite concentrations (0–100 μM).
Depletion of CM of specific immunomodulating factors
To neutralize the activity of specific factors, and not significantly alter the CM volume, or introduce exogenous antibodies into the macrophage cultures we use an absorption method that we had done before.29,30 To the CM 1 μg/mL of rabbit anti-TGF-β, anti-neuropeptide Y, anti-α-MSH, anti-PEDF, anti-SOM, or anti-CGRP antibody was added, and unimmunized rabbit isotype IgG was used as a control. The CM with antibody was incubated at room temperature for 60 min, 10 μL of Protein-G coupled sepharose beads (Thermo Scientific, Waltham, MA) was added, and incubated in an end-over-end rotation for an additional 30 min at room temperature. The beads were removed by centrifugation, and the supernatant, depleted CM, was collected for the assays.
Statistical analysis
One-way ANOVA with Bonferroni’s multiple comparison test was used to compare groups and differences between controls and assay conditions as described in the text. Significance was determined when P ≤ 0.05.
RESULTS
The effects of RPE CM on endotoxin stimulated J774 cells
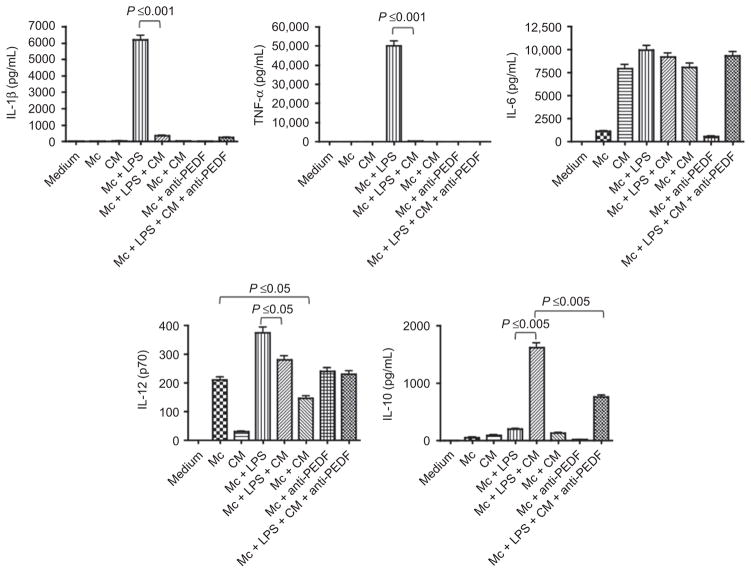
The cytokine analysis of the RPE conditioned media (RPE CM) showed that the eyecups produced the cytokine IL-6, but not proinflammatory IL-1 and TNF, nor the anti-inflammatory cytokine IL-10 (Figure 1). In addition, we found that the RPE CM contains 283 ± 37 ng/mL of total TGF-β (data not shown). Therefore, the RPE eyecups produce a limited set of cytokines detected in our assays that may additionally influence the macrophage response.
Figure 1.
The effects of healthy retinal pigmented epithelial eye cup conditioned media (CM) on J774 macrophage cells (Mc) activated with or without endotoxin (LPS). The macrophage cells were treated with RPE CM plus 1 μg/mL of LPS and the culture supernatant was assayed after 48 h of incubation for IL-1b, TNF-a, IL-6, IL-12p70, and IL-10 using multiplex analysis. The activity of PEDF in the CM was neutralized by depleting PEDF in the CM using the absorption procedure described in the methods. The effects of five different eyecup CM were tested. Presented are mean pg/mL ± standard error of the mean for each cytokine produced by the macrophages or present in the CM. Significance is indicated by P ≤ 0.05 as indicated.
The RPE CM significantly suppressed IL-1β and TNF-α production in endotoxin-stimulated J774 cells (Figure 1). In contrast, the RPE CM significantly induced IL-10 production (Figure 1). The J774 cells spontaneously produce IL-12p40 but this is significantly suppressed by the RPE CM treatment. Interestingly, the RPE CM did not induce a response in the unstimulated (resting) J774 macrophages other than suppress IL-12 production (Figure 1). We previously reported that PEDF was responsible for RPE CM induced IL-10 production in macrophages.15 To see if PEDF in the RPE CM was the inducer of IL-10 production by RPE CM treated J774 cells, we absorbed PEDF from the RPE CM and assayed the PEDF-depleted RPE CM on IL-10 production by the endotoxin-stimulated J774 cells. As expected we found a significant suppression in IL-10 production by the RPE CM treated, endotoxin-stimulated J774 cells (Figure 1). Moreover, PEDF depletion had no influence on the production of other cytokines by the RPE CM-treated endotoxin stimulated J774 cells. These findings show that the RPE secrete soluble mediators that change the pattern of cytokine expression by endotoxin-stimulated macrophage predominately away from a pro-inflammatory phenotype.
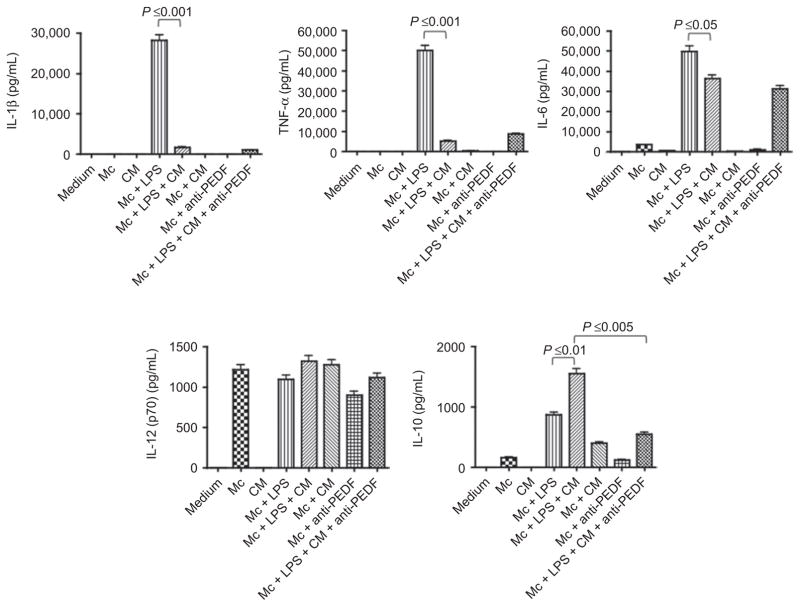
The effects of neural retina eyecup conditioned media (NR CM) on endotoxin stimulated J774 cells
Since the neural retina is a potential source of several immunomodulating neuropeptides and cytokines we assayed the CM of in situ neural retinal layers that were collected by pealing the intact neural retinal layers from the underlying RPE and placing it into the media. The NR CM was used to treat endotoxin-stimulated J774 cells and after 48 h of incubation we assayed the supernatant for IL-1β and TNF-α, IL-10, IL-6, and IL-12p70. We found that unlike the RPE eyecup CM the NR CM did not contain any of the assayed cytokines (Figure 2) except for TGF-β. The NR CM contains 2.1 ± 0.2 ng/mL of TGF-β (data not shown), which is significantly less than the RPE. Similar to RPE CM the NR CM suppressed endotoxin induction of IL-1β and TNF-α, and enhanced IL-10 production that was significantly neutralized by anti-PEDF antibody. In contrast to RPE CM, the NR CM suppressed IL-6 production, and had no influence on constitutive IL-12p70 production by the J774 cells. The differences between the effects of the RPE CM and NR CM are slight with the NR CM like the RPE CM predominately suppressing a proinflammatory pattern of cytokine expression by the endotoxin-stimulated macrophages.
Figure 2.
The effects of healthy neuroretina conditioned media (CM) on J774 macrophage cells (Mc) activated with or without endotoxin (LPS). The macrophage cells were treated with NR CM plus 1 μg/mL of LPS and the culture supernatant was assayed after 48 h of incubation for IL-1b, TNF-a, IL-6, IL-12p70, and IL-10 using multiplex analysis. The activity of PEDF in the CM was neutralized by depleting PEDF in the CM using the absorption procedure described in the methods. The effects of five different eyecup CM were tested. Presented are mean pg/mL ± standard error of the mean for each cytokine produced by the macrophages or present in the CM. Significance is indicated by P ≤ 0.05 as indicated.
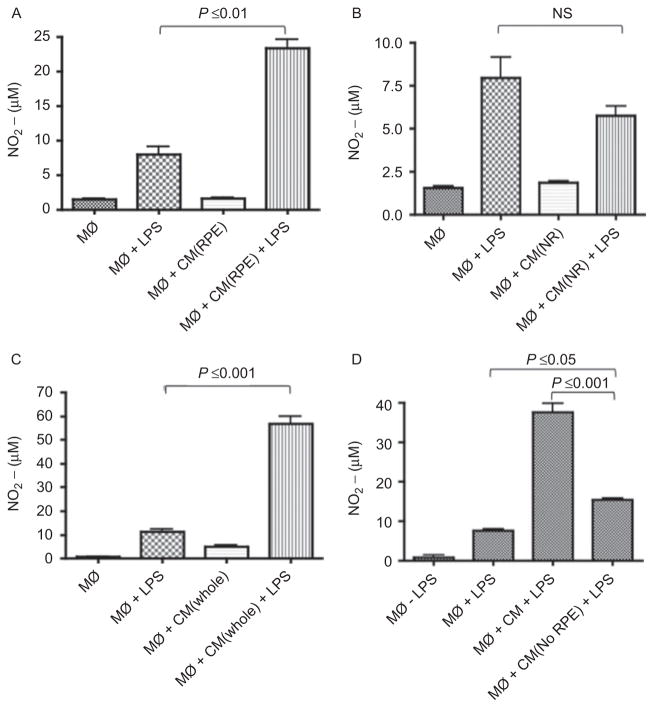
The effects of RPE CM and NR CM on endotoxin-stimulated nitric oxide production
One of the consequences of stimulating macrophages with endotoxin is the induction of NO generation. Our previous publication demonstrated that the CM of the RPE eyecup did not suppress but enhanced NO generation by an endotoxin-stimulated macrophage cell line.15 Therefore, we examined whether this result is reproduced with the endotoxin-stimulated J774 cells, and what is the effect of NR CM on NO generation by the macrophage cell line. We treated endotoxin-stimulated J774 cells with RPE, NR, or whole posterior chamber CM, and after 24 h we assayed the culture supernatant for nitrite by Griess reagent (Figure 3A and 3B). The RPE CM significantly enhanced NO generation in endotoxin-stimulated J774 cells. In contrast, the NR CM had no effect on endotoxin-stimulated NO generation. Neither RPE CM nor NR CM induced NO generation by the unstimulated J774 cells. When the endotoxin-stimulated cells were treated with CM of whole retinal eye cups there was a significant enhancement in NO generation (Figure 3C). The induction of NO generation by the whole retina CM is similar to the effects of the RPE CM on the macrophages, suggesting that promoting NO generation is a mechanism of retinal regulation of macrophage activity, and that there is a soluble RPE factor mediating the induction.. When we depleted the RPE eyecups of all RPE cells, leaving the choroid the CM was unable to enhance NO generation by the endotoxin-stimulated J774 cells (Figure 3D). There is still some stimulating activity by the RPE depleted eyecups. This could be due to the residual RPE cells (1% remaining) or that that the choroid is contributing to the stimulated NO generation, but not as strongly as an intact RPE layer.
Figure 3.
The effects of the conditioned media (CM) on LPS-stimulated nitric oxide (NO) generation. Macrophages were stimulated with LPS and treated as described in Figure 1 and the methods with the CM from (A) RPE, (B) NR, (C) whole retinal eye cups (whole), and (D) RPE eye cups depleted of RPE cells. The 24 h macrophage culture supernatants were assayed with Griess reagent for NO. Presented are the effect of five different eyecup CM on the mean μM ± standard error of the mean of the nitrite generated by treated macrophages. Significant differences and P values are indicated between treatment groups. NS = not significant.
RPE produced α-MSH augments nitric oxide generation by endotoxin-stimulated J774 cells
Since it has already been published that RPE production of TGF-β and thrombospondin-1 are responsible of the induction of ACAID like macrophages,16 and that we previously found that there was a balance between SOM and PEDF that neutralized PEDF suppression of nitric oxide production,15 we evaluated other soluble immunosuppressive molecules that we know are present in the eye and produced by the RPE that could be responsible for the enhanced NO generation by the RPE CM treated J774 cells. The RPE CM was depleted of SOM, PEDF, α-MSH, TGF-β, neuropeptide Y, or CGRP by absorption before the RPE CM was used to treat the endotoxin-stimulated J774 cells. Depleting the RPE CM of TGF-β, CGRP, SOM, PEDF, or neuropeptide Y did not effect the enhancement of NO generation by the RPE-CM (data not shown). Only when we depleted the RPE CM of α-MSH did we significantly neutralize RPE CM augmentation of NO generation by endotoxin-stimulated J774 cells (Figure 4). We also noted that without α-MSH the CM was suppressive of endotoxin-stimulated NO generation (Figure 4). Therefore, RPE produces at least one soluble factor, α-MSH, to mediate an alternative activation of endotoxin-stimulated macrophages.
Figure 4.
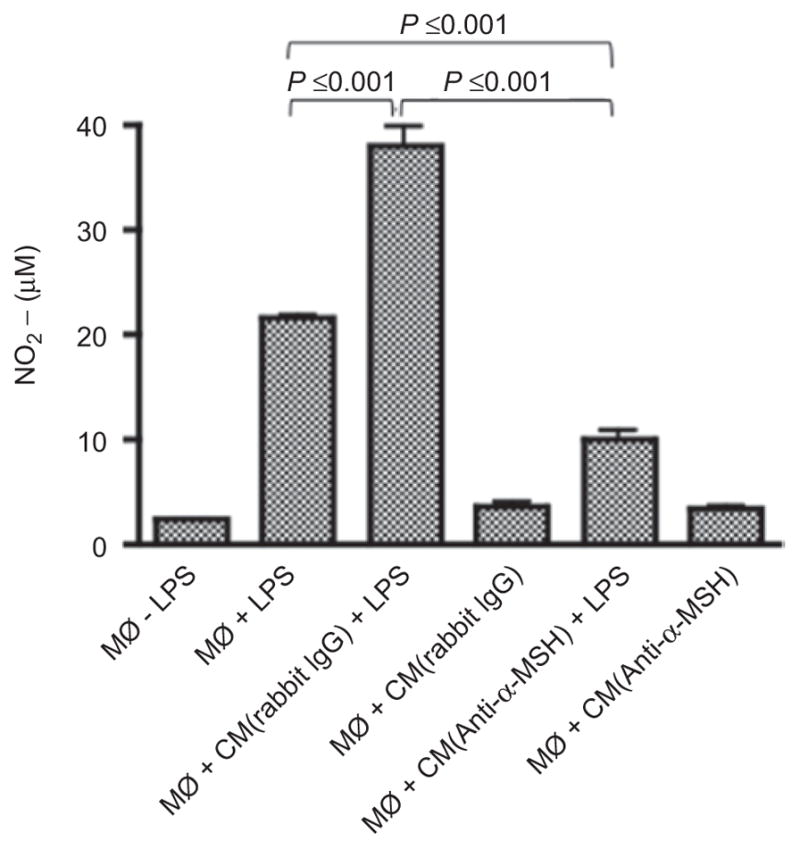
The neuropeptide a-MSH is the soluble factor in RPE CM enhanced NO production by LPS-stimulated macrophages. The experimental methods were the same as in Figure 1 for preparing the RPE CM; however, the RPE CM was depleted of a-MSH (CM[anti-a-MSH]) using the absorption procedure described in the methods. Control for the absorption procedure was done using unimmunized rabbit IgG (CM[rabbit IgG]). Presented are the results of one representative experiment repeated twice with similar relative changes in NO generation as the mean nitrite (μM) ± standard deviation from two different RPE eyecup preparations per group.
DISCUSSION
Characterization of the role of RPE in mediating immune regulation has been considered for sometime. It has been demonstrated that the posterior segment of the eye is immune privileged. The placement of foreign antigen into the subretinal space induces immune deviation that is similar to the mechanism of ACAID. Like ACAID posterior induced systemic immune tolerance involves antigen uptake and processing by a F4/80 positive antigen presenting macrophages that migrate to the spleen.9,31 The induction of these tolerating antigen-presenting macrophages is through RPE produced TGF-β and thrombospondin-1.16 While abundant amounts of TGF-β were present in the CM, we could not demonstrate a role for TGF-β in RPE mediated alternatively activated J774 macrophage cells. Some RPE mediated regulation of immunity involves direct contact induction of naive regulatory T cells through galectin-118,20,21; however, RPE regulation of macrophages, innate immune cells, is through soluble factors. It has been previously demonstrated that RPE derived PEDF can suppress endotoxin-induced IL-12 and NO production while promoting production of IL-10 by the monocytic leukemic cell line RAW 264.7.15 It was found that the effects of PEDF on NO generation was neutralized by SOM: however, neutralization of SOM did not affect the augmentation of NO generation by RPE CM treated J774 cells. While we saw RPE CM suppression of the constitutive IL-12 production by the endotoxin-simulated J774 cells, we could not link this suppression to PEDF. These are possibly revealing a difference in the PEDF and SOM response between the two cells lines. As was demonstrated before with the RAW cells, the PEDF released into both the NR CM, and the RPE CM does mediated IL-10 production by the endotoxin-stimulated J774 cells.
We found that RPE CM and NR CM suppressed IL-1β and TNF-α production by the endotoxin-stimulated J774 cells. This was an expected result since suppression of proinflammatory cytokine production is a consistent feature of ocular immune privilege. What was unexpected was finding the RPE CM enhancing NO generation by the endotoxin-stimulated J774 cells, while the same soluble RPE factors suppressed proinflammatory cytokines and promoted anti-inflammatory IL-10 production by the stimulated macrophages. We were able to demonstrate that the activity enhancing the generation of NO is from the RPE, and that it is the neuropeptide α-MSH. The finding of α-MSH being a mediator of NO generation is just as surprising, since most of the literature characterizes α-MSH neuroimmunomodulation as anti-inflammatory, suppressing endotoxin, IL-1, and TNF-α-stimulated proinflammatory functionalities in macrophages while promoting IL-10 production.32,33 The enhanced NO generation by α-MSH in the RPE CM, suggests that α-MSH is part of an RPE mediated mechanism that potentially promotes an alternative activation of macrophages in the retina under immune privileged conditions.
The types of activation signals macrophages receive can polarized their functionality and associated patterns of cytokines, chemokines, and enzymes.34–36 The stimulation of macrophages with endotoxin as we did in this manuscript is considered a classical activation that polarizes the macrophages to a M1 response. This is the response that we get when the endotoxin-stimulated J774 cells were not treated with the RPE CM or the NR CM. When the cells were treated with RPE CM or NR CM the cells had a cytokine pattern that is more like the M2 or alternatively activated macrophages. However, there is no evidence that we have activated the J774 cells through known M2 stimulating cytokines IL-4 and IL-13. While an M2 (Th2 stimulating) macrophage response is a reasonable expectation of activated macrophages in the normal immunosuppressive microenvironment, the simultaneous enhancement of Nitric Oxide generation; however, does not line up clearly with the descriptions of M2 cell subsets.35
What we discovered is that the RPE treated macrophages expressed NOS2 along with IL-10 production and some constitutive IL-12 and IL-6 production. These macrophages have the characteristics that are similar to myeloid suppressor cells37; however, such a functionality is not possible to demonstrate with J774 cells.38 It is speculated that suppressor macrophages induce apoptosis in adjacent activated T cells by nitrating tyrosine or cysteine residues on signaling proteins in the T cells.39 This enhanced nitrating activity is caused by the enhanced oxidative activity within the suppressor macrophage. Also, because arginine is the substrate for NOS2 the active suppressor macrophages can locally deplete arginine levels, further stressing adjacent T cells. The activation of such suppressor cells can happen through IL-6 and Toll-like receptor stimulation,40,41 and our results suggest that if such cells are activated in the retina this would be promoted by α-MSH, a neuropeptide constitutively expressed within the immune privileged ocular microenvironment.30
While it is to be seen whether myeloid suppressor cells are in residence in the retina, the possibility that they are induced within the normal immune privileged ocular microenvironment is very interesting. The ability of the RPE and the NR to suppress proinflammatory activity of endotoxin-stimulated macrophages is important in controlling the immune response. The potential for the RPE to mediate the induction of myeloid suppressor cells would further demonstrate the ability of immune privileged tissues to manipulate immunity to turn on itself for regulation. This means that there is a possibility that three potential types of macrophages are induced within the healthy posterior segment of the eye: a tolergenic, ACAID-mediating macrophage associated with RPE produced TGF-β and thrombospondin; a M2 macrophage associated with PEDF; and a potential suppressor or alternatively activated macrophage induced by α-MSH.
It is known that localized immunosuppression within the anterior chamber of the eye is mediated by the constitutive presence of specific neuropeptides. Our new findings further support the role of neuropeptides in modulating immunity throughout the ocular microenvironment, and that the localized regulation of immunity within the ocular microenvironment is a result of active mechanisms that engage and manipulate immune cells to regulate themselves.
Acknowledgments
Supported in part by grants from NIH EY10752, DOD W81XWH-04-1-0892, and the Massachusetts Lion’s Eye Research Fund.
Footnotes
Declaration of interest: The authors report no conflict of interest.
References
- 1.Streilein JW. Ocular immune privilege: the eye takes a dim but practical view of immunity and inflammation. J Leukoc Biol. 2003;74(2):179–185. doi: 10.1189/jlb.1102574. [DOI] [PubMed] [Google Scholar]
- 2.Taylor AW. Ocular immunosuppressive microenvironment. Chem Immunol Allergy. 2007;92:71–85. doi: 10.1159/000099255. [DOI] [PubMed] [Google Scholar]
- 3.Bok D. Evidence for an inflammatory process in age-related macular degeneration gains new support. Proc Natl Acad Sci USA. 2005;102(20):7053–7054. doi: 10.1073/pnas.0502819102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boulton M, Dayhaw-Barker P. The role of the retinal pigment epithelium: topographical variation and ageing changes. Eye. 2001;15(Pt 3):384–389. doi: 10.1038/eye.2001.141. [DOI] [PubMed] [Google Scholar]
- 5.Steinberg RH. Interactions between the retinal pigment epithelium and the neural retina. Doc Ophthalmol. 1985;60(4):327–346. doi: 10.1007/BF00158922. [DOI] [PubMed] [Google Scholar]
- 6.Finnemann SC. Focal adhesion kinase signaling promotes phagocytosis of integrin-bound photoreceptors. Embo J. 2003;22(16):4143–4154. doi: 10.1093/emboj/cdg416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gal A, Li Y, Thompson DA, et al. Mutations in MERTK, the human orthologue of the RCS rat retinal dystrophy gene, cause etinitis pigmentosa. Nat Genet. 2000;26(3):270–271. doi: 10.1038/81555. [DOI] [PubMed] [Google Scholar]
- 8.Strauss O, Stumpff F, Mergler S, et al. The Royal College of Surgeons rat: an animal model for inherited retinal degeneration with still unknown genetic defect. Acta Anat (Basel) 1998;162(2–3):101–111. doi: 10.1159/000046474. [DOI] [PubMed] [Google Scholar]
- 9.Jiang LQ, Jorquera M, Streilein JW. Subretinal space and vitreous cavity as immunologically privileged sites for retinal llografts. Invest Ophthalmol Vis Sci. 1993;34(12):3347–3354. [PubMed] [Google Scholar]
- 10.Wenkel H, Streilein JW. Evidence that retinal pigment epithelium functions as an immune-privileged tissue. Invest Ophthalmol Vis Sci. 2000;41(11):3467–3473. [PubMed] [Google Scholar]
- 11.Ng TF, Osawa H, Hori J, et al. Allogeneic neonatal neuronal retina grafts display partial immune privilege in the subcapsular space of the kidney. J Immunol. 2002;169(10):5601–5606. doi: 10.4049/jimmunol.169.10.5601. [DOI] [PubMed] [Google Scholar]
- 12.Streilein JW, Ma N, Wenkel H, et al. Immunobiology and privilege of neuronal retina and pigment epithelium transplants. Vision Res. 2002;42(4):487–495. doi: 10.1016/s0042-6989(01)00185-7. [DOI] [PubMed] [Google Scholar]
- 13.Griffith TS, Ferguson TA. The role of FasL-induced apoptosis in immune privilege. Immunol Today. 1997;18(5):240–244. doi: 10.1016/s0167-5699(97)81663-5. [DOI] [PubMed] [Google Scholar]
- 14.Jorgensen A, Wiencke AK, la Cour M, et al. Human retinal pigment epithelial cell-induced apoptosis in activated T cells. Invest Ophthalmol Vis Sci. 1998;39(9):1590–1599. [PubMed] [Google Scholar]
- 15.Zamiri P, Masli S, Streilein JW, et al. Pigment epithelial growth factor suppresses inflammation by modulating macrophage activation. Invest Ophthalmol Vis Sci. 2006;47(9):3912–3918. doi: 10.1167/iovs.05-1267. [DOI] [PubMed] [Google Scholar]
- 16.Zamiri P, Masli S, Kitaichi N, et al. Thrombospondin plays a vital role in the immune privilege of the eye. Invest Ophthalmol Vis Sci. 2005;46(3):908–919. doi: 10.1167/iovs.04-0362. [DOI] [PubMed] [Google Scholar]
- 17.Tanihara H, Yoshida M, Matsumoto M, et al. Identification of transforming growth factor-beta expressed in cultured human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1993;34(2):413–419. [PubMed] [Google Scholar]
- 18.Ishida K, Panjwani N, Cao Z, et al. Participation of pigment epithelium in ocular immune privilege. 3. Epithelia cultured from iris, ciliary body, and retina suppress T-cell activation by partially non-overlapping mechanisms. Ocul Immunol Inflamm. 2003;11(2):91–105. doi: 10.1076/ocii.11.2.91.15914. [DOI] [PubMed] [Google Scholar]
- 19.Gregerson DS, Heuss ND, Lew KL, et al. Interaction of retinal pigmented epithelial cells and CD4 T cells leads to T-cell anergy. Invest Ophthalmol Vis Sci. 2007;48(10):4654–4663. doi: 10.1167/iovs.07-0286. [DOI] [PubMed] [Google Scholar]
- 20.Sugita S, Keino H, Futagami Y, et al. B7+ iris pigment epithelial cells convert T cells into CTLA-4+, B7-expressing CD8+ regulatory T cells. Invest Ophthalmol Vis Sci. 2006;47(12):5376–5384. doi: 10.1167/iovs.05-1354. [DOI] [PubMed] [Google Scholar]
- 21.Sugita S, Ng TF, Lucas PJ, et al. B7+ iris pigment epithelium induce CD8+ T regulatory cells; both suppress CTLA-4+ T cells. J Immunol. 2006;176(1):118–127. doi: 10.4049/jimmunol.176.1.118. [DOI] [PubMed] [Google Scholar]
- 22.Wenkel H, Streilein JW. Analysis of immune deviation elicited by antigens injected into the subretinal space. Invest Ophthalmol Vis Sci. 1998;39(10):1823–1834. [PubMed] [Google Scholar]
- 23.Streilein JW. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat Rev Immunol. 2003;3(11):879–889. doi: 10.1038/nri1224. [DOI] [PubMed] [Google Scholar]
- 24.Kezic J, McMenamin PG. Differential turnover rates of monocyte-derived cells in varied ocular tissue microenvironments. J Leukoc Biol. 2008;84(3):721–729. doi: 10.1189/jlb.0308166. [DOI] [PubMed] [Google Scholar]
- 25.Kezic J, Xu H, Chinnery HR, et al. Retinal microglia and uveal tract dendritic cells and macrophages are not CX3CR1 dependent in their recruitment and distribution in the young mouse eye. Invest Ophthalmol Vis Sci. 2008;49(4):1599–1608. doi: 10.1167/iovs.07-0953. [DOI] [PubMed] [Google Scholar]
- 26.Xu H, Chen M, Mayer EJ, et al. Turnover of resident retinal microglia in the normal adult mouse. Glia. 2007;55(11):1189–1198. doi: 10.1002/glia.20535. [DOI] [PubMed] [Google Scholar]
- 27.Broderick C, Duncan L, Taylor N, et al. IFN-gamma and LPS-mediated IL-10-dependent suppression of retinal microglial activation. Invest Ophthalmol Vis Sci. 2000;41(9):2613–2622. [PubMed] [Google Scholar]
- 28.Zamiri P, Zhang Q, Streilein JW. Vulnerability of allogeneic retinal pigment epithelium to immune T-cell-mediated damage in vivo and in vitro. Invest Ophthalmol Vis Sci. 2004;45(1):177–184. doi: 10.1167/iovs.03-0211. [DOI] [PubMed] [Google Scholar]
- 29.Taylor AW, Streilein JW, Cousins SW. Immunoreactive vasoactive intestinal peptide contributes to the immunosuppressive activity of normal aqueous humor. J Immunol. 1994;153(3):1080–1086. [PubMed] [Google Scholar]
- 30.Taylor AW, Streilein JW, Cousins SW. Identification of alpha-melanocyte stimulating hormone as a potential immunosuppressive factor in aqueous humor. Curr Eye Res. 1992;11(12):1199–1206. doi: 10.3109/02713689208999545. [DOI] [PubMed] [Google Scholar]
- 31.Sonoda KH, Sakamoto T, Qiao H, et al. The analysis of systemic tolerance elicited by antigen inoculation into the vitreous cavity: vitreous cavity-associated immune deviation. Immunology. 2005;116(3):390–399. doi: 10.1111/j.1365-2567.2005.02239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luger TA, Scholzen TE, Brzoska T, et al. New insights into the functions of alpha-MSH and related peptides in the immune system. Ann N Y Acad Sci. 2003;994:133–140. doi: 10.1111/j.1749-6632.2003.tb03172.x. [DOI] [PubMed] [Google Scholar]
- 33.Lipton JM, Catania A. Anti-inflammatory actions of the neuroimmunomodulator alpha-MSH. Immunology Today. 1997;18(3):140–145. doi: 10.1016/s0167-5699(97)01009-8. [DOI] [PubMed] [Google Scholar]
- 34.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3(1):23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 35.Mantovani A, Sica A, Sozzani S, et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25(12):677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 36.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagaraj S, Gabrilovich DI. Myeloid-derived suppressor cells. Adv Exp Med Biol. 2007;601:213–223. doi: 10.1007/978-0-387-72005-0_22. [DOI] [PubMed] [Google Scholar]
- 38.Youn JI, Nagaraj S, Collazo M, et al. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181(8):5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5(8):641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 40.Delano MJ, Scumpia PO, Weinstein JS, et al. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J Exp Med. 2007;204(6):1463–1474. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bunt SK, Yang L, Sinha P, et al. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 2007;67(20):10019–10026. doi: 10.1158/0008-5472.CAN-07-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]